Hong Kong Med J 2023 Oct;29(5):421–31 | Epub 19 Oct 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Cutaneous manifestations, viral load, and prognosis among hospitalised patients with COVID-19: a cohort study
Christina SM Wong, MRCP, FRCP1 #; Ivan FN Hung, MD, FRCP2 #; Mike YW Kwan, MSc (Applied Epidemiology), FHKAM (Paediatrics)3; Martin MH Chung, MRCP, FHKAM (Medicine)1; Mandy WM Chan, MRCP, FHKAM (Medicine)1; Adrian KC Cheng, MRCP, FHKAM (Medicine)1; YM Lau, MB, BS, MRCP1; CK Yeung, MD, FRCP1; Henry HL Chan, PhD, FRCP1; CS Lau, MD, FRCP4
1 Division of Dermatology, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
2 Division of Infectious Diseases, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
3 Paediatric Infectious Disease Unit, Department of Paediatrics and Adolescent Medicine, Princess Margaret Hospital, Hong Kong SAR, China
4 Division of Rheumatology and Clinical Immunology, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
# Equal contribution
Corresponding author: Prof Christina SM Wong (wongsm11@hku.hk)
Abstract
Introduction: Various cutaneous manifestations
have been reported as symptoms of coronavirus
disease 2019 (COVID-19), which may facilitate
early clinical diagnosis and management. This study
explored the incidence of cutaneous manifestations
among hospitalised patients with COVID-19 and
investigated its relationships with viral load, co-morbidities,
and outcomes.
Methods: This retrospective study included
adult patients admitted to a tertiary hospital for
COVID-19 from July to September 2020. Clinical
information, co-morbidities, viral load (cycle
threshold [Ct] value), and outcomes were analysed.
Results: In total, 219 patients with confirmed
COVID-19 were included. Twenty patients presented
with new onset of rash. The incidence of new rash was
9.1% (95% confidence interval=6.25%-14.4%). The
most common manifestations were maculopapular
exanthem (n=6, 42.9%, median Ct value: 24.8),
followed by livedo reticularis (n=4, 28.6%, median
Ct value: 21.3), varicella-like lesions (n=2, 14.3%,
median Ct value: 19.3), urticaria (n=1, 7.1%, median
Ct value: 14.4), and acral chilblain and petechiae
(n=1, 7.1%, median Ct value: 33.1). The median
Ct values for patients with and without rash were
22.9 and 24.1, respectively (P=0.58). There were no
significant differences in mortality or hospital stay
between patients with and without rash. Patients with
rash were more likely to display fever on admission
(P<0.01). Regardless of cutaneous manifestations,
patients with older age, hypertension, and chronic
kidney disease stage ≥3 had significantly higher viral load and mortality (P<0.05).
Conclusion: This study revealed no associations
between cutaneous manifestation and viral load
or clinical outcomes. Older patients with multiple
co-morbidities have risks of high viral load and
mortality; they should be closely monitored.
New knowledge added by this study
- Patients with coronavirus disease 2019 (COVID-19) could display various cutaneous manifestations. The incidence of new rash in our cohort was 13.6%. The most common manifestation attributed to COVID-19 was maculopapular exanthem, followed by livedo reticularis.
- Informal extrapolation of our results to the general population in Hong Kong suggested that 0.91% solely involve rash presentation; these patients would remain undiagnosed without severe acute respiratory syndrome coronavirus 2 testing. This lack of diagnosis is a potential health threat and could facilitate viral spread.
- Rash is self-limiting in patients with COVID-19, potentially because of a more robust immune response among patients with rash.
- Older patients with multiple co-morbidities should undergo early screening and receive close monitoring if they develop symptoms of COVID-19; early treatment beginning at symptom onset can improve clinical outcomes.
Introduction
Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2), was first identified in December
2019 in Wuhan, Hubei, China.1 2 According to World
Health Organization Coronavirus (COVID-19)
data, as of 7 May 2022, 188 countries and territories
had reported more than 510.2 million cumulative
confirmed cases and more than 6.23 million deaths3;
in Hong Kong, there were 330 670 confirmed cases
and 9308 (2.81%) deaths.4 Common symptoms of
COVID-19 include fever, sore throat, cough, malaise,
dyspnoea, and anosmia or aguesia.1 Although most
people have mild symptoms, some develop acute
respiratory distress syndrome, which may lead to
cytokine storm, multiorgan failure, septic shock, and
even death.5
There is evidence that rash is an early symptom or
the only symptom in patients who are ‘asymptomatic’
or paucisymptomatic.6 7 8 9 Early detection of this
‘silent’ sign and corresponding diagnosis are
important for epidemiologic management because
asymptomatic or paucisymptomatic cases may
function as sources of community spread. Various
dermatologic manifestations of COVID-19 have
been reported including maculopapular eruption,
urticarial eruption, livedo reticularis, pernio/chilblain, vasculitis, vesicular eruption, and papulo-necrotic
eruption.10 11 12 13 14 15 The incidences of cutaneous
manifestations in patients with COVID-19 have
varied among case series (from 0.2% to 20.4%10 11 12 13),
possibly because of the under-recognition of
asymptomatic or paucisymptomatic cases.
The spread of SARS-CoV-2 mainly involves
droplets; it can also occur via direct contact and
is speculated to occur through faecal excretion.2
The primary target of SARS-CoV-2 is the upper
respiratory mucosa, where angiotensin-converting
enzyme 2 (ACE2) serves as a functional receptor
for viral spikes and eventual viral entry into host
cells. Gene expression of the SARS-CoV-2 cellular
receptor ACE2 has been demonstrated in multiple
human tissues, including skin and adipose tissue.16 17 18
Therefore, the proposed mechanisms by which
SARS-CoV-2 affect cutaneous tissues include
direct attacks on epidermal basal cells and vascular
endothelial cells (possibly targeting ACE2 expressed
on skin keratinocytes) and indirect impacts through
the antiviral inflammatory response.16 17 18
There is speculation that patients with rash
occurrence may have a better prognosis because
they display better antiviral immunity.19 Early in
the COVID-19 pandemic, little was known about
relationships among cutaneous manifestations, viral
load, co-morbidities, and clinical outcomes. A recent
systematic review showed inconclusive results about
the relationship between COVID-19 severity and
viral load; however, it suggested that older age and
higher SARS-CoV-2 viral load were directly related.20
Likewise, some rashes such as maculopapular rash
and chilblain-like lesions were found to be strongly
associated with paucisymptomatic disease course
and lower severity of COVID-19 while skin changes
such as acro-ischaemia, livedo reticularis and
purpura may be useful indicators of higher severity
of COVID-19.21 22 In 2020, according to the Public
Health Ordinance of Hong Kong, all patients with
SARS-CoV-2–positive test results were hospitalised
for quarantine, regardless of symptoms.4 Here,
we explored the incidences and patterns of
clinical and cutaneous manifestations among
hospitalised patients with confirmed COVID-19,
then investigated associations with viral load, co-morbidities,
and prognosis.
Methods
This retrospective cohort study was conducted from
1 July to 30 September 2020 in an acute tertiary
hospital, Queen Mary Hospital (ie, a major public
hospital within one of seven hospital clusters)
serving one-fifth of the population of 7.5 million in
Hong Kong. Electronic hospital records were used
to identify adult patients aged ≥18 years who were
admitted during the study period for suspected
COVID-19.
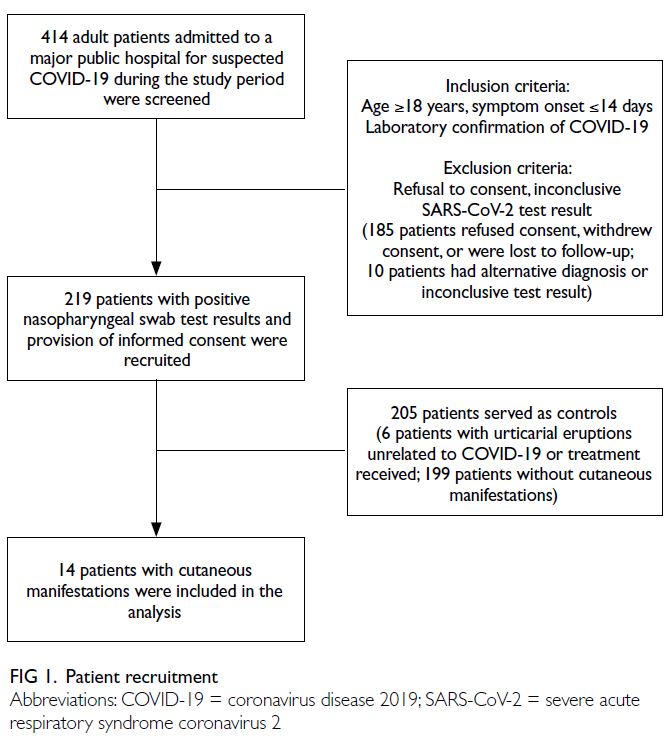
The flow of patient recruitment is illustrated
in Figure 1. Patients included in this study were
adults with laboratory confirmation of COVID-19
by real-time reverse transcription polymerase chain
reaction (rRT-PCR) assay from a nasopharyngeal
swab. Clinical information was collected from
electronic clinical photographs of patients who had
provided informed consent to receive treatment.
A physical examination was performed by a
dermatologist within 48 hours of rash onset to
confirm clinical signs; follow-up was conducted
monthly until 3 months after discharge. Rashes were
considered COVID-19–related if they were new,
could not be explained by the patient’s previous
or pre-existing skin conditions or an alternative
diagnosis (eg, drug eruption or other viral exanthem
of varicella, parvovirus, enterovirus, influenza,
parainfluenza, adenovirus, or respiratory syncytial
virus detected in nasopharyngeal swab [performed
as clinically indicated and excluded]), occurred
along with the SARS-CoV-2–positive rRT-PCR
test results, and resolved when other symptoms
improved.
Clinical and laboratory data
Clinical and laboratory data, including patient
demographics, initial COVID-19 viral load according
to cycle threshold (Ct) value, treatment received,
co-morbidities (diabetes mellitus, hypertension, and
chronic kidney disease [CKD]), and pre-existing skin
diseases, were retrieved from electronic medical
records for analysis. For the detection of viral
nucleic acids, rRT-PCR is considered a gold standard
diagnostic assay. The Ct value refers to the number
of rRT-PCR cycles needed to amplify viral RNA to a
detectable level; it is inversely related to viral load.23
Thus, the Ct value can indicate the relative quantity
of viral RNA in a specimen (lower Ct values reflect
greater quantities of viral RNA). In this study, Ct
values of <26, 26-30, and ≥31 were regarded as high,
intermediate, and low viral load, respectively.24 25
Statistical analysis
Continuous variables were expressed as medians
(interquartile ranges) or means (± standard
deviations), as appropriate. The Mann-Whitney U
test and Kruskal-Wallis test were used to compare
median values between two groups and among
≥3 groups, respectively. Categorical variables,
expressed as proportions, were compared using the
Chi squared test or Fisher’s exact test, as appropriate.
To identify factors independently associated
with outcomes, variables with P values <0.1 in
univariate analyses were subsequently entered into
binary logistic regression multivariate analyses; odds
ratios (ORs) and 95% confidence intervals (CIs) were
calculated. All statistical analyses were performed using SPSS (Windows version 26.0; IBM Corp,
Armonk [NY], United States). Two-tailed P values
<0.05 were considered statistically significant.
Results
From 1 July to 30 September 2020, 414 patients
with suspected COVID-19 were admitted to our
hospital. This study included 219 patients who
had SARS-CoV-2–positive rRT-PCR results in
analyses of nasopharyngeal swab samples (from 213
recovered patients and six patients who had died).
One hundred and ninety-five patients were excluded
because of non–COVID-19 diagnosis, unconfirmed
status, non-Asian ethnicity, or refusal to consent
(Fig 1).
The mean patient age was 54.7 ± 17.5
years (range, 18-99), the male-to-female ratio
was approximately 1:1, and 90.4% of the patients
were Chinese (Table 1). The mean duration of
hospitalisation was 9.87 ± 6.99 days and the overall
mortality rate was 2.7%. The mean SARS-CoV-2
rRT-PCR Ct values for nasopharyngeal swab on
admission was 24.2 ± 7.1. The median time to the
first post-discharge visit was 38 days (range, 28-42)
and the median duration of follow-up was 14 weeks
(range, 13.1-15.5).
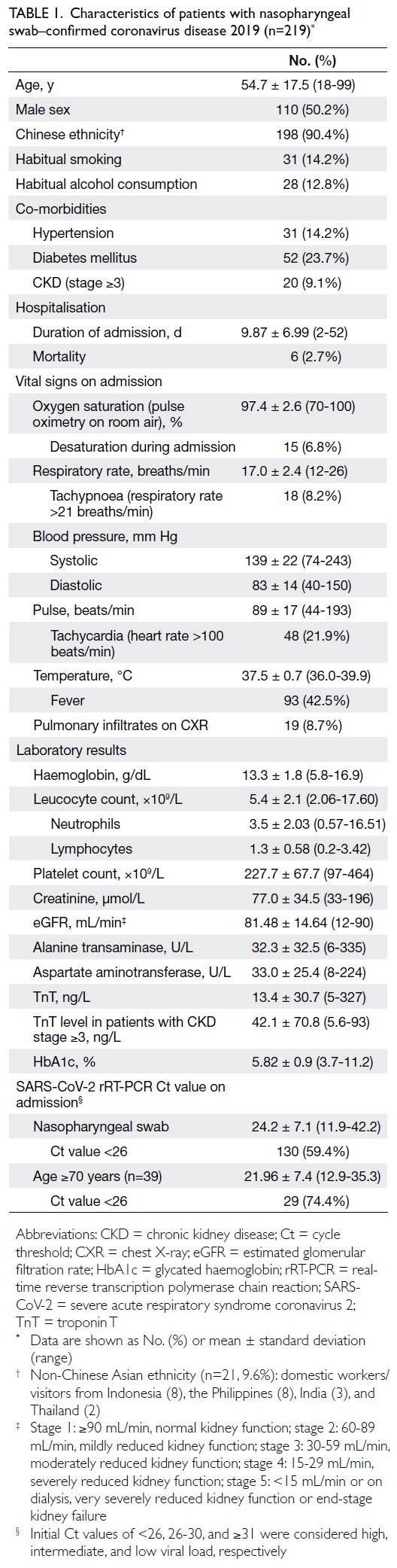
Table 1. Characteristics of patients with nasopharyngeal swab–confirmed coronavirus disease 2019 (n=219)
Clinical presentation of coronavirus disease
2019
The three most frequent symptoms were upper
respiratory symptoms: cough (51.5%), fever (42.5%),
and sputum production (27.8%). Among the 219
patients with positive SARS-CoV-2 test results, 58
(26.5%) were asymptomatic and had undergone
compulsory SARS-CoV-2 testing in accordance with
the Public Health Ordinance. Of the 58 patients,
75.9% reported contact with identifiable index cases,
such as household members, domestic helpers, or
work colleagues.
Cutaneous manifestations of coronavirus
disease 2019
Twenty patients presented with new rash. The
incidence of new rash was 9.1% in this 3-month study
period (95% CI=6.25%-14.4%). At the time of this
study, there were no biomarkers or diagnostic tests
for COVID-19–related cutaneous manifestations.
Any new cutaneous manifestation not attributable
to a previous/pre-existing skin disease or alternative
diagnosis was considered COVID-19–related.
Upon review by a dermatologist, six patients were
diagnosed with localised urticarial eruptions after
interferon injection treatment; 6.4% of patients
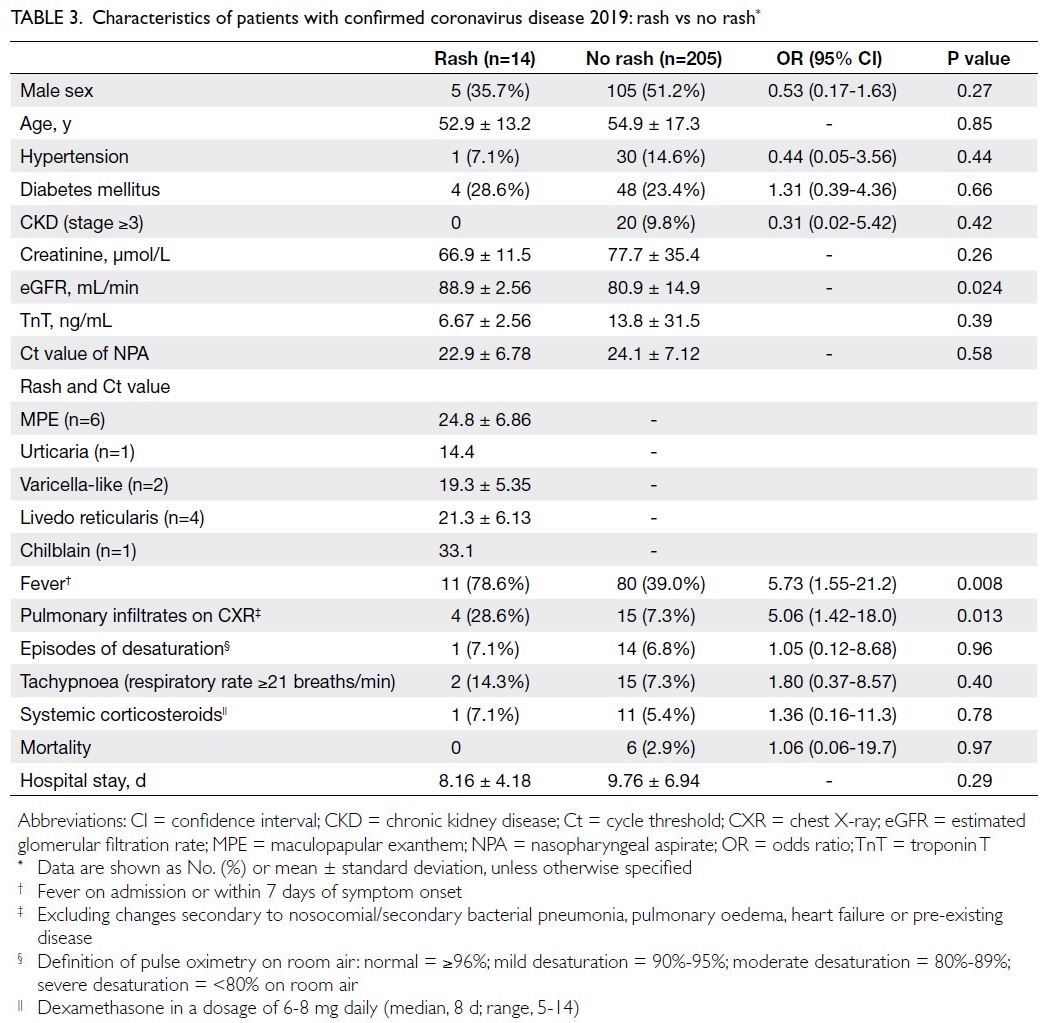
(14/219) displayed various forms of COVID-19–related rash (Fig 2 and Table 2).
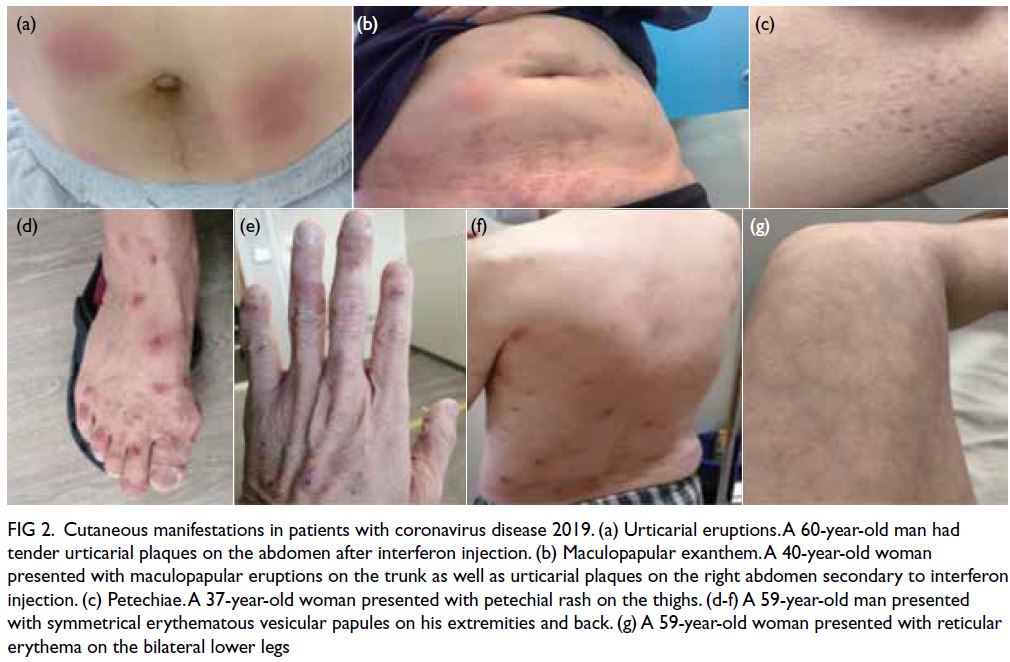
Figure 2. Cutaneous manifestations in patients with coronavirus disease 2019. (a) Urticarial eruptions. A 60-year-old man had tender urticarial plaques on the abdomen after interferon injection. (b) Maculopapular exanthem. A 40-year-old woman presented with maculopapular eruptions on the trunk as well as urticarial plaques on the right abdomen secondary to interferon injection. (c) Petechiae. A 37-year-old woman presented with petechial rash on the thighs. (d-f) A 59-year-old man presented with symmetrical erythematous vesicular papules on his extremities and back. (g) A 59-year-old woman presented with reticular erythema on the bilateral lower legs
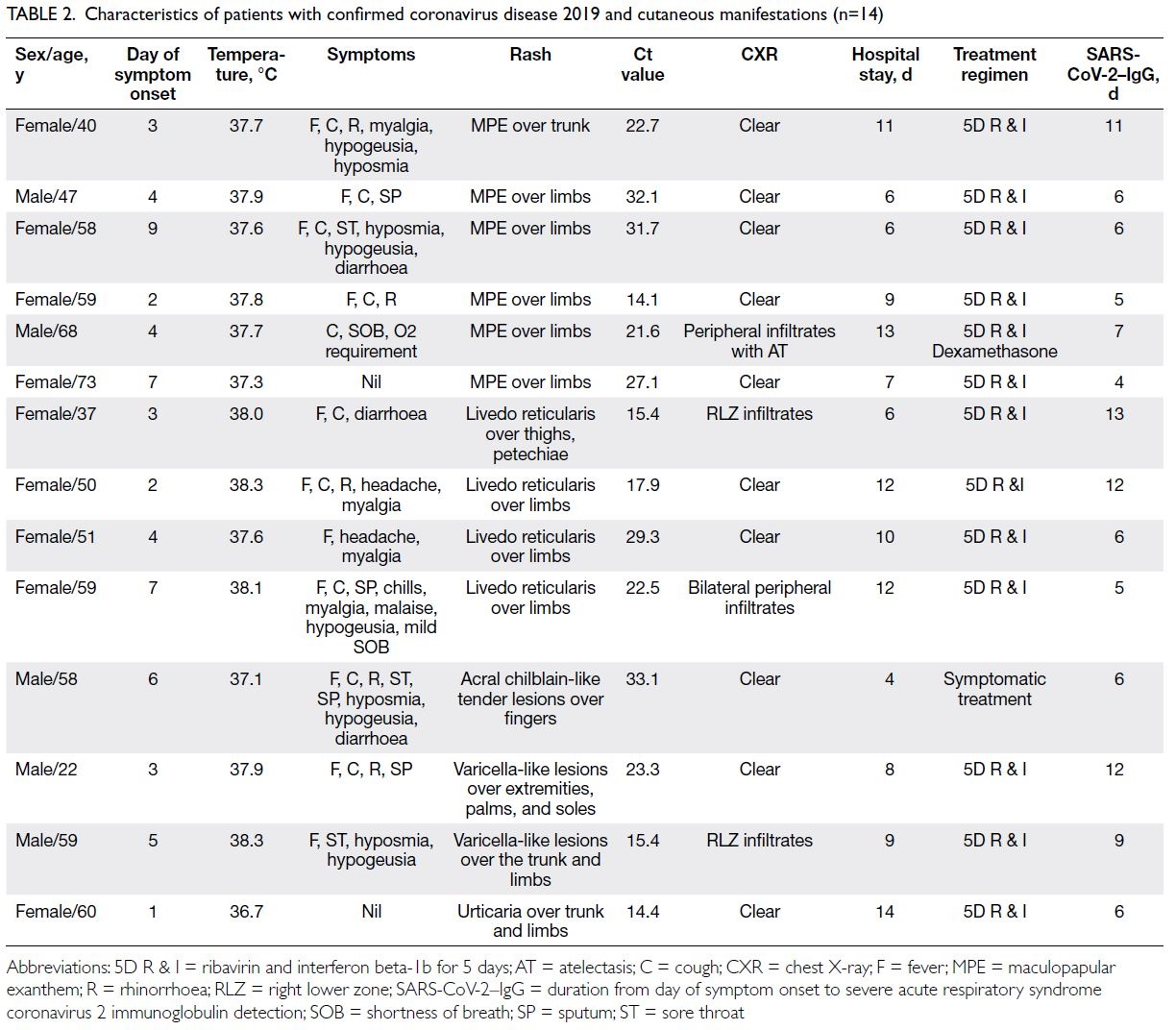
Table 2. Characteristics of patients with confirmed coronavirus disease 2019 and cutaneous manifestations (n=14)
The most common manifestations were
maculopapular exanthem (n=6, 42.9%, median Ct
value: 24.8), followed by livedo reticularis (n=4,
28.6%, median Ct value: 21.3), varicella-like lesions
(n=2, 14.3%, median Ct value: 19.3), urticaria (n=1,
7.1%, median Ct value: 14.4), and acral chilblain and petechiae (n=1, 7.1%, median Ct value: 33.1) [Fig 2].
The median Ct values for patients with and without
rash were 22.9 and 24.1, respectively (P=0.58). The
timing of symptom onset ranged from day 1 to day
9 (median, 4; mean, 4.28 ± 2.26). Skin symptoms
were the sole symptoms in two patients with
COVID-19 (0.91%), highlighting the importance of
carefully evaluating patients who only display initial
cutaneous symptoms or signs.
Outcomes and prognostic factors
Characteristics of patients with confirmed
coronavirus disease 2019: rash vs no rash
Compared with patients without rash, patients with
rash were more likely to exhibit fever (OR=5.73;
P=0.008) and display pulmonary infiltrates on chest
X-ray (OR=5.06; P=0.013). Among patients with
pulmonary infiltrates (n=19), four of them had rash.
The episodes of desaturation requiring supplemental
oxygen were less common in patients with rash (25%,
1/4) than in those without (93.3%, 14/15; OR=0.02,
95% CI=0.001-0.49; P=0.02). Furthermore, among
these 19 patients with pulmonary infiltrates, systemic
corticosteroids were less frequently required by
patients with rash (25%, 1/4) than by those without
(73.3%, 11/15; OR=0.12, 95% CI=0.01-1.53; P=0.10),
but it was not statistically significant. There were no
significant differences in age, sex, co-morbidities or Ct values between patients with and without rash.
The estimated glomerular filtration rate (eGFR)
was slightly lower in older patients without rash
(P=0.024); 10.2% of these patients had CKD stage
≥3. In terms of outcomes, patients with and without
rash had mortalities of 0.0% and 2.9%, respectively
(P=0.97). The length of hospitalisation was similar in
both groups (Table 3).
Characteristics of patients with coronavirus
disease 2019: co-morbidities and viral load
Patients aged ≥70 years had a significantly higher viral
load (as reflected by a lower Ct value), compared with
those aged <70 years (mean Ct value: 21.97 vs 24.65,
P=0.03). Regardless of age, patients with hypertension
and CKD stage ≥3 had a significantly higher viral load
and lower initial Ct value on admission (OR=2.65,
95% CI=1.08-6.45 and OR=3.65, 95% CI=1.18-11.3,
respectively; both P<0.05).
All six patients who died were men; their mean
age was 87.0 ± 7.3 years. The rates of hypertension,
diabetes mellitus, a glycated haemoglobin level of
≥6.5%, CKD stage ≥3, and higher viral load (ie, lower
Ct value on admission) were significantly greater
among patients who died than among those who
survived (Table 4). Older age, hypertension, and low
eGFR were associated with a higher risk of mortality
(all P<0.05) [Table 5].
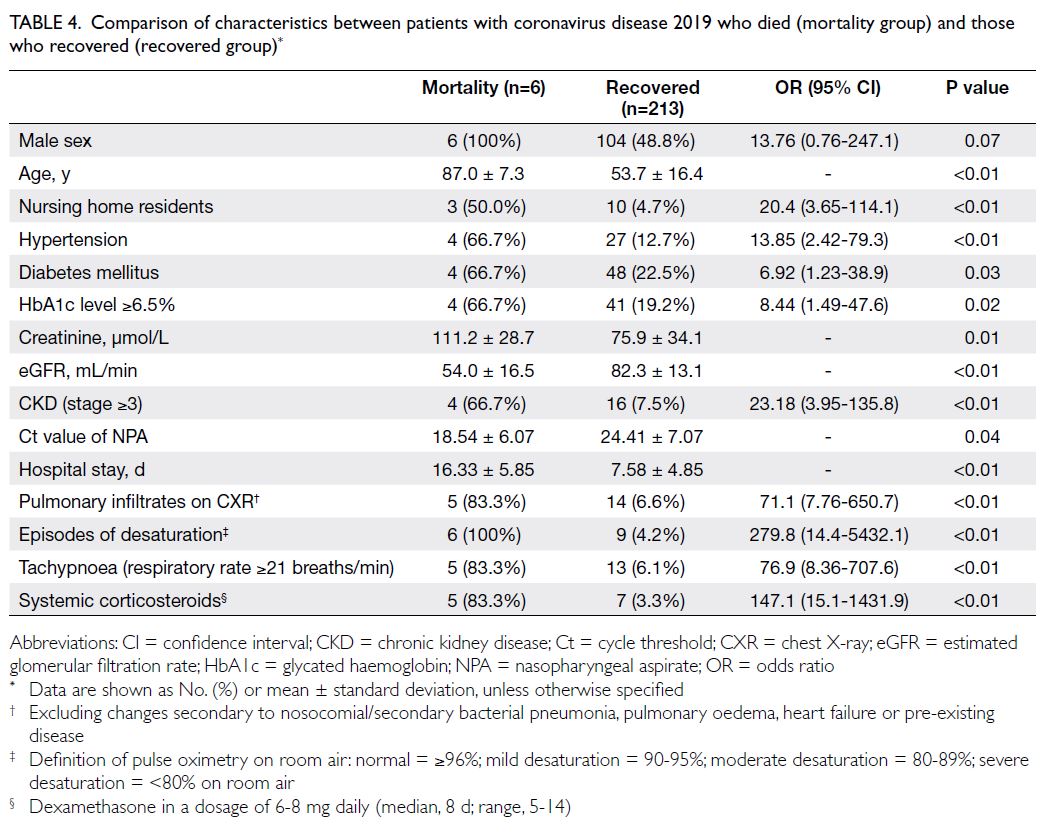
Table 4. Comparison of characteristics between patients with coronavirus disease 2019 who died (mortality group) and those who recovered (recovered group)
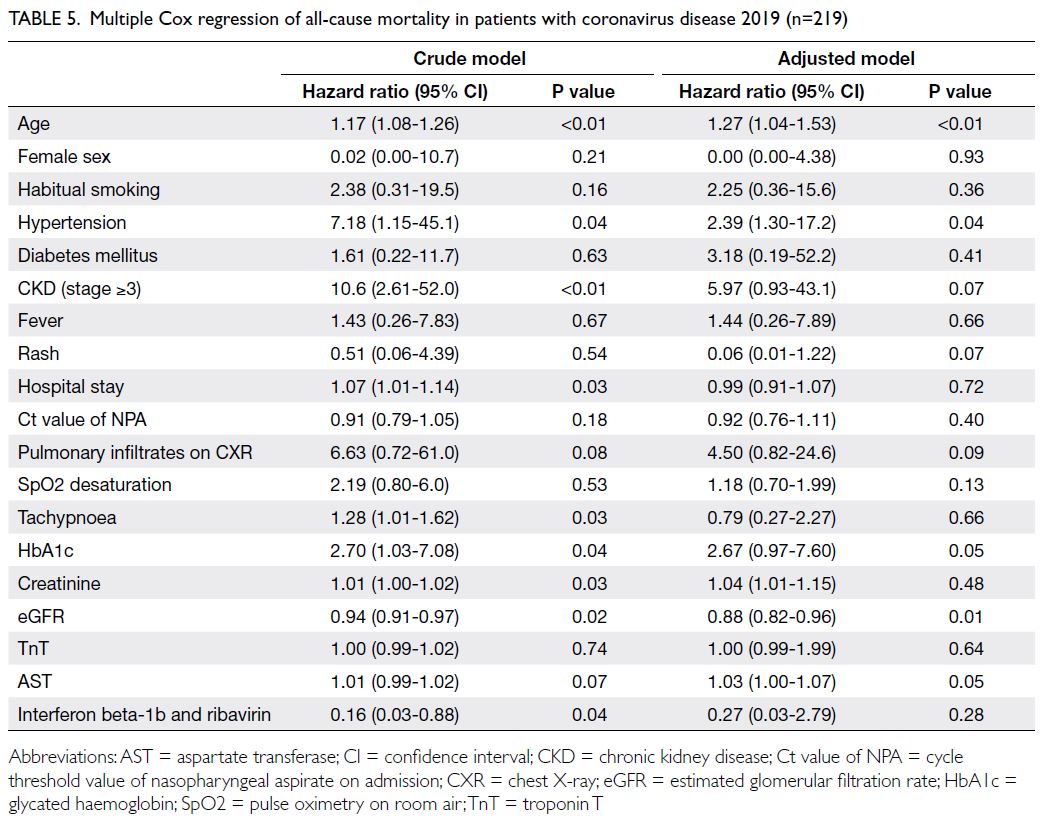
Table 5. Multiple Cox regression of all-cause mortality in patients with coronavirus disease 2019 (n=219)
Treatment received
Treatment varied in this cohort because there was no
standard of care in the early days of the COVID-19
pandemic. Symptomatic treatment was administered
to 53 patients (24.2%); 166 patients (75.8%) received
early treatment within the first week of symptom
onset, including interferon beta-1b and ribavirin,
which were administered based on the results of a
triple therapy clinical study.21 Emollients and topical
corticosteroids of mild to moderate potency (1%
hydrocortisone cream and 0.1% mometasone furoate
cream) were prescribed for symptomatic relief.
Follow-up and dermatological outcome
During follow-up, we observed that urticarial eruption after interferon injection resolved within
10 to 14 days upon completion of treatment. With
respect to COVID-19–related skin eruptions,
most lesions (maculopapular exanthem, livedo
reticularis, and urticaria) were self-limiting and
spontaneously resolved without specific treatment;
there were no severe sequelae. Two patients with
varicella-like lesions had mild post-inflammatory
hyperpigmentation without scarring.
Discussion
Cutaneous manifestations of the COVID-19
pandemic have been gaining increasing attention
because they may be useful in the early diagnosis of
COVID-19, triage of patients with SARS-CoV-2–positive test results, and risk stratification. There
is speculation that the mechanism involves the
direct action of SARS-CoV-2 on tissues, the
complement/interferon-driven immune response,
and the coagulation system; alternatively, it involves
nonspecific skin symptoms of systemic viral
infection.16 17 22 26 27 28 Although more investigations
are needed, it is possible that some symptoms are
clinical signs of milder COVID-19, whereas others
are indicators of more severe clinical illness.
Maculopapular exanthem: the most common
cutaneous manifestation
Our study showed that patients with confirmed
COVID-19 could display various cutaneous
manifestations. The most common manifestation
attributed to COVID-19 was maculopapular
exanthem, followed by livedo reticularis. Because most skin lesions were transient and self-limiting,
skin biopsy was only performed in one patient. In
that 40-year-old female patient, skin biopsy of the
left trunk revealed low to moderate numbers of
perivascular lymphocytes and histiocytes, as well as
sparse eosinophils, in the superficial dermis; focal
parakeratosis was present in the epidermis. There
was no evidence of vasculitis or interfacial changes.
These findings were compatible with maculopapular
exanthem.
In previous reports, erythema multiforme–like lesions, chilblain-like acral eruptions, and livedo
erythema were identified in children and young adult
patients with asymptomatic or mild disease.26 28 29 In
contrast, maculopapular rash and acro-ischaemic
lesions were often observed among adult patients
with more severe disease. Among our patients
who presented with rash, there were no instances of mortality; the duration of hospitalisation was
similar regardless of rash status. The results of a
previous study has suggested that the cutaneous
manifestation is the only manifestation of COVID-19
in some patients30; thus, careful documentation of
any cutaneous symptoms during the COVID-19
pandemic may be necessary for early recognition
and diagnosis.30 Additionally, urticaria with fever has
diagnostic implications because this combination
may be an early symptom of subsequently confirmed
SARS-CoV-2 infection.19 In our cohort, patients
with cutaneous manifestations were more likely to
present with fever. Although most of our patients
had symptoms other than rash alone, two patients
(0.9%) presented with rash only (one with urticaria
and one with maculopapular exanthem); the clinical
significance of these symptoms should not be
ignored. Informal extrapolation of these results to
the general population in Hong Kong suggested that
2477 cases (2/219; ie, 0.91% × 272 235 confirmed
cases)4 solely involve rash presentation; these
patients would remain undiagnosed if they did not
undergo SARS-CoV-2 testing. This lack of diagnosis
is a potential health threat and could facilitate viral
spread.
Incidence of cutaneous manifestations
In our cohort, the incidence of new rash was 13.6%.
In the study by Guan et al31 in China, the prevalence
of rash was much lower in patients with COVID-19
(0.2%; 2/1099). In that study, patients with rash may
have been underdiagnosed because patients with
suspected COVID-19 were managed by general
practitioners or hospitalists who had less familiarity
with cutaneous manifestations.31 In contrast,
our patients underwent prompt assessment by
in-hospital dermatologists to detect cutaneous
manifestations. In an Italian study, the prevalence
of rash presentation was much higher (20.4%),13
presumably because asymptomatic patients were
excluded through a lack of testing. However, if
we exclude the 58 asymptomatic patients in our
cohort (all of whom underwent compulsory testing
in accordance with the Public Health Ordinance),
the incidence of new rash in our study was 16.7%
(95% CI=14.5-18.8), which remains lower than the
incidence in the Italian study. We speculate that this
difference is related to the early initiation of combined
treatment (ribavirin and interferon beta-1b) in our
cohort, which may modify or halt the SARS-CoV-2–induced inflammatory process.21 Importantly, the genomic characteristics of SARS-CoV-2 spread
are under investigation worldwide; this approach
helps identify transmission routes in various
regions. In a case series in the United States, SARS-CoV-2 genomes in one region were predominantly
associated with isolates that originated in Europe
(>80%), similar to the distributions of viral strains
in other regions in the United States32; a smaller
subgroup of SARS-CoV-2 genomes displayed
similarity to strains that originated in Asia (15%),
indicating multiple sources of viral spread within
the community.32 Differences in the prevalences of
cutaneous manifestations may represent variations
in SARS-CoV-2 genomic characteristics among
regions; in Hong Kong, a cosmopolitan city with many
travellers from mainland China and other countries,
the prevalences of cutaneous manifestations may be
the result of viral strains from all provinces of China
as well as Europe and other regions. Further studies
are needed concerning genomic variations and
clinical manifestations.
Prognostic factors
In terms of viral load and prognosis, higher viral load on admission was significantly associated with
greater mortality in patients with older age, history of
hypertension, and CKD stage ≥3. Univariate analysis
showed that the risk of mortality was the greatest
among patients with older age, hypertension, higher
glycated haemoglobin level, and renal impairment.
Multivariate Cox regression analysis confirmed
that older age, hypertension, and low eGFR were
significantly associated with greater mortality risk.
Conversely, patients with renal impairment
were less likely to present with rash, suggesting that
the immune response is weaker in patients with renal
impairment. However, the length of hospitalisation
was similar regardless of cutaneous manifestations;
the presence of cutaneous manifestations was not
associated with other co-morbidities. There was
no clear association between Ct values and rash
occurrence. Additional studies with larger sample
sizes may be necessary to explore the relationship
between rash subtype and viral load.
Rash as immunological response
The results of a previous study suggested that cutaneous manifestations of COVID-19 were related to immunological responses rather than the direct
results of viral invasion17; cutaneous manifestations
may be an early sign of immunological responses
elsewhere in the body, similar to pulmonary
infiltrates secondary to cytokine storm. The present
study showed that the incidence of pulmonary
infiltrates was considerably higher among patients
with rash (28.6%) than among those without (7.3%)
[Table 3]; conversely, patients with rash were less
likely to display further deterioration, such as
oxygen desaturation and a requirement for oxygen
supplementation (P=0.016). Only one patient
with rash (25%) received dexamethasone, whereas
multiple patients without rash required such
treatment (73.3%) [P=0.11]. Another explanation
is that, overall, patients with rash tended to seek
medical attention earlier than those without, which
would increase the likelihood of prompt treatment.
A previous study has indicated that patients
with cutaneous manifestations may have a better
prognosis because those patients develop a more
robust immune response.17
In patients with new pulmonary infiltrates as
well as evidence of respiratory decompensation/failure (eg, desaturation and/or tachypnoea),
systemic corticosteroids have been used to prevent
tissue destruction from cytokine storm after other
causes had been ruled out. In this context, patients
receiving systemic corticosteroids had more severe
disease that involved evidence or features of
respiratory decompensation and carried a greater
risk of mortality.
In the present study, after the exclusion of
patients with nosocomial/secondary bacterial
pneumonia, heart failure, or pulmonary changes
related to prior disease, 19 patients (8.7%) had new
pulmonary infiltrates on admission. All 19 patients
received interferon beta-1b and ribavirin treatment;
12 patients received dexamethasone (daily dosage
range, 6-8 mg; mean duration, 8.63 ± 2.53 days) [Table 4]. Among the 12 patients receiving dexamethasone,
five patients (41.7%) died despite the use of systemic
corticosteroids, together with empirical antibiotics,
interferon beta-1b, and ribavirin; in contrast, only
one death (14.3%) occurred among seven patients
receiving interferon beta-1b and ribavirin without
corticosteroids (OR=4.28, 95% CI=0.38-47.6; P=0.23).
The mean interval from symptom onset to systemic
corticosteroid initiation was shorter among patients
who recovered than among those who died (5.14 ± 2.14 days vs 8.61 ± 2.30 days; P=0.0026). These results
suggest that the early use of systemic corticosteroids
may lead to a better survival outcome.
Mortality
Although no deaths occurred among patients with cutaneous manifestations, the mortality rate did not significantly differ from the rate of 2.9% among
patients without rash. Most patients received
treatment within the first week after diagnosis of
COVID-19 (according to detection of SARS-CoV-2–specific immunoglobulin G within 14 days after
symptom onset; mean, 7.71 ± 3.05 days; range, 4-13),
which may have improved disease outcomes and
shortened hospitalisation. These findings highlighted
the importance of early treatment beginning at
symptom onset (ie, in the first week) and supported
the use of interferon therapy described in a previous
report.21
Limitations
First, this study had a small number of patients.
Second, there was potential selection bias because
only hospitalised patients with SARS-CoV-2–positive test results were included in the analysis;
patients with COVID-19 who did not undergo
screening or seek medical consultation were not
diagnosed, and thus they were excluded from the
study. Third, Ct value analysis was not conducted
according to rash subtype and severity because of
the limited number of patients. Fourth, some viral
laboratory tests (eg, test for human herpesvirus 6)
were not routinely available in our hospital, which
may have hindered the interpretation of possible
causes of rash or the identification of coexisting
infections. Nevertheless, most other possible viral
infections were excluded from this study. Additional
studies with larger sample sizes and comparisons
with treatment outcomes are needed.
Conclusion
This study did not demonstrate direct relationships
among rash, viral load, and mortality. Furthermore,
cutaneous manifestations may be early signs of
immunological responses (similar to pulmonary
infiltrates). Patients with older age, hypertension,
and renal impairment have greater mortality risk
and higher viral load. These high-risk groups should
be prioritised in early screening and vaccination
efforts to avoid poor clinical outcomes.
Author contributions
Concept or design: CSM Wong, IFN Hung.
Acquisition of data: CSM Wong, MMH Chung, MWM Chan, AKC Cheng, YM Lau.
Analysis or interpretation of data: CSM Wong, IFN Hung. Drafting of the manuscript: CSM Wong, IFN Hung, CK Yeung, HHL Chan.
Critical revision of the manuscript for important intellectual content: CSM Wong, IFN Hung, MYW Kwan, CK Yeung, HHL Chan, CS Lau.
Acquisition of data: CSM Wong, MMH Chung, MWM Chan, AKC Cheng, YM Lau.
Analysis or interpretation of data: CSM Wong, IFN Hung. Drafting of the manuscript: CSM Wong, IFN Hung, CK Yeung, HHL Chan.
Critical revision of the manuscript for important intellectual content: CSM Wong, IFN Hung, MYW Kwan, CK Yeung, HHL Chan, CS Lau.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgement
The authors thank all patients for their participation.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Institutional Review
Board of The University of Hong Kong/Hospital Authority
Hong Kong West Cluster (Ref No.: UW20-725) and was
conducted in full compliance with the ICH E6 guideline for
Good Clinical Practice and the principles of the Declaration
of Helsinki. Appropriate patient consent was obtained for
clinical information and images to be publicly reported. All
participants’ clinical data and reports were deidentified to
maintain anonymity.
References
1. Chen N, Zhou M, Dong X, et al. Epidemiological and
clinical characteristics of 99 cases of 2019 novel coronavirus
pneumonia in Wuhan, China: a descriptive study. Lancet
2020;395:507-13. Crossref
2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med
2020;382:727-33. Crossref
3. World Health Organization. WHO coronavirus (COVID-19)
dashboard. Situation by region, country, territory & area.
Available from: https://covid19.who.int/table. Accessed 7 May 2022.
4. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Latest situation of coronavirus
disease (COVID-19) dashboard in Hong Kong. Available
from: https://chp-dashboard.geodata.gov.hk/covid-19/en.html. Accessed 7 May 2022.
5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect 2020;80:607-13. Crossref
6. Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 2020;82:e177. Crossref
7. Hassan K. Urticaria and angioedema as a prodromal cutaneous manifestation of SARS-CoV-2 (COVID-19) infection. BMJ Case Rep 2020;13:e236981. Crossref
8. Andina D, Noguera-Morel L, Bascuas-Arribas M, et al.
Chilblains in children in the setting of COVID-19
pandemic. Pediatr Dermatol 2020;37:406-11. Crossref
9. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like
exanthem as a specific COVID-19-associated skin
manifestation: multicenter case series of 22 patients. J Am
Acad Dermatol 2020;83:280-5. Crossref
10. De Giorgi V, Recalcati S, Jia Z, et al. Cutaneous
manifestations related to coronavirus disease 2019
(COVID-19): a prospective study from China and Italy. J
Am Acad Dermatol 2020;83:674-5. Crossref
11. Tammaro A, Adebanjo GA, Parisella FR, Pezzuto A, Rello J.
Cutaneous manifestations in COVID-19: the experiences
of Barcelona and Rome. J Eur Acad Dermatol Venereol 2020;34:e306-7. Crossref
12. Marraha F, Al Faker I, Gallouj S. A review of the
dermatological manifestations of coronavirus disease 2019
(COVID-19). Dermatol Res Pract 2020;2020:9360476. Crossref
13. Recalcati S. Cutaneous manifestations in COVID-19: a first
perspective. J Eur Acad Dermatol Venereol 2020;34:e212-3. Crossref
14. Galván Casas C, Català A, Carretero Hernández G, et al.
Classification of the cutaneous manifestations of
COVID-19: a rapid prospective nationwide consensus
study in Spain with 375 cases. Br J Dermatol 2020;183:71-7. Crossref
15. Zhao Q, Fang X, Pang Z, Zhang B, Liu H, Zhang F.
COVID-19 and cutaneous manifestations: a systematic
review. J Eur Acad Dermatol Venereol 2020;34:2505-10. Crossref
16. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G,
van Goor H. Tissue distribution of ACE2 protein, the
functional receptor for SARS coronavirus. A first step in
understanding SARS pathogenesis. J Pathol 2004;203:631-7. Crossref
17. Novak N, Peng W, Naegeli MC, et al. SARS-CoV-2,
COVID-19, skin and immunology—what do we know so
far? Allergy 2021;76:698-713. Crossref
18. Li MY, Li L, Zhang Y, Wang XS. Expression of the
SARS-CoV-2 cell receptor gene ACE2 in a wide variety of
human tissues. Infect Dis Poverty 2020;9:45. Crossref
19. Rahimi H, Tehranchinia Z. A comprehensive review of
cutaneous manifestations associated with COVID-19.
Biomed Res Int 2020;2020:1236520. Crossref
20. Dadras O, Afsahi AM, Pashaei Z, et al. The relationship
between COVID-19 viral load and disease severity: a
systematic review. Immun Inflamm Dis 2022;10:e580. Crossref
21. Hung IF, Lung KC, Tso EY, et al. Triple combination of
interferon beta-1b, lopinavir-ritonavir, and ribavirin in the
treatment of patients admitted to hospital with COVID-19:
an open-label, randomised, phase 2 trial. Lancet
2020;395:1695-704. Crossref
22. Wollina U, Karadağ AS, Rowland-Payne C, Chiriac A,
Lotti T. Cutaneous signs in COVID-19 patients: a review.
Dermatol Ther 2020;33:e13549. Crossref
23. Infectious Diseases Society of America; Association for
Molecular Pathology. IDSA and AMP joint statement on
the use of SARS-CoV-2 PCR cycle threshold (Ct) values
for clinical decision-making. Available from: https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-amp-statement.pdf. Accessed 12 Apr 2022.
24. Aranha C, Patel V, Bhor V, Gogoi D. Cycle threshold
values in RT-PCR to determine dynamics of SARS-CoV-2
viral load: an approach to reduce the isolation period for
COVID-19 patients. J Med Virol 2021;93:6794-7. Crossref
25. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A.
SARS-CoV-2, SARS-CoV, and MERS-CoV viral load
dynamics, duration of viral shedding, and infectiousness:
a systematic review and meta-analysis. Lancet Microbe
2021;2:e13-22. Crossref
26. Genovese G, Moltrasio C, Berti E, Marzano AV. Skin
manifestations associated with COVID-19: current
knowledge and future perspectives. Dermatology
2021;237:1-12. Crossref
27. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against
type I IFNs in patients with life-threatening COVID-19.
Science 2020;370:eabd4585. Crossref
28. Chua GT, Wong JS, Lam I, et al. Clinical characteristics and
transmission of COVID-19 in children and youths during 3 waves of outbreaks in Hong Kong. JAMA Netw Open
2021;4:e218824. Crossref
29. Chua GT, Wong JS, Chung J, et al. Paediatric multisystem
inflammatory syndrome temporally associated with SARS-CoV-2: a case report. Hong Kong Med J 2022;28:76-8. Crossref
30. Leung TY, Chan AY, Chan EW, et al. Short- and potential
long-term adverse health outcomes of COVID-19: a rapid
review. Emerg Microbes Infect 2020;9:2190-9. Crossref
31. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med
2020;382:1708-20. Crossref
32. Zhang W, Govindavari JP, Davis BD, et al. Analysis of
genomic characteristics and transmission routes of patients
with confirmed SARS-CoV-2 in southern California during
the early stage of the US COVID-19 pandemic. JAMA
Netw Open 2020;3:e2024191. Crossref