Carbon Dioxide Sensing—Biomedical Applications to Human Subjects
Abstract
:1. Introduction
2. Review of CO Sensing Techniques
2.1. Scope of the Review
- Infrared absorption of CO—Section 2.2.
- Hydration of dissolved CO into carbonic acid—Section 2.3.
- Reduction of CO into CO and CO—Section 2.4.
- Acoustic properties of gaseous CO—Section 2.5.
2.2. Infrared Absorption of CO
2.2.1. Non Dispersive Infra-Red (NDIR) Sensors
2.2.2. Photoacoustic Sensors
2.3. Hydration of CO into Carbonic Acid
2.3.1. Wet Conductometric Sensors
2.3.2. The Stow-Severinghaus Electrode
2.3.3. ISFET Sensors
2.3.4. Dye-Based Sensors
- Substrate: the substrate material does not seem to have any major influence on the sensing performance and is indifferently made of polyethylene terephthalate (PET) films, glass slides, or optical fibers. Nonetheless, polyethylene naphthalate (PEN) appears to be superior to PET, because its lower diffusivity towards CO yields shorter response times [128]. In addition, the adhesion of the polymer film on the glass substrate can be difficult without a cumbersome surface preparation [112,127] while PEN foils may just be roughened to enhance the sensitive layer adhesion [128].
- Polymer: hydroxy propyl methylcellulose (HPMC) may be a better choice than ethyl cellulose for being more hydrophilic than the latter, allowing water molecules to be entrapped with the dye and favoring the hydration of CO into bicarbonate ions [121].
- Covering membrane: Hyflon or even Cytop if a minimal response time is not mandatory may be used to limit the sensor poisoning and humidity loss [128].
2.3.5. Optical Fiber Sensors
2.4. Reduction of CO
2.4.1. Adsorption by Metal Oxide Thin Film
2.4.2. Adsorption by Graphene
2.4.3. Electrochemical Cells
2.4.4. Ionic Liquids-Based Sensors
2.5. Acoustic Properties of CO
2.5.1. Time of Flight Acoustic Sensors
2.5.2. Acoustic Attenuation Sensors
2.6. Comparison Table
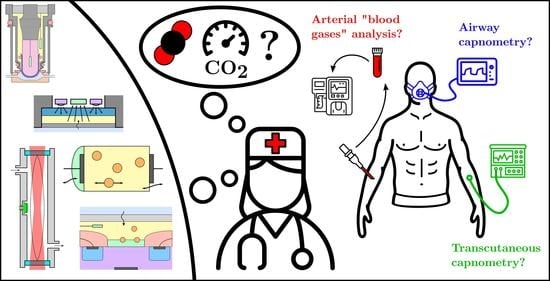
3. Current Applications to Biomedical CO Sensing
- inside the body with blood or tissues sampling—Section 3.1,
- in the exhaled air with airway capnometry—Section 3.2,
- on the skin with transcutaneous capnometry—Section 3.3.
3.1. Blood Gas Analysis
3.1.1. Clinical Significance
3.1.2. Probing Modalities
3.2. Airway Capnometry
3.2.1. Clinical Significance
3.2.2. Probing Modalities
3.3. Transcutaneous CO Sensing
3.3.1. Clinical Significance
3.3.2. Probing Modalities
4. Future Applications to Transcutaneous Monitoring
4.1. Past Attempts
4.1.1. Non-Dispersive Infrared and Transcutaneous Sensing
4.1.2. Mass Spectroscopy and Transcutaneous Sensing
4.2. Transcutaneous Sensing Constraints
- The correlation between the pCO measured at the skin surface—that is tcpCO—and that of arterial blood—that is paCO, which will determine the ability of the sensor to give clues about the patient’s status through a tcpCO reading.
- The exhalation rate of CO through the skin into the sensor, which will determine the response time of the sensor.
4.2.1. Correlation between paCO and tcpCO
4.2.2. Exhalation Rate
- –
- The exhalation rate of CO through the skin increases with an increasing temperature.
- –
- The CO production rate of the tissues increases with temperature (due to an increase in tissues metabolism).
- –
- Skin humidity, or the presence of various chemicals can change the CO exhalation rate through the skin [336].
4.2.3. Impact on the Sensor Form Factor in a Closed-Chamber Design
4.3. Recommendations for a Closed-Chamber Design
4.4. Other Perspectives
4.4.1. Rate-Based Approach
4.4.2. Diffuse Reflectance Spectometry (DRS)
4.5. Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CIL | Carbamate Ionic Liquid |
| CO | Carbon monoxide |
| CO | Carbon dioxide |
| COHb | Carbamino-Haemoglobin |
| COPD | Chronic Obstructive Pulmonary Disease |
| CTAH | Cetyltrimethyl ammonium hydroxide |
| DLR | Dual Lifetime Referencing |
| DRS | Diffuse Reflectance Spectometry |
| FDA | Food and Drug Administration |
| FET | Field-Effect Transistor |
| FRET | Fluorescent Resonance Energy Transfer |
| HO | Water |
| HHb | Deoxy-Haemoglobin |
| HPMC | Hydroxy propyl methylcellulose |
| HPTS | 1-hydroxy-pyrene-3,6,8-trisulfonate |
| IFE | Inner Filter Effect |
| ISFET | Ion-Selective Field-Effect Transistor |
| LED | Light Emitting Diode |
| MEMS | Micro Electro-Mechanical Systems |
| MOSFET | Metal Oxyde Semiconductor Field-Effect Transistor |
| N | Di-nitrogen |
| NDIR | Non Dispersive Infra-Red |
| O | Di-oxygen |
| pv-aCO | Carbon dioxide venous-to-arterial pressure difference |
| paCO | Arterial carbon dioxide partial pressure |
| paO | Arterial dioxygen partial pressure |
| pCO | Carbon dioxide partial pressure |
| PEN | Polyethylene naphthalate |
| PET | Polyethylene terephthalate |
| petCO | End-tidal carbon dioxide partial pressure |
| PPG | Photoplethysmography |
| PTFE | Polytetrafluoroethylene |
| tcpCO | Transcutaneous carbon dioxide partial pressure |
| TMAH | Tetramethyl ammonium hydroxide |
| TPB | Tributyl phosphate |
References
- Wagner, P.D. The Physiological Basis of Pulmonary Gas Exchange: Implications for Clinical Interpretation of Arterial Blood Gases. Eur. Respir. J. 2015, 45, 227–243. [Google Scholar] [CrossRef]
- Scheer, B.V.; Perel, A.; Pfeiffer, U.J. Clinical Review: Complications and Risk Factors of Peripheral Arterial Catheters Used for Haemodynamic Monitoring in Anaesthesia and Intensive Care Medicine. Crit. Care 2002, 6, 199. [Google Scholar] [CrossRef] [Green Version]
- Nanji, A.A.; Whitlow, K.J. Is it Necessary to Transport Arterial Blood Samples on Ice for pH and Gas Analysis? Can. Anaesth. Soc. J. 1984, 31, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Severinghaus, J.W.; Astrup, P.B. History of Blood Gas Analysis. III. Carbon Dioxide Tension. J. Clin. Monit. 1986, 2, 60–73. [Google Scholar] [CrossRef]
- Severinghaus, J.; Astrup, P. History of Blood Gas Analysis. VI. Oximetry. J. Clin. Monit. 1986, 2, 270–288. [Google Scholar] [CrossRef]
- Huttmann, S.E.; Windisch, W.; Storre, J.H. Techniques for the Measurement and Monitoring of Carbon Dioxide in the Blood. Ann. Am. Thorac. Soc. 2014, 11, 645–652. [Google Scholar] [CrossRef]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse Oximetry: Fundamentals and Technology Update. Med. Devices Evid. Res. 2014, 7, 231–239. [Google Scholar] [CrossRef]
- Jubran, A. Pulse Oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef] [Green Version]
- McSwain, S.D.; Hamel, D.S.; Smith, P.B.; Gentile, M.A.; Srinivasan, S.; Meliones, J.N.; Cheifetz, I.M. End-Tidal and Arterial Carbon Dioxide Measurements Correlate Across All Levels of Physiologic Dead Space. Respir. Care 2010, 55, 288–293. [Google Scholar]
- Donnellan, M.E. Capnography: Gradient PaCO2 and PetCO2. In Applied Technologies in Pulmonary Medicine; Karger Publishers: Basel, Switzerland, 2011; pp. 126–131. [Google Scholar]
- Conway, A.; Tipton, E.; Liu, W.H.; Conway, Z.; Soalheira, K.; Sutherland, J.; Fingleton, J. Accuracy and Precision of Transcutaneous Carbon Dioxide Monitoring: A Systematic Review and Meta-Analysis. Thorax 2018, 74, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Bendjelid, K.; Schütz, N.; Stotz, M.; Gerard, I.; Suter, P.; Romand, J.A. Transcutaneous pCO2 Monitoring in Critically Ill Adults: Clinical Evaluation of a New Sensor. Crit. Care Med. 2005, 33, 2203–2206. [Google Scholar] [CrossRef]
- Acock, B.; Wall, G.W. A Simple Conductimetric CO2 Analyzer With Automatic Recalibration: I. Design, Implementation, and Functionality. Agron. J. 1995, 87, 70–75. [Google Scholar] [CrossRef]
- Shitashima, K. Evolution of Compact Electrochemical In-Situ pH-pCO2 Sensor Using ISFET-pH Electrode. In Proceedings of the Oceans 2010 MTS/IEEE Seattle, Seattle, WA, USA, 20–23 September 2010. [Google Scholar] [CrossRef]
- Fanget, S.; Hentz, S.; Puget, P.; Arcamone, J.; Matheron, M.; Colinet, E.; Andreucci, P.; Duraffourg, L.; Myers, E.; Roukes, M. Gas Sensors Based on Gravimetric Detection—A Review. Sens. Actuators B Chem. 2011, 160, 804–821. [Google Scholar] [CrossRef]
- Zosel, J.; Oelßner, W.; Decker, M.; Gerlach, G.; Guth, U. The Measurement of Dissolved and Gaseous Carbon Dioxide Concentration. Meas. Sci. Technol. 2011, 22, 072001. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon Dioxide Sensors for Intelligent Food Packaging Applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Llobet, E. Gas Sensors Using Carbon Nanomaterials: A Review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.; Sadistap, S. Carbon Dioxide (CO2) Sensors for the Agri-food Industry—A Review. Food Bioprocess Technol. 2009, 2, 115–121. [Google Scholar] [CrossRef]
- Barrington, J. Design and Fabrication of Optical Fibre Long Period Gratings for CO2 Sensing. Ph.D. Thesis, Cranfield University, Cranfield, UK, 2018. [Google Scholar]
- Rebber, M.; Willa, C.; Koziej, D. Organic–Inorganic Hybrids for CO2 Sensing, Separation and Conversion. Nanoscale Horiz. 2020, 5, 431–453. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Sharma, J.; Gartia, M.R. Nanomaterial-Based CO2 Sensors. Nanomaterials 2020, 10, 2251. [Google Scholar] [CrossRef]
- Bhowmick, T.; Ambardekar, V.; Ghosh, A.; Dewan, M.; Bandyopadhyay, P.P.; Nag, S.; Majumder, S.B. Multilayered and Chemiresistive Thin and Thick Film Gas Sensors for Air Quality Monitoring. In Multilayer Thin Films; Basu, S., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 8. [Google Scholar] [CrossRef] [Green Version]
- Decker, M.; Oelßner, W.; Zosel, J. Electrochemical CO2 Sensors with Liquid or Pasty Electrolyte. In Carbon Dioxide Sensing: Fundamentals, Principles, and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 87–116. [Google Scholar] [CrossRef]
- Skoog, D.; West, D.; Holler, F.; Crouch, S. Fundamentals of Analytical Chemistry; Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Harvey, D.T. Analytical Chemistry 2.1. 2016. Available online: https://open.umn.edu/opentextbooks/textbooks/486 (accessed on 7 November 2021).
- Bernath, P.F. Spectra of Atoms and Molecules (Topics in Physical Chemistry); Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Gordon, I.; Rothman, L.; Hill, C.; Kochanov, R.; Tan, P.B.; Birk, M.; Boudon, V.; Campargue, K.C.; Drouin, B.; Flaud, J.M.; et al. The HITRAN2016 Molecular Spectroscopic Database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Smith, R.; Ho, W.; Saffell, J.R.; Tatam, R.P. A Low Cost, Optically Efficient Carbon Dioxide Sensor Based on Nondispersive Infra-Red (NDIR) Measurement at 4.2 μm. In Optical Sensing and Detection II; Berghmans, F., Mignani, A.G., Moor, P.D., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8439, pp. 352–361. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Smith, R.; Ho, W.O.; Saffell, J.R.; Tatam, R.P. Non-Dispersive Infra-Red (NDIR) Measurement of Carbon Dioxide at 4.2 μm in a Compact and Optically Efficient Sensor. Sens. Actuators B Chem. 2013, 186, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ming, A.; Ren, Y.; Tan, Q.; Ou, W.; Sun, X.; Wang, W.; Chen, D.; Xiong, J. CMOS MEMS Infrared Source Based on Black Silicon. In Proceedings of the 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, 17–20 April 2016; pp. 200–204. [Google Scholar] [CrossRef]
- Popa, D.; Udrea, F. Towards Integrated Mid-Infrared Gas Sensors. Sensors 2019, 19, 2076. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Li, Y.; Li, Q. A Miniaturized Carbon Dioxide Gas Sensor Based on Infrared Absorption. Opt. Lasers Eng. 2010, 48, 1206–1212. [Google Scholar] [CrossRef]
- Jing, Y.; Cheng, Y.; Yuan, Y.; Li, X.; Zhang, Z.; Xu, M.; Wang, D.; Mu, J.; Mei, Y.; Zhang, Y. Design and Optimization of an Integrated MEMS Gas Chamber with High Transmissivity. Digit. Commun. Netw. 2020, 7, 82–91. [Google Scholar] [CrossRef]
- Kohsiek, W. Fast-Response Water Vapor and Carbon Dioxide Sensor. In Fiber Optic Sensors: Engineering and Applications; Bruinsma, A.J., Culshaw, B., Eds.; International Society for Optics and Photonics: Boston, MA, USA, 1991; Volume 1511, pp. 114–119. [Google Scholar] [CrossRef]
- Gibson, D.; Macgregor, C. A Novel Solid State Non-Dispersive Infrared CO2 Gas Sensor Compatible With Wireless and Portable Deployment. Sensors 2013, 13, 7079–7103. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Wang, Y.; Xiong, B.; Li, T. MEMS-Based Thermoelectric Infrared Sensors: A Review. Front. Mech. Eng. 2017, 12, 557–566. [Google Scholar] [CrossRef]
- Lee, K.N.; Lee, D.S.; Jung, S.W.; Jang, Y.H.; Kim, Y.K.; Seong, W.K. A High-Temperature MEMS Heater Using Suspended Silicon Structures. J. Micromech. Microeng. 2009, 19, 115011. [Google Scholar] [CrossRef]
- Vincent, T.; Gardner, J. A Low Cost MEMS Based NDIR System for the Monitoring of Carbon Dioxide in Breath Analysis at ppm Levels. Sens. Actuators B Chem. 2016, 236, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Graf, P.; Meyer, J.; Pentina, A.; Brunner, D.; Perez-Cruz, F.; Hüglin, C.; Emmenegger, L. Integration and Calibration of Non-Dispersive Infrared (NDIR) CO2 Low-Cost Sensors and Their Operation in a Sensor Network Covering Switzerland. Atmos. Meas. Tech. 2020, 13, 3815–3834. [Google Scholar] [CrossRef]
- Wang, J.N.; Xue, Q.S.; Lin, G.Y.; Ma, Q.J. Mid-Infrared Carbon Dioxide Sensor With Wireless and Anti-Condensation Capability for Use in Greenhouses. Spectrosc. Lett. 2018, 51, 266–273. [Google Scholar] [CrossRef]
- Fietzek, P.; Fiedler, B.; Steinhoff, T.; Körtzinger, A. In situ Quality Assessment of a Novel Underwater pCO2 Sensor Based on Membrane Equilibration and NDIR Spectrometry. J. Atmos. Ocean. Technol. 2014, 31, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Gas Sensing Solutions Ltd. Reducing the Impact of Condensation on CO2 Sensor; Technical Report AN008; Gas Sensing Solutions Ltd.: Glasgow, UK, 2020. [Google Scholar]
- Jung, D.; Bank, S.; Lee, M.; Wasserman, D. Next Generation Mid-Infrared Sources. J. Opt. 2017, 19, 123001. [Google Scholar] [CrossRef] [Green Version]
- Hodgkinson, J.; Tatam, R.P. Optical Gas Sensing: A Review. Meas. Sci. Technol. 2012, 24, 012004. [Google Scholar] [CrossRef] [Green Version]
- Moumen, S.; Raible, I.; Krauß, A.; Wöllenstein, J. Infrared Investigation of CO2 Sorption by Amine Based Materials for the Development of a NDIR CO2 Sensor. Sens. Actuators B Chem. 2016, 236, 1083–1090. [Google Scholar] [CrossRef]
- Schaden, S.; Haberkorn, M.; Frank, J.; Baena, J.R.; Lendl, B. Direct Determination of Carbon Dioxide in Aqueous Solution Using Mid-Infrared Quantum Cascade Lasers. Appl. Spectrosc. 2004, 58, 667–670. [Google Scholar] [CrossRef]
- Bozóki, Z.; Pogány, A.; Szabó, G. Photoacoustic Instruments for Practical Applications: Present, Potentials, and Future Challenges. Appl. Spectrosc. Rev. 2011, 46, 1–37. [Google Scholar] [CrossRef]
- Borri, S.; Patimisco, P.; Galli, I.; Mazzotti, D.; Giusfredi, G.; Akikusa, N.; Yamanishi, M.; Scamarcio, G.; De Natale, P.; Spagnolo, V. Intracavity Quartz-Enhanced Photoacoustic Sensor. Appl. Phys. Lett. 2014, 104, 091114. [Google Scholar] [CrossRef]
- Rousseau, R.; Maurin, N.; Trzpil, W.; Bahriz, M.; Vicet, A. Quartz Tuning Fork Resonance Tracking and Application in Quartz Enhanced Photoacoustics Spectroscopy. Sensors 2019, 19, 5565. [Google Scholar] [CrossRef] [Green Version]
- Scholz, L.; Perez, A.; Bierer, B.; Eaksen, P.; Wollenstein, J.; Palzer, S. Miniature Low-Cost Carbon Dioxide Sensor for Mobile Devices. IEEE Sens. J. 2017, 17, 2889–2895. [Google Scholar] [CrossRef]
- Huber, J.; Wöllenstein, J.; Kolb, S.; Dehé, A.; Jost, F. Miniaturized Photoacoustic CO2 Sensors for Consumer Applications. Proc. Sens. 2015, E6, 688–692. [Google Scholar] [CrossRef]
- Pernau, H.F.; Schmitt, K.; Huber, J.; Rademacher, S.; Eberhardt, A.; Wöllenstein, J. Resonant Photoacoustic CO2 Spectroscopy With LED Light Source. In Proceedings of the 30th Anniversary Eurosensors Conference—Eurosensors 2016, Budapest, Hungary, 4–7 September 2016; Volume 168, pp. 1325–1328. [Google Scholar] [CrossRef]
- Lhermet, H.; Verdot, T.; Teulle, A.; Berthelot, A.; Gliere, A.; Desloges, B.; Souchon, F.; Fournier, M.; Fedeli, J.M.; Coutard, J.G. Micro-Photoacoustic Cell With Integrated Microphone for Sub-Ppm Gas Sensing. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 68–71. [Google Scholar] [CrossRef]
- Jensen, M.A.; Rechnitz, G.A. Response Time Characteristics of the pCO2 Electrode. Anal. Chem. 1979, 51, 1972–1977. [Google Scholar] [CrossRef]
- Mills, A. Optical Sensors for Carbon Dioxide and Their Applications. In Sensors for Environment, Health and Security; Baraton, M.I., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 347–370. [Google Scholar]
- Millero, F.J.; Graham, T.B.; Huang, F.; Bustos-Serrano, H.; Pierrot, D. Dissociation Constants of Carbonic Acid in Seawater as a Function of Salinity and Temperature. Mar. Chem. 2006, 100, 80–94. [Google Scholar] [CrossRef]
- Wang, X.; Conway, W.; Burns, R.; McCann, N.; Maeder, M. Comprehensive Study of the Hydration and Dehydration Reactions of Carbon Dioxide in Aqueous Solution. J. Phys. Chem. A 2010, 114, 1734–1740. [Google Scholar] [CrossRef]
- Baker, J.M.; Spaans, E.J.A.; Reece, C.F. Conductimetric Measurement of CO2 Concentration: Theoretical Basis and Its Verification. Agron. J. 1996, 88, 675–682. [Google Scholar] [CrossRef]
- Roxana Varlan, A.; Sansen, W. Micromachined Conductometric p(CO2) Sensor. Sens. Actuators B Chem. 1997, 44, 309–315. [Google Scholar] [CrossRef]
- Mirtaheri, P.; Grimnes, S.; Martinsen, Ø.; Tønnessen, T. A New Biomedical Sensor for Measuring PCO2. Physiol. Meas. 2004, 25, 421–436. [Google Scholar] [CrossRef]
- Mirtaheri, P.; Omtveit, T.; Klotzbuecher, T.; Grimnes, S.; Martinsen, Ø.G.; Tønnessen, T.I. Miniaturization of a Biomedical Gas Sensor. Physiol. Meas. 2004, 25, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Tronstad, C.; Pischke, S.; Holhjem, L.; Tønnessen, T.; Martinsen, Ø.; Grimnes, S. Early Detection of Cardiac Ischemia Using a Conductometric pCO2 Sensor: Real-Time Drift Correction and Parameterization. Physiol. Meas. 2010, 31, 1241–1255. [Google Scholar] [CrossRef] [Green Version]
- Wall, G.W.; Acock, B.; Milliken, G.A. A Simple Conductimetric CO2 Analyzer with Automatic Recalibration: II. Factors Affecting Calibration. Agron. J. 1995, 87, 76–80. [Google Scholar] [CrossRef]
- Lis, K.; Acker, H.; Lübbers, D.; Halle, M. A PCO2 Surface Electrode Working on the Principle of Electrical Conductivity. Pflügers Arch. Eur. J. Physiol. 1979, 381, 289–291. [Google Scholar] [CrossRef]
- Neethirajan, S.; Freund, M.; Jayas, D.; Shafai, C.; Thomson, D.; White, N. Development of Carbon Dioxide (CO2) Sensor for Grain Quality Monitoring. Biosyst. Eng. 2010, 106, 395–404. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Bradley, A.F. Electrodes for Blood pO2 and pCO2 Determination. J. Appl. Physiol. 1958, 13, 515–520. [Google Scholar] [CrossRef]
- Beran, A.V.; Huxtable, R.F.; Sperling, D.R. Electrochemical Sensor for Continuous Transcutaneous PCO2 Measurement. J. Appl. Physiol. 1976, 41, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, J.S.; Choi, S.H.; Lee, D.K.; Nam, H.; Cha, G.S. A Planar pCO2 Sensor with Enhanced Electrochemical Properties. Anal. Chem. 2000, 72, 4468–4473. [Google Scholar] [CrossRef]
- Severinghaus, J.W. A Combined Transcutaneous P(O2)-P(CO2) Electrode with Electrochemical HCO3- Stabilization. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 1027–1032. [Google Scholar] [CrossRef]
- Zhao, P.; Cai, W.J. An Improved Potentiometric pCO2 Microelectrode. Anal. Chem. 1997, 69, 5052–5058. [Google Scholar] [CrossRef]
- Suzuki, H.; Arakawa, H.; Sasaki, S.; Karube, I. Micromachined Severinghaus-Type Carbon Dioxide Electrode. Anal. Chem. 1999, 71, 1737–1743. [Google Scholar] [CrossRef]
- Beyenal, H.; Davis, C.C.; Lewandowski, Z. An Improved Severinghaus-Type Carbon Dioxide Microelectrode for Use in Biofilms. Sens. Actuators B Chem. 2004, 97, 202–210. [Google Scholar] [CrossRef]
- Donaldson, T.L.; Palmer, H.J. Dynamic Response of the Carbon Dioxide Electrode. AIChE J. 1979, 25, 143–151. [Google Scholar] [CrossRef]
- McGuire, M.A.; Teskey, R.O. Microelectrode Technique for In Situ Measurement of Carbon Dioxide Concentrations in Xylem Sap of Trees. Tree Physiol. 2002, 22, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.J.; Reimers, C.E. The Development of pH and pCO2 Microelectrodes for Studying the Carbonate Chemistry of Pore Waters Near the Sediment-Water Interface. Limnol. Oceanogr. 1993, 38, 1762–1773. [Google Scholar] [CrossRef]
- Whitehead, M.D.; Pollitzer, M.J.; Parker, D.; Halsall, D.; Delpy, D.T.; Reynolds, E.O.R. Transcutaneous Estimation of Arterial pO2 and pCO2 in Newborn Infants with a Single Electrochemical Sensor. Lancet 1980, 315, 1111–1114. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Hu, B.; Van Den Vlekkert, H.H.; De Rooij, N.F. Carbon Dioxide Gas-Sensing Electrode Based on a pH-ISFET with Back-Side Contacts. Sens. Actuators 1989, 17, 275–278. [Google Scholar] [CrossRef]
- Bergveld, P. The Operation of an ISFET as an Electronic Device. Sens. Actuators 1981, 1, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Jamasb, S. Continuous Monitoring of pH and Blood Gases Using Ion-Sensitive and Gas-Sensitive Field Effect Transistors Operating in the Amperometric Mode in Presence of Drift. Biosensors 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Star, A.; Han, T.R.; Joshi, V.; Gabriel, J.C.; Grüner, G. Nanoelectronic Carbon Dioxide Sensors. Adv. Mater. 2004, 16, 2049–2052. [Google Scholar] [CrossRef]
- Mohri, S.; Shimizu, J.; Goda, N.; Miyasaka, T.; Fujita, A.; Nakamura, M.; Kajiya, F. Measurements of CO2, Lactic Acid and Sodium Bicarbonate Secreted by Cultured Cells Using a Flow-Through Type pH/CO2 Sensor System Based on ISFET. Sens. Actuators B Chem. 2006, 115, 519–525. [Google Scholar] [CrossRef]
- Ekwińska, M.A.; Jaroszewicz, B.; Domański, K.; Grabiec, P.; Zaborowski, M.; Tomaszewski, D.; Pałko, T.; Przytulski, J.; ukasik, W.; Dawgul, M.; et al. Transcutaneous Blood Capnometry Sensor Head Based on a Back-Side Contacted ISFET. In Mechatronics: Recent Technological and Scientific Advances; Springer: Berlin/Heidelberg, Germany, 2012; pp. 607–614. [Google Scholar]
- Lai, C.S.; Lu, T.F.; Yang, C.M.; Lin, Y.C.; Pijanowska, D.; Jaroszewicz, B. Body Effect Minimization Using Single Layer Structure for pH-ISFET Applications. Sens. Actuators B Chem. 2010, 143, 494–499. [Google Scholar] [CrossRef]
- Sinha, S.; Bhardwaj, R.; Sahu, N.; Ahuja, H.; Sharma, R.; Mukhiya, R. Temperature and Temporal Drift Compensation for Al2O3-Gate ISFET-Based pH Sensor Using Machine Learning Techniques. Microelectron. J. 2020, 97, 104710. [Google Scholar] [CrossRef]
- Shimanoe, K.; Goto, K.; Obata, K.; Nakata, S.; Sakai, G.; Yamazoe, N. Development of FET-Type CO2 Sensor Operative at Room Temperature. Sens. Actuators B Chem. 2004, 102, 14–19. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Hung, W.C.; Chen, P.H.; Huang, I.Y. High-Performance Extended Gate Field-Effect-Transistor-Based Dissolved Carbon Dioxide Sensing System with a Packaged Microreference Electrode. J. Micro/Nanolithogr. MEMS MOEMS 2014, 13, 033017. [Google Scholar] [CrossRef] [Green Version]
- MacInnes, D.A.; Belcher, D. The Thermodynamic Ionization Constants of Carbonic Acid. J. Am. Chem. Soc. 1933, 55, 2630–2646. [Google Scholar] [CrossRef]
- Uttamlal, M.; Walt, D.R. A Fiber-Optic Carbon Dioxide Sensor for Fermentation Monitoring. Bio/Technology 1995, 13, 597–601. [Google Scholar] [CrossRef]
- Vurek, G.G.; Feustel, P.J.; Severinghaus, J.W. A Fiber Optic PCO2 Sensor. Ann. Biomed. Eng. 1983, 11, 499–510. [Google Scholar] [CrossRef] [PubMed]
- DeGrandpre, M.D.; Baehr, M.M.; Hammar, T.R. Calibration-Free Optical Chemical Sensors. Anal. Chem. 1999, 71, 1152–1159. [Google Scholar] [CrossRef]
- Ge, X.; Kostov, Y.; Rao, G. High-Stability Non-Invasive Autoclavable Naked Optical CO2 Sensor. Biosens. Bioelectron. 2003, 18, 857–865. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, M.; Xu, K.; Chen, J.; Liao, Y. A High Precision, Fast Response, and Low Power Consumption in situ Optical Fiber Chemical pCO2 Sensor. Talanta 2008, 76, 353–359. [Google Scholar] [CrossRef]
- Hakonen, A.; Hulth, S. A High-Precision Ratiometric Fluorosensor for pH: Implementing Time-Dependent Non-Linear Calibration Protocols for Drift Compensation. Anal. Chim. Acta 2008, 606, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Kostov, Y.; Henderson, R.; Selock, N.; Rao, G. A Low-Cost Fluorescent Sensor for pCO2 Measurements. Chemosensors 2014, 2, 108–120. [Google Scholar] [CrossRef]
- Wang, H.; Vagin, S.I.; Rieger, B.; Meldrum, A. An Ultrasensitive Fluorescent Paper-Based CO2 Sensor. ACS Appl. Mater. Interfaces 2020, 12, 20507–20513. [Google Scholar] [CrossRef] [PubMed]
- Opitz, N.; Lübbers, D.W. Compact CO2 Gas Analyzer with Favourable Signal-to-Noise Ratio and Resolution Using Special Fluorescence Sensors (Optodes) Illuminated by Blue Led’s. In Oxygen Transport to Tissue—VI; Springer: Boston, MA, USA, 1984; pp. 757–762. [Google Scholar] [CrossRef]
- Zhujun, Z.; Seitz, W.R. A Carbon Dioxide Sensor Based on Fluorescence. Anal. Chim. Acta 1984, 160, 305–309. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Weis, L.J.; Leiner, M.J.P.; Ziegler, W.E. Fiber-Optic Fluorosensor for Oxygen and Carbon Dioxide. Anal. Chem. 1988, 60, 2028–2030. [Google Scholar] [CrossRef]
- He, X.; Rechnitz, G.A. Linear Response Function for Fluorescence-Based Fiber-Optic CO2 Sensors. Anal. Chem. 1995, 67, 2264–2268. [Google Scholar] [CrossRef]
- Marazuela, M.; Moreno Bondi, M.; Orellana, G. Enhanced Performance of a Fibre-Optic Luminescence CO2 Sensor Using Carbonic Anhydrase. Sens. Actuators B Chem. 1995, 29, 126–131. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Wild, L. Breath-by-Breath Measurement of Carbon Dioxide Using a Plastic Film Optical Sensor. Sens. Actuators B Chem. 1997, 39, 419–425. [Google Scholar] [CrossRef]
- Malins, C.; MacCraith, B.D. Dye-doped Organically Modified Silica Glass for Fluorescence Based Carbon Dioxide Gas Detection. Analyst 1998, 123, 2373–2376. [Google Scholar] [CrossRef]
- Wolfbeis, O.; Kovacs, B.; Goswami, K.; Klainer, S. Fiber-Optic Fluorescence Carbon Dioxide Sensor for Environmental Monitoring. Microchim. Acta 1998, 129, 181–188. [Google Scholar] [CrossRef]
- Segawa, H.; Ohnishi, E.; Arai, Y.; Yoshida, K. Sensitivity of Fiber-Optic Carbon Dioxide Sensors Utilizing Indicator Dye. Sens. Actuators B Chem. 2003, 94, 276–281. [Google Scholar] [CrossRef]
- Oter, O.; Ertekin, K.; Topkaya, D.; Alp, S. Emission-Based Optical Carbon Dioxide Sensing with HPTS in Green Chemistry Reagents: Room-Temperature Ionic Liquids. Anal. Bioanal. Chem. 2006, 386, 1225–1234. [Google Scholar] [CrossRef]
- Fernández-Sánchez, J.F.; Cannas, R.; Spichiger, S.; Steiger, R.; Spichiger-Keller, U.E. Optical CO2-Sensing Layers for Clinical Application Based on pH-Sensitive Indicators Incorporated into Nanoscopic Metal-Oxide Supports. Sens. Actuators B Chem. 2007, 128, 145–153. [Google Scholar] [CrossRef]
- Oter, O.; Ertekin, K.; Derinkuyu, S. Ratiometric Sensing of CO2 in Ionic Liquid Modified Ethyl Cellulose Matrix. Talanta 2008, 76, 557–563. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. Fiber-Optic Carbon Dioxide Sensor Based on Fluorinated Xerogels Doped with HPTS. Sens. Actuators B Chem. 2008, 129, 120–125. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. Highly Sensitive and Linear Optical Fiber Carbon Dioxide Sensor Based on Sol-Gel Matrix Doped with Silica Particles and HPTS. Sens. Actuators B Chem. 2009, 143, 205–210. [Google Scholar] [CrossRef]
- Dansby-Sparks, R.N.; Jin, J.; Mechery, S.J.; Sampathkumaran, U.; Owen, T.W.; Yu, B.D.; Goswami, K.; Hong, K.; Grant, J.; Xue, Z.L. Fluorescent-Dye-Doped Sol-Gel Sensor for Highly Sensitive Carbon Dioxide Gas Detection below Atmospheric Concentrations. Anal. Chem. 2010, 82, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Syu, J.J. Optical Sensor for Dual Sensing of Oxygen and Carbon Dioxide Based on Sensing Films Coated on Filter Paper. Appl. Opt. 2017, 56, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Perez de Vargas-Sansalvador, I.M.; Erenas, M.M.; Diamond, D.; Quilty, B.; Capitan-Vallvey, L.F. Water Based-Ionic Liquid Carbon Dioxide Sensor for Applications in the Food Industry. Sens. Actuators B Chem. 2017, 253, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.S.; Achterberg, E.P.; Connelly, D.P.; Schuster, U.; Mowlem, M. Developments in Marine pCO2 Measurement Technology; towards Sustained in situ Observations. TrAC Trends Anal. Chem. 2017, 88, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, N.; Amao, Y. Optical Sensor for Carbon Dioxide Combining Colorimetric Change of a pH Indicator and a Reference Luminescent Dye. Anal. Bioanal. Chem. 2003, 376, 642–646. [Google Scholar] [CrossRef]
- Amao, Y.; Nakamura, N. Optical CO2 Sensor with the Combination of Colorimetric Change of α-naphtholphthalein and Internal Reference Fluorescent Porphyrin Dye. Sens. Actuators B Chem. 2004, 100, 347–351. [Google Scholar] [CrossRef]
- Amao, Y.; Nakamura, N. An Optical Sensor with the Combination of Colorimetric Change of α-naphtholphthalein and Internal Reference Luminescent Dye for CO2 in Water. Sens. Actuators B Chem. 2005, 107, 861–865. [Google Scholar] [CrossRef]
- Amao, Y.; Komori, T. Optical CO2 Sensor of the Combination of Colorimetric Change of α-naphtholphthalein in poly(isobutyl methacrylate) and Fluorescent Porphyrin in Polystyrene. Talanta 2005, 66, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Vargas-Sansalvador, I.M.; Carvajal, M.A.; Roldán-Muñoz, O.M.; Banqueri, J.; Fernández-Ramos, M.D.; Capitán-Vallvey, L.F. Phosphorescent Sensing of Carbon Dioxide Based on Secondary Inner-Filter Quenching. Anal. Chim. Acta 2009, 655, 66–74. [Google Scholar] [CrossRef]
- Aguayo-López, M.L.; Capitán-Vallvey, L.F.; Fernández-Ramos, M.D. Optical Sensor for Carbon Dioxide Gas Determination, Characterization and Improvements. Talanta 2014, 126, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, M.D.; Aguayo-López, M.L.; Pérez de Vargas-Sansalvador, I.; Capitán-Vallvey, L.F. Ionic Liquids on Optical Sensors for Gaseous Carbon Dioxide. Anal. Bioanal. Chem. 2018, 410, 5931–5939. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, M.D.; Aguayo-López, M.; De Los Reyes Berbel, E.; Santoyo-González, F.; Capitan-Vallvey, L.F. NIR Optical Carbon Dioxide Gas Sensor Based on Simple azaBODIPY pH Indicators. Analyst 2019, 144, 3870–3877. [Google Scholar] [CrossRef]
- Klimant, I.; Huber, C.; Liebsch, G.; Neurauter, G.; Stangelmayer, A.; Wolfbeis, O.S. Dual Lifetime Referencing (DLR)—A New Scheme for Converting Fluorescence Intensity into a Frequency-Domain or Time-Domain Information. In New Trends in Fluorescence Spectroscopy: Applications to Chemical and Life Sciences; Springer: Berlin/Heidelberg, Germany, 2001; pp. 257–274. [Google Scholar] [CrossRef]
- von Bültzingslöwen, C.; McEvoy, A.; Mcdonagh, C.; Maccraith, B.; Klimant, I.; Krause, C.; Wolfbeis, O. Sol-gel Based Optical Carbon Dioxide Sensor Employing Dual Luminophore Referencing for Application in Food Packaging Technology. Analyst 2002, 127, 1478–1483. [Google Scholar] [CrossRef]
- Burke, C.S.; Markey, A.; Nooney, R.I.; Byrne, P.; McDonagh, C. Development of an Optical Sensor Probe for the Detection of Dissolved Carbon Dioxide. Sens. Actuators B Chem. 2006, 119, 288–294. [Google Scholar] [CrossRef]
- Cajlakovic, M.; Bizzarri, A.; Ribitsch, V. Luminescence Lifetime-Based Carbon Dioxide Optical Sensor for Clinical Applications. Anal. Chim. Acta 2006, 573–574, 57–64. [Google Scholar] [CrossRef]
- Fritzsche, E.; Gruber, P.; Schutting, S.; Fischer, J.P.; Strobl, M.; Müller, J.D.; Borisov, S.M.; Klimant, I. Highly Sensitive Poisoning-Resistant Optical Carbon Dioxide Sensors for Environmental Monitoring. Anal. Methods 2017, 9, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Staudinger, C.; Strobl, M.; Fischer, J.P.; Thar, R.; Mayr, T.; Aigner, D.; Müller, B.J.; Müller, B.; Lehner, P.; Mistlberger, G.; et al. A Versatile Optode System for Oxygen, Carbon Dioxide, and pH Measurements in Seawater with Integrated Battery and Logger. Limnol. Oceanogr. Methods 2018, 16, 459–473. [Google Scholar] [CrossRef]
- Pfeifer, D.; Russegger, A.; Klimant, I.; Borisov, S.M. Green to Red Emitting BODIPY Dyes for Fluorescent Sensing and Imaging of Carbon Dioxide. Sens. Actuators B Chem. 2020, 304, 127312. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Szmacinski, H.; Karakelle, M. Optical Sensing of pH and pCO2 Using Phase-Modulation Fluorimetry and Resonance Energy Transfer. Anal. Chim. Acta 1993, 272, 179–186. [Google Scholar] [CrossRef]
- Neurauter, G.; Klimant, I.; Wolfbeis, O.S. Microsecond Lifetime-Based Optical Carbon Dioxide Sensor Using Luminescence Resonance Energy Transfer. Anal. Chim. Acta 1999, 382, 67–75. [Google Scholar] [CrossRef]
- Valeur, B. Resonance Energy Transfer and Its Applications. In Molecular Fluorescence; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2001; Chapter 9; pp. 247–272. [Google Scholar] [CrossRef]
- von Bültzingslöwen, C.; McEvoy, A.K.; McDonagh, C.; MacCraith, B.D. Lifetime-Based Optical Sensor for High-Level pCO2 Detection Employing Fluorescence Resonance Energy Transfer. Anal. Chim. Acta 2003, 480, 275–283. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Materials for Fluorescence-Based Optical Chemical Sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Kautsky, H. Quenching of Luminescence by Oxygen. Trans. Faraday Soc. 1939, 35, 216–219. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of Fluorescence by Oxygen. A Probe for Structural Fluctuations in Macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef]
- Huber, C.; Klimant, I.; Krause, C.; Werner, T.; Mayr, T.; Wolfbeis, O.S. Optical Sensor for Seawater Salinity. Fresenius J. Anal. Chem. 2000, 368, 196–202. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.; Dong, X.; Yang, J.; Long, H.; So, P.; Chan, C. Miniature pH Optical Fiber Sensor Based on Fabry-Perot Interferometer. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 331–335. [Google Scholar] [CrossRef]
- Ma, W.; Xing, J.; Wang, R.; Rong, Q.; Zhang, W.; Li, Y.; Zhang, J.; Qiao, X. Optical Fiber Fabry–Perot Interferometric CO2 Gas Sensor Using Guanidine Derivative Polymer Functionalized Layer. IEEE Sens. J. 2018, 18, 1924–1929. [Google Scholar] [CrossRef]
- Quintero, S.M.M.; Cremona, M.; Triques, A.L.C.; d’Almeida, A.R.; Braga, A.M.B. Swelling and Morphological Properties of poly(vinyl alcohol) (PVA) and poly(acrylic acid) (PAA) Hydrogels in Solution with High Salt Concentration. Polymer 2010, 51, 953–958. [Google Scholar] [CrossRef]
- Hill, K.O.; Meltz, G. Fiber Bragg Grating Technology Fundamentals and Overview. J. Light. Technol. 1997, 15, 1263–1276. [Google Scholar] [CrossRef] [Green Version]
- Herber, S.; Olthuis, W.; Bergveld, P.; van den Berg, A. Exploitation of a pH-Sensitive Hydrogel Disk for CO2 Detection. Sens. Actuators B Chem. 2004, 103, 284–289. [Google Scholar] [CrossRef]
- Hu, P.; Dong, X.; Wong, W.C.; Chen, L.; Ni, K.; Chiu Chan, C. Photonic Crystal Fiber Interferometric pH Sensor Based on Polyvinyl Alcohol/Polyacrylic Acid Hydrogel Coating. Appl. Opt. 2015, 54, 2647–2652. [Google Scholar] [CrossRef]
- Hromadka, J.; Tokay, B.; Correia, R.; Morgan, S.P.; Korposh, S. Carbon Dioxide Measurements Using Long Period Grating Optical Fibre Sensor Coated with Metal Organic Framework HKUST-1. Sens. Actuators B Chem. 2018, 255, 2483–2494. [Google Scholar] [CrossRef]
- Melo, L.; Burton, G.; Davies, B.; Risk, D.; Wild, P. Highly Sensitive Coated Long Period Grating Sensor for CO2 Detection at Atmospheric Pressure. Sens. Actuators B Chem. 2014, 202, 294–300. [Google Scholar] [CrossRef]
- Chong, X.; Kim, K.; Ohodnicki, P.R.; Li, E.; Chang, C.; Wang, A.X. Ultrashort Near-Infrared Fiber-Optic Sensors for Carbon Dioxide Detection. IEEE Sens. J. 2015, 15, 5327–5332. [Google Scholar] [CrossRef]
- Kersey, A.D. Multiplexed fiber optic sensors. In Distributed and Multiplexed Fiber Optic Sensors II; Dakin, J.P., Kersey, A.D., Eds.; International Society for Optics and Photonics: Boston, MA, USA, 1993; Volume 1797, pp. 161–185. [Google Scholar] [CrossRef]
- Perez-Herrera, R.A.; Lopez-Amo, M. Fiber Optic Sensor Networks. Opt. Fiber Technol. 2013, 19, 689–699. [Google Scholar] [CrossRef]
- Hotan, W.; Göpel, W.; Haul, R. Interaction of CO2 and CO with Nonpolar Zinc Oxide Surfaces. Surf. Sci. 1979, 83, 162–180. [Google Scholar] [CrossRef]
- Au, C.T.; Hirsch, W.; Hirschwald, W. Adsorption and Interaction of Carbon Dioxide, Formic Acid and Hydrogen/Carbon Dioxide Mixtures on (1010) Zinc Oxide Surfaces Studied by Photoelectron Spectroscopy (XPS and UPS). Surf. Sci. 1988, 199, 507–517. [Google Scholar] [CrossRef]
- Freund, H.J.; Roberts, M.W. Surface Chemistry of Carbon Dioxide. Surf. Sci. Rep. 1996, 25, 225–273. [Google Scholar] [CrossRef] [Green Version]
- Seiferth, O.; Wolter, K.; Dillmann, B.; Klivenyi, G.; Freund, H.J.; Scarano, D.; Zecchina, A. IR Investigations of CO2 Adsorption on Chromia Surfaces: Cr2O3 (0001)/Cr(110) Versus Polycrystalline α-Cr2O3. Surf. Sci. 1999, 421, 176–190. [Google Scholar] [CrossRef]
- Lopes Martins, J.B.; Longo, E.; Rodríguez Salmon, O.D.; Espinoza, V.A.; Taft, C.A. The Interaction of H2, CO, CO2, H2O and NH3 on ZnO Surfaces: An Oniom Study. Chem. Phys. Lett. 2004, 400, 481–486. [Google Scholar] [CrossRef]
- Herrán, J.; Mandayo, G.; Castaño, E. Semiconducting BaTiO3-CuO Mixed Oxide Thin Films for CO2 Detection. Thin Solid Films 2009, 517, 6192–6197. [Google Scholar] [CrossRef]
- Noei, H.; Wöll, C.; Muhler, M.; Wang, Y. Activation of Carbon Dioxide on ZnO Nanoparticles Studied by Vibrational Spectroscopy. J. Phys. Chem. C 2011, 115, 908–914. [Google Scholar] [CrossRef]
- Tang, Q.L.; Luo, Q.H. Adsorption of CO2 at ZnO: A Surface Structure Effect from DFT + U Calculations. J. Phys. Chem. C 2013, 117, 22954–22966. [Google Scholar] [CrossRef]
- Usseinov, A.B.; Akilbekov, A.T.; Kotomin, E.A.; Popov, A.I.; Seitov, D.D.; Nekrasov, K.A.; Giniyatova, S.G.; Karipbayev, Z.T. The First Principles Calculations of CO2 Adsorption on (1010) ZnO Surface. AIP Conf. Proc. 2019, 2174, 020181. [Google Scholar] [CrossRef]
- Gankanda, A.; Cwiertny, D.M.; Grassian, V.H. Role of Atmospheric CO2 and H2O Adsorption on ZnO and CuO Nanoparticle Aging: Formation of New Surface Phases and the Impact on Nanoparticle Dissolution. J. Phys. Chem. C 2016, 120, 19195–19203. [Google Scholar] [CrossRef]
- Çolak, H.; Karaköse, E. Synthesis and Characterization of Different Dopant (Ge, Nd, W)-Doped ZnO Nanorods and Their CO2 Gas Sensing Applications. Sens. Actuators B Chem. 2019, 296, 126629. [Google Scholar] [CrossRef]
- Álvarez-Ramos, M.E.; Necochea-Chamorro, J.I.; Carrillo-Torres, R.C.; Sánchez-Zeferino, R. Room Temperature CO2 Sensing Using Au-Decorated ZnO Nanorods Deposited on an Optical Fiber. Mater. Sci. Eng. B 2020, 262, 114720. [Google Scholar] [CrossRef]
- Kannan, P.K.; Saraswathi, R.; Rayappan, J.B.B. CO2 Gas Sensing Properties of DC Reactive Magnetron Sputtered ZnO Thin Film. Ceram. Int. 2014, 40, 13115–13122. [Google Scholar] [CrossRef]
- Haeusler, A.; Meyer, J.U. A Novel Thick Film Conductive Type CO2 Sensor. Sens. Actuators B Chem. 1996, 34, 388–395. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Balamurugan, C.; Lee, D.W. Enhanced CO2 Gas-Sensing Performance of ZnO Nanopowder by La Loaded during Simple Hydrothermal Method. Sens. Actuators B Chem. 2016, 229, 288–296. [Google Scholar] [CrossRef]
- Dhahri, R.; Leonardi, S.G.; Hjiri, M.; Mir, L.E.; Bonavita, A.; Donato, N.; Iannazzo, D.; Neri, G. Enhanced Performance of Novel Calcium/Aluminum Co-Doped Zinc Oxide for CO2 Sensors. Sens. Actuators B Chem. 2017, 239, 36–44. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Zhang, H.; Shi, S. CO2 Sensing Behavior of Calcium-Doped ZnO Thin Film: A Study to Address the Cross-Sensitivity of CO2 in H2 and CO Environment. Langmuir 2019, 35, 10267–10275. [Google Scholar] [CrossRef]
- Cavanagh, L.; Binions, R. BaSnO3 Thick Film as a Carbon Dioxide Sensor. J. Electrochem. Soc. 2011, 159, 1006–1009. [Google Scholar] [CrossRef]
- Chen, G.; Han, B.; Deng, S.; Wang, Y.; Wang, Y. Lanthanum Dioxide Carbonate La2O2CO3 Nanorods as a Sensing Material for Chemoresistive CO2 Gas Sensor. Electrochim. Acta 2014, 127, 355–361. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Shaban, M.; El Sayed, A.M. Enhanced Gas Sensing Properties of Spin-coated Na-doped ZnO Nanostructured Films. Sci. Rep. 2017, 7, 41716. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari Shohany, B.; Motevalizadeh, L.; Ebrahimizadeh Abrishami, M. Investigation of ZnO Thin-Film Sensing Properties for CO2 Detection: Effect of Mn Doping. J. Theor. Appl. Phys. 2018, 12, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Kometani, K.; Mizuhara, Y.; Takita, Y. Capacitive-Type Gas Sensor for the Selective Detection of Carbon Dioxide. Sens. Actuators B Chem. 1993, 13, 470–472. [Google Scholar] [CrossRef]
- Shokry Hassan, H.; Kashyout, A.B.; Morsi, I.; Nasser, A.A.A.; Ali, I. Synthesis, Characterization and Fabrication of Gas Sensor Devices Using ZnO and ZnO: In Nanomaterials. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Tanvir, N.B.; Wilbertz, C.; Steinhauer, S.; Köck, A.; Urban, G.; Yurchenko, O. Work Function Based CO2 Gas Sensing Using Metal Oxide Nanoparticles at Room Temperature. Mater. Today Proc. 2015, 2, 4190–4195. [Google Scholar] [CrossRef]
- Tanvir, N.B.; Yurchenko, O.; Urban, G. Optimization Study for Work Function Based CO2 Sensing Using CuO-nanoparticles in Respect to Humidity and Temperature. Procedia Eng. 2015, 120, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Kometani, K.; Nishi, Y.; Takita, Y. Improved Sensitivity of CuO–BaTiO3 Capacitive-Type CO2 Sensor by Additives. Sens. Actuators B Chem. 1995, 28, 49–54. [Google Scholar] [CrossRef]
- Heiland, G. Homogeneous Semiconducting Gas Sensors. Sens. Actuators 1981, 2, 343–361. [Google Scholar] [CrossRef]
- Sears, W.M.; Colbow, K.; Consadori, F. General Characteristics of Thermally Cycled Tin Oxide Gas Sensors. Semicond. Sci. Technol. 1989, 4, 351–359. [Google Scholar] [CrossRef]
- Nakata, S.; Kaneda, Y.; Nakamura, H.; Yoshikawa, K. Detection and Quantification of CO Gas Based on the Dynamic Response of a Ceramic Sensor. Chem. Lett. 1991, 20, 1505–1508. [Google Scholar] [CrossRef] [Green Version]
- Wlodek, S.; Colbow, K.; Consadori, F. Signal-Shape Analysis of a Thermally Cycled Tin-Oxide Gas Sensor. Sens. Actuators B Chem. 1991, 3, 63–68. [Google Scholar] [CrossRef]
- Hiranaka, Y.; Abe, T.; Murata, H. Gas-Dependent Response in the Temperature Transient of SnO2 Gas Sensors. Sens. Actuators B Chem. 1992, 9, 177–182. [Google Scholar] [CrossRef]
- Nakata, S.; Akakabe, S.; Nakasuji, M.; Yoshikawa, K. Gas Sensing Based on a Nonlinear Response: Discrimination Between Hydrocarbons and Quantification of Individual Components in a Gas Mixture. Anal. Chem. 1996, 68, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Reedy, B.J. Temperature Modulation in Semiconductor Gas Sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Vergara, A.; Martinelli, E.; Llobet, E.; D’Amico, A.; Di Natale, C. Optimized Feature Extraction for Temperature-Modulated Gas Sensors. J. Sens. 2009, 2009, 716316. [Google Scholar] [CrossRef] [Green Version]
- Gosangi, R.; Gutierrez-Osuna, R. Active Temperature Modulation of Metal-Oxide Sensors for Quantitative Analysis of Gas Mixtures. Sens. Actuators B Chem. 2013, 185, 201–210. [Google Scholar] [CrossRef]
- Vergara, A.; Llobet, E.; Brezmes, J.; Ivanov, P.; Cané, C.; Gràcia, I.; Vilanova, X.; Correig, X. Quantitative Gas Mixture Analysis Using Temperature-Modulated Micro-Hotplate Gas Sensors: Selection and Validation of the Optimal Modulating Frequencies. Sens. Actuators B Chem. 2007, 123, 1002–1016. [Google Scholar] [CrossRef]
- Madrolle, S.; Grangeat, P.; Jutten, C. A Linear-Quadratic Model for the Quantification of a Mixture of Two Diluted Gases with a Single Metal Oxide Sensor. Sensors 2018, 18, 1785. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.J.; Shim, C.H.; Song, K.D.; Lee, D.S.; Huh, J.S.; Lee, D.D. Classification of Workplace Gases Using Temperature Modulation of Two SnO2 Sensing Films on Substrate. Sens. Actuators B Chem. 2002, 86, 251–258. [Google Scholar] [CrossRef]
- Radhakrishnan, J.K.; Kumara, M. Effect of Temperature Modulation, on the Gas Sensing Characteristics of ZnO Nanostructures, for Gases O2, CO and CO2. Sens. Int. 2021, 2, 100059. [Google Scholar] [CrossRef]
- Mizuno, N.; Yoshioka, T.; Kato, K.; Iwamoto, M. CO2-Sensing Characteristics of SnO2 Element Modified by La2O3. Sens. Actuators B Chem. 1993, 13, 473–475. [Google Scholar] [CrossRef]
- Ishihara, T.; Kometani, K.; Hashida, M.; Takita, Y. Application of Mixed Oxide Capacitor to the Selective Carbon Dioxide Sensor: I. Measurement of Carbon Dioxide Sensing Characteristics. J. Electrochem. Soc. 1991, 138, 173–176. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal Oxide Composites in Conductometric Gas Sensors: Achievements and Challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Amin, K.R.; Bid, A. Graphene as a Sensor. Curr. Sci. 2014, 107, 430–436. [Google Scholar]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible Graphene-Based Wearable Gas and Chemical Sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.C. Carbon Dioxide Gas Sensor Using a Graphene Sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Xie, G.; Wu, M.; Tai, H. Gas Sensors for CO2 Detection Based on RGO–PEI Films at Room Temperature. Chin. Sci. Bull. 2014, 59, 1999–2005. [Google Scholar] [CrossRef]
- Akhter, F.; Alahi, M.E.E.; Siddiquei, H.R.; Gooneratne, C.P.; Mukhopadhyay, S.C. Graphene Oxide (GO) Coated Impedimetric Gas Sensor for Selective Detection of Carbon Dioxide (CO2) with Temperature and Humidity Compensation. IEEE Sens. J. 2021, 21, 4241–4249. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced Graphene Oxide for Room-Temperature Gas Sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef]
- Muruganathan, M.; Sun, J.; Imamura, T.; Mizuta, H. Electrically Tunable van der Waals Interaction in Graphene–Molecule Complex. Nano Lett. 2015, 15, 8176–8180. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Muruganathan, M.; Mizuta, H. Room Temperature Detection of Individual Molecular Physisorption Using Suspended Bilayer Graphene. Sci. Adv. 2016, 2, e1501518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Paronyan, T.M.; Harutyunyan, A.R. Sub-ppt Gas Detection with Pristine Graphene. Appl. Phys. Lett. 2012, 101, 053119. [Google Scholar] [CrossRef]
- Smith, A.D.; Elgammal, K.; Fan, X.; Lemme, M.C.; Delin, A.; Råsander, M.; Bergqvist, L.; Schröder, S.; Fischer, A.C.; Niklaus, F.; et al. Graphene-Based CO2 Sensing and its Cross-Sensitivity with Humidity. RSC Adv. 2017, 7, 22329–22339. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Elgammal, K.; Smith, A.D.; Östling, M.; Delin, A.; Lemme, M.C.; Niklaus, F. Humidity and CO2 Gas Sensing Properties of Double-Layer Graphene. Carbon 2018, 127, 576–587. [Google Scholar] [CrossRef]
- Zaki, S.E.; Basyooni, M.A.; Shaban, M.; Rabia, M.; Eker, Y.R.; Attia, G.F.; Yilmaz, M.; Ahmed, A.M. Role of Oxygen Vacancies in Vanadium Oxide and Oxygen Functional Groups in Graphene Oxide for Room Temperature CO2 Gas Sensors. Sens. Actuators A Phys. 2019, 294, 17–24. [Google Scholar] [CrossRef]
- Nemade, K.R.; Waghuley, S.A. Highly Responsive Carbon Dioxide Sensing by Graphene/Al2O3 Quantum Dots Composites at Low Operable Temperature. Indian J. Phys. 2014, 88, 577–583. [Google Scholar] [CrossRef]
- Nemade, K.R.; Waghuley, S.A. Role of Defects Concentration on Optical and Carbon Dioxide Gas Sensing Properties of Sb2O3/Graphene composites. Opt. Mater. 2014, 36, 712–716. [Google Scholar] [CrossRef]
- Amarnath, M.; Gurunathan, K. Highly Selective CO2 Gas Sensor Using Stabilized NiO-In2O3 Nanospheres Coated Reduced Graphene Oxide Sensing Electrodes at Room Temperature. J. Alloys Compd. 2021, 857, 157584. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and Application of Nanoporous Graphene Oxide Film for CO2, H2, and C2H2 Gases Sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Rumyantsev, S.; Liu, G.; Shur, M.S.; Potyrailo, R.A.; Balandin, A.A. Selective Gas Sensing with a Single Pristine Graphene Transistor. Nano Lett. 2012, 12, 2294–2298. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, M.; Chamberland, A. Solid-State Detectors for the Potentiometric Determination of Gaseous Oxides: I. Measurement in Air. J. Electrochem. Soc. 1977, 124, 1579–1583. [Google Scholar] [CrossRef]
- Maruyama, T.; Sasaki, S.; Saito, Y. Potentiometric Gas Sensor for Carbon Dioxide Using Solid Electrolytes. Solid State Ion. 1987, 23, 107–112. [Google Scholar] [CrossRef]
- Kishi, S.; Yuasa, M.; Tetsuya, K.; Lantto, V.; Shimanoe, K.; Yamazoe, N. A Stable Solid-Reference Electrode of BiCuVOx/Perovskite-Oxide for Potentiometric Solid Electrolyte CO2 Sensor. J. Ceram. Soc. Jpn. 2007, 115, 706–711. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.Y.; Guo, X.M. Characteristics and Performance of NASICON-Based CO2 Sensor Using Bi8Nb2O17 plus Pt as Solid-Reference Electrode. Sens. Actuators B Chem. 2013, 178, 163–168. [Google Scholar] [CrossRef]
- Lee, I.; Akbar, S.A.; Dutta, P.K. High Temperature Potentiometric Carbon Dioxide Sensor with Minimal Interference to Humidity. Sens. Actuators B Chem. 2009, 142, 337–341. [Google Scholar] [CrossRef]
- Lee, I.; Akbar, S.A. Potentiometric Carbon Dioxide Sensor Based on Thin Li3PO4 Electrolyte and Li2CO3 Sensing Electrode. Ionics 2014, 20, 563–569. [Google Scholar] [CrossRef]
- Lee, H.K.; Choi, N.J.; Moon, S.E.; Heo, J.; Yang, W.S.; Kim, J. Durability Improvement of Solid Electrolyte CO2 Sensor against Humidity Variations. J. Nanosci. Nanotechnol. 2015, 15, 404–407. [Google Scholar] [CrossRef]
- Obata, K.; Shimanoe, K.; Miura, N.; Yamazoe, N. Influences of Water Vapor on NASICON-Based CO2 Sensor Operative at Room Temperature. Sens. Actuators B Chem. 2003, 93, 243–249. [Google Scholar] [CrossRef]
- Kida, T.; Miyachi, Y.; Shimanoe, K.; Yamazoe, N. NASICON Thick Film-Based CO2 Sensor Prepared by a Sol–Gel Method. Sens. Actuators B Chem. 2001, 80, 28–32. [Google Scholar] [CrossRef]
- Liu, J.; Weppner, W. Beta′′-Alumina Solid Electrolytes for Solid State Electrochemical CO2 Gas Sensors. Solid State Commun. 1990, 76, 311–313. [Google Scholar] [CrossRef]
- Ma, N.; Ide, S.; Suematsu, K.; Watanabe, K.; Shimanoe, K. Novel Solid Electrolyte CO2 Gas Sensors Based on c-Axis-Oriented Y-Doped La9.66Si5.3B0.7O26.14. ACS Appl. Mater. Interfaces 2020, 12, 21515–21520. [Google Scholar] [CrossRef]
- Struzik, M.; Garbayo, I.; Pfenninger, R.; Rupp, J.L.M. A Simple and Fast Electrochemical CO2 Sensor Based on Li7La3Zr2O12 for Environmental Monitoring. Adv. Mater. 2018, 30, 1804098. [Google Scholar] [CrossRef]
- Choi, N.J.; Lee, H.K.; Moon, S.E.; Yang, W.S.; Kim, J. Stacked-Type Potentiometric Solid-State CO2 Gas Sensor. Sens. Actuators B Chem. 2013, 187, 340–346. [Google Scholar] [CrossRef]
- Barrosse-Antle, L.; Bond, A.; Compton, R.; O’Mahony, A.; Rogers, E.; Silvester, D. Voltammetry in Room Temperature Ionic Liquids: Comparisons and Contrasts with Conventional Electrochemical Solvents. Chem. Asian J. 2010, 5, 202–230. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Methods and Approaches of Utilizing Ionic Liquids as Gas Sensing Materials. RSC Adv. 2015, 5, 58371–58392. [Google Scholar] [CrossRef] [PubMed]
- Behera, K.; Pandey, S.; Kadyan, A.; Pandey, S. Ionic Liquid-Based Optical and Electrochemical Carbon Dioxide Sensors. Sensors 2015, 15, 30487–30503. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T. Ionic Liquids in Gas Sensors and Biosensors. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 10; pp. 287–318. [Google Scholar]
- Fapyane, D.; Revsbech, N.P. Fast Responding Amperometric CO2 Microsensor with Ionic Liquid–Aprotic Solvent Electrolytes. ACS Sens. 2020, 5, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.A.; Salehi-Khojin, A.; Masel, R.I. A Microfabricated Carbon Dioxide Sensor for Portable Applications. In Proceedings of the IEEE Sensors 2010 Conference, Waikoloa, HI, USA, 1–4 November 2010; pp. 365–368. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Wang, X.; Zhu, Z.; Ma, H.; Zhang, T.; Jin, J. Poly(Ionic Liquid)-Wwrapped Single-Walled Carbon Nanotubes for Sub-ppb Detection of CO2. Chem. Commun. 2012, 48, 8222–8224. [Google Scholar] [CrossRef]
- Honda, M.; Takei, Y.; Ishizu, K.; Imamoto, H.; Itoh, T.; Maeda, R.; Matsumoto, K.; Shimoyama, I. Low-Power-Consumption CO2 Gas Sensor Using Ionic Liquids for Green Energy Management. In Proceedings of the Sensors, 2012 IEEE, Taipei, Taiwan, 28–31 October 2012; pp. 1–4. [Google Scholar] [CrossRef]
- Ishizu, K.; Takei, Y.; Honda, M.; Noda, K.; Inaba, A.; Itoh, T.; Maeda, R.; Matsumoto, K.; Shimoyama, I. Carbon Dioxide Gas Sensor with Ionic Gel. In Proceedings of the 2013 Transducers Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013; pp. 1633–1636. [Google Scholar] [CrossRef]
- Willa, C.; Yuan, J.; Niederberger, M.; Koziej, D. When Nanoparticles Meet Poly(Ionic Liquid)s: Chemoresistive CO2 Sensing at Room Temperature. Adv. Funct. Mater. 2015, 25, 2537–2542. [Google Scholar] [CrossRef] [Green Version]
- Willa, C.; Schmid, A.; Briand, D.; Yuan, J.; Koziej, D. Lightweight, Room-Temperature CO2 Gas Sensor Based on Rare-Earth Metal-Free Composites-An Impedance Study. ACS Appl. Mater. Interfaces 2017, 9, 25553–25558. [Google Scholar] [CrossRef] [Green Version]
- Revsbech, N.P.; Garcia-Robledo, E.; Sveegaard, S.; Andersen, M.H.; Gothelf, K.V.; Larsen, L.H. Amperometic Microsensor for Measurement of Gaseous and Dissolved CO2. Sens. Actuators B Chem. 2019, 283, 349–354. [Google Scholar] [CrossRef]
- Chen, L.; Huang, D.; Ren, S.; Chi, Y.; Chen, G. Carbon Dioxide Gas Sensor Based on Ionic Liquid-Induced Electrochemiluminescence. Anal. Chem. 2011, 83, 6862–6867. [Google Scholar] [CrossRef]
- Buzzeo, M.C.; Klymenko, O.V.; Wadhawan, J.D.; Hardacre, C.; Seddon, K.R.; Compton, R.G. Kinetic Analysis of the Reaction between Electrogenerated Superoxide and Carbon Dioxide in the Room Temperature Ionic Liquids 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide and Hexyltriethylammonium Bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. B 2004, 108, 3947–3954. [Google Scholar] [CrossRef]
- Xiao, C.; Zeng, X. In Situ EQCM Evaluation of the Reaction between Carbon Dioxide and Electrogenerated Superoxide in Ionic Liquids. J. Electrochem. Soc. 2013, 160, H749–H756. [Google Scholar] [CrossRef]
- Wu, J.; Yin, M.j.; Seefeldt, K.; Dani, A.; Guterman, R.; Yuan, J.; Zhang, A.P.; Tam, H.Y. In Situ μ-Printed Optical Fiber-Tip CO2 Sensor Using a Photocrosslinkable Poly(Ionic Liquid). Sens. Actuators B Chem. 2018, 259, 833–839. [Google Scholar] [CrossRef]
- Mineo, P.G.; Livoti, L.; Lo Schiavo, S.; Cardiano, P. Fast and Reversible CO2 Quartz Crystal Microbalance Response of Vinylimidazolium-Based Poly(Ionic Liquid)s. Polym. Adv. Technol. 2012, 23, 1511–1519. [Google Scholar] [CrossRef]
- Zhou, R.; Vaihinger, S.; Geckeler, K.E.; Göpel, W. Reliable CO2 Sensors with Silicon-Based Polymers on Quartz Microbalance Transducers. Sens. Actuators B Chem. 1994, 19, 415–420. [Google Scholar] [CrossRef]
- Gomes, M.; Duarte, A.C.; Oliveira, J.P. Detection of CO2 Using a Quartz Crystal Microbalance. Sens. Actuators B Chem. 1995, 26, 191–194. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, K.K.; Kupnik, M.; Khuri-Yakub, B.T. Functionalization Layers for CO2 Sensing Using Capacitive Micromachined Ultrasonic Transducers. Sens. Actuators B Chem. 2012, 174, 87–93. [Google Scholar] [CrossRef]
- Hoffmann, R.; Schreiter, M.; Heitmann, J. The Concept of Thin Film Bulk Acoustic Resonators as Selective CO2 Gas Sensors. J. Sens. Sens. Syst. 2017, 6, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Siefker, Z.A.; Hodul, J.N.; Zhao, X.; Bajaj, N.; Brayton, K.M.; Flores-Hansen, C.; Zhao, W.; Chiu, G.T.C.; Braun, J.E.; Rhoads, J.F.; et al. Manipulating Polymer Composition to Create Low-Cost, High-Fidelity Sensors for Indoor CO2 Monitoring. Sci. Rep. 2021, 11, 13237. [Google Scholar] [CrossRef]
- Zuckerwar, A. Handbook of the Speed of Sound in Real Gases: Theory; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Joos, M.; Müller, H.; Lindner, G. An Ultrasonic Sensor for the Analysis of Binary Gas Mixtures. Sens. Actuators B Chem. 1993, 16, 413–419. [Google Scholar] [CrossRef]
- Gerlach, G. Acoustic CO2 Sensors. In Carbon Dioxide Sensing; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2019; Chapter 9; pp. 215–245. [Google Scholar] [CrossRef]
- Cicek, A.; Trak, D.; Arslan, Y.; Korozlu, N.; Kaya, O.A.; Ulug, B. Ultrasonic Gas Sensing by Two-Dimensional Surface Phononic Crystal Ring Resonators. ACS Sens. 2019, 4, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.B.; Bass, H.E.; Sutherland, L.C. Atmospheric Absorption of Sound: Theoretical Predictions. J. Acoust. Soc. Am. 1972, 51, 1565–1575. [Google Scholar] [CrossRef]
- Bass, H.E.; Sutherland, L.; Zuckerwar, A.J. Atmospheric Absorption of Sound—Update. J. Acoust. Soc. Am. 1990, 88, 2019–2021. [Google Scholar] [CrossRef]
- Petculescu, A. Future Trends in Acoustic Gas Monitoring and Sensing. J. Optoelectron. Adv. Mater. 2006, 8, 217. [Google Scholar]
- Dain, Y.; Lueptow, R.M. Acoustic Attenuation in a Three-Gas Mixture: Results. J. Acoust. Soc. Am. 2001, 110, 2974–2979. [Google Scholar] [CrossRef]
- Cottet, A.; Neumeier, Y.; Scarborough, D.; Bibik, A.; Lieuwen, T. Acoustic Absorption Measurements for Characterization of Gas Mixing. J. Acoust. Soc. Am. 2004, 116, 2081–2088. [Google Scholar] [CrossRef] [Green Version]
- Ejakov, S.; Phillips, S.; Dain, Y.; Lueptow, R.; H Visser, J. Acoustic Attenuation in Gas Mixtures with Nitrogen: Experimental Data and Calculations. J. Acoust. Soc. Am. 2003, 113, 1871–1879. [Google Scholar] [CrossRef] [Green Version]
- Petculescu, A.; Hall, B.; Fraenzle, R.; Phillips, S.; Lueptow, R. A Prototype Acoustic Gas Sensor Based on Attenuation (L). J. Acoust. Soc. Am. 2006, 120. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, B.; Du, M.; Wang, X. Gas Composition Recognition Based on Analyzing Acoustic Relaxation Absorption Spectra: Wavelet Decomposition and Support Vector Machine Classifier. In Proceedings of the 2018 2nd International Conference on Electrical Engineering and Automation (ICEEA 2018), Chengdu, China, 25–26 March 2018; pp. 126–130. [Google Scholar] [CrossRef] [Green Version]
- Zuckerwar, A.J.; Griffin, W.A. Effect of Water Vapor on Sound Absorption in Nitrogen at low Frequency/Pressure Ratios. J. Acoust. Soc. Am. 1981, 69, 150–154. [Google Scholar] [CrossRef]
- Wysocki, G.; Kosterev, A.; Tittel, F. Influence of Molecular Relaxation Dynamics on Quartz-enhanced Photoacoustic Detection of CO2 at λ = 2 μm. Appl. Phys. B Lasers Opt. 2006, 85, 301–306. [Google Scholar] [CrossRef]
- Eberl, M.; Jost, F.; Kolb, S.; Schaller, R.; Dettmann, W.; Gassner, S.; Skorupa, F. Miniaturized Photoacoustic CO2 Gas Sensors—A New Approach for the Automotive Sector. In Proceedings of the AmE 2019—Automotive meets Electronics, 10th GMM-Symposium, Dortmund, Germany, 12–13 March 2019; pp. 1–5. [Google Scholar]
- Hahn, C.E.W. Techniques for Measuring the Partial Pressures of Gases in the Blood. I. In Vitro Measurements. J. Phys. E Sci. Instrum. 1980, 13, 470–482. [Google Scholar] [CrossRef]
- Atamanchuk, D.; Tengberg, A.; Thomas, P.J.; Hovdenes, J.; Apostolidis, A.; Huber, C.; Hall, P.O. Performance of a Lifetime-Based Optode for Measuring Partial Pressure of Carbon Dioxide in Natural Waters. Limnol. Oceanogr. Methods 2014, 12, 63–73. [Google Scholar] [CrossRef]
- Joshi, S.; Jones, L.A.; Sabri, Y.M.; Bhargava, S.K.; Sunkara, M.V.; Ippolito, S.J. Facile Conversion of Zinc Hydroxide Carbonate to CaO-ZnO for Selective CO2 Gas Detection. J. Colloid Interface Sci. 2020, 558, 310–322. [Google Scholar] [CrossRef]
- Kaneyasu, K.; Otsuka, K.; Setoguchi, Y.; Sonoda, S.; Nakahara, T.; Aso, I.; Nakagaichi, N. A Carbon Dioxide Gas Sensor Based on Solid Electrolyte for Air Quality Control. Sens. Actuators B Chem. 2000, 66, 56–58. [Google Scholar] [CrossRef]
- Jones, N.L. An obsession with CO2. Appl. Physiol. Nutr. Metab. 2008, 33, 641–650. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Bayliss, D.A. Neural Control of Breathing and CO2 Homeostasis. Neuron 2015, 87, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumb, A. Control of Breathing. In Nunn’s Applied Respiratory Physiology, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 4; pp. 61–82. [Google Scholar]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef] [Green Version]
- Curley, G.; Laffey, J.G.; Kavanagh, B.P. Bench-to-Bedside Review: Carbon Dioxide. Crit. Care 2010, 14, 220. [Google Scholar] [CrossRef] [Green Version]
- Geers, C.; Gros, G. Carbon Dioxide Transport and Carbonic Anhydrase in Blood and Muscle. Physiol. Rev. 2000, 80, 681–715. [Google Scholar] [CrossRef] [Green Version]
- Lumb, A. Carbon Dioxide. In Nunn’s Applied Respiratory Physiology, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 9; pp. 159–177. [Google Scholar]
- Larkin, B.G.; Zimmanck, R.J. Interpreting Arterial Blood Gases Successfully. AORN J. 2015, 102, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Lopez, D.; Thomas, H.M. Pulmonary Rehabilitation in COPD Patients with Elevated PCO2. Am. Rev. Respir. Dis. 1988, 138, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Cukic, V. The Changes of Arterial Blood Gases in COPD During Four-Year Period. Med. Arch. 2014, 68, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, S.; Rubini, F.; Zanotti, E.; Ambrosino, N.; Bruschi, C.; Vitacca, M.; Fracchia, C.; Rampulla, C. Survival and Prediction of Successful Ventilator Weaning in COPD Patients Requiring Mechanical Ventilation for More Than 21 Days. Eur. Respir. J. 1994, 7, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, T.; Ohi, M.; Oga, T.; Machida, K.; Chihara, Y.; Harada, Y.; Takahashi, K.; Sumi, K.; Handa, T.; Niimi, A.; et al. Importance of the PaCO2 from 3 to 6 Months after Initiation of Long-Term Non-Invasive Ventilation. Respir. Med. 2010, 104, 1850–1857. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.L.; Saxen, M.A. Respiratory Effects of a Balanced Anesthetic Technique—Revisited Fifteen Years Later. Anesth. Prog. 1994, 41, 1–5. [Google Scholar]
- Schneider, A.G.; Eastwood, G.M.; Bellomo, R.; Bailey, M.; Lipcsey, M.; Pilcher, D.; Young, P.; Stow, P.; Santamaria, J.; Stachowski, E.; et al. Arterial Carbon Dioxide Tension and Outcome in Patients Admitted to the Intensive Care Unit After Cardiac Arrest. Resuscitation 2013, 84, 927–934. [Google Scholar] [CrossRef]
- Zavorsky, G.S.; Cao, J.; Mayo, N.E.; Gabbay, R.; Murias, J.M. Arterial Versus Capillary Blood Gases: A Meta-Analysis. Respir. Physiol. Neurobiol. 2007, 155, 268–279. [Google Scholar] [CrossRef]
- Byrne, A.L.; Bennett, M.; Chatterji, R.; Symons, R.; Pace, N.L.; Thomas, P.S. Peripheral Venous and Arterial Blood Gas Analysis in Adults: Are They Comparable? A Systematic Review and Meta-Analysis. Respirology 2014, 19, 168–175. [Google Scholar] [CrossRef]
- Malinoski, D.J.; Todd, S.R.; Slone, D.S.; Mullins, R.J.; Schreiber, M.A. Correlation of Central Venous and Arterial Blood Gas Measurements in Mechanically Ventilated Trauma Patients. Arch. Surg. 2005, 140, 1122–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treger, R.; Pirouz, S.; Kamangar, N.; Corry, D. Agreement between Central Venous and Arterial Blood Gas Measurements in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. 2010, 5, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; Farber, H.W.; O’Donnell, C.; Cabral, H.; Eagan, J.S.; Philippides, G.J. The Accuracy of the Central Venous Blood Gas for Acid-Base Monitoring. J. Intensive Care Med. 2010, 25, 104–110. [Google Scholar] [CrossRef]
- Scheeren, T.W.; Wicke, J.N.; Teboul, J.L. Understanding the Carbon Dioxide Gaps. Curr. Opin. Crit. Care 2018, 24, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Charbel, F.T.; Du, X.; Hoffman, W.E.; Ausman, J.I. Brain Tissue PO2, PCO2, and pH During Cerebral Vasospasm. Surg. Neurol. 2000, 54, 432–437. [Google Scholar] [CrossRef]
- Brooks, A.J.; Hammond, J.S.; Girling, K.; Beckingham, I.J. The Effect of Hepatic Vascular Inflow Occlusion on Liver Tissue pH, Carbon Dioxide, and Oxygen Partial Pressures: Defining the Optimal Clamp/Release Regime for Intermittent Portal Clamping. J. Surg. Res. 2007, 141, 247–251. [Google Scholar] [CrossRef]
- McKinley, B.A.; Butler, B.D. Comparison of Skeletal Muscle PO2, PCO2, and pH with Gastric Tonometric PCO2 and pH in Hemorrhagic Shock. Crit. Care Med. 1999, 27, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Mythen, M.G. Does Gastric Tonometry-Guided Therapy Reduce Total Mortality in Critically Ill Patients? Crit. Care 2015, 19, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallat, J.; Vallet, B. Regional Capnography. In Monitoring Tissue Perfusion in Shock: From Physiology to the Bedside; Pinto Lima, A.A., Silva, E., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 181–192. [Google Scholar] [CrossRef]
- Ganter, M.; Zollinger, A. Continuous Intravascular Blood Gas Monitoring: Development, Current Techniques, and Clinical Use of a Commercial Device. BJA Br. J. Anaesth. 2003, 91, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Luukkonen, A.A.; Lehto, T.M.; Hedberg, P.S.; Vaskivuo, T.E. Evaluation of a Hand-Held Blood Gas Analyzer for Rapid Determination of Blood Gases, Electrolytes and Metabolites in Intensive Care Setting. Clin. Chem. Lab. Med. (CCLM) 2016, 54, 585–594. [Google Scholar] [CrossRef]
- Menzel, M.; Soukup, J.; Henze, D.; Engelbrecht, K.; Senderreck, M.; Scharf, A.; Rieger, A.; Grond, S. Experiences with Continuous Intra-Arterial Blood Gas Monitoring: Precision and Drift of a Pure Optode-System. Intensive Care Med. 2003, 29, 2180–2186. [Google Scholar] [CrossRef]
- Badnjevic, A.; Beganovic, E.; Music, O. Facts About Solution Based and Cartridge Based Devices for Blood Gas Analysis. In Proceedings of the 2011 18th International Conference on Systems, Signals and Image Processing, Sarajevo, Bosnia and Herzegovina, 16–18 June 2011; pp. 1–5. [Google Scholar]
- Gravenstein, J.; Jaffe, M.; Gravenstein, N.; Paulus, D. Capnography, 2nd ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Kodali, B.S. Capnography, 11th ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Siobal, M.S. Monitoring Exhaled Carbon Dioxide. Respir. Care 2016, 61, 1397–1416. [Google Scholar] [CrossRef] [Green Version]
- Long, B.; Koyfman, A.; Vivirito, M.A. Capnography in the Emergency Department: A Review of Uses, Waveforms, and Limitations. J. Emerg. Med. 2017, 53, 829–842. [Google Scholar] [CrossRef]
- Amaddeo, A.; Fauroux, B. Oxygen and Carbon Dioxide Monitoring During Sleep. Paediatr. Respir. Rev. 2016, 20, 42–44. [Google Scholar] [CrossRef]
- Hochwald, O.; Borenstein-Levin, L.; Dinur, G.; Jubran, H.; Ben-David, S.; Kugelman, A. Continuous Noninvasive Carbon Dioxide Monitoring in Neonates: From Theory to Standard of Care. Pediatrics 2019, 144, e20183640. [Google Scholar] [CrossRef]
- Jaffe, M.B. Mainstream or Sidestream Capnography? Respironics White Paper; Respironics Novametrix, Inc.: Wallingford, CT, USA, 2002. [Google Scholar]
- Barter, L.S. Capnography. In Advanced Monitoring and Procedures for Small Animal Emergency and Critical Care; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; Chapter 26; pp. 340–348. [Google Scholar] [CrossRef]
- Balogh, A.L.; Petak, F.; Fodor, G.H.; Tolnai, J.; Csorba, Z.; Babik, B. Capnogram Slope and Ventilation Dead Space Parameters: Comparison of Mainstream and Sidestream Techniques. Br. J. Anaesth. 2016, 117, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, K.; Yukioka, H.; Fujimori, M.; Maeda, C.; Noguchi, H.; Ishihara, S.; Yamanaka, I.; Tase, C. Utility of Colorimetric End-Tidal Carbon Dioxide Detector for Monitoring During Prehospital Cardiopulmonary Resuscitation. Am. J. Emerg. Med. 1999, 17, 203–206. [Google Scholar] [CrossRef]
- Mari, A.; Nougue, H.; Mateo, J.; Vallet, B.; Vallée, F. Transcutaneous PCO2 Monitoring in Critically Ill Patients: Update and Perspectives. J. Thorac. Dis. 2019, 11, S1558–S1567. [Google Scholar] [CrossRef] [PubMed]
- Vallée, F.; Nougué, H.; Mari, A.; Vodovar, N.; Dubreuil, G.; Damoisel, C.; Dépret, F.; Mateo, J. Variations of Cutaneous Capnometry and Perfusion Index During a Heating Challenge is Early Impaired in Septic Shock and Related to Prognostic in Non-Septic Shock. Shock 2019, 51, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, R.D.; Hirst, K.R.; Wittnebel, L.; Wettstein, R. AARC Clinical Practice Guideline: Transcutaneous Monitoring of Carbon Dioxide and Oxygen: 2012. Respir. Care 2012, 57, 1955–1962. [Google Scholar] [CrossRef]
- Vallée, F.; Mateo, J.; Dubreuil, G.; Poussant, T.; Tachon, G.; Ouanounou, I.; Payen, D. Cutaneous Ear Lobe PCO2 at 37 °C to Evaluate Microperfusion in Patients with Septic Shock. Chest 2010, 138, 1062–1070. [Google Scholar] [CrossRef]
- Steventon, A.; Bardsley, M.; Billings, J.; Dixon, J.; Doll, H.; Hirani, S.; Cartwright, M.; Rixon, L.; Knapp, M.; Henderson, C.; et al. Effect of Telehealth on Use of Secondary Care and Mortality: Findings From the Whole System Demonstrator Cluster Randomised Trial. BMJ 2012, 344, e3874. [Google Scholar] [CrossRef] [Green Version]
- Kruse, C.; Pesek, B.; Anderson, M.; Brennan, K.; Comfort, H. Telemonitoring to Manage Chronic Obstructive Pulmonary Disease: Systematic Literature Review. JMIR Med. Inf. 2019, 7, e11496. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.E.; Park, J.E.; Park, H.Y.; Lee, H.Y.; Park, D.A. Comparative Effectiveness of Telemonitoring Versus Usual Care for Heart Failure: A Systematic Review and Meta-analysis. J. Card. Fail. 2018, 24, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Garfan, S.; Alamoodi, A.H.; Zaidan, B.B.; Al-Zobbi, M.; Hamid, R.A.; Alwan, J.K.; Ahmaro, I.Y.; Khalid, E.T.; Jumaah, F.M.; Albahri, O.S.; et al. Telehealth Utilization During the COVID-19 Pandemic: A Systematic Review. Comput. Biol. Med. 2021, 138, 104878. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the Medical Revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef] [Green Version]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable Flexible Sweat Sensors for Healthcare Monitoring: A Review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in Cardiology: Here to Stay. Heart Rhythm 2020, 17, 889–895. [Google Scholar] [CrossRef]
- Dervieux, E.; Bodinier, Q.; Uhring, W.; Théron, M. Measuring Hemoglobin Spectra: Searching for Carbamino-Hemoglobin. J. Biomed. Opt. 2020, 25, 105001. [Google Scholar] [CrossRef]
- Eletr, S.; Jimison, H.; Ream, A.K.; Dolan, W.M.; Rosenthal, M.H. Cutaneous Monitoring of Systemic Pco2 on Patients in the Respiratory Intensive Care Unit Being Weaned From the Ventilator. Acta Anaesthesiol. Scand. 1978, 22, 123–127. [Google Scholar] [CrossRef]
- Greenspan, G.H.; Block, A.J.; Haldeman, L.W.; Lindsey, S.; Martin, C.S. Transcutaneous Noninvasive Monitoring of Carbon Dioxide Tension. Chest 1981, 80, 442–446. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ge, X.; Kostov, Y.; Tolosa, L.; Rao, G. A Novel Approach Toward Noninvasive Monitoring of Transcutaneous CO2. Med. Eng. Phys. 2014, 36, 136–139. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, M.; Ge, X.; Kostov, Y.; Luu, P.; Tolosa, L.; Woo, H.; Viscardi, R.; Falk, S.; Potts, R.; Rao, G. A Rate-Based Transcutaneous CO2 Sensor for Noninvasive Respiration Monitoring. Physiol. Meas. 2015, 36, 883–894. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Adangwa, P.; Young Lim, J.; Kostov, Y.; Tolosa, L.; Pierson, R.; Herr, D.; Rao, G. Development and Characterization of a Point-of Care Rate-Based Transcutaneous Respiratory Status Monitor. Med. Eng. Phys. 2018, 56, 36–41. [Google Scholar] [CrossRef]
- Iitani, K.; Tyson, J.; Rao, S.; Ramamurthy, S.S.; Ge, X.; Rao, G. What do Masks Mask? A Study on Transdermal CO2 Monitoring. Med. Eng. Phys. 2021, 98, 50–56. [Google Scholar] [CrossRef]
- Grangeat, P.; Gharbi, S.; Accensi, M.; Grateau, H. First Evaluation of a Transcutaneous Carbon Dioxide Monitoring Wristband Device during a Cardiopulmonary Exercise Test. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3352–3355. [Google Scholar] [CrossRef]
- Grangeat, P.; Gharbi, S.; Koenig, A.; Comsa, M.P.; Accensi, M.; Grateau, H.; Ghaith, A.; Chacaroun, S.; Doutreleau, S.; Verges, S. Evaluation in Healthy Subjects of a Transcutaneous Carbon Dioxide Monitoring Wristband during Hypo and Hypercapnia Conditions. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4640–4643. [Google Scholar] [CrossRef]
- Shaw, L.A.; Messer, A.C. Cutaneous Respiration in Man: II. The Effect of Temperature and of Relative Humidity upon the Rate of Carbon Dioxide Elimination and Oxygen Absorption. Am. J. Physiol.-Leg. Content 1930, 95, 13–19. [Google Scholar] [CrossRef]
- Scheuplein, R.J. Permeability of the Skin: A Review of Major Concepts and Some New Developments. J. Investig. Dermatol. 1976, 67, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, B.; Boerboom, A.J.; Kistemaker, J. A Mass Spectrometer for Continuous Analysis of Gaseous Compounds Excreted by Human Skin. J. Appl. Physiol. 1966, 21, 1903–1906. [Google Scholar] [CrossRef] [PubMed]
- Levshankov, A.I.; Pushkina, M.A.; Slutskaia, M.E.; Uvarov, B.S. Determination of Local Gas Exchange on the Body Surface by the Method of Mass Spectrometry. Meditsinskaia Tekhnika 1983, 1, 21–26. [Google Scholar]
- Ernstene, A.C.; Volk, M.C. Cutaneous Respiration in Man: IV. The Rate of Carbon Dioxide Elimination and Oxygen Absorption in Normal Subjects. J. Clin. Investig. 1932, 11, 363–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlroy, M.B.; Simbruner, G.; Sonoda, Y. Transcutaneous Blood Gas Measurements Using a Mass Spectrometer. Acta Anaesthesiol. Scand. 1978, 22, 128–130. [Google Scholar] [CrossRef]
- Hansen, T.N.; Sonoda, Y.; McIlroy, M.B. Transfer of Oxygen, Nitrogen, and Carbon Dioxide through Normal Adult Human Skin. J. Appl. Physiol. 1980, 49, 438–443. [Google Scholar] [CrossRef]
- Targett, R.C.; Kocher, O.; Muramatsu, K.; McIlroy, M.B. Skin Gas Tensions and Resistances Measured by Mass Spectrometry in Adults. J. Appl. Physiol. 1984, 56, 1431–1435. [Google Scholar] [CrossRef]
- Lawrence, J.C.; Bull, J.P. Thermal Conditions which Cause Skin Burns. Eng. Med. 1976, 5, 61–63. [Google Scholar] [CrossRef]
- Moritz, A.R.; Henriques, F.C. Studies of Thermal Injury: II. The Relative Importance of Time and Surface Temperature in the Causation of Cutaneous Burns. Am. J. Pathol. 1947, 23, 695–720. [Google Scholar]
- Wimberley, P.D.; Grønlund Pedersen, K.; Olsson, J.; Siggaard-Andersen, O. Transcutaneous Carbon Dioxide and Oxygen Tension Measured at Different Temperatures in Healthy Adults. Clin. Chem. 1985, 31, 1611–1615. [Google Scholar] [CrossRef]
- Food and Drug Administration. Cutaneous Carbon Dioxide (PcCO2) and Oxygen (PcO2) Monitors—Class II Special Controls Guidance Document for Industry and FDA; Technical Report; U.S. Department Of Healsth and Human Services: Washington, DC, USA, 2002. [Google Scholar]
- Fitzgerald, L.R. Cutaneous Respiration in Man. Physiol. Rev. 1957, 37, 325–336. [Google Scholar] [CrossRef]
- Shaw, L.A.; Messer, A.C. Cutaneous Respiration in Man: I. Factors Affecting the Rate of Carbon Dioxide Elimination and Oxygen Absorption. Am. J. Physiol.-Leg. Content 1930, 95, 107–118. [Google Scholar] [CrossRef]
- Barratt, W. On the Normal and Pathological Elimination of Carbonic Acid and of Water by the Skin. J. Physiol. 1897, 21, 192–208. [Google Scholar] [CrossRef]
- Whitehouse, A.G.R.; Hancock, W.; Haldane, J.S. The Osmotic Passage of Water and Gases through the Human Skin. Proc. R. Soc. Lond. Ser. B 1932, 111, 412–429. [Google Scholar] [CrossRef]
- Thiele, F.A.J.; Van Kempen, L.H.J. A Micro Method for Measuring the Carbon Dioxide Release by Small Skin Areas. Br. J. Dermatol. 1972, 86, 463–471. [Google Scholar] [CrossRef]
- Frame, G.W.; Strauss, W.G.; Maibach, H.I. Carbon Dioxide Emission of the Human Arm and Hand. J. Investig. Dermatol. 1972, 59, 155–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eöry, A. In-Vivo Skin Respiration (CO2) Measurements in the Acupuncture Loci. Acupunct. Electro-Ther. Res. 1984, 9, 217–223. [Google Scholar] [CrossRef]
- Montgomery, L.D.; Williams, B.A. Effect of Ambient Temperature on the Thermal Profile of the Human Forearm, Hand, and Fingers. Ann. Biomed. Eng. 1976, 4, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Grønlund, J. Evaluation of Factors Affecting Relationship Between Transcutaneous PO2 and Probe Temperature. J. Appl. Physiol. 1985, 59, 1117–1127. [Google Scholar] [CrossRef]
- Palmisano, B.W.; Severinghaus, J.W. Transcutaneous Pco2 and Po2: A multicenter study of Accuracy. J. Clin. Monit. 1990, 6, 189–195. [Google Scholar] [CrossRef]
- Herrell, N.; Martin, R.J.; Pultusker, M.; Lough, M.; Fanaroff, A. Optimal Temperature for the Measurement of Transcutaneous Carbon Dioxide Tension in the Neonate. J. Pediatr. 1980, 97, 114–117. [Google Scholar] [CrossRef]
- Tremper, K.K.; Mentelos, R.A.; Shoemaker, W.C. Clinical And Experimental Transcutaneous PCO2 Monitoring. J. Clin. Eng. 1981, 6, 143–147. [Google Scholar] [CrossRef]
- Briand, D.; Oprea, A.; Courbat, J.; Bârsan, N. Making Environmental Sensors on Plastic Foil. Mater. Today 2011, 14, 416–423. [Google Scholar] [CrossRef]
- Köstler, S.; Kraker, E.; Sagmeister, M.; Lamprecht, B.; Bizzari, A.; Stadlober, B.; Ribitsch, B.; Abel, T.; Mayr, T. C7.3—From Optochemical Sensors for Industrial Processes to Large-Area Printing of Sensor Systems Integrated with Organic Electronics. In Proceedings of the Sensor + Test Conferences 2011, Nürnberg, Germany, 7–9 June 2011. [Google Scholar]
- Xing, X.; Ma, Z.; Zhang, M.; Zhou, Y.; Dong, W.; Song, M. An Unobtrusive and Calibration-Free Blood Pressure Estimation Method Using Photoplethysmography and Biometrics. Sci. Rep. 2019, 9, 8611. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Shi, P.; Hu, S.; Yu, H. A Revised Point-to-Point Calibration Approach with Adaptive Errors Correction to Weaken Initial Sensitivity of Cuff-Less Blood Pressure Estimation. Sensors 2020, 20, 2205. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Moret-Bonillo, V.; Alvarez-Estévez, D.; Fernández-Leal, A.; Hernández-Pereira, E. Intelligent Approach for Analysis of Respiratory Signals and Oxygen Saturation in the Sleep Apnea/Hypopnea Syndrome. Open Med. Inform. J. 2014, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.; Gokalp, H.; Fursse, J.; Jones, R.W. Dynamic Threshold Analysis of Daily Oxygen Saturation for Improved Management of COPD Patients. IEEE J. Biomed. Health Inform. 2016, 20, 1352–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domján, G.; Kaffka, K.J.; Jákó, J.M.; Vályi-Nagy, I.T. Rapid Analysis of Whole Blood and Blood Serum Using Near Infrared Spectroscopy. J. Infrared Spectrosc. 1994, 2, 67–78. [Google Scholar] [CrossRef]
- Marasco, C.; Perez, A.; Ehrman, J.; Hoilett, O. Metabolic Rate Measurement Apparatus and Method Thereof. U.S. Patent 2020/0113516 Al, 16 April 2020. [Google Scholar]
- Freilich, J. Prophetic patents. UC Davis L. Rev. 2019, 53, 663. [Google Scholar] [CrossRef]
- Tamura, T. Current Progress of Photoplethysmography and SpO2 for Health Monitoring. Biomed. Eng. Lett. 2019, 9, 21–36. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Labrie, D.; Chylek, P. Refractive Indices of Water and Ice in the 0.65- to 2.5-μm Spectral Range. Appl. Opt. 1993, 32, 3531–3540. [Google Scholar] [CrossRef] [Green Version]
















| Criterion → ↓ Technique | Lifetime | Calibration Need | Humidity Cross-Sensitivity | Accuracy | Response Time | Miniaturisation Potential | References |
|---|---|---|---|---|---|---|---|
| NDIR (Section 2.2.1) | ≥15 years | No | No | 0.03% | ≤1 s | Moderate (down to mm) | [32,33,34,36] |
| Photoacous. (Section 2.2.2) | ? (∞) | No | No | 1% | ≤1 s | Low (∼cm) | [51,257,258] |
| Wet Conduct. (Section 2.3.1) | ? | Yes (0.4% drift/h) | Yes (dries out) | ? | 5 s | High (down to 100 µm thickness) | [60,62] |
| S-S elec. (Section 2.3.2) | 80 days | Yes (0.03 mV/h) | Yes (dries out) | 0.03% (0–100 mmHg) | 30 s | High (down to 10 µm thickness) | [67,69,73,259] |
| ISFET (Section 2.3.3) | ≥30 days | Yes (0.23 mV/h) | Yes | ? | 60 s | High (down to 100 µm thickness) | [79,88] |
| Dye-based (Section 2.3.4) | 19 months | No | Yes | 0.1% | ≤1 s | Excellent (down to 1 µm thickness) | [56,103,112,122,123,260] |
| Optical fiber (Section 2.3.5) | ? | ? | Yes | 4.1% | 40–75 s | Excellent (down to 55 nm coating thickness) | [145,146,147] |
| Metal Oxide Ads. (Section 2.4.1) | ≥5 months | No | Yes (low) | 1.6% | 30 s | Excellent (down to 125 nm thickness) | [155,163,164,171,175,189,261] |
| Graphene (Section 2.4.2) | ≥90 days | No | Yes | 0.8% | 3 s | Excellent (down to 3 nm thickness) | [194,196,207] |
| Electro. Cells (Section 2.4.3) | ≥2 years | No | No | 4% | 2–4 s | Excellent (down to 1 µm thickness) | [213,214,221,262] |
| Ionic liquids (Section 2.4.4) | ≥4 months | No | No | 2.05% | 40 s | Excellent (down to 430 nm thickness) | [226,231,233,234,238] |
| Time of flight (Section 2.5.1) | ? (∞) | No | Yes (low) | 0.3% (0–10% range) | ≤1 s | Low (∼cm) | [245] |
| Acous. Att. (Section 2.5.2) | ? (∞) | No | ? | ? | ≤1 s | Low (∼cm) | [250,254] |
| Exhalation Rate [cm·m·h] | Temp. [°C] | Num. of Subjects | Ref. |
|---|---|---|---|
| 25–120 | 22–36 (air) | 2 | Shaw (1929) [336] |
| 10–160 | 25–37 (air) | 1 | Shaw (1930) [323] |
| 40–125 | 25–30 (air) | 2 | Ernstene (1932) [327] |
| 12–143 | 23–37 (air) | 1 | Whitehouse (1932) [338] |
| 180–2500 † | - | 1 | Adamczyk (1966) [325] |
| 25–87 | - | 5 | Thiele (1972) [339] |
| 11–28 | 25–35 (air) | 3 | Frame (1972) [340] |
| 50 | - | 27 | Levshankov (1983) [326] |
| 131–206 | 36 (skin) | 14 | Eöry (1984) [341] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervieux, E.; Théron, M.; Uhring, W. Carbon Dioxide Sensing—Biomedical Applications to Human Subjects. Sensors 2022, 22, 188. https://doi.org/10.3390/s22010188
Dervieux E, Théron M, Uhring W. Carbon Dioxide Sensing—Biomedical Applications to Human Subjects. Sensors. 2022; 22(1):188. https://doi.org/10.3390/s22010188
Chicago/Turabian StyleDervieux, Emmanuel, Michaël Théron, and Wilfried Uhring. 2022. "Carbon Dioxide Sensing—Biomedical Applications to Human Subjects" Sensors 22, no. 1: 188. https://doi.org/10.3390/s22010188