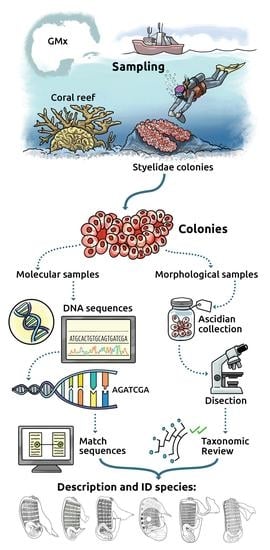
Species Delimitations
A preliminary phylogeny based on p-distances served as the species hypotheses we tested using delimitation analyses. Not all sequences were long enough to be included in the alignment for species delimitation (e.g., CAGoM-2090 and 2100 belonging to
Botryllus humilis Monniot, 1988). Their assignment is therefore based on the preliminary phylogeny. The species delimitation results are summarized in
Figure 2 (Bayesian Tree) and
Supplementary Figure S1 (Maximum Likelihood Tree), and are presented in detail in
Supplementary Table S3. Pairwise p-distances of all sequences present in the alignment used to create species delimitation trees are shown in
Supplementary Table S4. A
Symplegma clade’s two sequences were each considered a putative species by ASAP and bPTP, and although they were singletons, morphological characters justified their recognition as two valid species (
Symplegma sp. and
Symplegma papillata sp. nov). Sequences in one clade were identified through megablast to the GenBank database as
Botrylloides niger, a well-known circum-tropical species [
34].
There were two samples with ambiguous assignments. CAGoM-2211 was grouped with Botryllus tunnelli sp. nov. using ASAP but was classified as a separate species by bPTP; thus, we decided to not include it in this species. CAGoM-2456 was placed in Botryllus humilis Monniot, 1988 using bPTP, but was placed as a separate species by ASAP. It is 97–98% identical to other Botryllus humilis sequences from GenBank, which means that it likely belongs in Botryllus humilis despite the ASAP results, and we decided to classify it as such.
The three Symplegma spp. in this study did not have close matches to any sequences on GenBank, nor to unpublished sequences being used for a holistic revision of the Symplegma genus (Gissi et al., unpublished data).
Genus Botryllus Gaertner, 1774
Holotype: CAGoM-2339 La Bonanza Reef (ARMS), artificial substrate (PVC), 4 m depth, 26 July 2019, col. Lilian Palomino-Alvarez.
Paratype: CAGoM-2521, La Bonanza Reef (ARMS), artificial substrate (PVC), 4 m depth, 25 October 2019, col. Lilian Palomino-Alvarez.
Etymology: This species was found in Arrecife La Bonanza from Parque Nacional Arrecife Puerto Morelos under the charge of Dr. María del Carmen García Rivas, to whom we are grateful for all the support provided for the realization of this work.
The colonies are about 3–5 cm across and 1 mm thick. The systems have 3–4 zooids each with sets of zooid pairs attached only by loose tunic to the systems in CAGoM-2339. The zooids are arranged in a “schlosseri-type” configuration (as described in Brunetti 2009 [
35]). Finger-shaped ampullae, which are peach-colored to brown-colored, are visible in a few sections on the edges of the colony. The vascular network is easily visible in the tunic because there are large sections of the colonies with no zooids present. After formalin preservation, the colony appears reddish-brown with a transparent tunic (
Figure 3A).
In CAGoM-2339 zooids were contracted and six of the most relaxed zooids were 0.78 mm long on average and buds were 0.4 mm. The zooids are wider than usual for Botryllus. The anterior region is flat with the oral siphon in the middle and includes the 1st and 2nd stigmatal rows. Zooids are reddish-brown, and the body is usually heavily pigmented so that internal structures are not visible from the outside. The pigment cells can also be clustered in the transverse vessels. In colony CAGoM-2521, ovaries are visible in the dorsal view, despite the dark and wide anterior region.
The ten oral tentacles are of three size orders, in this position: L S M S M S L S M S. There are four stigmatal rows on both sides of the body. The second row is dorsally incomplete, usually reduced by one or two stigmata. There are 13 stigmata per half-row (DL3v3v3v4E) in the second and third rows of stigmata.
The stomach is cylindrical, meaning that the cardiac end is the same width as the pyloric end. There are 7–8 poorly marked stomach folds, with some of the folds incomplete or not even visible in some zooids. The pyloric caecum is bent at a right angle and the tip is very swollen. The caecum would be ¾ the length of the stomach if the caecum were not curved (
Figure 3B). The intestine, rectum, and anus are shaped like a figure-eight, which is an unusual orientation for
Botrylloides and
Botryllus. The rectum does not rise vertically towards the anterior of the body but remains in a horizontal position and the anus is tucked into the intestinal loop, at the same stigmatal row as the anterior-most portion of the intestinal loop.
Ovaries were present in CAGoM-2521 but not in CAGoM-2339. Ovaries are at the level of the second and third row of stigmata on both sides or only on the left side of the body, with one oocyte each. Testes were not present in either colony. Buds were present in the colony CAGoM-2339, attached to adult zooids.
Holotype: CAGoM-2207 Alacranes Reef (ALA01), on dead coral and sponges, 9 m depth, 18 July 2019, col. Lilian Palomino-Alvarez.
Paratype: CAGoM-2266, Bajos del Norte Reef (BN01), on dead coral, 15m depth, 20 July 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-2257, Bajos del Norte Reef (BN01), on dead coral, 15m depth, 20 July 2019, col. Lilian Palomino-Alvarez.
Etymology: The name of the species is due to the unusual hooked shape of the caecum.
Sample CAGoM-2266 was growing on coral and sponges. Colonies are about 4–5 cm across and 2 mm thick. The zooids are arranged in a “schlosseri-type” configuration 35]. In life, the colony CAGoM-2257 was dark and translucent with white dots on the ventral and dorsal sides of the oral siphon, while CAGoM-2266 was orange and translucent, and CAGoM-2207 was white with red atrial languets that form the ceiling of the cloaca (
Figure 4A). After formalin preservation, all colonies appear light brown (
Figure 4B,C).
Zooids were contracted, and 19 of the most relaxed (CAGoM-2207) were 1 mm long on average (
Figure 4D). The body wall is uncolored and transparent. There are six oral tentacles of two size orders (L S L S L S). Each zooid has 13–14 rows of stigmata on the right side of the body, and only 11 rows visible on the left side (CAGoM-2207). The second row, dorsally complete, was only visible in four zooids in CAGoM-2207. There are approximately 13–14 stigmata per half-row on the right and left sides of the body, with the following formula: DL3v4v4v3E3v4v4v3DL.
The stomach is short and square-shaped and there are eight isodiametric folds. The pyloric caecum is curved into a closed C-shape (looks like a candy cane but the tip is longer than the stalk), and moderately swollen in the tip. The caecum would be ½ the length of the stomach in CAGoM-2266 and ¾ in CAGoM-2207 if the caecum was not curved (
Figure 4E). The intestine has a dilation in the first loop. The anus opens at the third or fourth stigmatal row. Ovaries and testes were not visible in these zooids, while primary and secondary buds were present.
Holotype: CAGoM-2087, Bajo 10 Reef (ARMS), artificial substrate, 7 m depth, 30 April 2019, col. Lilian Palomino-Alvarez.
Paratype: CAGoM-2093, Bajo 10 Reef (ARMS), artificial substrate, 7 m depth, 30 April 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-2084, CAGoM-2085, Bajo 10 Reef (ARMS), artificial substrate, 7 m depth, 30 April 2019, col. Lilian Palomino-Alvarez. CAGoM-2579, Bajo 10 Reef (ARMS), artificial substrate, 7 m depth, 12 February 2020, col. Lilian Palomino-Alvarez. CAGoM-2695, Puerto Morelos Reef (ARMS), 4 m depth, 12 February 2020, col. Lilian Palomino-Alvarez.
Etymology: The name of the species is dedicated to Mr. Edward H. Harte for his generous donation on 19 September 2000 that enabled the creation of the Harte Research Institute for Gulf of Mexico Studies (HRI) at Texas A&M University-Corpus Christi, Texas, USA. HRI has continuously supported research on biodiversity and conservation of the Southern Gulf of México, including the taxonomic work and scientific collections of the Biodiversidad marina de Yucatán (BDMY) research group at UMDI-Sisal, FC-UNAM, Yucatán México where the authors Lilian Palomino-Alvarez and Nuno Simões work.
In life, the colonies are salmon or dark purple; after formalin preservation, they turn reddish-brown (
Figure 5A–D). The rim of the cloaca and the oral siphons in CAGoM-2087 are dark purple. Under magnification, it is possible to see reddish-brown lines radiating outward from the cloacas formed by pigment cells that outline the pronounced atrial languet of each zooid in the system (
Figure 5E). The zooids are arranged in a “schlosseri-type” configuration 35] and the systems appear to rise above the colony surface. The colony is 1 mm thick. Adult zooids, embryos, and testes are visible through the colony tunic. Ampullae are finger-shaped and can have a peach color (CAGoM-2087) or are colorless (CAGoM-2093).
Zooids are an average of 1.1 mm long, and are pink and opaque when preserved so that the stigmatal rows are not easily visible from the outside (
Figure 5F). Dark purple pigment cells are spread across the body wall, most often at medium density. These pigment cells are also visible when the zooids are in the colony. There are orange pigment clusters on either side of the endostyle, corresponding roughly to stigmatal rows. In CAGoM-2093 the stomach is dark brown, and visible through the body wall even when the zooids are still inside the colony.
There are eight oral tentacles of three size orders variable in their position. Zooids have 8–9 stigmatalrows on both sides of the body and the second row is dorsally complete on both the left and right sides. There are 12–13 stigmata per half-row on each side, organized as DL4v2-3v2-3v3-4E on the right and as DL4v2-3v2-3v3E on the left.
The stomach is cylindrical, with eight stomach folds without longitudinal grooves, and the last one is often smaller than the others. The pyloric caecum is large and would be longer than the stomach if the caecum was not curved. It resembles a candy cane, with the curved distal section ½ to ⅔ as long as the proximal section, and this is at a right angle with respect to the longitudinal axis of the stomach. The tip of the pyloric caecum is slightly swollen (
Figure 5G). The intestinal loop is closed and dips posteriorly towards the stomach or covers a portion of the stomach before turning anteriorly at a 90-degree angle to form the rectum. The anterior edge of the intestinal loop is situated from the 6th to the 8th stigmatal row, and the anus opens between the 4th row and the 5th transverse vessel. The anus position is quite variable with respect to the anterior edge of the intestinal loop, between 1–4 rows anterior.
Ovaries are anterior to testes, present only in CAGoM-2087. Testes are peach-colored and form a rosette with 2–3 follicles in CAGoM-2093 to 4–12 follicles in CAGoM-2087. The left side testis is anterior to the right side testis and overlaps the anterior-most intestinal loop. Buds are visible in both colonies, in a layer underneath the adult zooids in CAGoM-2087, and on the external edges of the systems in CAGoM-2093. There are embryos on one or both sides of the zooid, and many zooids have both embryos and testes at the same time in CAGoM-2087.
Examined material: CAGoM-2071, Bajo 10 Reef (ARMS), 7 m depth, 03 April 2019 col. Lilian Palomino-Alvarez. CAGoM-2102 Bajo 10 Reef (ARMS), 7 m depth, 03 April 2019 col. Lilian Palomino-Alvarez. CAGoM-2407, CAGoM-2427 Bajo 10 Reef (ARMS), 7 m depth, 03 July 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-2076, CAGoM-2081, CAGoM-2082, CAGoM-2083, CAGoM-2086, CAGoM-2089, CAGoM-2103, CAGoM-2105, CAGoM-2109, CAGoM-2111, CAGoM-2114, CAGoM-2118, CAGoM-2119, CAGoM-2120, CAGoM-2126, Bajo 10 Reef (ARMS), 7 m depth, 03 April 2019 col. Lilian Palomino-Alvarez. Artificial substrate. CAGoM-2170, Mahahual Reef (MAH6), 9 m depth, on dead-dead coral. 15 May 2019 col. Lilian Palomino-Alvarez. CAGoM-2385, CAGoM-2398, CAGoM-2405, CAGoM-2407, CAGoM-2413, CAGoM-2414, CAGoM-2422, Bajo 10 Reef (ARMS), 7 m depth, 03-07-2019 col. Lilian Palomino-Alvarez. Artificial substrate. CAGoM-2456, Bajo 10 Reef (ARMS), 7 m depth, 25 October 2019 col. Lilian Palomino-Alvarez. Artificial substrate. CAGoM-2564, Bajo 10 Reef (ARMS), 7 m depth, 12 February 2020 col. Lilian Palomino-Alvarez. Artificial substrate.
Sample CAGoM-2071 and CAGoM-2102 are large colonies, while CAGoM-2407 is small (three systems). The colonies are 1–2 mm thick with small networks of hydrozoans on the surface. The systems are variable: CAGoM-2071 contains ladder-like, oval, and circular systems, CAGoM-2102 contains ladder-like systems only, and CAGoM-2407 oval systems only. The oval and circular systems in CAGoM-2071 are too large for all zooids in each system to be involved in the construction of the cloaca. The systems in CAGoM-2407 are oval, but the colony is small. Small colonies of either “leachii-type” or “schlosseri-type” can appear oval. In life, colony CAGoM-2071 was rose-colored and colony CAGoM-2102 was purple with brown, but a variety of colors was observed in this species, such as pink and orange with brown. After formalin preservation, colonies CAGoM-2071 and CAGoM-2407 are tan/light brown, whereas colony CAGoM-2102 is reddish-brown.
Zooids were in various states of relaxation, although none were fully relaxed. A small number of zooids, the most relaxed, from each colony were measured with an average length of 1.16 mm. Removed from the tunic the zooids are yellow, but the body wall is transparent enough that the intestine, ovaries, and testes are visible from the outside. CAGoM-2102′s zooids have dark red atrial languets and pink bodies in the tunic (
Figure 6A,B). Removed from the tunic, the posterior ends of zooids are yellow because the stomach is visible through the body wall. Common pigmentation features of the CAGoM-2071 and CAGoM-2407 zooids are two light brown or orange pigment clusters (one on either side of the oral siphon, at the base of large oral tentacles) and two brown pigment clusters, one on either side of the distal end of the atrial languet. Diffuse and faint orange pigment cells appear on either side of the endostyle, corresponding to stigmatal rows 2–5 or 2–6, and there are yellow pigment cells spread across the body wall, at high or medium density. Three common pigmentation features of the CAGoM-2102 zooids are (1) magenta pigment cells on the atrial languet at medium density with two longitudinal lines down the length of the languet or variable patterning, (2) Dark orange pigment cells overlain with dark purple pigment cells on either side of endostyle, corresponding roughly to stigmatal rows, most often stigmatal rows 2–6, and (3) two light brown or peach pigment clusters, one on either side of the oral siphon (as in CAGoM-2071 and CAGoM-2407). The zooids in this colony can have pigmentation elsewhere, including on the anterior-most portion of the zooid, on the posterior edge of the atrial opening, covering the transverse vessels, on either side of the distal end of the atrial languet, covering a portion of the stomach, or spread across the body wall at low density.
The 12 oral tentacles are of three size orders, in one of these two positions: L S M S M S M S M S M S or L S M S L M S M S M S M. Each zooid has 8 rows of stigmata on each side of the body. The second row has fewer (1–2) stigmata between the dorsal lamina and the first longitudinal vessel. There are 15–16 (more often 15) stigmata per half-row on both sides of the body, in the following arrangement: DL5-6v2-3v2-3v4-5E.
The stomach is cylindrical, with 8–9 stomach folds, and when there are 9 folds the last one is often smaller than the others. The folds have shallow longitudinal ruts. The pyloric caecum is large (it would be longer than the stomach if the caecum were not bent). The caecum is bent (not perpendicular) from the longitudinal axis of the stomach, and the moderately swollen tip is at a right angle with respect to the stalk or bent slightly downward from the stalk at almost 90 degrees. The length of the tip of the caecum is very close to the length of the stalk (
Figure 6E).
The anterior edge of the first intestinal loop is somewhere between the 5th and the 7th transverse vessel, most commonly at the 6th stigmatal row. The intestine loop and rectum have variable orientations in these colonies: sometimes the rectum makes an obtuse angle with the intestinal loop, or sometimes an acute angle, following the curve of the body. The anus opens somewhere between the 6th row and the 7th transverse vessel, most commonly at the 7th row. The anus opens at the same level or posterior to the first intestinal loop, which is unusual in Botrylloides and Botryllus.
Ovaries were occasionally visible only in the CAGoM-2071 zooids. When present with testes, the right ovary is anterior to the testis, while the left ovary is dorsal. Testes were present in the CAGoM-2071 and CAGoM-2102 colonies. In all zooids, the left testis overlaps the anterior—most intestinal loop and is anterior to the right testis. Testes had a single follicle (regressed), all the way to 9 follicles (the biggest, pear-shaped). Buds were present in all three colonies, attached to adult zooids.
Holotype: CAGoM-2420, Bajo 10 Reef (ARMS), 7 m depth, 30 July 2019 col. Lilian Palomino-Alvarez. Artificial substrate.
Paratype: CAGoM-2077, Bajo 10 Reef (ARMS), 7 m depth, 03 April 2019 col. Lilian Palomino-Alvarez. Artificial substrate. CAGoM-2391, Bajo 10 Reef (ARMS), 7 m depth, 30 July 2019 col. Lilian Palomino-Alvarez. Artificial substrate.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-2092 Bajo 10 Reef (ARMS), 7 m depth, 30 April 2019, col. Lilian Palomino-Alvarez. CAGoM-2336, CAGoM-2372, Puerto Morelos (ARMS), 4 m epth, 26 July 2019, col. Lilian Palomino-Alvarez. CAGoM-2583, Bajo 10 Reef (ARMS), 7 m depth, 30 July 2019, col. Lilian Palomino-Alvarez.
Etymology: Named after Charles and Gretchen Lambert who devoted their careers to advancing the fields of ascidian biology and taxonomy.
The colonies are about 5–7 cm across and less than 1 mm thick. Well-defined star-shaped systems are separated from each other in a transparent tunic. Colonies from Bajo de 10 Reef were heavily fouled with an encrusting bryozoan. In life and preserved, colonies are brown and white or bright yellow, due to the zooids’ color pattern. Pigment cells are scattered in the body wall at low density (
Figure 7A,B), dark brown in endostyle/anterior regions, and light brown otherwise. Zooids also have two elongated patches of white pigment on each side of the atrial languets, resembling the shape of a human tooth. The vascular network is easily visible through the tunic, as well as long and thin light brown ampullae in the margins of the colony (
Figure 7C). The zooids are arranged in a “schlosseri-type” configuration 35], each system with 9–14 zooids.
The average zooid length is 0.8 mm (
Figure 7D). There are 10–12 oral tentacles of three size orders variable in their position. In colony CAGoM-2391 there are small projections (like papillae) that are smaller than the smallest oral tentacles and they are very common in the pre-pharyngeal region. Zooids have four rows of stigmata on both sides of the body and the second stigmatal row is dorsally complete on both sides. There are 11–12 stigmata per half-row on each side of the body. On the right side, the stigmata are organized as DL4v2-3v2-3v3-4E, while on the left side, DL3v2v3v3E.
The stomach is cylindrical, with an unusual longitudinal axis orientation: it is oriented vertically, with the cardiac end pointing toward the posterior end of the zooid. There are seven stomach folds, with one wider than the others, which may be the result of two folds that merged during development. The pyloric caecum is perpendicular with respect to the longitudinal axis of the stomach, slightly curved with a swollen tip. The proximal intestine continues along the posterior/anterior axis of the stomach before it angles downward to form the intestinal loop and curves upward into the rectum. Usually, the anterior edge of the intestinal loop is at the 3rd stigmatal row but in some zooids, it is at the 2nd row. The anus position is quite variable with respect to the anterior edge of the intestinal loop and usually opens at the 4th stigmatal row or transverse vessel.
Ovaries and testes are not present in these samples. Buds attached by vascular stolons to adult zooids, sometimes on both sides of the body, are visible in the tunic. Their body wall is not heavily pigmented, so internal organs are visible through the body wall.
Holotype: CAGoM-2283, Bajos del Norte (BN04), on dead coral and bryozoans, 18 m depth, 21 July 2019, col. Lilian Palomino-Alvarez.
Etymology: This species was found in the Bajos del Norte reef, Yucatán Peninsula.
In life, the colonies are black due to zooid pigmentation (
Figure 8A). After formalin preservation, they turn brown (
Figure 8B). Colonies are less than 1 mm thick. The tunic is transparent, and the zooids are perfectly visible. The zooids are arranged in a “leachii-type” configuration 35]: not all zooids are involved in the construction of the cloaca and the systems are linear in shape. Finger-shaped ampullae line the margins of the colony.
The average zooid length is 1.2 mm (
Figure 8C). Dark pigmentation exists throughout the body wall but mostly in the anterior area. The oral siphon is a short tube with a smooth margin and the atrial opening is reduced with a short atrial languet (the atrial languet could also be missing). There are 10 very short oral tentacles of two size orders in the following position: S S S L S L L L S. Zooids have 9 stigmatal rows on the right side, with the last row sometimes with irregular stigmata, while the second row is dorsally incomplete on the right side of the body. There are 16–18 stigmata per half-row on both sides with the formula: DL5v3v3v5E on the right side, while on the left side: DL5v3v3v5E.
The stomach is small and globular with eight folds, the last smaller than the others. The cardiac ends do not have swellings and the folds have longitudinal grooves (bisected into two halves). The pyloric caecum is perpendicular to the stomach’s longitudinal axis wall and slightly curved. It is 2/3 the length of the stomach (if not bent), and the tip is round and swollen (
Figure 8D). The anterior-most portion of the intestinal loop is often horizontal and turns vertically into the rectum at a 90° angle. The anus opens at the 8th to 9th stigmatal row.
Ovaries and testes were not found. Buds were present, attached to adult zooids that were located within the systems.
Holotype: CAGoM-2470, Bajo 10 Reef (ARMS), 7 m depth, 25 October 2019, col. Lilian Palomino-Alvarez.
Paratypes: CAGoM-1570 Bajo 10 Reef (PIL), artificial substratepthm depth, 18 February 2019, col. Lilian Palomino-Alvarez. CAGoM-2034 Mahahual Reef (MPIL), artificial substrate, 8 m depth, 01 March 2019, col. Lilian Palomino-Alvarez. CAGoM-2298 Puerto Morelos Reef (PM01), on dead coral, 7 m depth, 15 July 2019, col. Lilian Palomino-Alvarez. CAGoM-2506 Bajo 10 Reef, Artificial substrate, 7 m depth, 25 October 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-01191 Cayo Arcas Reef (T-VIII), on dead-dead coral, 9 m depth, 23 April 2018, col. Lilian Palomino-Alvarez. CAGoM-2201 Alacranes Reef (ALA01), on dead coral and sponges, 9m depth, 18 July 2019, col. CAGoM-2392, CAGoM-2396, CAGoM-2421, Bajo 10 Reef (ARMS), 7 m depth, 30 July 2019, col. Lilian Palomino-Alvarez. CAGoM-2574, Bajo 10 Reef (ARMSepthm depth, 12 February 2020, col. Lilian Palomino-Alvarez. CAGoM-2586 La Bonanza Reef (ARMSE), artificial substrate (PVC), 4 m depth, 21 February 2020, col. Lilian Palomino-Alvarez. CAGoM-2654 Cozumel (COZ-01), on live coral, 8.2 m depth, 27 August 2020, col. Lilian Palomino-Alvarez.
Etymology: In memory of Dr. Wes Tunnell, whose hard work, dedication, and endless curiosity for the Southern Gulf of Mexico coral reef systems have contributed significant knowledge to better understand and manage these complex ecosystems. Dr. Tunnell’s unconditional support of the marine biodiversity research efforts in Mexico helped shape the lives of many students and colleagues.
Colonies were attached to both coral debris and artificial substrate. In life holotype and paratype had a dark brown colony with cream-colored patches, especially in the anterior region around the oral siphons. One other colony was yellow with red patches in each system. The zooids are arranged in a “schlosseri-type” configuration 35]. Zooids grow right up to the edge of the colony and there are few peach-colored ampullae at the outer edges of the colony, seen only in small sections of the zooid-free tunic (
Figure 9A,B).
Many of the zooids were contracted, and six of the most relaxed zooids had an average length of 1.2 mm. Zooids are pink-colored, and the body is heavily pigmented, so internal structures are not easily visible from the outside; however, the pronounced atrial languets are pigment-free (
Figure 9C). Pigmented cells are spread evenly around the body wall at low density. Orange pigment cells are clustered on either side of the endostyle. These clusters correspond roughly to stigmatal rows, although there are sometimes fewer clusters than rows. There were between 6–9 pairs of pigment clusters in these zooids, most often 7. These pigment clusters corresponded to stigmatal rows 2–7, 2–8, 2–10, 2–11, or 3–8, most often 2–8.
The eight oral tentacles are of two or three size-orders, with high variability in their organization. The oral tentacles are large with respect to the size of the oral siphon, so they overlap with each other and can be difficult to count. Each zooid has 8–9 rows of stigmata on each side of the body (
Figure 9D). The second row was only visible in a bud, being dorsally incomplete on the right side, while it was not visible on the left side. There is an average of 15 stigmata per half-row on the left side of the body, arranged in the following formula: DL5v3v3v4E. On the right side, the stigmata between the dorsal lamina andthe 1st longitudinal vessel were not visible, but the other stigmata were arranged in a similar way: DL?v3v2-3v4E.
The stomach is cylindrical, with eight folds, the last one occasionally smaller than the others. The folds do not have longitudinal grooves but do have shallow longitudinal ruts. The pyloric caecum is curled into a C-shape, and is long (would be longer than the stomach if the caecum were not curved). The tip of the caecum is slightly swollen (
Figure 9E). The intestinal loop dips down to touch the cardiac portion of the stomach before turning vertically into the rectum at an acute angle. The intestinal loop reaches anywhere from the 6th to the 8th stigmatal row, most commonly the 7th row. The anus opens anywhere from the 3rd to the 5th transverse vessel, most commonly at the 5th row. This anus placement is further anterior than in most botryllid species unless the species have zooids with 4 stigmatal rows.
The testes were present in CAGoM-2506 but with a single follicle each, the left one overlapping the cardiac ends of the anterior-most stomach folds, while ovaries were not visible. In two other samples not barcoded but taken from the same site and with similar morphology (CAGoM-1570 and CAGoM-2034), the ovaries were anterior and dorsal to testes on both sides of the body. They appear in first-order buds with one oocyte on each side, but adult zooids have up to two developing embryos on each side of the body. Testes have 10 to 12 circular follicles of different sizes on each side. In all zooids, the left testis overlaps the anterior-most intestinal loop and it is anterior to the right side testis. One (CAGoM-2034) or two (CAGoM-1570) larvae were found on each side of the body. In most of the colonies observed, the larvae were not well-developed and had pigment spots, especially on the trunk. Due to this pigment, it was only possible to see four ectodermal ampullae.
Holotype: CAGoM-2272 Bajos del Norte Reef (BN03), 17 m depth, 21 July 2019, on dead coral, col. Lilian Palomino-Alvarez.
Paratype: CAGoM-0367 Cayo Arcas Reepthr), 1 m depth, 21 August 2016, col. Lilian Palomino-Alvarez. Over dead coral. CAGoM-951 Cayo Arcas Reef (T-II), on coral, 7.6 m depth, 23 April 2018, col. Lilian Palomino-Alvarez. CAGoM-2280 Bajos del Norte Reef epth), 14 m depth, on dead coral, 21 July 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-0376 Cayo Arcas Reef (PMX), 13 m depth, 21 August 2016, col. Lilian Palomino-Alvarez. Platform. CAGoM-0980 Cayo Arcas Reef (T-XVII), 13 m depth, on dead coral, 23 April 2018, col. Lilian Palomino-Alvarez.
Etymology: The name is dedicated to the institution “Universidad Nacional Autónoma de México” (UNAM) for its significant role in the development of teaching, scientific research, and the spread of culture in Mexico.
Living colonies are 3–4 cm long and ~1 mm thick. The zooids (10–12) are arranged in systems with a “schlosseri-type” configuration 35]. In life, the zooids are dark (CAGoM-2280) or orange (CAGoM-2272), easily visible through the transparent tunic. The surface of the tunic shows a white network linking the oral siphons and cloacas (
Figure 10A–C). After formalin preservation, the colony appears reddish-brown with a light yellowish tunic (
Figure 10D).
Many of the zooids were contracted, and 20 of the most relaxed zooids had an average length of 1.2 mm (
Figure 10E). The reddish-brown zooids have white pigment around the oral siphon. The body wall is heavily pigmented so that internal structures are not visible from the outside of the zooid. Zooids in CAGoM-2280 have a low density of pigmented cells spread evenly in the body wall and also clustered in a ring around the oral siphon. Orange pigment cells are clustered on either side of the endostyle, corresponding roughly to stigmatal rows but in very pigmented zooids, the number and location of the clusters could not always be determined. There were between 6–8 pairs of pigment clusters, most often 6, corresponding to stigmatal rows 2–7, 2–8, or 2–9, most often 2–7.
There are eight to nine oral tentacles of three size orders, in one of these two positions (L S M S M S M S) or (L S M S L M S M). Each zooid has 9–10 rows of stigmata on the right side of the body and 10 rows on the left side. The second row was only visible in the buds, where there were no stigmata visible between the dorsal lamina and the first longitudinal vessel. However, we believe that stigmata might still appear in this space in fully grown zooids, given there were stigmata between the second and third longitudinal vessels, and the lack of stigmata usually extends into this space when the row is incomplete. There are approximately 14–15 stigmata per half-row on the right side, with the following formula: DL5v2-3v3v4E. On the left side, stigmata between the 2nd and 3rd longitudinal vessels were not visible, and thus we present a partial formula: DL5v3v?v4E.
The stomach is short and cylindrical with eight folds, almost always of the same size. The pyloric caecum is curved into a C-shape and is long (would be ¾ the length of the stomach if the caecum was not curved) (
Figure 10F,G). The caecum projects perpendicular to the longitudinal axis of the stomach, but in CAGoM-367 it is bent towards the intestinal loop, and the tip is visible on the other side of the body. Usually, the width of the caecum is uniform but in some zooids, the tip is slightly swollen. The first loop of the intestine is closed, and the anterior edge of the loop is at the 8th or the 9th row of stigmata, while the medium portion of the intestine is almost parallel to the stomach. In CAGoM-367 zooids, the middle of the intestine is perpendicular to the stomach. Then the intestine bends vertically forming the rectum and the anus opens at the 6th, 7th, or 8th row, usually one row more anterior to the edge of the first intestinal loop.
Ovaries were not visible in zooids. A testis was visible in a single zooid of CAGoM-2280, on the left side of the body with a single follicle. This testis was just anterior to the intestinal loop. Buds were present in the colony, attached to adult zooids.
Genus Botrylloides Milne Edwards, 1841
Holotype: CAGoM-2227, Alacranes Reef (ALA04), on dead coral, 7 m depth, 18 July 2019, col. Lilian Palomino-Alvarez.
Paratypes: CAGoM-2270, Bajos del Norte Reef (BN02), on dead coral, 10 m depth, 20 July 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-2261, Bajos del Norte Reef (BN01), 10 m depth, 20 July 2019 col. Lilian Palomino-Alvarez. Dead coral.
Etymology: The holotype was found in Alacranes Reef, Yucatán, which is the largest coral reef within the Gulf of Mexico and a biodiversity hotspot in the region.
In life, the colonies are greenish and appear as meandering lines (
Figure 11A) because zooids are arranged in a “leachii-type” configuration 35]. After formalin preservation, the colonies are brick-red (
Figure 11B,C). Both colonies are 1 mm thick. In CAGoM-2227, there are sections in the colony without zooids and these areas are larger than cloacal openings would normally be. Finger-shaped ampullae line the exterior of one half of the colony, with three color patterns when preserved: (1) peach-colored, (2) yellow, and (3) dark purple at the base but missing from the tips. The zooids are yellow with gold pigment clusters in the anterior half, while the posterior half has a transparent body wall. Additionally, there are brick-red pigment cells scattered across the body wall at low density, in clusters evenly spaced forming a wide ring around the oral siphon, and clustered on either side of the endostyle, corresponding roughly to stigmatal rows although these clusters are smaller and more diffuse than the orange pigment clusters in other botryllid species. Buds have orange and purple pigment cells spread at low density throughout the body wall.
In the colony CAGoM-2270, the preserved zooids’ body walls are translucent and a section of the reddish-brown pharynx is visible. The anterior and posterior regions of the zooid are colored pinkish, in contrast to the reddish-brown center. The endostyle is outlined in brick-red pigment. As in CAGoM-2227, brick-red pigment cells are scattered across the body wall at low density, but in contrast, the brick-red pigment cells are not visible around the oral siphon or clustered on either side of the endostyle.
The average zooid length is 1.55 mm in CAGoM-2227 and 1.62 mm in CAGoM-2270 (
Figure 11D). In both colonies, the oral siphon is more pronounced than in other
Botrylloides and
Botryllus, and the anterior portion of the zooid surrounding the oral siphon is concave. In CAGoM-2270, the oral siphon is split into thin projections, giving the siphon the appearance of a crown. In both colonies, the atrial opening of the zooids is very large, and the dorsal lip is bowl-shaped. There are eight oral tentacles of three size orders. Zooids have 11–12 stigmatal rows on the right side of the body, with the last row sometimes irregular, while the second row is dorsally complete on both sides. There are 15–17 stigmata per half-row on each side of the body, with the following formula DL5v3v3v4 E on the right side, and DL5v3v3v4E on the left.
The stomach is conical, meaning that the cardiac end is wider than the pyloric end, with most often nine folds (eight in some zooids) and the last one occasionally smaller than the others. The folds do not have longitudinal grooves and the cardiac ends are swollen. The pyloric caecum is at a perpendicular angle to the longitudinal axis of the stomach. It is small, ¼ to ⅓ the length of the stomach, and the tip of the caecum is slightly swollen (
Figure 11E). The first intestinal loop is closed, with the descending section touching the cardiac portion of the stomach before turning vertically into the rectum at an obtuse angle. The anterior edge of the first intestinal loop is anywhere from the 8th to 11th transverse vessel. The anus opens anywhere from the 7th to 10th stigmatal row and most often the 9th row and the position is quite variable with respect to the anterior edge of the intestinal loop.
Ovaries were not found. Most of the testes had a single follicle, although one testis had three. Buds were present in CAGoM-2227, attached to adult zooids.
Botrylloides ampullarius sp. nov. (Figure 12) Holotype: CAGoM-2425, Bajo 10 Reef (ARMS), growing on a blade of macroscopic algae, 7 m depth, 30 July 2019, col. Lilian Palomino-Alvarez.
Paratypes: CAGoM-1497, CAGoM-1542, Bajo 10 Reef (ARMS), 7 m depth, 18 February 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-2420, Bajo 10 Reef (ARMS), 7 m depth, 30 July 2019, col. Lilian Palomino-Alvarez.
Etymology: The name of the species is due to the large number of ampullae in colonies.
In life, the colony is brown with some yellow, and it is 1–2 mm thick (
Figure 12A). The zooids are arranged in a “schlosseri-type” configuration 35]. There were 6–7 zooids in each system, all sharing a cloaca. Vascular networks with ampullae are visible on the underside of the colony. Finger-shaped ampullae are dense along the margin of the colony, and circular ampullae appear in a more interior region of the colony than the finger-shaped ampullae. After formalin preservation, the colony is dark brown to black. Ampullae are dull gold or yellow/brown (
Figure 12B,C). There are two other colonies (CAGoM-1497 and CAGoM-1542) without barcode sequences collected in the same location with similar morphological characteristics to the type specimen (CAGoM-2425): those colonies are purple with orange and with 8–12 zooids in each oval system.
The average zooid length is 1.48 mm (
Figure 12D). The zooid body wall is covered with yellow pigment cells, either at high or low density, but this pigment is light enough that the internal structures are visible from the outside. The atrial languet nearly always has yellow and brown pigment cells at high density. Dark pigment cells are often found in clusters around the oral siphon, in the atrial languet of some zooids, in the body wall more commonly on the dorsal than the ventral side, and are nearly always found in transverse vessels.
The eight oral tentacles are of two size orders, alternating. Given that this is difficult to see, other arrangements may exist. Each zooid has 9–10 rows of stigmata on each side of the body, most commonly 9. The second row is dorsally complete on the left side, but not visible on the right side. There are 14–15 (more often 15) stigmata per half-row on the right side of the body in the following arrangement: DL5v3v2-3v4E, while on the left side 14 stigmata are more common with the following arrangement: DL4-5v3v3v3-4E.
The stomach is conical, with folds swollen and dark at the cardiac end. There are eight stomach folds, with the 8th fold commonly smaller than the others. The small pyloric caecum is a stalk with a moderately swollen tip, projecting at a perpendicular angle to the longitudinal axis of the stomach (
Figure 12E). The intestinal loop dips down to touch the cardiac portion of the stomach before turning vertically at an acute angle to form the rectum. The anterior edge of the first intestinal loop is anywhere from the 7th to the 8th transverse vessel, most commonly at the 7th. The anus opens most commonly two rows anterior to the first intestinal loop, anywhere from the 5th transverse vessel to the 7th row of stigmata, most commonly at the 6th row.
Ovaries and testes were not found in this colony. Buds were present, attached to adult zooids.
Holotype: CAGoM-2212, Alacranes Reef (ALA02), on algae, 6 m depth, 18 July 2019, col. Lilian Palomino-Alvarez.
Paratypes: CAGoM-0955, Cayo Arcas Reef (T-II), on coral, 7.6 m depth, 23 April 2018, col. Lilian Palomino-Alvarez. CAGoM-2234, Alacranes Reef (ALA05), on dead coral, 12 m depth, 20 July 2019, col. Lilian Palomino-Alvarez. CAGoM-2254, Alacranes Reef (ALA07), on dead coral, 9 m depth, 19 July 2019, col. Lilian Palomino-Alvarez.
Samples with barcode only and determined by species delimitation analysis are as follows: ARC06, CAGoM-0393, CAGoM-0394, Cayo Arcas Reef (ZR), artificial substrate, 7 m depth, 23 August 2016 col. Lilian Palomino-Alvarez. CAGoM-0955, Cayo Arcas Reef (T-I), on coral, 2.6 m depth, 21 April 2018, col. Lilian Palomino-Alvarez. CAGoM-1150 Cayo Arcas Reef (TEX-1, on coral, 4 m depth, 19 August 2018, col. Lilian Palomino-Alvarez. CAGoM-2434, Bajo 10 Reef (ARMS), 7 m depth, 25 October 2019, col. Lilian Palomino-Alvarez.
Etymology: In memory of Catalina San German, an extraordinary woman and example of life who supported the first author.
Colonies are very irregular in shape, 1–2 mm thick, and grow on dead coral or sponge, with sections covered with coral fragments or algae. The zooids are arranged in a “leachii-type” configuration 35] around large and circular cloacas, slightly raised from the surface of the colony. In the living colony, the atrial languet is yellow and the anterior ventral region of the zooid is white or gray and white, giving the colony a general gray color with patches of yellow and white. All the color is from the zooids, the tunic being an uncolored and transparent membrane (
Figure 13A–C). After formalin preservation, the colony is dark brown and zooids are yellow or brown, opaque in the anterior region, and transparent posteriorly. In CAGoM-2254, clusters of orange pigment cells are also present on either side of the endostyle, corresponding roughly to stigmatal rows. These clusters start at row 2 and end between rows 6 and 11, most commonly 7 or 8. These orange pigment cells were not visible in CAGoM-2212, CAGoM-2234, or CAGoM-0955 but were likely obscured by the presence of dark purple pigment cells. Vascular networks with numerous ampullae are visible on the underside, between systems, and along the margin of the colonies. CAGoM-2212 has unusually long ampullae along the margin of one side of the colony, as long as the zooids in some cases (
Figure 13D), while CAGoM-2254 contains a 4 × 5 mm section in the margin of the colony filled with ampullae but no zooids. The numerous ampullae in preserved colonies range in color from white to peach or dark brown.
Adult zooid length is 1.5–1.9 mm (
Figure 13E). The eight oral tentacles are of three size-orders, in one of these two dispositions: L S M S M S M S or L S M S L M S M. There are 10 to 12 rows of stigmata and always more rows on the right than on the left side. The second row is dorsally incomplete on the left side, and we could not confirm if this is the case on the right side due to the pigmentation of the body. There are usually 14–15 stigmata per half-row on both sides with the following formula: DL5v3v3v3-4E.
The stomach lays horizontally and there are 8–9 stomach folds, with the 9th fold always smaller than the others. The folds are not well marked along the complete length of the stomach but form conspicuous elongated dilations in the cardiac end. The small pyloric caecum is digitiform, projecting from a wide typhlosole at a slight angle (
Figure 13F). The primary loop is shallow and most of the intestine runs parallel to the stomach, but the end of the loop touches the cardiac portion of the stomach before turning vertically into the rectum. The anterior edge of the first intestinal loop is anywhere between the 10th and 11th transverse vessel, most commonly at the 11th. The anus is smooth and opens anywhere from the 8th to the 11th row of stigmata, most commonly at the 11th, at the posterior margin of the atrial aperture.
Ovaries and testes were not seen together in the same zooid. Among 30 dissected zooids from CAGoM-2254, a single ovary was seen, on the right side of the body. The testis with 12–16 pear-shaped follicles was visible in CAGoM-0955. The posterior portion of this testis overlapped with the anterior-most intestinal loop. Buds were present, attached to adult zooids.
Botrylloides niger Herdman, 1886
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-0372, Cayo Arcas Reef (Pr), artificial substrate, 1 m depth, 21 August 2016 col. Lilian Palomino-Alvarez.
This is a well-known species and will not be described here.
Genus Symplegma Herdman, 1886
Holotype: CAGoM-2271, Bajos del Norte Reef (BN03), 15.7 m depth, 20 July 2019 col. Lilian Palomino-Alvarez. Dead coral.
Etymology: The name of the species is due to the presence of various papillae in the prepharyngeal area, which is uncommon in this genus.
In life, the tunic is transparent and the zooids are perfectly visible with yellow pigment in the ventral (throughout the endostyle) and dorsal area, between the siphons, and between the rows of stigmata (
Figure 14A,B). After formalin preservation, the zooids are yellow but still transparent. The vascular network is visible in the tunic, and contains pigment cells that are the same color as the zooids.
The average zooid length is 2.4 mm (
Figure 14C,D). There are 12 oral tentacles of three size-orders. The dorsal tubercle area is in the form of a wide and deep “V” with five or more small papillae posterior to the tubercle aperture (
Figure 14E). The right side of the zooid has 11 rows of stigmata and the left side has 10. The second row is incomplete on the right side (undetermined on the left). There are 20 stigmata in each half row: DL4v4v4v4v4E (right side) and DL4v4v4v4v4E (left side).
The abdomen is small and posterior. The stomach is globular with six folds, two of them shorter and near the pyloric caecum. The pyloric caecum is C-shaped, small (¼ the length of the stomach if it was not bent), and the tip is not swollen (
Figure 14F). There are connections between the curve of the caecum and the middle of the intestine and between the caecum tip and the stomach. Sometimes the anus opens at the same level as the intestinal loop or one row of stigmata posterior to that level. The base of the rectum is not isodiametric as it twists away from the stomach. Testes and ovaries are not visible in this colony.
Holotype: CAGoM-02404, Bajo de 10 Reef (ARMS), 7 m depth, 30 July 2019 col. Lilian Palomino-Alvarez. Artificial Substrate.
Paratype: CAGoM-02424, Bajo de 10 Reef (ARMS), 7 m depth, 30 July 2019 col. Lilian Palomino-Alvarez. Artificial Substrate.
Samples with barcode only and determined by species delimitation analysis are as follows: CAGoM-02094, CAGoM-02099, CAGoM-02133 Bajo de 10 Reef (ARMS), 7 m depth, 03 April 2019 col. Lilian Palomino-Alvarez. Artificial Substrate. CAGoM-02382, Bajo de 10 Reef (ARMS), 7 m depth, 30 July 2019 col. Lilian Palomino-Alvarez. Artificial Substrate.
Etymology: The name of the species is dedicated to the locality of the species, the old village of Sisal in Yucatán, México.
The colony is delicate, with an uncolored tunic, turning the magenta zooids perfectly visible (
Figure 15A,B). The finger-shaped ampullae that line the margins of the colony have the same pigmentation. After formalin preservation, the colony appears pink with a yellowish, opaque tunic; the ampullae contain both white and peach-colored pigment cells but are lighter colored than the zooids, as is common in preserved colonies. The vascular network is easily visible in the sections of the colony that lack zooids. Fragments of coral, bryozoan, or circular serpulid tubes are embedded in the base of the colony.
The average length of three relaxed zooids was 2.07 mm (
Figure 15C,D). There are at least 16 oral tentacles, in two size categories; the longer is twice as long as the shorter. The peritubercular area resembles a calyx with three vertical bars and the dorsal tubercle aperture at the tip of the middle bar (
Figure 15E). Each zooid has 12 stigmatal rows on the right side, with the second row dorsally incomplete (the second row on the left side was not visible). There are ~24 stigmata per half-row, with the following formula on the right side: DL5-6v4-5v4v4v6E, and on the left side: DL5v4-5v4-5v4v5E and the first dorsal vessel of the left side touches the dorsal lamina at the level of the 6th–7th row.
The stomach is square-shaped and flat with 10–17 stomach folds, most often 16 with 8 on each side. There is a large curved pyloric caecum (¾ the length of the stomach if the caecum were not curved) with a moderately swollen tip, and two tissue connections to the intestinal loop (
Figure 15F). The stomach and intestine together take up half the portion of the left body cavity. The first intestinal loop can reach all the way to the 5th or 6th stigmatal row.
Ovaries were not visible, while the testes were well developed, between the 5th and 7th row of stigmata on the right side and the 3rd and 5th rows on the left side. There are 5–7 marked pear-shaped testis lobes in each half. Buds were not visible in the colony.
Holotype: CAGoM-2104, Bajo de 10 Reef (ARMS), 7 m depth, 03 April 2019 col. Lilian Palomino-Alvarez. Artificial Substrate.
The colony has a thin and transparent tunic and the zooids are perfectly visible through the surface. Zooids are purple with a yellow triangle dorsally and two additional small pigment clusters on each side anterior to the oral siphon. The vascular network is visible in the tunic and contains pigment cells the same color as the zooids. The ampullae are finger-like and contain purple pigment cells only (
Figure 16A). After formalin preservation, the colony is golden yellow (
Figure 16B).
The average zooid length is 1.4 mm (
Figure 16C). Zooids are opaque, with a yellow stomach and white intestine faintly visible through the body wall. There is often a diffuse white substance in the body wall in the center of the zooid which disappears with staining. There are at least 12 oral tentacles of three size orders. The oral tentacles have a narrow base and are long and curved. The dorsal tubercle has a round aperture in the center of a swollen region (
Figure 16D). The right side of the zooid has 9 stigmatal rows. The formula of the stigmata on the right side is DL5v?v4v4v4-5E, while on the left side it is DL5v?v5v3-4v4E. The 1st longitudinal vessel connects with the dorsal lamina at either the 4th transverse vessel or the 5th stigmatal row.
The stomach is elongated with most often 9, sometimes 10 stomach folds. The pyloric caecum rises at the posterior end of the stomach at a perpendicular angle to the typhlosole. The pyloric caecum is medium-sized (½ the length of the stomach if it was not bent) and bent at a right angle half along its length, with a tip that is not swollen. It has a single tissue connection between its tip and the intestinal loop. The first and second intestinal loops are very close, the first reaching the 6–8th stigmatal row while the anus opens at the 4th or 5th row. The anus position is quite variable with respect to the anterior edge of the intestinal loop, always 1–4 rows ahead.
Testes and ovaries were not visible in this colony. Buds were present, with the same color as the zooids in living colonies.