Changes in Chemical Composition and Accumulation of Cryoprotectants as the Adaptation of Anholocyclic Aphid Cinara tujafilina to Overwintering
Abstract
:1. Introduction
2. Results
2.1. Chemical Compositions
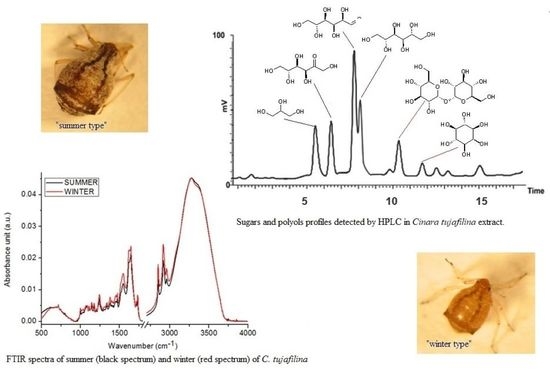
2.2. Sugar and Polyols Analysis
3. Discussion
4. Materials and Methods
4.1. Aphids and Sample Collection
4.2. Determination of Chemical Compositions
4.3. Determination of Sugars and Polyols
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danks, H.V. The elements of seasonal adaptations in insects. Can. Entomol. 2007, 139, 1–44. [Google Scholar] [CrossRef]
- Hullé, M.; Coeur d’Acier, A.; Bankhead-Dronnet, S.; Harrington, R. Aphids in the face of global changes. Comptes Rendus Biol. 2010, 333, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Liu, H.; Qiao, G.; Huang, X. Investigating the impact of climate warming on phenology of aphid pests in China using long-term historical data. Insects 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, K.B.; Storey, J.M. Biochemistry of cryoprotectants. In Insects at Low Temperature; Denlinger, D., Lee, R.E., Eds.; Chapman and Hall: New York, NY, USA, 1991; pp. 64–93. [Google Scholar]
- Hodkova, M.; Hodek, I. Photoperiod, diapause and cold-hardiness. Eur. J. Entomol. 2004, 101, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Koštál, V.; Šlachta, M.; Šimek, P. Cryoprotective role of polyols independent of the increase in supercooling capacity in diapausing adults of Pyrrhocoris apterus (Heteroptera: Insecta). Comp. Biochem. Physiol. B 2001, 130, 365–374. [Google Scholar] [CrossRef]
- Koštál, V.; Zahradníčková, H.; Šimek, P.; Zelený, J. Multiple component system of sugars and polyols in the overwintering spruce bark beetle, Ips typographus. J. Insect Physiol. 2007, 53, 580–586. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Aphid Ecology, 2nd ed.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Strathdee, A.T.; Howling, G.G.; Bale, J.S. Cold hardiness of overwintering aphid eggs. J. Insect Physiol. 1995, 41, 653–657. [Google Scholar] [CrossRef]
- Nelson, J.; Denlinger, D.L.; Somers, D.E. Photoperiodism: The Biological Calendar; Oxford University Press Inc.: Oxford, UK, 2009. [Google Scholar]
- Vuong, P.T.; Kim, J.; Song, Y. Overwintering two aphid species, Lipaphis pseudobrassicae and Myzus persicae (Homoptera: Aphididae), in southern greenhouse area in Korea. J. Asia Pac. Entomol. 2003, 6, 63–67. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Trees: An Identification and Information Guide; CAB International & The Natural History Museum: London, UK, 1994. [Google Scholar]
- Furuta, K. Annual alternating population size of the thuja aphid, Cinara tujafilina (Del Guercio), and the impacts of syrphids and disease. J. Appl. Entomol. 1988, 105, 344–354. [Google Scholar] [CrossRef]
- Carter, C.I.; Maslen, N.R. Conifer Lachnids in Britain. For. Comm. Bull. 1982, 58, 4–75. [Google Scholar]
- Colombo, M.; Parisini, M. Nuovi acquisizioni sulla biologia e sul controllo di Cinara (Del Guercio) (Aphidodea, Lachnidae). Boll. Zool. Agrar. Bachicolt. 1984, 18, 191–194. [Google Scholar]
- Durak, R. The overwintering strategy of the anholocyclic aphid Cinara tujafilina. Physiol. Entomol. 2014, 39, 313–321. [Google Scholar] [CrossRef]
- Storey, K.; Storey, J. Insect cold hardiness protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Saeidi, F.; Moharramipour, S.; Barzegar, M. Seasonal patterns of cold hardiness and cryoprotectant profiles in Brevicoryne brassicae (Hemiptera: Aphididae). Environ. Entomol. 2012, 41, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Rickards, J.; Kelleher, M.J.; Storey, K.B. Strategies of freeze avoidance in larvae of the goldenrod gall moth, Epiblema scudderiana: Winter profiles of a natural population. J. Insect Physiol. 1987, 33, 443–450. [Google Scholar] [CrossRef]
- Hansen, T.E.; Viyk, M.O.; Luyk, A.K. Biochemical changes and cold hardiness in overwintering bark-beetles Ips typographus. Entomol. Rev. 1982, 59, 9–12. [Google Scholar]
- Saeidi, F.; Moharramipour, S.; Mikani, A. Effect of cold acclimation and rapid cold hardiness on cold tolerance and cryoprotectants of the greenbug Schizaphis graminum (Hemiptera: Aphididae). J. Entomol. Soc. Iran 2017, 37, 193–205. [Google Scholar]
- Li, Y.P.; Goto, M.; Ito, S.; Sato, Y.; Sasaki, K.; Goto, N. Physiology of diapause and cold hardiness in the overwintering pupae of the fall webworm Hyphantria cunea in Japan. J. Insect Physiol. 2001, 47, 1181–1187. [Google Scholar] [CrossRef]
- Khani, A.; Moharramipour, S.; Barzegar, M. Cold tolerance and trehalose accumulation in overwintering larvae of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). Eur. J. Entomol. 2007, 104, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Moriwaki, N.; Matsuhita, K.; Nishina, M.; Kono, Y. High concentrations of trehalose in aphid hemolymph. Appl. Entomol. Zool. 2003, 38, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Koštál, V.; Šimek, P. Biochemistry and physiology of aestivo-hibernation in the adult apple blossom weevil, Anthonomus pomorum (Coleoptera: Curculionidae). J. Insect Physiol. 1996, 42, 727–733. [Google Scholar] [CrossRef]
- Watanabe, M. Cold tolerance and myo-inositol accumulation in overwintering adults of a lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2002, 99, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Tanaka, K. Seasonal change of the thermal response in relation to myo-inositol metabolism in adults of Aulacophora nigripennis (Coleoptera Chrysomelidae). J. Insect Physiol. 1999, 45, 167–172. [Google Scholar] [CrossRef]
- Moriwaki, N.; Matsuhita, K.; Nishina, M.; Nishina, M.; Kono, Y. High myo-inositol concentration in the hemolymph of planthoppers. Appl. Entomol. Zool. 2003, 38, 359–364. [Google Scholar] [CrossRef]
- Vesala, L.; Salminen, T.S.; Kostál, V.; Zahradníčkov, H.; Hoikkala, H. Myo-inositol as a main metabolite in overwintering flies: Seasonal metabolomic profiles and cold stress tolerance in a northern drosophilid fly. J. Exp. Biol. 2012, 215, 2891–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrix, D.; Salvucci, M. Polyol metabolism in homopterans at high temperatures: Accumulation of mannitol in aphids (Aphididae: Homoptera) and sorbitol in whiteflies (Aleyrodidae: Homoptera). Comp. Biochem. Physiol. A 1998, 120, 487–494. [Google Scholar] [CrossRef]
- Fischer, M.K.; Völkl, W.; Hoffmann, K.H. Honeydew production and honeydew sugar composition of polyphagous black bean aphid, Aphis fabae (Hemiptera: Aphididae) on various host plants and implications for ant-attendance. Eur. J. Entomol. 2005, 102, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Schlöder, P.; Steele, J.E.; Wegener, G. The regulation of trehalose metabolism in insects. Experientia 1996, 52, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, D.R.; Storey, K.B. Enzyme activity profiles in an overwintering population of freeze-avoiding gall moth larvae, Epiblema scudderiana. Can. J. Zool. 1994, 72, 1079–1084. [Google Scholar] [CrossRef]
- Doležal, P.; Sehnal, F. Effects of photoperiod and temperature on the development and diapause of the bark beetle Ips typographus. J. Appl. Entomol. 2007, 131, 165–173. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Crowe, J.H. The mechanism of cryoprotection of proteins by solutes. Cryobiology 1988, 25, 244–255. [Google Scholar] [CrossRef]
- Kandror, O.; DeLeon, A.; Goldberg, A.L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 2002, 99, 9727–9732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.I.; Clegg, J.S. Influence of trehalose on the molecular chaperone activity of a small heat shock/a-crystallin protein. Cell Stress Chaperones 2001, 6, 126–135. [Google Scholar] [CrossRef]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Perez, A.; Burg, M.B. Role of organic osmolytes in adaptation of renal cells to high osmolality. J. Membr. Biol. 1991, 119, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ditrich, T.; Koštal, V. Comparative analysis of overwintering physiology in nine species of semi-aquatic bugs (Heteroptera: Gerromorpha). Physiol. Entomol. 2011, 36, 261–270. [Google Scholar] [CrossRef]
- Ammar, E.; Alessandro, R.T.; Hall, D.G. Ultrastructural and chemical studies on waxy secretions and wax-producing structures on the integument of the woolly oak aphid Stegophylla brevirostris Quednau (Hemiptera: Aphididae). J. Microsc. Ultrastruct. 2013, 1, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Coury, C.; Dillner, A.M. A method to quantify organic functional groups and inorganic compounds in ambient aerosols using attenuated total reflectance FTIR spectroscopy and multivariate chemometric techniques. Atmos. Environ. 2008, 42, 5923–5932. [Google Scholar] [CrossRef]
- Lasch, P.; Boese, M.; Pacifico, A.; Diem, M. FT-IR spectroscopic investigations of single cells on the subcellular level. Vib. Spectrosc. 2002, 28, 147–157. [Google Scholar] [CrossRef]
- Pickeringa, C.; Handsa, J.R.; Fullwooda, L.M.; Smith, J.A.; Bakera, M.J. Rapid discrimination of maggots utilising ATR-FTIR spectroscopy. Forensic Sci. Int. 2015, 249, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheeseman, S.; Truong, V.; Vongsvivut, J.; Tobin, M.J.; Crawford, R.; Ivanova, E.P. Applications of Synchrotron-Source IR Spectroscopy for the Investigation of Insect Wings; Intechopen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef] [Green Version]








| Wavenumber (cm−1) | Summer Type of C. tujafilina (a.u.) ± SEM | Winter Type of C. tujafilina (a.u.) ± SEM |
|---|---|---|
| 1018 | 0.00256 ± 0.00007 | 0.00351 ± 0.00006 |
| 1172 | 0.00451 ± 0.00007 | 0.00521 ± 0.00007 |
| 1252 | 0.00488 ± 0.00004 | 0.00613 ± 0.00003 |
| 1325 | 0.00407 ± 0.00010 | 0.00483 ± 0.00010 |
| 1394 | 0.00488 ± 0.00006 | 0.00612 ± 0.00009 |
| 1480 | 0.00489 ± 0.00007 | 0.00617 ± 0.00007 |
| 1513 | 0.00880 ± 0.00003 | 0.01189 ± 0.00008 |
| 1637 | 0.01975 ± 0.00010 | 0.02231 ± 0.00005 |
| 1741 | 0.00338 ± 0.00005 | 0.00400 ± 0.00006 |
| 2827 | 0.00548 ± 0.00003 | 0.00692 ± 0.00005 |
| 2919 | 0.01869 ± 0.00006 | 0.02327 ± 0.00010 |
| 2957 | 0.01342 ± 0.00005 | 0.01671 ± 0.00003 |
| Wavenumber (cm−1) | October (a.u.) ±SEM | November (a.u.) ±SEM | December (a.u.) ±SEM |
|---|---|---|---|
| 1018 #,*,& | 0.00230 ± 0.00004 | 0.00390 ± 0.00009 | 0.00351 ± 0.00006 |
| 1172 #,*,& | 0.00212 ± 0.00007 | 0.00379 ± 0.00006 | 0.00521 ± 0.00007 |
| 1252 #,*,& | 0.00428 ± 0.00006 | 0.00645 ± 0.00007 | 0.00613 ± 0.00003 |
| 1325 #,*,& | 0.00408 ± 0.00009 | 0.00590 ± 0.00007 | 0.00483 ± 0.00010 |
| 1394 #,& | 0.00606 ± 0.00010 | 0.00798 ± 0.00004 | 0.00612 ± 0.00009 |
| 1480 #,*,& | 0.00553 ± 0.00005 | 0.00769 ± 0.00010 | 0.00617 ± 0.00007 |
| 1513 #,*,& | 0.01162 ± 0.00003 | 0.01549 ± 0.00006 | 0.01189 ± 0.00008 |
| 1637 #,*,& | 0.02186 ± 0.00008 | 0.02484 ± 0.00008 | 0.02231 ± 0.00005 |
| 1741 #,*,& | 0.00123 ± 0.00010 | 0.00208 ± 0.00009 | 0.00400 ± 0.00006 |
| 2827 #,*,& | 0.00556 ± 0.00009 | 0.00751 ± 0.00010 | 0.00692 ± 0.00005 |
| 2919 #,*,& | 0.01447 ± 0.00007 | 0.01990 ± 0.00002 | 0.02327 ± 0.00010 |
| 2957 #,*,& | 0.01296 ± 0.00006 | 0.01577 ± 0.00003 | 0.01671 ± 0.00003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, R.; Depciuch, J.; Kapusta, I.; Kisała, J.; Durak, T. Changes in Chemical Composition and Accumulation of Cryoprotectants as the Adaptation of Anholocyclic Aphid Cinara tujafilina to Overwintering. Int. J. Mol. Sci. 2021, 22, 511. https://doi.org/10.3390/ijms22020511
Durak R, Depciuch J, Kapusta I, Kisała J, Durak T. Changes in Chemical Composition and Accumulation of Cryoprotectants as the Adaptation of Anholocyclic Aphid Cinara tujafilina to Overwintering. International Journal of Molecular Sciences. 2021; 22(2):511. https://doi.org/10.3390/ijms22020511
Chicago/Turabian StyleDurak, Roma, Joanna Depciuch, Ireneusz Kapusta, Joanna Kisała, and Tomasz Durak. 2021. "Changes in Chemical Composition and Accumulation of Cryoprotectants as the Adaptation of Anholocyclic Aphid Cinara tujafilina to Overwintering" International Journal of Molecular Sciences 22, no. 2: 511. https://doi.org/10.3390/ijms22020511