State of the Art Review of Cell Therapy in the Treatment of Lung Disease, and the Potential for Aerosol Delivery
Abstract
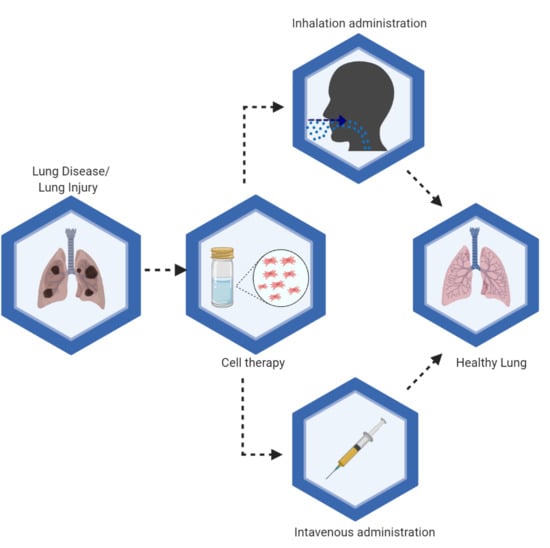
:1. Introduction
1.1. Stem Cells
1.2. Mesenchymal Stem Cells
1.3. Preconditioning and Potentiation of MSCs
1.4. Conditioned Media (CM)
1.5. MSC Secretome
1.6. Extracellular Vesicles (EVs)
1.7. Exosomes
1.8. Cell Therapies
2. Pulmonary and Respiratory Diseases and Infections
2.1. Acute Respiratory Distress Syndrome (ARDS)
2.2. Sepsis
2.3. COVID-19
3. Route of Administration
3.1. Intravenous Administration
3.2. Intratracheal Instillation
3.3. Inhalation
3.4. Aerosolization
3.5. Nebulizers
4. Pre-Clinical Studies
4.1. ARDS
4.2. Sepsis
4.3. Aerosolization of Stem Cells In Vivo And In Vitro
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Medicines. Advanced Therapy Medicinal Products: Overview. 2017. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview (accessed on 20 May 2020).
- Rousseau, C.F.; Maciulaitis, R.; Śladowski, D.; Narayanan, G. Cell and Gene Therapies: European View on Challenges in Translation and How to Address Them. Front. Med. 2018, 5, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann-Fritsch, F.; Marino, D.; Reichmann, E. About ATMPs, SOPs and GMP: The Hurdles to Produce Novel Skin Grafts for Clinical Use. Transfus. Med. Hemotherapy 2016, 43, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, E.; Rémuzat, C.; Auquier, P.; Toumi, M. Advanced therapy medicinal products: Current and future perspectives. J. Mark. Access Health Policy 2016, 4, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, R.M.T.; Hoekman, J.; Hövels, A.M.; Broekmans, A.W.; Leufkens, H.G.; Klungel, O.H. Challenges in Advanced Therapy Medicinal Product Development: A Survey among Companies in Europe. Mol. Ther. Methods Clin. Dev. 2018, 11, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seoane-Vazquez, E.; Shukla, V.; Rodriguez-Monguio, R. Innovation and competition in advanced therapy medicinal products. EMBO Mol. Med. 2019, 11, 9992. [Google Scholar] [CrossRef]
- Iglesias-López, C.; Agustí, A.; Obach, M.; Vallano, A. Regulatory Framework for Advanced Therapy Medicinal Products in Europe and United States. Front. Pharmacol. 2019, 10, 921. [Google Scholar] [CrossRef] [Green Version]
- Gilead. U.S. FDA Approves Kite’s Tecartus™, the First and Only CAR T Treatment for Relapsed or Refractory Mantle Cell Lymphoma. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2020/7/us-fda-approves-kites-tecartus-the-first-and-only-car-t-treatment-for-relapsed-or-refractory-mantle-cell-lymphoma (accessed on 13 August 2020).
- European Medicines. EU/3/19/2220. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3192220 (accessed on 13 August 2020).
- Tuch, B. Stem cells—A clinical update. Aust. Fam. Physician 2006, 35, 719. [Google Scholar]
- O’Brien, T.E.; Barry, F.P. Stem Cell Therapy and Regenerative Medicine. Mayo Clin. Proc. 2009, 84, 859–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, H.-X.; Tang, Y.; Hu, M.; Zhao, X. Stem Cells: General Features and Characteristics. In Stem Cells in Clinic and Research; IntechOpen: London, UK, 2011; pp. 3–20. [Google Scholar]
- Barfoot, J.; Bruce, D.; Laurie, G.; Bauer, N.; Paterson, J.; Bownes, M. Stem Cell: Science and Ethics, Stemcell—Resourse, 3rd ed.; BBSRC: Edinburgh, UK, 2016; pp. 1–56. [Google Scholar]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Chagastelles, P.C.; Nardi, N.B. Biology of stem cells: An overview. Kidney Int. Suppl. 2011, 1, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu, S.C.; Antunes, M.A.; Pelosi, P.; Morales, M.M.; Rocco, P.R.M. Mechanisms of cellular therapy in respiratory diseases. Intensive Care Med. 2011, 37, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Kavanagh, B.P.; Laffey, J. What’s new in cell therapies in ARDS? Intensive Care Med. 2015, 42, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, C.-M.; An, S.; Cheng, Q.; Huang, Y.-F.; Wang, Y.-T.; Gou, Y.; Xiao, L.; Yu, W.-J.; Wang, J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2013, 20, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Deans, R.J.; Moseley, A.B. Mesenchymal stem cells: Biology and potential clinical uses. Exp. Hematol. 2000, 28, 875–884. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-González, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Maumus, M.; Jorgensen, C.; Noel, D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 2013, 95, 2229–2234. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.H.A.; Antunes, M.A.; Dos Santos, C.C.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Iannaccone, G.; Scacciavillani, R.; Del Buono, M.G.; Camilli, M.; Ronco, C.; Lavie, C.J.; Abbate, A.; Crea, F.; Massetti, M.; Aspromonte, N. Weathering the Cytokine Storm in COVID-19: Therapeutic Implications. CardioRenal Med. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J. Biomed. Technol. Res. 2014, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Fang, X.; Krasnodembskaya, A.D.; Howard, J.P.; Matthay, M.A. Concise Review: Mesenchymal Stem Cells for Acute Lung Injury: Role of Paracrine Soluble Factors. Stem Cells 2011, 29, 913–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Han, Y.-S.; Lee, S.H. Potentiation of biological effects of mesenchymal stem cells in ischemic conditions by melatonin via upregulation of cellular prion protein expression. J. Pineal Res. 2017, 62, 12385. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, U.; Gumina, R.J.; Wolfe, B.; Kuppusamy, M.L.; Kuppusamy, P.; Boudoulas, K.D. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. J. Cell Biochem. 2013, 114, 2612–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Hu, X.-Y.; Xie, X.-J.; Xu, Q.-Y.; Wang, Y.-P.; Liu, X.-B.; Xiang, M.-X.; Wang, Y.S.; Wang, J.-A. Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J. Zhejiang Univ. Sci. B 2010, 11, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, C.-W. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, T.M.; Silva, J.D.; Cruz, F.F.; Alegria, S.; Xisto, D.G.; Assis, E.F.; Castro-Faria-Neto, H.C.; Dos Santos, C.C.; Morales, M.M.; Rocco, P.R.M. Insult-dependent effect of bone marrow cell therapy on inflammatory response in a murine model of extrapulmonary acute respiratory distress syndrome. Stem Cell Res. Ther. 2013, 4, 123. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Liu, J.; Zhao, J.; Xiao, L.; An, S.; Gou, Y.; Quan, H.; Cheng, Q.; Zhang, Y.-L.; He, W.; et al. Effects of Hypoxia on the Immunomodulatory Properties of Human Gingiva–Derived Mesenchymal Stem Cells. J. Dent. Res. 2014, 94, 69–77. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Chiang, C.-H.; Hung, S.-C.; Chian, C.-F.; Tsai, C.-L.; Chen, W.-C.; Zhang, H. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS ONE 2017, 12, 0187637. [Google Scholar] [CrossRef]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef] [Green Version]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.; Liles, W.C.; Waworuntu, R.; Mulligan, M.S. Pretreatment with bone marrow–derived mesenchymal stromal cell–conditioned media confers pulmonary ischemic tolerance. J. Thorac. Cardiovasc. Surg. 2016, 151, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-Y.; Lee, J.-H.; Kim, H.J.; Park, M.K.; Huh, J.W.; Ro, J.Y.; Oh, Y.-M.; Lee, S.-D.; Lee, Y.-S. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am. J. Physiol. Cell Mol. Physiol. 2012, 302, 891–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shologu, N.; Scully, M.; Laffey, J.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizoso, F.J.; Eiró, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, M.M.; Cancemi, P.; Geraci, F. Mesenchymal and Induced Pluripotent Stem Cells-Derived Extracellular Vesicles: The New Frontier for Regenerative Medicine? Cells 2020, 9, 1163. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.-G.; Gudapati, V.; Lim, H.; Lee, J.W. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin. Boil. Ther. 2016, 16, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Ferrarotti, I.; Saracino, L.; Perteghella, S.; Torre, M.L.; Corsico, A. Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use. Cells 2020, 9, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangadaran, P.; Ahn, B.-C. Extracellular Vesicle- and Extracellular Vesicle Mimetics-Based Drug Delivery Systems: New Perspectives, Challenges, and Clinical Developments. Pharmaceutical 2020, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control Release 2019, 309, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kishore, R.; Khan, M. More than tiny sacks: Stem cell exosomes as cell-free modality for cardiac repair. Circ. Res. 2016, 118, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Vakhshiteh, F.; Atyabi, F.; Ostad, S.N. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int. J. Nanomed. 2019, 14, 2847–2859. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal Stem Cell Exosomes, in Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 40, pp. 82–88. [Google Scholar]
- Kordelas, L.; Rebmann, V.; Ludwig, A.-K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Kim, I. A brief overview of cell therapy and its product. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 201–202. [Google Scholar] [CrossRef] [Green Version]
- Dodson, B.P.; Levine, A.D. Challenges in the translation and commercialization of cell therapies. BMC Biotechnol. 2015, 15, 70. [Google Scholar] [CrossRef] [Green Version]
- Bartel, R.L. Stem Cells and Cell Therapy. In Translational Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 107–112. [Google Scholar]
- Meij, P.; Canals, J.M.; Lowery, M.; Scott, M. Advanced Therapy Medicinal Products. In Proceedings of the 2019 PDA Europe Conference on Advanced Therapy Medicinal Products (ATMPs), Vilnius, Lithuania, 4–5 June 2019. [Google Scholar]
- Arrighi, N. Stem Cells: Therapeutic Innovations Under Control; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kazmi, B.; Inglefield, C.J.; Lewis, M.P. Autologous cell therapy: Current treatments and future prospects. Wounds 2009, 21, 234–242. [Google Scholar] [PubMed]
- Gage, F.H. “Cell therapy”, Nature, Research Support, Non-U S Gov’t Research Support, U S Gov’t. PHS Rev. 1998, 392, 18–24. [Google Scholar]
- Tögel, F.; Cohen, A.; Zhang, P.; Yang, Y.; Hu, Z.; Westenfelder, C. Autologous and Allogeneic Marrow Stromal Cells Are Safe and Effective for the Treatment of Acute Kidney Injury. Stem Cells Dev. 2009, 18, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Łęgosz, P.; Sarzyńska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezoagli, E.; Murphy, E.J.; Laffey, J.; O’Toole, D. The Safety and Efficiency of Addressing ARDS Using Stem Cell Therapies in Clinical Trials. In Stem Cell-Based Therapy for Lung Disease; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 219–238. [Google Scholar]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Ooi, S.; Wang, L. Immunogenicity and Tumorigenicity of Pluripotent Stem Cells and their Derivatives: Genetic and Epigenetic Perspectives. Curr. Stem Cell Res. Ther. 2014, 9, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.; Broaddus, V.C.; Martin, T.; King, T.; Schraufnagel, D.; Murray, J.; Nadel, J. Murray and Nadel’s Textbook of Respiratory Medicine E-Book: 2-Volume Set; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Schraufnagel, D.E. Breathing in America: Diseases, Progress, and Hope; American Thoracic Society: New York, NY, USA, 2010. [Google Scholar]
- Griffiths, M.J.D.; McAuley, D.F.; Perkins, G.D.; Barrett, N.; Blackwood, B.; Boyle, A.; Chee, N.; Connolly, B.; Dark, P.; Finney, S.J.; et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir. Res. 2019, 6, 420. [Google Scholar] [CrossRef] [Green Version]
- Rawal, G.; Yadav, S.; Kumar, R. Acute Respiratory Distress Syndrome: An Update and Review. J. Transl. Int. Med. 2018, 6, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 1996, 334, 1469–1470. [Google Scholar] [CrossRef]
- Bhatia, M.; Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004, 202, 145–156. [Google Scholar] [CrossRef]
- Leaver, S.K.; Evans, T.W. Acute respiratory distress syndrome. BMJ 2007, 335, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Luh, S.-P.; Chiang, C. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): The mechanism, present strategies and future perspectives of therapies. J. Zhejiang Univ. Sci. B 2007, 8, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierrakos, C.; Karanikolas, M.; Scolletta, S.; Karamouzos, V.; Velissaris, D. Acute Respiratory Distress Syndrome: Pathophysiology and Therapeutic Options. J. Clin. Med. Res. 2012, 4, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteve, F.; Lopez-Delgado, J.C.; Javierre, C.; Skaltsa, K.; Carrio, M.L.L.; Rodríguez-Castro, D.; Torrado, H.; Farrero, E.; Diaz-Prieto, A.; Ventura, J.L.L.; et al. Evaluation of the PaO2/FiO2 ratio after cardiac surgery as a predictor of outcome during hospital stay. BMC Anesthesiol. 2014, 14, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Q.; Wang, S.; Liu, R.; Wang, H.; Zheng, J.; Yu, K. Risk factors for outcomes of acute respiratory distress syndrome patients: A retrospective study. J. Thorac. Dis. 2019, 11, 673–685. [Google Scholar] [CrossRef]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef] [Green Version]
- De Hemptinne, Q.; Remmelink, M.; Brimioulle, S.; Salmon, I.; Vincent, J.-L. ARDS: A clinicopathological confrontation. Chest 2009, 135, 944–949. [Google Scholar] [CrossRef]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis and Septic Shock: Current Treatment Strategies and New Approaches. Eurasian J. Med. 2017, 49, 53–58. [Google Scholar] [CrossRef]
- U.o.B. Columbia. Sepsis Leading Cause of Death Worldwide. Faculty of Medicine. Available online: https://www.med.ubc.ca/news/sepsis-leading-cause-of-death-worldwide/ (accessed on 8 May 2020).
- Hajj, J.; Blaine, N.; Salavaci, J.; Jacoby, D.S. The “Centrality of Sepsis”: A Review on Incidence, Mortality, and Cost of Care. Health 2018, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-Y.; Hong, S.-B. Sepsis and Acute Respiratory Distress Syndrome: Recent Update. Tuberc. Respir. Dis. 2016, 79, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Hostiuc, S.; Dermengiu, D.; Ceausu, M.; Rusu, M.C.; Curca, G.C. Pathology and immunopathology of the lung in sepsis. Rom. J. Leg. Med. 2011, 19, 83–88. [Google Scholar] [CrossRef]
- Keane, C.; Jerkic, M.; Laffey, J. Stem Cell–based Therapies for Sepsis. Anesthesiology 2017, 127, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Jung, H.; Kim, D.-H.; Kim, E.-Y.; Chung, J.-W.; Cho, Y.-S.; Park, S.G.; Park, B.-C.; Ko, Y.; Bae, K.-H.; et al. Regulation of adipogenic differentiation by LAR tyrosine phosphatase in human mesenchymal stem cells and 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122, 4160–4167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Cheng, Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Raj, K.; Rohit; Ghosh, A.; Singh, S. Coronavirus as silent killer: Recent advancement to pathogenesis, therapeutic strategy and future perspectives. VirusDisease 2020, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Weiss, D.J. Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010–2012. Ann. Am. Thorac. Soc. 2013, 10, 45–97. [Google Scholar] [CrossRef]
- Lamas, C.C.; De Lorenzo, A.R. COVID-19 and the cardiovascular system. Heart Vessel. Transplant. 2020, 4, 37. [Google Scholar] [CrossRef]
- Zu, Z.Y.; Di Jiang, M.; Xu, P.P.; Chen, W.; Ni, Q.Q.; Lu, G.M.; Zhang, L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiologia 2020, 296, 15–25. [Google Scholar] [CrossRef] [Green Version]
- CDC COVID-19 Response Team; Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–16 March, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Mungroo, M.R.; Khan, N.A.; Siddiqui, R. Novel Coronavirus: Current Understanding of Clinical Features, Diagnosis, Pathogenesis, and Treatment Options. Pathogens 2020, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Chow, N.; Fleming-Dutra, K.; Gierke, R.; Hall, A.; Hughes, M.; Pilishvili, T.; Ritchey, M.; Roguski, K.; Skoff, T.; Ussery, E. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 382–386. [Google Scholar] [CrossRef]
- Hoang, V.T.; Dao, T.L.; Gautret, P. Recurrence of positive SARS-CoV-2 in patients recovered from COVID-19. Published ahead of print. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.E.; Adab, P.; Cheng, K.K. Covid-19: Risk factors for severe disease and death. BMJ 2020, 368, m1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 1. [Google Scholar] [PubMed]
- Medicine, J.H. What Coronavirus Does to the Lungs. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/what-coronavirus-does-to-the-lungs (accessed on 20 July 2020).
- Susan, R.W. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus/Susan R. Weiss, Sonia Navas-Martin. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biologic perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Barton, L.M.; Duval, E.J.; Stroberg, E.; Ghosh, S.; Mukhopadhyay, S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020, 153, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Rajarshi, K.; Chatterjee, A.; Ray, S. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol. Rep. 2020, 26, e00467. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, J.A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.G.; Singh, D.; Hadfield, R.M. Inhaled corticosteroids and COVID-19: A systematic review and clinical perspective. Eur. Respir. J. 2020, 55, 2001009. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D. PHAR 7633, Routes of Drug Administration, 7th ed.; Boomer Press: Aurora, CO, USA, 2014; pp. 1–14. [Google Scholar]
- Zhou, Q.; Jin, J.-F.; Zhu, L.-L.; Chen, M.; Xu, H.-M.; Wang, H.-F.; Feng, X.-Q.; Zhu, X.-P. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 9, 923–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Zhao, L. Passive lung-targeted drug delivery systemsviaintravenous administration. Pharm. Dev. Technol. 2013, 19, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef] [Green Version]
- Klepikov, I. The Effect of Intravenous Infusion on the Dynamics of Acute Pneumonia. EC Pulmonol. Respir. Med. 2017, 4, 15–20. [Google Scholar]
- Osier, M.; Oberdörster, G. Intratracheal inhalation vs intratracheal instillation: Differences in particle effects. Fundam. Appl. Toxicol. 1997, 40, 220–227. [Google Scholar] [CrossRef]
- Baran, K. Toxicity Testing, Inhalation. Encycl. Toxicol. 2014, 4, 669–672. [Google Scholar]
- Xisto, D.G.; Abreu, S.C.; Antunes, M.A.; Crossetti, J.; Capelozzi, V.L.; Morales, M.M.; Rocco, P.R.M. Intratracheal Versus Intravenous Bone Marrow Mononuclear Cell Therapy in Experimental Chronic Allergic Asthma: Which Is The Best Administration Route? In Proceedings of the American Thoracic Society 2011 International Conference, Denver, CO, USA, 13–18 May 2011; American Thoracic Society: New York, NY, USA, 2011; p. 3588. [Google Scholar]
- Garcia-Contreras, L.; Ibrahim, M.; Verma, R. Inhalation drug delivery devices: Technology update. Med. Devices Évid. Res. 2015, 8, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Hickey, A.J. Back to the Future: Inhaled Drug Products. J. Pharm. Sci. 2013, 102, 1165–1172. [Google Scholar] [CrossRef]
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef] [PubMed]
- Ari, A. Aerosol Therapy in Pulmonary Critical Care. Respir. Care 2015, 60, 858–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashkin, D. A review of nebulized drug delivery in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2585–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, K.L.; Wilken, L.; Hart, M.K. Pulmonary Disease Aerosol Delivery Devices 2017. Available online: https://www.aarc.org/wp-content/uploads/2018/01/aerosol-guide-for-hcp-3rd.pdf (accessed on 1 July 2020).
- Chandel, A.; Goyal, A.K.; Ghosh, G.; Rath, G. Recent advances in aerosolised drug delivery. Biomed. Pharmacother. 2019, 112, 108601. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K. Dosage Forms and Their Routes of Administration; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 9–29. [Google Scholar]
- Stein, S.W.; Thiel, C.G. The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol Med. Pulm. Drug Deliv. 2016, 30, 20–41. [Google Scholar] [CrossRef]
- Horie, S.; Masterson, C.; Devaney, J.; Laffey, J. Stem cell therapy for acute respiratory distress syndrome. Curr. Opin. Crit. Care 2016, 22, 14–20. [Google Scholar] [CrossRef]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Cardenes, N.; Aranda-Valderrama, P.; Carney, J.P.; Torres, J.S.; Alvarez, D.; Kocydirim, E.; Smith, J.A.W.; Ting, A.E.; Lagazzi, L.; Yu, Z.; et al. Cell therapy for ARDS: Efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir. Res. 2019, 6, e000308. [Google Scholar] [CrossRef]
- Ionescu, L.; Byrne, R.N.; Van Haaften, T.; Vadivel, A.; Alphonse, R.S.; Rey-Parra, G.J.; Weissmann, G.; Hall, A.; Eaton, F.; Thébaud, B. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am. J. Physiol. Cell. Mol. Physiol. 2012, 303, L967–L977. [Google Scholar] [CrossRef] [Green Version]
- Su, V.Y.-F.; Lin, C.-S.; Hung, S.-C.; Yang, K.-Y. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 2208. [Google Scholar] [CrossRef] [Green Version]
- Gotts, J.E.; Matthay, M.A. Cell-based Therapy in Sepsis. A Step Closer. Am. J. Respir. Crit. Care Med. 2018, 197, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, S.M.K.; Bhat, V. Could stem cells be the future therapy for sepsis? Blood Rev. 2016, 30, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Gurudutta, G.; Satija, N.K.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Tripathi, R. Stem cell therapy: A novel & futuristic treatment modality for disaster injuries. Indian J. Med. Res. 2012, 135, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Anderson, P.; González, M.A.; Rico, L.; Büscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Markel, T.A.; Herrmann, J.L.; Abarbanell, A.M.; Kelly, M.L.; Meldrum, D.R. Stem Cells in Sepsis. Ann. Surg. 2009, 250, 19–27. [Google Scholar] [CrossRef]
- Horak, J.; Nalos, L.; Martinkova, V.; Tegl, V.; Vistejnova, L.; Kuncova, J.; Kohoutova, M.; Jarkovska, D.; Dolejsova, M.; Benes, J.; et al. Evaluation of Mesenchymal Stem Cell Therapy for Sepsis: A Randomized Controlled Porcine Study. Front. Immunol. 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laroye, C.; Lemarié, J.; Boufenzer, A.; Labroca, P.; Cunat, L.; Alauzet, C.; Groubatch, F.; Cailac, C.; Jolly, L.; Bensoussan, D.; et al. Clinical-grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs. Intensive Care Med. Exp. 2018, 6, 24. [Google Scholar] [CrossRef]
- Abreu, S.C.; Xisto, D.G.; De Oliveira, T.B.; Blanco, N.G.; De Castro, L.L.; Kitoko, J.Z.; Olsen, P.C.; Lopes-Pacheco, M.; Morales, M.M.; Weiss, D.J.; et al. Serum from Asthmatic Mice Potentiates the Therapeutic Effects of Mesenchymal Stromal Cells in Experimental Allergic Asthma. Stem Cells Transl. Med. 2018, 8, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Kardia, E.; Halim, N.S.S.A.; Yahaya, B.H. Aerosol-Based Cell Therapy for Treatment of Lung Diseases. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; Volume 1516, pp. 243–255. [Google Scholar]
- Halim, N.S.S.; Ch’Ng, E.S.; Kardia, E.; Ali, S.A.; Radzi, R.; Yahaya, B.H. Aerosolised Mesenchymal Stem Cells Expressing Angiopoietin-1 Enhances Airway Repair. Stem Cell Rev. Rep. 2018, 15, 112–125. [Google Scholar] [CrossRef]
- Averyanov, A.; Konoplyannikov, A.; Zabozlaev, F.; Danilevskaya, O.; Konoplyannikov, M.; Kuzovlev, O.; Koroleva, I. Comparative effects of inhaled and intravenous mesenchymal stem cells in bleomycin-induced pulmonary fibrosis in rabbits. Eur Respir. Soc. 2013, 44, 4–14. [Google Scholar]
- Alhasan, L.; Qi, A.; Rezk, A.R.; Yeo, L.Y.; Chan, P.P.Y. Assessment of the potential of a high frequency acoustomicrofluidic nebulisation platform for inhaled stem cell therapy. Integr. Biophys. 2016, 8, 12–20. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.D.; Horgan, E.; Ali, A.; Masterson, C.; Laffey, J.G.; MacLoughlin, R.; O’Toole, D. Nebulized Mesenchymal Stem Cell Derived Conditioned Medium Retains Antibacterial Properties Against Clinical Pathogen Isolates. J. Aerosol Med. Pulm. Drug Deliv. 2019, 33, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Averyanov, A.V.; Konoplyannikov, A.G.; Antonov, N.S.; Osipova, G.L.; Vasil’Eva, O.S.; Sakharova, M.G.; Tatarskii, A.R.; Kobylyansky, V.I. Survival of Mesenchymal Stem Cells in Different Methods of Nebulization. Bull. Exp. Boil. Med. 2018, 164, 576–578. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.; Watpool, I.; Schlosser, K.; Stewart, D.J.; Mei, S.H.J.; Courtman, D.W.; Granton, J.; Marshall, J.C.; Dos Santos, C.C.; Walley, K.R.; et al. Cellular Immunotherapy for Septic Shock. A Phase I Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Perlee, D.; Van Vught, L.A.; Scicluna, B.; Maag, A.; Lutter, R.; Kemper, E.M.; Veer, C.V.T.; Punchard, M.A.; González, J.; Richard, M.P.; et al. Intravenous Infusion of Human Adipose Mesenchymal Stem Cells Modifies the Host Response to Lipopolysaccharide in Humans: A Randomized, Single-Blind, Parallel Group, Placebo Controlled Trial. Stem Cells 2018, 36, 1778–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Z.; Zhu, R.; Hou, W.; Fengchun, Z.; Yangyang, Z.; Luchan, D.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Chen, Y.; Luo, X.; He, X.; Zhang, Y.; Wang, J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef]
- Ercelen, N.O.; Bilgili, B.; Monteleone, B.; Gul, F.; Gulay, G.R.; Alpaydin, N.; Demir, O.T.; Simsek, M.; Turan, D.; Karadeniz, O.; et al. MSC Transplantation in Eight Severe COVID-19 Patients: Can Cytokine StormBe Reversed? J. Stem Cell Res. Ther. 2020, 10, 3. [Google Scholar] [CrossRef]
- Antebi, B.; Mohammadipoor, A.; Batchinsky, A.I.; Cancio, L.C. The promise of mesenchymal stem cell therapy for acute respiratory distress syndrome. J. Trauma Acute Care Surg. 2018, 84, 183–191. [Google Scholar] [CrossRef]
- Polverino, F. Best of Milan 2017—Repair of the emphysematous lung: Mesenchymal stromal cell and matrix. J. Thorac. Dis. 2017, 9, S1544–S1547. [Google Scholar] [CrossRef]
- Woods, N.; MacLoughlin, R. Defining a Regulatory Strategy for ATMP/Aerosol Delivery Device Combinations in the Treatment of Respiratory Disease. Pharmaceutics 2020. under review. [Google Scholar]






| NCT No. | Title | Status | Company Name | Disease/Conditions | Route of Administration | Intervention/Mechanism Target | Results | Phase |
|---|---|---|---|---|---|---|---|---|
| NCT01775774 | Human MSCs for acute respiratory distress syndrome | Completed | University of California | ARDS | Intravenous | Participants received 1 × 106, 5 × 106 and 10 × 106 cells/kg body weight of allogenic BM-HMSCs | A single dose of MSCs demonstrated to be safe and was tolerated well | 1 |
| NCT02611609 | A phase 1 and 2 study to assess multistem therapy in acute respiratory distress syndrome | Completed | Athersys, Inc | ARDS | Intravenous | MultiStem® | N/A | 1 and 2 |
| NCT03608592 | Human Umbilical Cord Mesenchymal stem cells (MSC) therapy in ARDS | Recruiting | Lv Haijin, Sun Yat-sen University | ARDS | Intravenous | 60 × 106 UC-MCSs intravenously administered in 2 h | N/A | N/A |
| NCT01902082 | Adipose-derived Mesenchymal stem cells in acute respiratory distress syndrome | Unknown | Shaoxing Second Hospital | ARDS | Intravenous | Participants will receive 1 × 106 Adipose-derived MSCs on day 2. | N/A | 1 |
| NCT02804945 | Mesenchymal Stem cells (MSCs) for the treatment of Acute Respiratory Distress Syndrome (ARDS) in patients with Malignancies | Completed | Anderson Cancer Center | ARDS | Intravenous infusion “MSCs by vein” | Participants received 3 × 106 intravenously administered on day 1 | N/A | 1 |
| NCT03042143 | Repair of Acute Respiratory Distress Syndrome by Stromal cell Administration (COVID-19) (Realist) | Recruiting | Belfast Health and Social Care Trust | COVID/ARDS | Intravenous | Participants will receive max. tolerated dose of human umbilical cord derived CD362 enriched MSCs given in 30–90 min. | N/A | 1 and 2 |
| NCT04347967 | Mesenchymal stem cells for the treatment of Acute Respiratory Distress Syndrome (ARDS) | Not recruiting yet | Meridigen Biotech Co. Ltd. | ARDS | Intravenous Infusion | Participants will receive low, medium, and high doses of UMC119-06 given to 3 different set of people | N/A | 1 |
| NCT03818854 | Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome (STAT) | Recruiting | University of California | ARDS | Intravenous | Participants will receive 10 × 106 cells given over 60–80 min | N/A | 2 |
| NCT04377334 | Mesenchymal Stem cells (MSCs) in inflammation-Resolution Programs of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS) | Not Recruiting Yet | University Hospital Tuebingen | ARDS/COVID | Intravenous | Participants will receive allogenic bone marrow derived HMSCs | N/A | 2 |
| NCT02444455 | Human Umbilical-Cord-Derived Mesenchymal Stem Cell Therapy in Acute Lung Injury (UCMSC-ALI) | Unknown | Affiliated Hospital to Acadmey of Military Medical Sciences | ARDS/ALI | Intravenous | Participants will/would receive 5 × 105 cells kg/body weight of human UC-MCSs on day 2, 7 and 14 | N/A | 1 and 2 |
| NCT02215811 | Treatment of Severe Acute Respiratory Distress syndrome with allogenic bone marrow-derived Mesenchymal Stromal cells | Unknown | Karolinska University | ARDS | N/A | Participants will/would receive biological MSCs | N/A | 1 |
| NCT02112500 | Mesenchymal stem cell in patients with acute severe respiratory failure (STELLAR) | Unknown | Asan Medical Center | Respiratory Distress Syndrome | Intravenous | Participants will/would receive biological MSCs | N/A | 2 |
| 2019-002688-89 | Phase 1/2 clinical study to assess the feasibility, safety, tolerability, and preliminary efficacy of the administration of HCR040, a drug whose active substance is HC016, allogeneic adipose-derived adult mesenchymal stem cells expanded and pulsed with H2O2, in patients with acute respiratory distress syndrome. (included patients COVID-19) | Ongoing | Histocell S.L | ARDS | Intravenous | Participants will receive HCR040 (allogenic adipose derived adult mesenchymal stem cells pulsed with H2O2) | No results available | 1 and 2 |
| 2020-001505-22 | Double-blind, randomized, parallel, placebo-controlled pilot clinical trial, nested in a prospective cohort observational study, for the evaluation of the efficacy and safety of two doses of WJ-MSC in patients with acute respiratory distress syndrome secondary to infection by COVID-19 | Ongoing | Banc de Sang I Teixits | ARDS | Intravenous | Participants will receive 2 doses of WJ-MSCs | N/A | 1 |
| NCT04447833 | Mesenchymal Stromal Cell Therapy for The Treatment of Acute Respiratory Distress Syndrome (ARDS-MSC-205) | Recruiting | Uppsala University | ARDS | Intravenous | 1st 3 participants will receive 1 × 106 and next 6 participants will receive 2 × 106 of allogenic BM-MSCs | N/A | 1 |
| NCT04456361 | Use of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Caused by COVID-19 | Active, not recruiting | Instituto de Medicina Regenerativa | ARDS, Human COVID-19 | Intravenous | Participants will receive 1 × 108 dose of Wharton jelly MSCs | N/A | 1 |
| NCT03807804 | Efficacy and Safety Study of HLCM051 (MultiStem® for Pneumonic Acute Respiratory Distress Syndrome (ONE-BRIDGE) | Recruiting | Healios K.K. | Respiratory Distress Syndrome | Intravenous | One dose of HCLM0S1 consisting of 9.0 × 108 of cells | N/A | 2 |
| NCT04371393 | MSCs in COVID-19 ARDS | Recruiting | Icahn School of Medicine at Mount Sinai | ARDS, COVID-19 | Intravenous | One dose of 2 × 106 MSCs cells/kg body weight | N/A | 3 |
| NCT02095444 | Using Human Menstrual Blood Cells to Treat Acute Lung Injury Caused by H7N9 Bird Flu Virus Infection | Unknown | S-Evans Biosciences Co., Ltd. | ALI/ARDS and multiple organ failure | Intravenous | Participants will receive 1 dose of 1 × 107 menstrual blood stem cells/kg body weight twice a week for 2 weeks | N/A | 1 and 2 |
| NCT04345601 | Mesenchymal Stromal Cells for the Treatment of SARS-CoV-2 Induced Acute Respiratory Failure (COVID-19 Disease) | Not recruiting yet | Baylor College of Medicine | ARDS/ COVID-19 | Intravenous | Participants will receive 1 × 108 MSCs. | N/A | Early phase 1 |
| NCT04452097 | Use of hUC-MSC Product (BX-U001) for the Treatment of COVID-19 With ARDS | Not recruiting yet | Baylx Inc. | ARDS/COVID-19 | Intravenous | Participants will receive 1 dose of 0.5 × 106, 1.0 × 106 or 1.5 × 106 cells/kg of body weight | N/A | 1 |
| NCT04400032 | Cellular Immuno-therapy for COVID-19 Acute Respiratory Distress Syndrome—Vanguard (CIRCA-19) | Not recruiting yet | Ottawa Hospital Research institute | COVID-19, ARDS | Intravenous | 75 × 106, 150 × 106 and 270 × 106 BM-MSCs given to 2 sets of groups | N/A | 1 |
| NCT04331613 | Safety and Efficacy of CAStem for Severe COVID-19 Associated With/Without ARDS | Recruiting | Chinese Academy of Sciences | COVID-19, Acute Respiratory Distress Syndrome, Pneumonia and Acute Lung Injury | Intravenous | 3 cohorts with 3 patients will receive 3 × 106, 5 × 106 and 10 × 106 | N/A | 1 and 2 |
| NCT04390152 | Safety and Efficacy of Intravenous Wharton’s jelly derived Mesenchymal stem cells in acute respiratory distress syndrome due to COVID-19 | Not recruiting yet | BioXcelleraltor | COVID-19, ARDS | Intravenous | Participants will receive 2 doses of 50 × 106 WJ MSC and hydroxychloroquine, lopinavir or azithromycin and ventilation support | N/A | 1 and 2 |
| NCT04345601 | Mesenchymal Stromal cells for the treatment of SARS-CoV-2 Induced Acute Respiratory Failure (COVID-19 Disease) | Not recruiting yet | Baylor College of Medicine | COVID-19, ARDS | Intravenous | Participants will be given 1 × 108 MSCs | N/A | 1 |
| NCT04390139 | Efficacy and safety of Evaluation of Mesenchymal stem cells for the treatment of patients with Respiratory Distress Due to COVID-19 (COVIDMES | Recruiting | Banc de Sang i Teixits | COVID-19, ARDS | Intravenous | Participants will receive 1 × 106 cells/kg body weight W-J MSCs on day 1 and day 3 | N/A | 1 and 2 |
| NCT04399889 | Human Cord Tissue- MSCs for COVID-19 | Not recruiting yet | Joanne Kurtzberg, MD | COVID-19, ARDS | Intravenous | Participants will receive hCT-MSCs | N/A | 1 and 2 |
| NCT04355728 | Use of UC-MSCs for COVID-19 Patients | Recruiting | Camillo Ricordi, University of Miami | COVID-19. ARDS, Acute Lung Injury | Intravenous | Participants will receive 1 × 108 UC-MSCs and standard treatment | N/A | 1 and 2 |
| NCT04377334 | Mesenchymal Stem Cells (MSCs) in inflammation-resolution programs of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS) | Not recruiting yet | University Hospital Tuebingen | COVID-19, ARDS | Intravenous | Participants will receive allogenic bone marrow-derived human mesenchymal stem cells | N/A | 2 |
| NCT04348461 | Battle Against COVID-19 Using Mesenchymal Stromal Cells | Not recruiting yet | Instituto de Investigacóin Sanitaria de la Fundación Jieménez Díaz | COVID-19, Respiratory Distress Syndrome | Intravenous | Participants to receive 2 doses of 1.5 × 106 ad-MSCs cells/kg body weight | N/A | 2 |
| NCT04367077 | MultiStem Administration for COVID-19 Induced ARDS (MACoVIA) (MACoVIA) | Recruiting | Athersys | COVID-19, ARDS | Intravenous | Participants to receive doses of MultiStem | N/A | 2 and 3 |
| NCT No. | Title | Status | Company Name | Disease/Conditions | Route of Administration | Intervention/Mechanism Target | Results | Phase |
|---|---|---|---|---|---|---|---|---|
| NCT02789995 | Dysfunctions of human muscle stem cells in Sepsis | Completed | Institut Pasteur | Sepsis | Intravenous Donation | Study of patients with or without sepsis, blood and bone marrow sample and muscle biopsy | N/A | N/A |
| NCT02421484 | Cellular immunotherapy for septic shock: A phase 1 trial (CISS) | Completed | Ottawa Hospital Research Institute | Sepsis | Intravenous | 0.3 × 106, 1 × 106 and 3 × 106 cells/kg body weight was administered to participants | Single dose of MSCs demonstrated to be safe and was tolerated well. | 1 |
| NCT03369275 | Cellular Immunotherapy for Septic Shock (CISS2) | Not yet recruiting | Ottawa Hospital Research Institute | Sepsis, Septic Shock | Intravenous | Participants will receive 3 × 108 BM- HMSCs | N/A | 2 |
| NCT01849237 | Russian clinical trial of mesenchymal stem cells in patients with septic shock and severe neutropenia | Unknown | National Research center for Hematology | Sepsis | Intravenous | Participants will receive 1–2 × 106 MSC intravenous infusions up to 10 h after septic shock. | N/A | 1 & 2 |
| NCT02883803 | Treatment of Severe Infections with Mesenchymal Stem Cells (CHOCMSC) | Not yet recruiting | Central Hospital Nancy France | Septic Shock | Intravenous | Participants will receive 1 × 106 cells/kg body weight after 12 h of septic shock | N/A | 2 |
| NCT02328612 | Randomized, Parallel Group, Placebo Control, Unicentric, Interventional Study to Assess the Effect of Expanded Human Allogeneic Adipose-derived Mesenchymal Adult Stem Cells on the Human Response to Lipopolysaccharide in Human Volunteers (CELLULA) | Completed | Tigenix S.A.U | Sepsis | Intravenous | 0.25 × 106, 1 × 106 and 4 × 106 cells/kg body weight were administered to different sets of participants | Intravenous infusion of the cells exhibited anti-inflammatory effects and proved to be safe and efficient | 1 |
| NCT No. | Title | Status | Company Name | Disease/Conditions | Route of Administration | Intervention/Mechanism Target | Results | Phase |
|---|---|---|---|---|---|---|---|---|
| NCT04333368 | Cell therapy using Umbilical cord-derived Mesenchymal Stromal Cells in SARS-CoV-2 related ARDS (STROMA-CoV2) | Recruiting | Hopitaux de Paris | COVID-19 | Intravenous | Participants will receive 1 × 106 UC-MSCs in 60 min. Via peripheral or central venous line | N/A | 1 and 2 |
| NCT04392778 | Clinical use of stem cells for the treatment of COVID-19 | Recruiting | SBÜ | COVID-19, Pneumonia, Multiple Organ Failure, CoronaVirus Infection | Intravenous | Participants will receive 3 × 106 MSC on Day 0, 3 and 6. | N/A | 1 and 2 |
| NCT04252118 | Mesenchymal stem cell treatment for pneumonia patients infected with COVID-19 | Recruiting | Beijing 302 Hospital | COVID-19 | Intravenous | Participants will receive 3 × 107 of MSCs on day 0, 3 and 6 | N/A | 1 |
| NCT04348435 | A randomized, double-blind, placebo-controlled clinical trial to determine the safety and efficacy of hope biosciences allogenic mesenchymal stem cell therapy (HB-adMSCs) to provide protection against COVID-19 | Enrolling by Invitation | Hope biosciences | COVID-19 | Intravenous | 2 × 108, 1 × 108 0.5 × 108 and 0.1 × 108 Allogenic HB-adMSCs given to 4 different sets participants on day 0, 2, 6, 10, and day 14 | N/A | 2 |
| NCT04366063 | Mesenchymal Stem cell Therapy for SARS-CoV-2 related Acute Respiratory Distress Syndrome | Recruiting | Royan Institute | COVID-19 | Intravenous | Participants will receive 2 doses of 100 × 106 MSC at day 0 and 2 and 2 doses of EVs at day 4 and 6 | N/A | 2 and 3 |
| NCT04288102 | Treatment with mesenchymal system cells for severe Coronavirus Disease 2019 (COVID-19) | Recruiting | Beijing 302 | COVID-19 | Intravenous | Participants will receive 4 × 107 MSCs 3 times a day on day 0, 3 and 6 | N/A | 2 |
| NCT04349631 | A Clinical Trial to Determine the safety and efficacy of Hope Biosciences Autologous Mesenchymal Stem Cell Therapy (HB-adMSCs) to provide protection Against COVID-19 | Enrolling by Invitation | Hope biosciences | COVID-19 | Intravenous | Participants will receive 5 IV infusions of autologous, AD-MSCs collected and infused and follow up on week 6, 14, 26 | N/A | 2 |
| NCT04324996 | A Phase I/II Study of universal off-the-shelf- NKG2D-ACE2 CAR-NK Cells for Therapy of COVID-19 | Recruiting | Chongqing Public Health Medical Center | COVID-19 | Intravenous | Participants will receive 1 × 108 cells administered per kilogram of body weight | N/A | 1 and 2 |
| NCT04273646 | Study of Human Umbilical Cord Mesenchymal Stem cells in the treatment of Severe COVID-19 | Not recruiting yet | Wuhan Union Hospital China | COVID-19, 2019 Novel Coronavirus pneumonia | Intravenous | Participants will receive 0.5 × 106 UC-MSCs cells/kg body weight administered on day 1, day 3, day 5, and day 7 | N/A | N/A |
| NCT04397471 | A study to collect bone marrow for process development and production of BM-MSC to treat severe COVID-19 Pneumonitis (COMET20d | Not recruiting yet | Cambridge Cellular Therapies Laboratory | COVID-19, Pneumonia | Donation | 30–80 mL sample of bone marrow collected from posterior superior iliac crests | N/A | N/A |
| NCT04382547 | Treatment of COVID-19 Associated Pneumonia with Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells | Enrolling by Invitation | Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus | COVID-19, Pneumonia | Intravenous | Participants will receive standard treatments and also allogenic pooled olfactory mucosa derived MSCs | N/A | 1 and 2 |
| NCT04366271 | Clinical Trial of Allogenic Mesenchymal Cells from Umbilical Cord Tissue with Patients with COVID-19 (MESCEL-COVID-19) | Recruiting | Hospital Infantil Universitario Nino Jesus, Madrid Spain | COVID-19 | Intravenous | Participants will receive 1 infusion of undifferentiated allogenic UC-MSCs | N/A | 2 |
| NCT04361942 | Treatment of Severe COVID-19 Pneumonia with Allogeneic Mesenchymal Stromal Cells (COVID_MSV) (COVID_MSV) | Recruiting | Red de Terapia Celular | COVID-19, Pneumonia | Intravenous | Participants will receive 1 × 106 MSCs | N/A | 2 |
| NCT04346368 | Bone Marrow-Derived Mesenchymal Stem cell treatment for severe patients with coronavirus disease 2019 (COVID-19 | Not recruiting yet | Guangzhou Institute of Respiratory Disease | COVID-19 | Intravenous | Participants will receive 1 × 106 MSCs kg/body weight on day 1 | N/A | 1 and 2 |
| NCT04416139 | Mesenchymal stem cells for acute respiratory distress Syndrome due to COVID-19 | Recruiting | Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran | COVID-19 | Intravenous | Participants will receive a single dose of 1 × 106 MSCs | N/A | 2 |
| NCT04336254 | Safety and Efficacy Study of Allogenic Human Dental Pulp Mesenchymal Stem Cells to treat severe COVID-19 Patients | Recruiting | Renmin Hospital of Wuhan University | COVID-19 | Intravenous | Participants will receive 3 × 107 human dental pulp stem cells on day 1, day 4 and day 7 | N/A | 1 and 2 |
| NCT04428801 | Autologous Adipose-derived Stem cells (Ad-MSCs) for COVID-19 | Not recruiting yet | Celltex Therapeutics Corporation | COVID-19 | Intravenous | Participants will receive 2 × 108 Ad-MSCs on day 0, day 3 and day 6 | N/A | 2 |
| NCT04269525 | Umbilical Cord (UC)-Derived Mesenchymal Stem Cells (MSCs) Treatment for the 2019-novel Coronavirus (nCOV) Pneumonia | Recruiting | ZhiYong Peng, Zhongnan Hospital | COVID-19 Pneumonia | Intravenous | Participants will receive UC-MSC infusions and day 1, day 3 and day 5 | N/A | 2 |
| NCT04429763 | Safety and Efficacy of Mesenchymal Stem Cells in the management of severe COVID-19 pneumonia | Not recruiting yet | Trustem | COVID-19 | Intravenous | Participants will receive 1 dose of 1 × 106 MSCs | N/A | 2 |
| NCT04366323 | Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Allogeneic Adult Mesenchymal Stem Cells of Expanded Adipose Tissue in Patients with Severe Pneumonia Due to COVID-19 | Recruiting | Andalusian Network for design and translation of Advanced Therapies | COVID-19 | Intravenous | Participants will receive 8 × 107 allogenic Ad-MSCs | N/A | 1 and 2 |
| NCT04352803 | Adipose Mesenchymal cells for Abadement of SARS-CoV-2 Respiratory Compromise in COVID-19 Disease | Not recruiting yet | Regeneris Medical | COVID-19, Cytokine Storm | Intravenous | Participants will receive 0.5 × 106 autologous ad-MSCs | N/A | 1 and 2 |
| NCT04398303 | ACT-20 in Patients with severe COVID-19 Pneumonia | Not recruiting yet | Aspire Health Science | COVID-19 Pneumonia | Intravenous | Participants will receive 1 × 106 allogenic human umbilical derived MSCs | N/A | 2 |
| NCT04339660 | Clinical Research of Human Mesenchymal Stem Cells in the Treatment of COVID-19 Pneumonia | Recruiting | Puren Hospital Affliated to Wuhan University of Science and Technology | COVID-19 | Intravenous | Participants will receive 1 × 106 per kg/bodyweight UC-MSCs | N/A | 1 |
| NCT04444271 | Mesenchymal Stem Cell Infusion for COVID-19 Infection | Recruiting | Armed Forces Bone Marrow | COVID-19 | Intravenous | Participants will receive 1 × 106 cells/kg body weight of MSCs | N/A | 1 and 2 |
| 2020-001682-36 | Double-blind, placebo-controlled phase I/II clinical trial to evaluate the safety and efficacy of allogeneic mesenchymal stem cells (MSV®-allo) in acute respiratory failure in patients with COVID-19 pneumonia. | Ongoing | CITOSPIN S.L. | COVID-19 | Intravenous | Adult allogeneic stem cell mesenchymal stem cells expanded in suspension | N/A | 1 |
| NCT0444520 | A Study of Cell Therapy in COVID-19 Subjects with Acute Kidney Injury Who Are Receiving Renal Replacement Therapy | Not recruiting yet | Sentien Biotechnologies, Inc. | COVID-19 | Integration | Allogenic SB-101 biologic combination device | N/A | 1 |
| NCT04461925 | Treatment of Coronavirus COVID-19 Pneumonia (Pathogen SARS-CoV-2) With Cryopreserved Allogeneic P_MMSCs and UC-MMSCs | Recruiting | Institute of Cell Therapy | COVID-19 and Pneumonia | Intravenous | Participants will receive 1 × 106 cells/kg body weight 3 times a day on day 1, 4 and 7 | N/A | 1 and 2 |
| NCT04445454 | Mesenchymal Stromal Cell Therapy for Severe Covid-19 Infection | Recruiting | University of Liege | COVID-19 | Intravenous | Participants will receive 3 doses of (1.5)–3.0 × 106/BM-MSC kg/body weight at 3–4 days interval | N/A | 1 and 2 |
| NCT04437823 | Efficacy of Intravenous Infusions of Stem Cells in the Treatment of COVID-19 Patients | Recruiting | Jinnah Hospital | COVID-19 | Intravenous | Participants will receive 5 × 105 of UC-MSCs on day 1,3 and 5 | N/A | 2 |
| NCT04456361 | Use of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Caused by COVID-19 | Active, not recruiting | Instituto de Medicina Regenerativa | COVID-19 | Intravenous | Participants will receive 1 × 108 of Wharton jelly derived UC-MSCs | N/A | 2 |
| NCT04457609 | Administration of Allogenic UC-MSCs as Adjuvant Therapy for Critically Ill COVID-19 Patients | Recruiting | Indonesia University | COVID-19 | Intravenous | Participants will 1st receive standardized treatment oseltamivir and azithromycin and then 1 × 106 UC-MSCs in 100 cc of 0.9% NaCl in 1 h | N/A | 2 and 3 |
| NCT04467047 | Safety and Feasibility of Allogenic MSC in the Treatment of COVID-19 (COVID19) | Not recruiting yet | Hospital de Clinicas de Porto Alegre | COVID-19 | Intravenous | Participants will receive 1 × 106 MSCs | N/A | 1 |
| NCT04466098 | Multiple Dosing of Mesenchymal Stromal Cells in Patients with ARDS (COVID-19) | Not recruiting yet | Masonic Cancer Center, University of Minnesota | COVID-19 | Intravenous | Participants will receive a thawed product consisting of 300 × 106 in DMSO in 1: w/ Dextran 40 + 5% human serum albumin | N/A | 2 |
| NCT04486001 | Study of Intravenous Administration of Allogeneic Adipose Stem Cells for COVID-19 (CoronaStem1) | Not recruiting yet | Personalized Stem Cells, Inc. | COVD-19 | Intravenous | Participants will receive adipose derived stem cells | N/A | 1 |
| NCT04490486 | Umbilical Cord Tissue (UC) Derived Mesenchymal Stem Cells (MSCs) Versus Placebo to Treat Acute Pulmonary Inflammation Due to COVID-19 (COVID-19) | Not recruiting yet | Joshua M Hare | COVID-19 | Intravenous | Participants will receive 1 × 108 UC-MSCS on day 0 and day 3 | N/A | 1 |
| NCT04456439 | Intermediate-size Expanded Access Program (EAP), Mesenchymal Stromal Cells (MSC) for Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease (COVID-19) | Available | Mesoblast International Sàrl | COVID-19 | Intravenous | Participants will receive 2 × 106 remestemcel-L within a 5-day period | N/A | N/A |
| NCT04313322 | Treatment of COVID-19 Patients using Wharton Jelly Mesenchymal Stem Cells | Recruiting | Stem Cells Arabia | COVID-19 | Intravenous | Participants will receive WJ-MSCs suspended in 25 mL of saline solution | N/A | 1 |
| NCT04371601 | Safety and effectiveness of mesenchymal stem cells in the treatment of pneumonia of coronavirus disease 2019 | Active, Not recruiting | Fuzhou General Hospital | COVID-19 Pneumonia | Intravenous | Participants will first receive standard treatment (oseltamivir and hormones) followed by 1 dose of 1 × 106 cells kg/body weight once daily for 4 days | N/A | 1 |
| NCT04362189 | Efficacy and Safety of Allogenic HB-adMSCs for the treatment of COVID-19 | Recruiting | Hope biosciences | COVID-19 | Intravenous | Participants will receive 1 × 108 of HB-adMSCs on day 0, 3, 7 and 10 | N/A | 2 |
| NCT04384445 | Organicell Flow for Patients With COVID-19 | Not yet recruiting | Organicell Regenerative Medicine | COVID-19 | Intravenous | Participants will receive 1 mL of organicell flow on days 0, 4, and 8 | N/a | 1 and 2 |
| NCT04341610 | ASC Therapy for Patients with Severe Respiratory COVID-19 (ASC COVID-19) | Withdrawn (Not approved by ethical committee) | Rigshospitalet, Denmark | Respiratory tract diseases (COVID-19) | Intravenous | 100 million allogenic adipose-derived mesenchymal stem cells in 100 mL saline | N/A | 1 and 2 |
| NCT04293692 | Therapy for Pneumonia Patients Infected by 2019 Novel Coronavirus | Withdrawn (Patients were transferred to designated hospitals for treatment as needed, the clinical trials cannot be conducted.) | Puren Hospital Affiliated to Wuhan University of Science and Technology | COVID | Intravenous | 0.5 × 106 UC-MSCs kg/body weight suspended in 100 mL saline on day 1, day 3, day 5 and day 7 | N/A | N/A |
| NCT No. | Title | Status | Company Name | Disease/Conditions | Route of Administration | Intervention/Mechanism Target | Results | Phase |
|---|---|---|---|---|---|---|---|---|
| NCT04313647 | A Tolerance Clinical Study on Aerosol Inhalation of Mesenchymal Stem Cells Exosomes in Healthy Volunteers | Recruiting | Ruijin Hospital | Safety and Tolerance | Inhalation | 2 × 108, 4 × 108, 8 × 108, 16 × 108, 20 × 108 nano vesicles/3 mL to be administered to different sets of participants | N/A | 1 |
| NCT04473170 | Study Evaluating the Safety and Efficacy of Autologous Non-Hematopoietic Peripheral Blood Stem Cells in COVID-19 (SENTAD-COVID) | Completed | Abu Dhabi Stem Cells Center | COVID-19 | Inhalation | Participants were put into groups; group A received NHPBSC through jet nebulization and group B received standard care | N/A | 1 and 2 |
| NCT04389385 | COVID-19 Specific T cell-derived Exosomes (CSTC-Exo) | Active, Not recruiting yet | TC Erciyes University | COVID-19 | Inhalation | Participants will receive specific T cell-derived exosomes (CSTC-Exo) Aerosol inhalation of CSTC-Exo (2.0 × 108 nanovesicles / 3 mL at Day 1, 2, 3, 4 and 5 times daily | N/A | 1 |
| NCT04491240 | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia (COVID-19EXO) | Enrolling by Invitation | State-Financed Health Facility “Samara Regional Medical Center Dinasty” | COVID-19 | Inhalation | 2 sets of participants will receive 0.5–2 × 1010 of nanoparticles (exosomes) | N/A | 1 and 2 |
| NCT04276987 | A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus | Not recruiting yet | Ruijin Hospital | COVID-19 | Inhalation | Participants will receive MSC-derived exosomes 5 times, aerosol inhalations of MSC-derived exosomes (2.0 × 108 nano vesicles/3 mL at Days 1, 2, 3, 4 and 5) | N/A | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brave, H.; MacLoughlin, R. State of the Art Review of Cell Therapy in the Treatment of Lung Disease, and the Potential for Aerosol Delivery. Int. J. Mol. Sci. 2020, 21, 6435. https://doi.org/10.3390/ijms21176435
Brave H, MacLoughlin R. State of the Art Review of Cell Therapy in the Treatment of Lung Disease, and the Potential for Aerosol Delivery. International Journal of Molecular Sciences. 2020; 21(17):6435. https://doi.org/10.3390/ijms21176435
Chicago/Turabian StyleBrave, Hosanna, and Ronan MacLoughlin. 2020. "State of the Art Review of Cell Therapy in the Treatment of Lung Disease, and the Potential for Aerosol Delivery" International Journal of Molecular Sciences 21, no. 17: 6435. https://doi.org/10.3390/ijms21176435