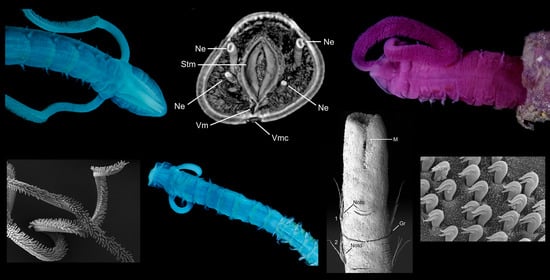
On the Systematics and Biodiversity of the Palaeoannelida
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Magelonidae Cunningham and Ramage, 1888
3.1.1. Systematics
3.1.2. Taxonomic History
3.1.3. Taxonomic Characters and External Morphology
3.1.4. Internal Morphology
3.1.5. Species Diversity and Distribution
3.1.6. Biology and Ecology
3.2. Family Oweniidae Rioja, 1917
3.2.1. Systematics
3.2.2. Taxonomic History
3.2.3. Taxonomic Characters
3.2.4. Internal Morphology
3.2.5. Species Diversity and Distribution
3.2.6. Biology and Ecology
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| SPECIES | TYPE LOCALITY | REALM | DEPTH (m) |
|---|---|---|---|
| Magelona agoensis Kitamori, 1967 | Ago Bay, Japan | Temperate Northern Pacific | 5 |
| Magelona alexandrae Magalhães, Bailey-Brock and Watling, 2018 | Hawaii, USA | Eastern Indo-Pacific | 39.6 |
| Magelona alleni Wilson, 1958 | Plymouth, England | Temperate Northern Atlantic | Intertidal |
| Magelona americana Hartman, 1965 | off New England, USA | Temperate Northern Atlantic | N.d. |
| Magelona annulata Hartmann-Schröder, 1962 | Isla Blanca, Chimbote Bay, Peru | Temperate South America | 8 |
| Magelona anuheone Magalhães, Bailey-Brock and Watling, 2018 | Easter Island | Eastern Indo-Pacific | Shallow |
| Magelona berkeleyi Jones, 1971 | Puget Sound, Washington, USA | Temperate Northern Pacific | 32–40 |
| Magelona californica Hartman, 1944 | Mission Bay, California, USA | Temperate Northern Pacific | ? |
| Magelona capax Hartman, 1965 | off the mouth of the Amazon River, Brazil | Tropical Atlantic | 1500 |
| Magelona capensis Day, 1961 | Agulhas Bank, South Africa | Temperate South Africa | 86 |
| Magelona cepiceps Mortimer and Mackie, 2006 | Mahé, Seychelles | Western Indo-Pacific | 33–45 |
| Magelona cerae Hartman and Reish, 1950 | Coos Bay, Oregon, USA | Temperate Northern Pacific | 37–73 |
| Magelona cincta Ehlers, 1908 | Algoa Bay, South Africa | Temperate South Africa | 40 |
| Magelona cinthyae Magalhães, Bailey-Brock and Watling, 2018 | Hawaii, USA | Eastern Indo-Pacific | 31–39 |
| Magelona conversa Mortimer and Mackie, 2003 | Mahé, Seychelles | Western Indo-Pacific | 26–42 |
| Magelona cornuta Wesenberg-Lund, 1949 | E by N of Ras Jagin, Gulf of Oman | Western Indo-Pacific | 12 |
| Magelona crenulata Bolívar and Lana, 1986 | Paranaguá Bay, Brazil | Temperate South America | 10 |
| Magelona crenulifrons Gallardo, 1968 | Nha Trang Bay, Viet Nam | Central Indo-Pacific | 6–48 |
| Magelona dakini Jones, 1978 | Careel Bay, New South Wales, Australia | Temperate Australasia | Shallow |
| Magelona debeerei Clarke, Paterson, Florence and Gibbons, 2010 | Beverly Hill, Namibia | Temperate South Africa | 37 |
| Magelona equilamellae Harmelin, 1964 | Rade de Villefranche and Golfe de Marseille, France | Temperate Northern Atlantic | 13–18 |
| Magelona falcifera Mortimer and Mackie, 2003 | Mahé, Seychelles | Western Indo-Pacific | 10–56 |
| Magelona fauchaldi Shakouri, Mortimer and Dehani, 2017 | Chabahar Bay, Iran, Gulf of Oman | Western Indo-Pacific | 1.5–20 |
| Magelona filiformis Wilson, 1959 | Mill Bay, Salcombe, England | Temperate Northern Atlantic | Intertidal |
| Magelona gemmata Mortimer and Mackie, 2003 | Mahé, Seychelles | Western Indo-Pacific | 48–62 |
| Magelona hartmanae Jones, 1978 | near Port Hueneme and Oxnard, California, USA | Temperate Northern Pacific | 12.7 |
| Magelona hobsonae Jones, 1978 | Eagle Cove, Puget Sound, Washington, USA | Temperate Northern Pacific | Intertidal |
| Magelona japonica Okuda, 1937 | Jinsen, Korean Archipelago | Temperate Northern Pacific | N.d. |
| Magelona johnstoni Fiege, Licher and Mackie, 2000 | St Andrews, Scotland | Temperate Northern Atlantic | N.d. |
| Magelona jonesi Hartmann-Schröder, 1980 | Western Australia | Central Indo-Pacific | N.d. |
| Magelona kamala Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 10–20 |
| Magelona koreana Okuda, 1937 | Jinsen, Korean Archipelago | Temperate Northern Pacific | N.d. |
| Magelona lenticulata Gallardo, 1968 | Nha Trang Bay, Viet Nam | Central Indo-Pacific | N.d. |
| Magelona longicornis Johnson, 1901 | West Seattle, Puget Sound, USA | Temperate Northern Pacific | N.d. |
| Magelona lusitanica Mortimer, Gil and Fiege, 2011 | SW Portuguese continental shelf | Temperate Northern Atlantic | 105–327 |
| Magelona magnahamata Aguado and San Martín, 2004 | Granito de Oro, Panamá | Tropical Eastern Pacific | N.d. |
| Magelona mahensis Mortimer and Mackie, 2006 | Mahé, Seychelles | Western Indo-Pacific | 11–48 |
| Magelona marianae Hernández-Alcántara and Solís-Weiss, 2000 | Punta Mita, Mexico | Temperate Northern Pacific | 23.5–46.4 |
| Magelona methae Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 8.4–10 |
| Magelona mickminni Nateewathana and Hylleberg, 1991 | Patong Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona minuta Eliason, 1962 | Öresund, Sweden | Temperate Northern Atlantic | 15–18 |
| Magelona mirabilis (Johnston, 1865) | St Andrews, Scotland, UK. | Temperate Northern Atlantic | N.d. |
| Magelona montera Mortimer, Cassà, Martin and Gil, 2012 | Eilat, Gulf of Aqaba, Red sea | Western Indo-Pacific | Intertidal |
| Magelona nonatoi Bolívar and Lana, 1986 | Rio de Janeiro, Brazil | Temperate South America | N.d. |
| Magelona noppi Nateewathana and Hylleberg, 1991 | Nopparat-Tara Beach, Krabi, Thailand | Western Indo-Pacific | Intertidal |
| Magelona obockensis Gravier, 1905 | Obock, Gulf of Aden, Red Sea | Western Indo-Pacific | Intertidal |
| Magelona pacifica Monro, 1933 | Gorgona Island, Panamá | Tropical Eastern Pacific | Intertidal |
| Magelona papillicornis F. Müller, 1858 | Santa Catarina Island, Brazil | Temperate South America | 0–0.8 |
| Magelona parochilis Zhou and Mortimer, 2013 | Yellow Sea China | Temperate Northern Pacific | Intertidal |
| Magelona paulolanai Magalhães, Bailey-Brock and Watling, 2018 | Hawaii, USA | Eastern Indo-Pacific | 30–40 |
| Magelona pectinata Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona petersenae Nateewathana and Hylleberg, 1991 | Bang Tao Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona pettiboneae Jones, 1963 | St Andrew Bay, Bay County, Florida, USA | Temperate Northern Atlantic | 30 |
| Magelona phyllisae Jones, 1963 | Lamont Geological Observatory Station V-15-68, Santa, Peru | Temperate South America | 181 |
| Magelona pitelkai Hartman, 1944 | Tomales Bay, California | Temperate Northern Pacific | Shallow |
| Magelona polydentata Jones, 1963 | Bimini Lagoon, Bahamas | Tropical Atlantic | Intertidal |
| Magelona posterelongata Bolívar and Lana, 1986 | Paraná Bay, Brazil | Temperate South America | Shallow |
| Magelona pulchella Mohammad, 1970 | Kuwait, Arabian Gulf | Western Indo-Pacific | Intertidal |
| Magelona pygmaea Nateewathana and Hylleberg, 1991 | Kamala Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona riojai Jones, 1963 | Antón Lizardo, Veracruz, Mexico | Tropical Atlantic | Intertidal |
| Magelona rosea Moore, 1907 | Buzzard’s Bay, Wood’s Hole, Massachusetts | Temperate Northern Atlantic | Intertidal |
| Magelona sacculata Hartman, 1961 | San Pedro shelf area, California, USA | Temperate Northern Pacific | 20–40 |
| Magelona sachalinensis Buzhinskaja, 1985 | Sakhalin Island, Sea of Japan | Temperate Northern Pacific | 30–175 |
| Magelona sinbadi Mortimer, Cassà, Martin and Gil, 2012 | Iran, Persian Gulf | Western Indo-Pacific | 20 |
| Magelona spinifera (Hernández-Alcántara and Solís-Weiss, 2000) | Santa María Bay, Mexico | Temperate Northern Pacific | 75 |
| Magelona symmetrica Mortimer and Mackie, 2006 | Mahé, Seychelles | Western Indo-Pacific | 20 |
| Magelona tehuanensis Hernández-Alcántara and Solís-Weiss, 2000 | Western Salina Cruz, Mexico | Tropical Eastern Pacific | 70 |
| Magelona tinae Nateewathana and Hylleberg, 1991 | Bang Tao Bay, Phuket Island, Thailand | Western Indo-Pacific | 10 |
| Magelona uebelackerae (Hernández-Alcántara and Solís-Weiss, 2000) | Texas, Gulf of Mexico | Temperate Northern Atlantic | 3 |
| Magelona variolamellata Bolívar and Lana, 1986 | Paraná, São Paulo and Rio de Janeiro, Brazil | Temperate South America | Shallow |
| Magelona wilsoni Glémarec, 1966 | South of Brittany, ‘Grande Vasière’, France | Temperate Northern Atlantic | 60–110 |
| Octomagelona bizkaiensis Aguirrezabalaga, Ceberio and Fiege, 2001 | Capbreton Canyon, Bay of Biscay | Temperate Northern Atlantic | 984–1040 |
| SPECIES | TYPE LOCALITY | REALMS | DEPTH (m) |
|---|---|---|---|
| Galathowenia africana Kirkegaard, 1959 | West Africa | Tropical Atlantic | 30–42 |
| Galathowenia annae Capa, Parapar and Hutchings, 2012 | Botany Bay, Sydney, Australia | Temperate Australasia | 16 |
| Galathowenia arafurensis Capa, Parapar and Hutchings, 2012 | Arafura Sea, Australia | Central Indo-Pacific | 88 |
| Galathowenia australis (Grube, 1866) | Saint Paul Island, Indian Ocean | Temperate Southern Africa | N.d. |
| Galathowenia eurystoma (Caullery, 1944) | Indonesia | Central Indo-Pacific | 31–1570 |
| Galathowenia fragilis (Nilsen and Holthe, 1985) | Norwegian Sea | Temperate Northern Atlantic | 800–2600 |
| Galathowenia haplosoma (Gibbs, 1972) | Cook Islands | Eastern Indo-Pacific | 1–5 |
| Galathowenia joinvillensis Hartmann-Schröder and Rosenfeldt, 1989 | Antarctic Ocean | Southern Ocean | 68–458 |
| Galathowenia kirkegaardi De León-González and Sanchez-Hernández, 2012 | Gulf of Mexico | Tropical Atlantic | 1–4.1 |
| Galathowenia lobopygidiata (Uschakov, 1950) | Okhotsk Sea | Temperate Northern Pacific | 110–1366 |
| Galathowenia longicollaris Hartmann-Schröder and Rosenfeldt, 1989 | Antarctic Ocean | Southern Ocean | 68 |
| Galathowenia oculata (Zachs, 1923) | White Sea | Arctic | 12–2500 |
| Galathowenia piltzi Blake, 2000 | Santa Maria Basin, California | Temperate Northern Pacific | 92 |
| Galathowenia pygidialis (Harman, 1960) | California and Baja California | Temperate Northern Pacific | >2000 |
| Galathowenia quelis Capa, Parapar and Hutchings, 2012 | North of Sydney, Australia | Temperate Australasia | 1–60 |
| Galathowenia scotiae (Hartman, 1978) | Weddell Sea | Southern Ocean | 42–1592 |
| Myriochele annenkovae Hartman, 1960 (1) | Northern Sea of Japan | Temperate Northern Pacific | 45 |
| Myriochele antarctica (Hartman, 1967) | South Orkney Islands | Southern Ocean | 604–3816 |
| Myriochele antarctica Cantone and Di Pietro, 2001 | Ross Sea, Terra Nova Bay | Southern Ocean | 126–136 |
| Myriochele australiensis Capa, Parapar and Hutchings, 2012 | East of Long Reef, Sydney, Australia | Temperate Australasia | 60 |
| Myriochele danielsseni Hansen, 1878 | Norwegian Sea | Temperate Northern Atlantic | 1187 |
| Myriochele gracilis Hartman, 1955 | Pacific Ocean, Southern California | Temperate Northern Pacific | 115 |
| Myriochele heeri Malmgren, 1867 | Spitsbergen, Norway | Temperate Northern Atlantic | 120–2600 |
| Myriochele heruensis Gibbs, 1971 | Solomon Islands | Eastern Indo-Pacific | 16 |
| Myriochele islandica (Parapar, 2003) | West Iceland | Temperate Northern Atlantic | 1187–1407 |
| Myriochele japonica (Imajima and Morita, 1987) | Japan | Temperate Northern Pacific | 62–125 |
| Myriochele malmgreni (Parapar, 2006) | Faroe Passage, Iceland | Temperate Northern Atlantic | 1187–1407 |
| Myriochele minor Caullery, 1944 | Indonesia | Central Indo-Pacific | 462–959 |
| Myriochele olgae Blake, 2000 | off Half Moon Bay, California | Temperate Northern Pacific | 100–200 |
| Myriochele pacifica McIntosh, 1885 | Central Pacific Ocean | Eastern Indo-Pacific | 4754 |
| Myriochele picta Southern, 1921 | Chilka Lake, India | Western Indo-Pacific | 1–3 |
| Myriochele riojai Parapar, 2003 | Bransfield Strait, Antarctic Ocean | Southern Ocean | 647–1416 |
| Myriochele robusta Parapar, 2003 | Bransfield Strait, Antarctic Ocean | Southern Ocean | 640–1500 |
| Myriochele striolata Blake, 2000 | Santa Maria Basin, California | Temperate Northern Pacific | 91–400 |
| Myriowenia californiensis Hartman, 1960 | Southern California | Temperate Northern Pacific | 106 |
| Myriowenia gosnoldi Hartman, 1965 | off Suriname | Tropical Atlantic | 520–550 |
| Owenia artifex (Verrill, 1885) | Massachusetts | Temperate Northern Atlantic | 122 |
| Owenia assimilator Caullery, 1944 (2) | n.d. | n.d. | N.d. |
| Owenia assimilis (Sars, 1851) | Norwegian Sea | Temperate Northern Atlantic | 2–460 |
| Owenia australis Ford and Hutchings, 2005 | New South Wales, Australia | Temperate Australasia | <100 |
| Owenia bassensis Ford and Hutchings, 2005 | Victoria, East Bass Strait, Australia | Temperate Australasia | <100 |
| Owenia borealis Koh, Bhaud and Jirkov, 2003 | North East Atlantic | Temperate Northern Atlantic | 41–1350 |
| Owenia caissara Silva and Lana, 2017 | Santa Catarina State, Brazil | Temperate South America | 0–5 |
| Owenia caudisetosa Hartmann-Schröder, 1959 | El Salvador | Tropical Atlantic | N.d. |
| Owenia collaris Hartman, 1955 | Santa Catalina Island | Temperate Northern Pacific | 0–150 |
| Owenia dichotoma Parapar and Moreira, 2015 | Lizard Island, Australia | Central Indo-Pacific | 12 |
| Owenia fusiformis Delle Chiaje, 1844 | Sicily, Mediterranean | Temperate Northern Atlantic | N.d. |
| Owenia gomsoni Koh and Bhaud, 2001 | South Korea, Yellow Sea | Temperate Northern Pacific | 0 |
| Owenia johnsoni Blake, 2000 | Tomales Bay, California | Temperate Northern Pacific | 3–20 |
| Owenia mirrawa Ford and Hutchings, 2005 | Northern Territory, Australia | Central Indo-Pacific | <100 |
| Owenia persica Martin, Koh, Bhaud, Dutrieux and Gil, 2006 | Persian Gulf | Western Indo-Pacific | 16 |
| Owenia petersenae Koh and Bhaud, 2003 | Wellington, New Zealand | Southern Ocean | 20–30 |
| Owenia picta Parapar and Moreira, 2015 | Lizard Island, Australia | Central Indo-Pacific | 2–12 |
| Owenia polaris Koh, Bhaud and Jirkov, 2003 | East Iceland | Temperate Northern Atlantic | 12–930 |
| Owenia vieitezi Díaz-Díaz, Parapar and Moreira, 2020 | Gulf of Venezuela, Caribbean Sea | Tropical Atlantic | 6–18 |
- (1)
- (2)
- Read and Fauchald [85] name it as Owenia assimilator Caullery, 1944 but Caullery [165] reports it as O. assimilator Andrews. Gil [156] affirms that Caullery was mistaken, because he was referring to Ammochares aedificator Andrews, 1891, a species that Read and Fauchald [85] report as synonym of Owenia fusiformis.
References
- Weigert, A.; Bleidorn, C. Current Status of Annelid Phylogeny. Org. Divers. Evol. 2016, 16, 345–362. [Google Scholar] [CrossRef]
- Helm, C.; Beckers, P.; Bartolomaeus, T.; Drukewitz, S.H.; Kourtesis, I.; Weigert, A.; Purschke, G.; Worsaae, K.; Struck, T.H.; Bleidorn, C. Convergent Evolution of the Ladder-like Ventral Nerve Cord in Annelida. Front. Zool. 2018, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Struck, T. Phylogeny. In Handbook of Zoology. Annelida. Basal Groups and Pleistoannelida, Sedentaria I; Purschke, G., Böggemann, M., Westheide, W., Eds.; DeGruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 37–68. [Google Scholar]
- Weigert, A.; Helm, C.; Meyer, M.; Nickel, B.; Arendt, D.; Hausdorf, B.; Santos, S.R.; Halanych, K.M.; Purschke, G.; Bleidorn, C.; et al. Illuminating the Base of the Annelid Tree Using Transcriptomics. Mol. Biol. Evol. 2014, 31, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.C.S.; Novo, M.; Kawauchi, G.Y.; Worsaae, K.; Pleijel, F.; Giribet, G.; Rouse, G.W. Articulating “Archiannelids”: Phylogenomics and Annelid Relationships, with Emphasis on Meiofaunal Taxa. Mol. Biol. Evol. 2015, 32, 2860–2875. [Google Scholar] [CrossRef]
- Struck, T.H.; Golombek, A.; Weigert, A.; Franke, F.A.; Westheide, W.; Purschke, G.; Bleidorn, C.; Halanych, K.M. The Evolution of Annelids Reveals Two Adaptive Routes to the Interstitial Realm. Curr. Biol. 2015, 25, 1993–1999. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S. Paraphyletic Status of Polychaeta Suggested by Phylogenetic Analysis Based on the Amino Acid Sequences of Elongation Factor 1-Alpha. Mol. Phylogenet. Evol. 1998, 9, 255–261. [Google Scholar] [CrossRef]
- Eeckhaut, I.; McHugh, D.; Mardulyn, P.; Tiedemann, R.; Monteyne, D.; Jangoux, M.; Milinkovitch, C. Myzostomida: A Link between Trochozoans and Flatworms? P. Roy. Soc. B Biol. Sci. 2000, 267, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Bleidorn, C.; Vogt, L.; Bartolomaeus, T. New Insights into Polychaete Phylogeny (Annelida) Inferred from 18S RDNA Sequences. Mol. Phylogenet. Evol. 2003, 29, 279–288. [Google Scholar] [CrossRef]
- Rousset, V.; Rouse, G.W.; Siddall, M.E.; Tillier, A.; Pleijel, F. The Phylogenetic Position of Siboglinidae (Annelida) Inferred from 18S RRNA, 28S RRNA and Morphological Data. Cladistics 2004, 20, 518–533. [Google Scholar] [CrossRef]
- Rousset, V.; Pleijel, F.; Rouse, G.W.; Erséus, C.; Siddall, M.E. A Molecular Phylogeny of Annelids. Cladistics 2007, 23, 41–63. [Google Scholar] [CrossRef]
- Struck, T.H.; Schult, N.; Kusen, T.; Hickman, E.; Bleidorn, C.; McHugh, D.; Halanych, K.M. Annelid Phylogeny and the Status of Sipuncula and Echiura. BMC Evol. Biol. 2007, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struck, T.H.; Nesnidal, M.P.; Purschke, G.; Halanych, K.M. Detecting Possibly Saturated Positions in 18S and 28S Sequences and Their Influence on Phylogenetic Reconstruction of Annelida (Lophotrochozoa). Mol. Phylogenet. Evol. 2008, 48, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H. The Impact of Paralogy on Phylogenomic Studies—A Case Study on Annelid Relationships. PLoS ONE 2013, 8, e62892. [Google Scholar] [CrossRef] [PubMed]
- Zrzavý, J.; Říha, P.; Piálek, L.; Janouškovec, J. Phylogeny of Annelida (Lophotrochozoa): Total-Evidence Analysis of Morphology and Six Genes. BMC Evol. Biol. 2009, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Parry, L.A.; Vinther, J.; Zhai, D.; Hou, X.; Ma, X. A Cambrian Crown Annelid Reconciles Phylogenomics and the Fossil Record. Nature 2020, 583, 249–252. [Google Scholar] [CrossRef]
- Capa, M.; Parapar, J.; Hutchings, P. Phylogeny of Oweniidae (Polychaeta) Based on Morphological Data and Taxonomic Revision of Australian Fauna. Zool. J. Linn. Soc. 2012, 166, 236–278. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, K.; Mills, K.; Jordana, E.; Pinedo, S.; Gil, J. A Further Review of European Magelonidae (Annelida), Including Redescriptions of Magelona equilamellae and Magelona filiformis. Zootaxa 2020, 4767, 89–114. [Google Scholar] [CrossRef]
- Rouse, G.W. Magelona Müller, 1858. In Polychaetes; Rouse, G.W., Pleijel, F., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 261–263. [Google Scholar] [CrossRef]
- Cunningham, J.T.; Ramage, G.A. The Polychaeta Sedentaria of the Firth of Forth. Trans. Roy. Soc. Edinburg 1888, 33, 635–684. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Brasil, A.C. Filogenia de Magelonidae Cunningham & Ramage, 1888 (Annelida–Polychaeta) com Base na Morfologia Externa. PhD Thesis, Universidade Federal do Paraná, Curitiva, Brazil, 2003. [Google Scholar]
- Mortimer, K. 4.2 Magelonidae. In Handbook of Zoology. Annelida. Basal Groups and Pleistoannelida, Sedentaria I; Purschke, G., Böggemann, M., Westheide, W., Eds.; DeGruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 112–131. [Google Scholar]
- McIntosh, W.C. On the Structure of Magelona. Proc. R. Soc. Lond. 1877, 25, 559–564. [Google Scholar]
- McIntosh, W.C. Beiträge zur Anatomie von Magelona. Z. Wiss. Zool. 1878, 31, 401–472. [Google Scholar]
- McIntosh, W.C. On the Circulatory System of Magelona. J. Anat. Physiol., London 1879, 13, 331–345. [Google Scholar]
- McIntosh, W.C. On the Structure of Magelona. Ann. Mag. Nat. Hist. (Ser. 8) 1911, 7, 417–457. [Google Scholar] [CrossRef]
- McIntosh, W.C. A monograph of the British marine annelids. In Polychaeta. Opheliidae to Ammocharidae; The Ray Society: London, UK, 1915; Volume 3, Part I. Text; pp. 1–368. [Google Scholar]
- McIntosh, W.C. A monograph of the British marine annelids. In Polychaeta. Opheliidae to Ammocharidae; The Ray Society: London, UK, 1916; Volume 3, Part II. Plates. [Google Scholar]
- Jones, M.L. Four New Species of Magelona (Annelida, Polychaeta) and a Redescription of Magelona longicornis Johnson. Am. Mus. Novit. 1963, 2164, 1–29. [Google Scholar]
- Jones, M.L. On the Morphology, Feeding, and Behavior of Magelona sp. Biol. Bull. 1968, 134, 272–297. [Google Scholar] [CrossRef]
- Jones, M.L. Magelona berkeleyi n. sp. from Puget Sound (Annelida: Polychaeta), with a Further Redescription of Magelona longicornis Johnson and a Consideration of Recently Described Species of Magelona. J. Fish. Res. Bd. Can. 1971, 28, 1445–1454. [Google Scholar] [CrossRef]
- Jones, M.L. Redescription of M. papillicornis F. Müller. In Essays on Polychaetous Annelids in Memory of Dr. Olga Hartman; Reish, D.J., Fauchald, K., Eds.; Allan Hancock Foundation: University of Southern California: Los Angeles, CA, USA, 1977; pp. 247–266. [Google Scholar]
- Jones, M.L. Three New Species of Magelona (Annelida, Polychaeta) and a Redescription of Magelona pitelkai Hartman. Proc. Biol. Soc. Wash. 1978, 91, 336–363. [Google Scholar]
- Nateewathana, A.; Hylleberg, J. Magelonid Polychaetes from Thailand, the Andaman Sea, with Descriptions of Eight New Species. Ophelia 1991, 5, 169–184. [Google Scholar]
- Mortimer, K.; Mackie, A.S.Y. The Magelonidae (Annelida: Polychaeta) from the Seychelles, with the Description of Three New Species. Hydrobiologia 2003, 496, 163–173. [Google Scholar] [CrossRef]
- Mortimer, K.; Mackie, A.S.Y. The Magelonidae (Annelida: Polychaeta) from the Seychelles. 2. Description of Four Additional Species, Three New to Science. Sci. Mar. 2006, 70, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, K. Magelonidae (Polychaeta) from the Arabian Peninsula: A Review of Known Species, with Notes on Magelona tinae from Thailand. Zootaxa 2010, 2628, 1–26. [Google Scholar] [CrossRef]
- Mortimer, K.; Cassà, S.; Martin, D.; Gil, J. New Records and New Species of Magelonidae (Polychaeta) from the Arabian Peninsula, with a Re-Description of Magelona pacifica and a Discussion on the Magelonid Buccal Region. Zootaxa 2012, 3331, 1–43. [Google Scholar] [CrossRef]
- Shakouri, A.; Mortimer, K.; Dehani, E. A New Species and New Records of Magelona (Annelida: Magelonidae) from Chabahar Bay, Gulf of Oman, South-Eastern Iran. J. Mar. Biol. Ass. UK 2017, 97, 1537–1552. [Google Scholar] [CrossRef]
- Mortimer, K.; Mackie, A.S.Y. Magelonidae (Polychaeta) from Hong Kong, China; with Discussions on Related Species and Redescriptions of Three Species. Zoosymposia 2009, 2, 179–199. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Mortimer, K. A New Species of Magelona (Polychaeta: Magelonidae) from Chinese Coastal Waters. J. Mar. Biol. Ass. UK 2013, 93, 1503–1510. [Google Scholar] [CrossRef]
- Uebelacker, J.M.; Jones, M.L. Family Magelonidae. In Taxonomic Guide to the Polychaetes of the Northern Gulf of Mexico; Uebelacker, J.M., Johnson, P.G., Eds.; Barry A. Vittor & Associates: Mobile, AL, USA, 1984; Volume 2, pp. 7.1–7.29. [Google Scholar]
- Hernández-Alcántara, P.; Solís-Weiss, V. Magelonidae from the Mexican Pacific and Northern Gulf of Mexico, with the Description of a New Genus (Meridithia) and Four New Species. Bull. Mar. Sci. 2000, 67, 625–644. [Google Scholar]
- Hernández-Alcántara, P.; Solís-Weiss, V. Magelonidae Cunningham & Ramage, 1888. In Poliquetos (Annelida: Poychaeta) de México y América Tropical; León-González, J.A., Bastida-Zavala, J.R., Carrera Parra, L.F., García-Garza, M.E., Peña-Rivera, A., Salazar-Vallejo, S.I., Solís-Weiss, V., Eds.; Universidad Autónoma de Nuevo León: Nuevo León, Mexico, 2009; Volume 2, pp. 277–289. [Google Scholar]
- Blake, J.A. Family Magelonidae Cunnigham & Ramage, 1888. In Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel; Blake, J.A., Hilbig, B., Scott, P.H., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1996; Volume 6, pp. 253–261. [Google Scholar]
- Bolívar, G.A.; Lana, P.C. Magelonidae (Annelida: Polychaeta) do Litoral Sudeste do Brasil. Neritica 1986, 1, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.C.M.; Vivan, J.M.; Pesserl, B.H.; Lana, P. Polychaetes of the North-Central Santa Catarina State, Brazil. Check List 2012, 8, 204–206. [Google Scholar] [CrossRef] [Green Version]
- Fiege, D.; Licher, F.; Mackie, A.S.Y. A Partial Review of the European Magelonidae (Annelida: Polychaeta): Magelona mirabilis Redefined and M. johnstoni sp. nov. Distinguished. J. Mar. Biol. Assoc. UK 2000, 80, 215–234. [Google Scholar] [CrossRef]
- Mortimer, K.; Gil, J.; Fiege, D. Portuguese Magelona (Annelida: Magelonidae) with a Description of a New Species, a Re-Description of Magelona wilsoni Glémarec, and a Key to Adult Magelonidae from European Waters. Ital. J. Zool. 2011, 78, 124–139. [Google Scholar] [CrossRef]
- Mills, K.; Mortimer, K. Redescription of Magelona minuta Eliason, 1962 (Annelida), with discussions on the validity of Magelona filiformis minuta. Zootaxa 2018, 4527, 541–559. [Google Scholar] [CrossRef]
- Mortimer, K.; Mackie, A.S.Y. Morphology, Feeding and Behaviour of British Magelona (Annelida: Magelonidae), with Discussions on the Form and Function of Abdominal Lateral Pouches. Mem. Mus. Vic. 2014, 71, 177–201. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.; Mortimer, K. Observations on the Tubicolous Annelid Magelona alleni (Magelonidae), with Discussions on the Relationship between Morphology and Behaviour of European Magelonids. J. Mar. Biol. Ass. UK 2019, 99, 715–727. [Google Scholar] [CrossRef]
- Mills, K.; Mortimer, K. Habitat and Distribution of the Shovelhead Worm Magelona equilamellae Harmelin, 1964 with Notes on the Morphologically Similar Magelona alleni Wilson, 1958. Bull. Porcupine Mar. Nat. Hist. Soc. 2019, 12, 63–67. [Google Scholar]
- Fauchald, K. Life Diagram Patterns in Benthic Polychaetes. Proc. Biol. Soc. Wash. 1983, 96, 160–177. [Google Scholar]
- Hartman, O. Abyssal Polychaetous Annelids from the Mozambique Basin off Southeast Africa, with a Compendium of Abyssal Polychaetous Annelids from World-Wide Areas. J. Fish. Res. Board Can. 1971, 28, 1407–1428. [Google Scholar] [CrossRef]
- Aguirrezabalaga, F.; Ceberio, A.; Fiege, D. Octomagelona bizkaiensis (Polychaeta: Magelonidae) a New Genus and Species from the Capbreton Canyon (Bay of Biscay, North-East Atlantic). J. Mar. Biol. Ass. UK 2001, 81, 221–224. [Google Scholar] [CrossRef]
- Capa, M.; Parapar, J.; Hutchings, P. 4.1. Oweniidae. In Handbook of Zoology. Annelida. Basal Groups and Pleistoannelida, Sedentaria I; Purschke, G., Böggemann, M., Westheide, W., Eds.; DeGruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 91–112. [Google Scholar] [CrossRef]
- Read, G.; Fauchald, K. (Eds.) World Polychaeta Database. Magelonidae Cunningham & Ramage, 1888. 2021. Available online: http://marinespecies.org/aphia.php?p=taxdetails&id=975 (accessed on 12 January 2021).
- Blake, J.A. Polychaeta Oweniidae from Antarctic Seas Collected by the United States Antarctic Research Program. In Proceedings of the First International Polychaete Conference; Hutchings, P.A., Ed.; Linnean Society of New South Wales: Sydney, Australia, 1984; pp. 112–117. [Google Scholar]
- Hartmann-Schröder, G.; Rosenfeldt, P. Die Polychaten Der “Polarstern”- Reise ANT III/2 in Die Antarktis) 1984. Teil 2: Cirratulidae Dis Serpulidae. Mitt. Hamb. Zool. Mus. Inst. 1989, 86, 65–106. [Google Scholar]
- Cantone, G.; Di Pietro, N. A New Species of Myriochele (Polychaeta, Oweniidae) from Antarctica, with Considerations on Antarctic Oweniids. Polar Biol. 1998, 19, 421–423. [Google Scholar] [CrossRef]
- Parapar, J. Revision of Five Species Referred to Myriochele and Galathowenia (Polychaeta: Oweniidae) from the Antarctic Seas Based upon Type Material. Proc. Biol. Soc. Wash. 2001, 114, 403–413. [Google Scholar]
- Parapar, J. Two New Species of Myriochele (Polychaeta: Oweniidae) from the Bransfield Strait (Antarctica). Antarct. Sci. 2003, 15, 219–226. [Google Scholar] [CrossRef]
- Blake, J.A.; Dean, D. Polychaetous Annelids Collected by the R/V Hero from Baffin Island, Davis Strait, and West Greenland in 1968. Bull. S. Calif. Acad. Sci. 1973, 72, 31–39. [Google Scholar]
- Nilsen, R.; Holthe, T. Arctic and Scandinavian Oweniidae (Polychaeta) with a Description of Myriochele fragilis sp.n., and Comments on the Phylogeny of the Family. Sarsia 1985, 70, 17–32. [Google Scholar] [CrossRef]
- Koh, B.S.; Bhaud, M.R.; Jirkov, I.A. Two New Species of Owenia (Annelida: Polychaeta) in the Northern Part of the North Atlantic Ocean and Remarks on Previously Erected Species from the Same Area. Sarsia 2003, 88, 175–188. [Google Scholar] [CrossRef]
- Parapar, J.O. Oweniidae (Annelida, Polychaeta) from Icelandic Waters, Collected by the BIOICE Project, with the Description of Myrioglobula islandica n. sp. Sarsia 2003, 88, 274–290. [Google Scholar] [CrossRef]
- Parapar, J. The Genera Myriochele and Myrioglobula (Polychaeta, Oweniidae) in Icelandic Waters with the Revision of Type Material of Myriochele heeri Malmgren, 1867, and the Description of a New Species. J. Nat. Hist. 2006, 40, 523–547. [Google Scholar] [CrossRef]
- Martin, D. Revisión de las especies de Owenidae (Annelida, Polychaeta) de la Peninsula Ibérica. Sci. Mar. 1989, 53, 47–52. [Google Scholar]
- Hartman, O. Atlas of the Sedentariate Polychaetous Annelids from California; Allan Hancock Foundation, University of Southern California: Los Angeles, CA, USA, 1969. [Google Scholar]
- Blake, J.A. Chapter 5. Family Oweniidae Rioja, 1917. In Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel; Blake, J.A., Hilbig, B., Scott, P.H., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 2000; Volume 7, pp. 97–127. [Google Scholar]
- Milligan, M.R. Family Oweniidae Rioja, 1917. In Taxonomic Guide to the Polychaetes of the Northern Gulf of Mexico; Uebelacker, J.M., Johnson, P.G., Eds.; Barry A. Vittor & Associates: Mobile, AL, USA, 1984; Volume 6, pp. 46.1–46.12. [Google Scholar]
- Villalobos-Guerrero, T.F. Oweniidae, Rioja 1917. In Poliquetos (Annelida: Poychaeta) de México y América Tropical; León-González, J.A., Bastida-Zavala, J.R., Carrera Parra, L.F., García-Garza, M.E., Peña-Rivera, A., Salazar-Vallejo, S.I., Solís-Weiss, V., Eds.; Universidad Autónoma de Nuevo León: Nuevo León, Mexico, 2009; Volume 2, pp. 391–402. [Google Scholar]
- Díaz-Díaz, O.; Parapar, J.; Moreira, J. A New Species of Genus Owenia Delle-Chiaje, 1844 (Annelida; Oweniidae) from the Coast of Venezuela. Cah. Biol. Mar. 2018, 59, 589–597. [Google Scholar]
- Koh, B.; Bhaud, M. Description of Owenia gomsoni n. sp. (Oweniidae, Annelida Polychaeta) from the Yellow Sea and Evidence That Owenia fusiformis Is Not a Cosmopolitan Species. Vie Milieu 2001, 51, 77–87. [Google Scholar]
- Imajima, M.; Morita, Y. Oweniidae (Annelida, Polychaeta) from Japan. Bull. Natl. Sci. Mus. 1987, 13, 85–102. [Google Scholar]
- Ford, E.; Hutchings, P. An Analysis of Morphological Characters of Owenia Useful to Distinguish Species: Description of Three New Species of Owenia (Oweniidae: Polychaeta) from Australian Waters. Mar. Ecol. 2005, 26, 181–196. [Google Scholar] [CrossRef]
- Parapar, J.; Moreira, J. The Oweniidae (Annelida; Polychaeta) from Lizard Island (Great Barrier Reef, Australia) with the Description of Two New Species of Owenia Delle Chiaje, 1844. Zootaxa 2015, 4019, 604–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dauvin, J.-C.; Thiébaut, E. Is Owenia fusiformis Delle Chiaje a Cosmopolitan Species? Mém. Mus. Nat. Paris 1994, 162, 383–404. [Google Scholar]
- Parapar, J. Resurrection of Galathowenia australis (Polychaeta, Oweniidae) Based upon Type Material. Cah. Biol. Mar. 2003, 44, 249–255. [Google Scholar]
- Martin, D.; Koh, B.-S.; Bhaud, M.; Gil, J.; Dutrieux, E. The Genus Owenia (Annelida, Polychaeta) in the Persian Gulf, with Description of a New Species, Owenia persica. Org. Divers. Evol. 2006, 6, 325–326. [Google Scholar] [CrossRef]
- Ménard, F.; Gentil, F.; Dauvin, J.-C. Population Dynamics and Secondary Production of Owenia fusiformis Delle Chiaje. Polychaeta from the Bay of Seine Eastern English Channel France. J. Exp. Mar. Biol. Ecol. 1989, 133, 151–168. [Google Scholar] [CrossRef]
- Smart, T.I.; Von Dassow, G. Unusual Development of the Mitraria Larva in the Polychaete Owenia collaris. Biol. Bull. 2009, 217, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Kirkergaard, J.B. Benthic Polychaeta from Depths Exceeding 6000 m. Galathea Rep. 1956, 2, 63–78. [Google Scholar]
- Read, G.; Fauchald, K. (Eds.) World Polychaeta Database. Oweniidae Rioja, 1917. 2021. Available online: http://marinespecies.org/aphia.php?p=taxdetails&id=975 (accessed on 12 January 2021).
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Rouse, G.W.; Fauchald, K. Cladistics and Polychaetes. Zool. Scr. 1997, 26, 139–204. [Google Scholar] [CrossRef]
- Weigert, A.; Golombek, A.; Gerth, M.; Schwarz, F.; Struck, T.H.; Bleidorn, C. Evolution of Mitochondrial Gene Order in Annelida. Mol. Phylogenet. Evol. 2016, 94, 196–206. [Google Scholar] [CrossRef]
- Parry, L.; Vinther, J.; Edgecombe, G.D. Cambrian stem-group annelids and a metameric origin of the annelid head. Biol. Lett. 2015, 11, 20150763. [Google Scholar] [CrossRef] [PubMed]
- Hartman, O. Polychaetous Annelids from California, Including the Descriptions of Two New Genera and Nine New Species. Allan Hancock Pac. Exp. 1944, 10, 239–307. [Google Scholar]
- Hartman, O.; Reish, D.J. The Marine Annelids of Oregon. Or. State Monogr. Stud. Zool. 1950, 6, 1–64. [Google Scholar]
- Gallardo, V.A. Polychaeta from the Bay of Nha Trang, South Viet Nam. Naga Report 1968, 4, 35–279. [Google Scholar]
- Eliason, A. Undersökningar Över Öresund. XXXXI. Weitere Untersuchungen Über Die Polychaetenfauna Des Öresunds. Acta Univ. Lund. Avd. 2 1962, 58, 1–98. [Google Scholar]
- Hartman, O. Polychaetous Annelids from California. Allan Hancock Pac. Exp. 1961, 25, 1–226. [Google Scholar]
- Hartman, O. Catalogue of the Polychaetous Annelids of the World. Supplement 1960-1965 and Index. Allan Hancock Found. Publ. Occas. Pap. 1965, 23, 1–197. [Google Scholar]
- Glémarec, M. Les Magelonidae des Coates de Bretagne. Description de Magelona wilsoni n. sp. Vie Milieu 1966, 17, 1077–1085. [Google Scholar]
- Kitamori, R. Magelonidae (Polychaetous Annelids) from Japan, Including the Description of a New Species. Bull. Tokai Reg. Fish. Res. Lab. 1967, 50, 49–54. [Google Scholar]
- Reish, D.J. Benthic Polychaetous Annelids from Bering, Chukchi, and Beaufort Seas. Proc. US Nat. Mus. 1965, 117, 131–157. [Google Scholar] [CrossRef] [Green Version]
- Hartmann-Schröder, G. Zweiter Beitrag Zur Polychaetenfauna von Peru. Kiel. Meeresforsch. 1962, 18, 109–147. [Google Scholar]
- Day, J.H. The Polychaet [Sic] Fauna of South Africa. Part 6. Sedentary Species Dredged off Cape Coasts with a Few New Records from the Shore. J. Linn. Soc. London 1961, 44, 463–560. [Google Scholar] [CrossRef]
- Harmelin, J.G. Étude de l’endofaune des “mattes” d’herbiers de Posidonia oceanica Delile. Recl. Trav. Stn. Mar. Endoume 1964, 35, 43–105. [Google Scholar]
- Magalhães, W.F.; Bailey-Brock, J.; Watling, L. Four New Species of Magelona (Annelida: Magelonidae) from Easter Island, Guam and Hawaii. Zootaxa 2018, 4457, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S. Spioniform Polychaetes from Japan. J. Fac. Sci. Hokkaido Univ. Ser. 1937, 6, 217–254. [Google Scholar]
- Clarke, D.T.; Paterson, G.L.J.; Florence, W.K.; Gibbons, M.J. A New Species of Magelona (Polychaeta: Magelonidae) from Southern Namibia. Afr. Nat. Hist. 2010, 6, 77–82. [Google Scholar]
- Wilson, D.P. The Larval Development of Three Species of Magelona (Polychaeta) from Localities near Plymouth. J. Mar. Biol. Assoc. UK 1982, 62, 385–401. [Google Scholar] [CrossRef]
- Mésnil, F. Études de morphologie externe chez les annélides. I. Les spionidens des côtes de la Manche. Bull. Sci. Fr. Belg. 1896, 29, 110–287. [Google Scholar] [CrossRef]
- Romieu, M. Contribution à l’histologie comparée du muscle strié. C. R. Acad. Sci. Paris 1923, 176, 864–867. [Google Scholar]
- Orrhage, L. Über das Vorkommen von Muskelzellen vom “Nematoden-Typus” bei Polychaeten als phylogenetisch-systematisches Merkmal. Zool. Bidr. Upps. 1962, 35, 321–327. [Google Scholar]
- Orrhage, L. Über Die Anatomie Des Zentralen Nervensystemes Der Sedentären Polychaeten. Ark. Zool. 1966, 19, 99–133. [Google Scholar]
- Bartolomaeus, T. Secondary Monociliarity in the Annelida: Monociliated Epidermal Cells in Larvae of Magelona mirabilis (Magelonidae). Microfauna Mar. 1995, 10, 327–332. [Google Scholar]
- Benham, W.B. The Blood of Magelona. Q. J. Microsc. Sci. 1896, 39, 1–17. [Google Scholar]
- Wells, R.M.; Dales, R.P. Oxygenational Properties of Haemerythrin in the Blood of Magelona papillicornis Muller (Polychaeta: Magelonidae). Comp. Biochem. Physiol. 1974, 49A, 57–64. [Google Scholar] [CrossRef]
- Purschke, G. Annelida: Basal groups and Pleistoannelida. In Structure and Evolution of Invertebrate Nervous Systems; Schmidt-Rhaesa, A., Harzsch, S., Purschke, G., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 254–312. [Google Scholar]
- Beckers, P.; Helm, C.; Bartolomaeus, T. The Anatomy and Development of the Nervous System in Magelonidae (Annelida)—Insights into the Evolution of the Annelid Brain. BMC Evol. Biol. 2019, 19, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippova, A.; Purschke, G.; Tzetlin, A.B.; Müller, M.C.M. Reconstruction of the Musculature of Magelona cf. mirabilis (Magelonidae) and Prionospio cirrifera (Spionidae) (Polychaeta, Annelida) by Phalloidin Labeling and CLSM. Zoomorphology 2005, 124, 1–8. [Google Scholar] [CrossRef]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [Green Version]
- Wissocq, J.-C. Muscles à striation oblique et à striation transversale chez les Annélides Polychètes. Ital. J. Zool. 1981, 48, 57–74. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Home Page. 2021. Available online: https://www.gbif.org (accessed on 13 January 2021).
- Wilson, D.P. The Polychaete Magelona alleni n. sp. and a Re- Assessment of Magelona cincta Ehlers. J. Mar. Biol. Assoc. UK 1958, 37, 617–626. [Google Scholar] [CrossRef]
- Wilson, D.P. The Polychaete Magelona filiformis n. sp. and Notes on Other Species of Magelona. J. Mar. Biol. Assoc. UK 1959, 38, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Hylleberg, J.; Nateewathana, A. Temporal and Spatial Distribution of Subtidal Magelonid Polychaetes at Phuket Island, Thailand, Andaman Sea. Ophelia 1991, 5, 573–578. [Google Scholar]
- Hannan, C.A. The Life Histories and Population Dynamics of the Polychaetes, Nothria elegans (Johnson) (Onuphidae) and Magelona sacculata Hartman (Magelonidae) in Monterey Bay, California. Master’s Thesis, San Jose State University, San Jose, CA, USA, 1980. [Google Scholar]
- Fajardo, M.; Andrade, D.; Bonicelli, J.; Bon, M.; Gómez, G.; Riascos, J.M.; Pacheco, A.S. Macrobenthic Communities in a Shallow Normoxia to Hypoxia Gradient in the Humboldt Upwelling Ecosystem. PLoS ONE 2018, 13, e0200349. [Google Scholar] [CrossRef] [PubMed]
- Gaston, G. Effects of Hypoxia on Macrobenthos of the Inner Shelf off Cameron, Louisiana. Estuar. Coast. Shelf Sci. 1985, 20, 603–613. [Google Scholar] [CrossRef]
- Sivadas, S.K.; Carvalho, R. Marine Annelida of India: Taxonomy and Status Evaluation and an Updated Checklist. J. Threat. Taxa 2020, 12, 16647–16714. [Google Scholar] [CrossRef]
- Meißner, K.; Darr, A. Distribution of Magelona Species (Polychaeta: Magelonidae) in the German Bight (North Sea): A Modelling Approach. Zoosymposia 2009, 2, 567–586. [Google Scholar] [CrossRef] [Green Version]
- Fauchald, K.; Jumars, P. The Diet of Worms: A Study of Polychaete Feedings Guilds. Oceanogr. Mar. Biol. Ann. Rev. 1979, 17, 193–284. [Google Scholar]
- Wolff, W.J. The Estuary as a Habitat: An Analysis of Data on the Soft-Bottom Macrofauna of the Estuarine Area of the Rivers Rhine, Meuse, and Scheldt. Zool. Verh. 1973, 126, 1–242. [Google Scholar]
- Hartmann-Schroder, G. Annelida, Borstenwurmer, Polychaeta. Tierwelt Dtschl. 1971, 58, 1–594. [Google Scholar]
- McMahon, R.F.; Jones, M.L. Observations on Feeding in Magelona sp. Biol. Bull. 1967, 133, 476. [Google Scholar]
- Hunt, O.D. The Food of the Bottom Fauna of the Plymouth Fishing Grounds. J. Mar. Biol. Ass. UK 1925, 13, 560–599. [Google Scholar] [CrossRef] [Green Version]
- Mare, M.F. A Study of a Marine Benthic Community with Special Reference to the Micro-Organisms. J. Mar. Biol. Ass. UK 1942, 25, 517–554. [Google Scholar] [CrossRef] [Green Version]
- Kühl, H. Über Vorkommen Und Nahrung der Larven von Magelona papillicornis O.F. Müller (Polychaeta Sedentaria) Im Mündungsgebiet von Elbe, Weser Und Ems. Ber. Dtsch. Wiss. Komm. Meeresfirsch. 1974, 23, 296–301. [Google Scholar]
- Rouse, G.W. Polychaete Sperm: Phylogenetic and Functional Considerations. Hydrobiologia 1999, 402, 215–224. [Google Scholar] [CrossRef]
- Rouse, G.W. Chapter 3. In Annelid Sperm and Spermiogenesis; Rouse, G.W., Pleijel, F., Eds.; Science Publishers Inc: Enfield, NH, USA, 2006; pp. 45–76. [Google Scholar]
- Johnson, K.B.; Brink, L.A. Predation on Bivalve Veligers by Polychaete Larvae. Biol. Bull. 1998, 194, 297–303. [Google Scholar] [CrossRef]
- Plate, S.; Husemann, E. Identification Guide to the Planktonic Larvae around the Island of Helgoland (German Bight). Helgol. Meeresunters. 1994, 48, 1–58. [Google Scholar] [CrossRef] [Green Version]
- Carson, H.S.; Hentschel, B.T. Estimating the Dispersal Potential of Polychaete Species in the Southern California Bight: Implications for Designing Marine Reserves. Mar. Ecol. Prog. Ser. 2006, 316, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Criales-Hernández, M.I.; Schwamborn, R.; Graco, M.; Ayón, P.; Hirche, H.-J.; Wolff, M. Zooplankton Vertical Distribution and Migration off Central Peru in Relation to the Oxygen Minimum Layer. Helgol. Mar. Res. 2008, 62, 85–100. [Google Scholar] [CrossRef]
- Dean, H.K. The Use of Polychaetes (Annelida) as Indicator Species of Marine Pollution: A Review. Rev. Biol. Trop. 2008, 56, 29. [Google Scholar]
- Pagliosa, P.R. Another Diet of Worms: The Applicability of Polychaete Feeding Guilds as a Useful Conceptual Framework and Biological Variable. Mar. Ecol. 2005, 26, 246–254. [Google Scholar] [CrossRef]
- Liwanow, N.A.; Porfirjewa, N.A. Die Organisation Der Pogonophoren Und Deren Beziehungen Zu Den Polychäten. Biol. Zbl. 1967, 86, 177–204. [Google Scholar]
- Gardiner, S.L. Fine Structure of the Ciliated Epidermis on the Tentacles of Owenia fusiformis (Polychaeta, Oweniidae). Zoomorphologie 1978, 91, 37–48. [Google Scholar] [CrossRef]
- Smith, P.R.; Ruppert, E.E.; Gardiner, S.L. A Deuterostome-like Nephridium in the Mitraria Larva of Owenia fusiformis (Polychaeta, Annelida). Biol. Bull. 1987, 172, 315–323. [Google Scholar] [CrossRef]
- Westheide, W. The Direction of Evolution within the Polychaeta. J. Nat. Hist. 1997, 31, 1–15. [Google Scholar] [CrossRef]
- Hausen, H. Chaetae and chaetogenesis in polychaetes (Annelida). Hydrobiologia 2005, 535, 37–52. [Google Scholar] [CrossRef]
- Rimskaya-Korsakova, N.N.; Kristof, A.; Malakhov, V.V.; Wanninger, A. Neural Architecture of Galathowenia oculata Zach, 1923 (Oweniidae, Annelida). Front. Zool. 2016, 13, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Rimskaya-Korsakova, N.; Dyachuk, V.; Temereva, E. Parapodial Glandular Organs in Owenia borealis (Annelida: Oweniidae) and Their Possible Relationship with Nephridia. J. Exp. Zool. Part B 2020, 334, 88–99. [Google Scholar] [CrossRef]
- Kirkegaard, J.B. The Polychaeta of West Africa Part I. Sedentary Species. Atlantide Rep. 1959, 5, 7–117. [Google Scholar]
- Kirkegaard, J.B. Bathyal Benthic Polychaetes from the N.E. Atlantic Ocean, S.W. of the British Isles. J. Mar. Biol. Ass. UK 1983, 63, 593–608. [Google Scholar] [CrossRef]
- Fauvel, P. Polychètes Sédentaires. Addenda aux Errantes, Archiannélides, Myzostomaires. Faune France 1927, 16, 1–494. [Google Scholar]
- Uschakov, P. Polychaeta of the Far Eastern Seas of the U.S.S.R.; Israel Program for Scientific Translations: Jerusalem, Israel, 1965. [Google Scholar]
- Hartman, O. Polychaeta Myzostomidae and Sedentaria of Antarctica. Antarctic Res. Ser. 1966, 7, 1–158. [Google Scholar]
- Day, J.H. A Monograph on the Polychaeta of Southern Africa 2. Sedentaria. Brit. Mus. (Nat. Hist.) Lond. 1967, 459–878. [Google Scholar]
- Hartmann-Schröder, G. Annelida, Borstenwürmer, Polychaeta. Tierwelt Dtschl. 1996, 58, 1–648. [Google Scholar]
- Gil, J. The European Fauna of Annelida Polychaeta. Ph.D. Thesis, University of Lisboa, Lisbon, Portugal, 2011. [Google Scholar]
- Rouse, G.W. Oweniidae. In Polychaetes; Rouse, G.W., Pleijel, F., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 185–188. [Google Scholar] [CrossRef]
- Watson, A.T. On the structure and habits of the Polychaeta of the family Ammocharidae. J. Linn. Soc. 1901, 28, 230–260. [Google Scholar] [CrossRef]
- Wilson, D.P. On the Mitraria Larva of Owenia fusiformis Delle Chiaje. Philos. T. R. Soc. Lon. B. 1932, 22, 231–334. [Google Scholar]
- Dales, R.P. The Feeding Mechanism and Structure of the Gut of Owenia fusiformis Delle Chiaje. J. Mar. Biol. Ass. UK 1957, 36, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Beckers, P.; Helm, C.; Purschke, G.; Worsaae, K.; Hutchings, P.; Bartolomaeus, T. The central nervous system of Oweniidae (Annelida) and its implications for the structure of the ancestral annelid brain. Front. Zool. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, P.A. Family Oweniidae. In Polychaetes & Allies: The Southern Synthesis; Fauna of Australia. Vol. 4A Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula; Beesley, P.L., Ross, G.J.B., Glasby, C.J., Eds.; CSIRO Publishing: Melbourne, Australia, 2000; pp. 173–176. [Google Scholar]
- Koh, B.-S.; Bhaud, M. Identification of New Criteria for Differentiating between Populations of Owenia fusiformis (Annelida Polychaeta) from Different Origins: Rehabilitation of Old Species and Erection of New Species. Vie Milieu 2003, 53, 65–95. [Google Scholar]
- Silva, L.; Lana, P. Strategies for tube construction in Owenia caissara (Oweniidae, Annelida) from southern Brazil. Zoology 2018, 129, 9–16. [Google Scholar] [CrossRef]
- Caullery, M. Polychètes Sédentaire de l’Expédition Du Siboga: Ariciidae, Spionidae, Chaetopteridae, Chlorhaemidae, Opheliidae, Oweniidae, Sabellariidae, Sternaspidae, Amphictenidae, Ampharetidae, Terebellidae. In Siboga-Expeditie Uitkomsten op Zoologisch, Bonatisch, Oceanographisch en Geologisch gebied Verzameld in Nederlandsch Oost-Indië 1899-1900; World Register of Marine Species: Ostend, Belgium, 1944; Volume XXIV 2 bis, pp. 1–204. [Google Scholar]
- Verrill, A.E. Notice of Recent Additions to the Marine Invertebrata of the Northeastern Coast of America, with Descriptions of New Genera and Species and Critical Remarks on Others. Part V. Annelida, Echinodermata, Hydroida, Tunicata. Proc. US Nat. Mus. 1885, 8, 424–448. [Google Scholar] [CrossRef]
- Rioja, E. Estudio de Los Poliquetos de La Península Ibérica. Mem. Real Acad. Ci. Exact. Madrid Ser. Cienc. Nat. 1931, 2, 1–471. [Google Scholar]
- Díaz-Díaz, O.F.; Parapar, J.; Moreira, J. Validation of Owenia vieitezi Díaz-Díaz, Parapar & Moreira, a polychaete from the coast of Venezuela (Annelida: Oweniidae). Zootaxa 2020, 4729, 145–146. [Google Scholar]
- Malmgren, A.J. Annulata Polychaeta Spetsbergiae, Grönlandiae et Scandinaviae Hactenus Cognita. Öfvers. K. Vet. Akad. Förh. 1867, 24, 127–235. [Google Scholar]
- Delle Chiaje, S. Descrizione e Notomia degli Animali Invertebrati della Sicilia Citeriore osservati vivi negli anni 1822–1830. Tomo Ottavo. Appendice, Osservazioni Critiche, Indice Generale; Stabilimento Tipografico di C. Batelli e Comp.: Naples, Italy, 1844. [Google Scholar]
- Berkeley, E. Morphological characters of Myriochele heeri Malmgren. Nature 1949, 164, 239. [Google Scholar] [CrossRef]
- Rullier, F. Étude Morphologique, Histologique et Physiologique de l’organe Nucal Chez Les Annélides Polychètes Sédentaires. Ann. Inst. Ocean. 1951, 25, 207–341. [Google Scholar]
- Gardiner, S.L.; Rieger, R.M. Rudimentary Cilia in Muscle Cells of Annelids and Echinoderms. Cell Tissue Res. 1980, 213, 247–252. [Google Scholar] [CrossRef]
- Tzetlin, A.B.; Filippova, A.V. Muscular system in polychaetes (Annelida). Hydrobiologia 2005, 535, 113–126. [Google Scholar] [CrossRef]
- Fransen, M.E. Coelomic and Vascular Systems. Chapter XII. In The Ultrastructure of Polychaeta. Microfauna Marina; Westheide, W., Hermans, C.O., Eds.; Fischer Gustav Verlag GmbH & Co. KG.: Sttutgart, Germany, 1988; Volume 4, pp. 199–213. [Google Scholar]
- Parapar, J.; Candás, M.; Cunha-Veira, X.; Moreira, J. Exploring annelid anatomy using micro-computed tomography: A taxonomic approach. Zool. Anz. 2017, 270, 19–42. [Google Scholar] [CrossRef]
- Tzetlin, A.B.; Purschke, G. Pharynx and intestine. Hydrobiologia 2005, 535, 199–205. [Google Scholar] [CrossRef]
- Purschke, G.; Tzetlin, A.B. Dorsolateral Ciliary Folds in the Polychaete Foregut: Structure, Prevalence and Phylogenetic Significance. Acta Zool. 1996, 77, 33–49. [Google Scholar] [CrossRef]
- Bubko, O.V.; Minichev, Y.S. The Nervous System of Oweniidae (Polychaeta). Zool. Zhurnal 1972, 51, 1288–1299. [Google Scholar]
- Coulon, J.; Bessone, R. Autoradiographic Detection of Indolamine and Catecholamine Neurons in the Nervous System of Owenia fusiformis (Polychaeta, Annelida). Cell Tissue Res. 1979, 198, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Purschke, G. Sense Organs in Polychaetes (Annelida). Hydrobiologia 2005, 535, 53–78. [Google Scholar] [CrossRef]
- Purschke, G.; Arendt, D.; Hausen, H.; Müller, M.C.M. Photoreceptor Cells and Eyes in Annelida. Arthropod Struct. Dev. 2006, 35, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.; Vöcking, O.; Kourtesis, I.; Hausen, H. Owenia fusiformis—A Basally Branching Annelid Suitable for Studying Ancestral Features of Annelid Neural Development. BMC Evol. Biol. 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rioja, E. Datos para el conocimiento de la fauna de anélidos poliquetos del Cantábrico. Trab. Mus. Nac. Cienc. Nat. Ser. Zool. 1917, 29, 1–111. [Google Scholar]
- Day, J.H. New Polychaeta from Beaufort, with a Key to All Species Recorded from North Carolina; NOAA tech. Rep. NMFS, circ. # 375; U.S. Dept. of Commerce, NOAA, Marine Fisheries Service: Seattle, WA, USA, 1973; pp. 1–140. [Google Scholar]
- Fauchald, K. The Polychaete Worms. Definitions and Keys to the Orders, Families and Genera. Nat. Hist. Mus. Los Angel. Cty. Sci. Ser. 1977, 28, 1–188. [Google Scholar]
- Pettibone, M.H. Annelida. In Synopsis and Classification of Living Organisms; Parker, S.P., Ed.; McGraw-Hill Book Co: New York, NY, USA, 1982; Volume 2, pp. 1–43. [Google Scholar]
- Hartman, O. Catalogue of the polychaetous annelids of the world. Allan Hancock Found. Publ. Occas. Pap. 1959, 23, 1–628. [Google Scholar]
- Hutchings, P.A.; Rainer, S. The Polychaete Fauna of Careel Bay, Pittwater, New South Wales, Australia. J. Nat. Hist. 1979, 13, 745–796. [Google Scholar] [CrossRef]
- Day, J.H.; Hutchings, P.A. Descriptive Notes on the Fauna and Flora of Merimbula, Pambula and Black Lakes, New South Wales. Aust. Zool. 1984, 21, 269–289. [Google Scholar]
- Hutchings, P.; Murray, A. Taxonomy of Polychaetes from the Hawkesbury River and the Southern Estuaries of New South Wales, Australia. Rec. Aust. Mus. Suppl. 1984, 3, 1–118. [Google Scholar] [CrossRef] [Green Version]
- Nygren, A. Cryptic Polychaete Diversity: A Review. Zool. Scr. 2014, 43, 172–183. [Google Scholar] [CrossRef]
- Hutchings, P.; Kupriyanova, E. Cosmopolitan Polychaetes—Fact or Fiction? Personal and Historical Perspectives. Invert. Syst. 2018, 32, 1. [Google Scholar] [CrossRef]
- Jolly, M.T.; Viard, F.; Gentil, F.; Thiébaut, E.; Jollivet, D. Comparative Phylogeography of Two Coastal Polychaete Tubeworms in the Northeast Atlantic Supports Shared History and Vicariant Events: Comparative Phylogeography Of Coastal Tubeworms. Mol. Ecol. 2006, 15, 1841–1855. [Google Scholar] [CrossRef] [PubMed]
- Gentil, F.; Dauvin, J.-C.; Ménard, F. Reproductive Biology of the Polychaete Owenia fusiformis Delle Chiaje in the Bay of Seine (Eastern English Channel). J. Exp. Mar Biol. Ecol. 1990, 142, 13–23. [Google Scholar] [CrossRef]
- McNulty, J.K.; López, N.N. Year-Round Production of Ripe Gametes by Benthic Polychaetes in Biscayne Bay, Florida. Bull. Mar. Sci. 1969, 19, 945–954. [Google Scholar]
- Thiébaut, E.; Dauvin, J.C. Modelling of population dynamics of Owenia fusiformis (Annelida, Polychaeta) in eastern Bay of Seine. In Report of the Workshop Modelling the Benthos Yerseke, The Netherlands; Herman, P.M.J., Ed.; Delta Institute Communication, Delta Institute for Hydrobiological Research: Yerseke, The Netherlands, 1991; Volume 538, pp. 189–190. [Google Scholar]
- Okada, K.A. Ch. 7. Annelida. In Invertebrate Embryology; Kume, M., Dan, K., Eds.; Bai Fu Kan Press: Tokyo, Japan, 1970; pp. 192–241. [Google Scholar]
- Oliver, J.S. Selection for Asexual Reproduction in an Antarctic Polychaete Worm. Mar. Ecol. Prog. Ser. 1984, 19, 3–38. [Google Scholar] [CrossRef]
- Aguirrezabalaga, F.; Gil, J.; Viéitez, J.M. Presence of Myriochele danielsseni Hansen, 1879 (Polychaeta, Oweniidae) en las costas de la Península Ibérica. Bol. R. Soc. Esp. Hist. Nat. 2000, 96, 57–68. [Google Scholar]
- Signor, P.W.; McMenamin, M.A.S. The early Cambrian worm tube Onuphionella from California and Nevada. J. Paleontol. 1988, 62, 233–240. [Google Scholar] [CrossRef]
- Gambi, M.C. Osservazioni su morfologia funzionale e comportamento trofico di Owenia fusiformis delle Chiaje (Polychaeta, Oweniidae) in rapporto ai fattori ambientali. Oebalia 1989, 15, 145–155. [Google Scholar]
- Dales, R.P. The Nature of the Pigments in the Crown of Sabellid and Serpulid Polychaetes. J. Mar. Biol. Assoc. UK 1962, 42, 259–274. [Google Scholar] [CrossRef] [Green Version]
- Ziegelmeier, E. Neue Untersuchungen über die Wohnröhren-Bauweise von Lanice conchilega (Polychaeta, Sedentaria). Helgol. Wiss. Meeresunters. 1969, 19, 216–229. [Google Scholar] [CrossRef]
- Künitzer, A. Zur Verbreitung und Populationsdynamik der Bodenfauna der Zentralen Nordsee. PhD. Thesis, Universität Bremen, Bremen, Germany, 1990. [Google Scholar]
- Dauvin, J.-C.; Gillet, P. Spatio-Temporal Variability in Population Structure of Owenia fusiformis Delle Chiaje (Annelida: Polychaeta) from the Bay of Seine (Eastern English Channel). J. Exp. Mar. Biol. Ecol. 1991, 152, 105–122. [Google Scholar] [CrossRef]
- Pinedo, S.; Sardá, R.; Rey, C.; Bhaud, M. Effect of Sediment Particle Size on Recruitment of Owenia fusiformis in the Bay of Blanes (NW Mediterranean Sea): An Experimental Approach to Explain Field Distribution. Mar. Ecol. Prog. Ser. 2000, 203, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Deart, Y.V.; Britayev, T.A. “New” Benthic Community Dominated by Oweniidae (Polychaeta, Oweniidae) at the Murman Coast: Structure and Causes of Appearance. Dokl. Biol. Sci. 2014, 454, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Bailey-Brock, J.H.; McGurr, M.M. Temporal Changes in the Polychaete Infaunal Community Surrounding a Hawaiian Mariculture Operation. Mar. Ecol. Prog. Ser. 2006, 307, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Kopp, D.; Le Bris, H.; Grimaud, L.; Nérot, C.; Brind’Amour, A. Spatial Analysis of the Trophic Interactions between Two Juvenile Fish Species and Their Preys along a Coastal–Estuarine Gradient. J. Sea Res. 2013, 81, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Fager, E.W. Marine Sediments: Effects of Tube-Dwelling Polychaete. Science 1964, 143, 356–359. [Google Scholar] [CrossRef]
- Eckman, J.E.; Nowell, A.R.M.; Jumars, P.A. Sediment destabilization by animal tubes. J. Mar. Res. 1981, 39, 361–374. [Google Scholar]
- Somaschini, A. A Mediterranean Fine-Sand Polychaete Community and the Effect of the Tube-Dwelling Owenia fusiformis Delle Chiaje on Community Structure. Int. Rev. Gesamten Hydrobiol. 1993, 78, 219–233. [Google Scholar] [CrossRef]
- Ambrogi, R.; Fontana, P.; Gambi, M.C. Population dynamics and estimate of secondary production of Owenia fusiformis Delle Chiaje (Polychaeta, Oweniidae) in the coastal area of the Po river Delta (Italy). In Biology and Ecology of Shallow Coastal Waters; Eleftheriou, A., Smith, C.J., Ansell, A.D., Eds.; Olsen & Olsen: Fredensborg, Denmark, 1995; pp. 207–214. [Google Scholar]
- Oyarzún, C.; Fernández, C.; Landaeta, M.; Cortés, N. Ontogenetic and Temporal Fluctuations in Feeding Habits of the Chilean Croaker Cilus gilberti (Perciformes, Sciaenidae) in Southern Chile. Cah. Biol. Mar. 2001, 42, 295–302. [Google Scholar]
- Hartman, O. Systematic account of some marine invertebrate animals from the deep basins of southern California. In The Benthic Fauna of the Deep Basins off Southern California. Part II; Hartman, O., Barnard, J.L., Eds.; Allan Hancock Pacific Expeditions, University of Southern California Press: Los Angeles, CA, USA, 1960; Volume 22, pp. 69–215. [Google Scholar]
- Annenkova, N.P. The Polychaete Fauna of the Northern Part of the Japan Sea. Issled. Rus. Mor. 1937, 23, 139–216. [Google Scholar]
- McIntosh, W.C. Report on the Annelida Polychaeta Collected by H.M.S. Challenger during the Years 1873-1876. Chall. Rep. (Zool.) 1885, 12, 1–554. [Google Scholar]























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parapar, J.; Mortimer, K.; Capa, M.; Moreira, J. On the Systematics and Biodiversity of the Palaeoannelida. Diversity 2021, 13, 41. https://doi.org/10.3390/d13020041
Parapar J, Mortimer K, Capa M, Moreira J. On the Systematics and Biodiversity of the Palaeoannelida. Diversity. 2021; 13(2):41. https://doi.org/10.3390/d13020041
Chicago/Turabian StyleParapar, Julio, Kate Mortimer, María Capa, and Juan Moreira. 2021. "On the Systematics and Biodiversity of the Palaeoannelida" Diversity 13, no. 2: 41. https://doi.org/10.3390/d13020041