Effects of Botulinum Toxin Therapy on Health-Related Quality of Life Evaluated by the Oromandibular Dystonia Rating Scale
Abstract
:1. Introduction
2. Results
2.1. Demographic Data and Results of Treatment
2.2. OMDRS Scores at Baseline and Endpoint
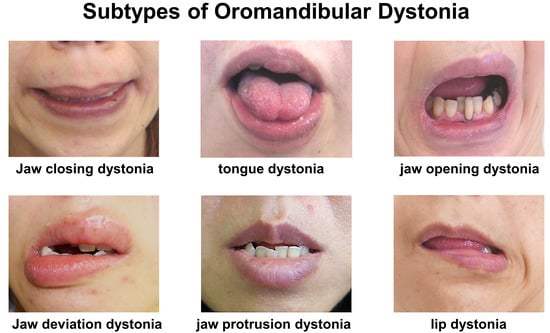
2.3. Differences in Subscales Scores of OMDRS among Subtypes of OMD
2.4. Differences in OMDRS Scores between Idiopathic and Tardive Cases
3. Discussion
3.1. Rating Scales for OMD
3.2. Results of This Study
3.3. Treatment Modalities of OMD
3.4. Limitations and Strengths
3.5. Future Directions
4. Conclusions
5. Materials and Methods
5.1. Participants
5.2. BoNT Therapy
5.3. Statistical Analysis
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus up date. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kaji, R.; Kubori, T.; Kohara, N.; Iizuka, T.; Kimura, J. Muscle afferent block for the treatment of oromandibular dystonia. Mov. Disord. 1998, 13, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Larsen, B.M.; Dalager, T.; Møller, E. Oromandibular dystonia–functional and clinical characteristics: A report on 21 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e21–e26. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.F.; Gurey, L.E.; Blitzer, A. Oromandibular dystonia: Long-term management with botulinum toxin. Laryngoscope 2013, 123, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Skármeta, N.P.; Espinoza-Mellado, P.; Chana, P. Orofacial dystonia and other oromandibular movement disorders. In Dystonia; Rizk, T.M.G., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Comella, C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon 2018, 147, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Dadgardoust, P.D.; Rosales, R.L.; Asuncion, R.M.; Dressler, D. Botulinum neurotoxin a therapy efficacy and safety for oromandibular dystonia: A meta-analysis. J. Neural Transm. 2019, 126, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Oromandibular dystonia screening questionnaire for differential diagnosis. Clin. Oral Investig. 2019, 23, 405–411. [Google Scholar] [CrossRef]
- Yoshida, K. Development and validation of a disease-specific oromandibular dystonia rating scale (OMDRS). Front. Neurol. 2020, 11, 583177. [Google Scholar] [CrossRef]
- Scorr, L.M.; Factor, S.A.; Parra, S.P.; Kaye, R.; Paniello, R.C.; Norris, S.A.; Perlmutter, J.S.; Bäumer, T.; Usnich, T.; Berman, B.D.; et al. Oromandibular dystonia: A clinical examination of 2020 cases. Front. Neurol. 2021, 12, 700714. [Google Scholar] [CrossRef]
- Yoshida, K. Prevalence and incidence of oromandibular dystonia: An oral and maxillofacial surgery service-based study. Clin. Oral Investig. 2021, 25, 5755–5764. [Google Scholar] [CrossRef]
- Yoshida, K. Behandlungsstrategien bei oromandibulärer Dystonie. Fortschr. Neurol. Psychiatr. 2021, 89, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Botulinum toxin therapy for oromandibular dystonia and other movement disorders in the stomatognathic system. Toxins 2022, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Botulinum Toxin Therapy for Oromandibular Dystonia. Scholarly Community Encyclopedia. 2022. Available online: https://encyclopedia.pub/entry/23511 (accessed on 1 August 2022).
- Nutt, J.G.; Muenter, M.D.; Aronson, A.; Kurland, L.T.; Melton, L.J., 3rd. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov. Disord. 1988, 3, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Steeves, T.D.; Day, L.; Dykeman, J.; Jette, N.; Pringsheim, T. The prevalence of primary dystonia: A systematic review and meta-analysis. Mov. Disord. 2012, 27, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Iizuka, T. Botulinum toxin treatment for upper airway collapse resulting from temporomandibular joint dislocation due to jaw-opening dystonia. Cranio 2006, 24, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Botulinum neurotoxin injection for the treatment of recurrent temporomandibular joint dislocation with and without neurogenic muscular hyperactivity. Toxins 2018, 10, 174. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum neurotoxin therapy for lingual dystonia using an individualized injection method based on clinical features. Toxins 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kaji, R.; Shibasaki, H.; Iizuka, T. Factors influencing the therapeutic effect of muscle afferent block for oromandibular dystonia and dyskinesia: Implications for their distinct pathophysiology. Int. J. Oral Maxillofac. Surg. 2002, 31, 499–505. [Google Scholar] [CrossRef]
- Merz, R.I.; Deakin, J.; Hawthorne, M.R. Oromandibular dystonia questionnaire (OMDQ-25): A valid and reliable instrument for measuring health-related quality of life. Clin. Otolaryngol. 2010, 35, 390–396. [Google Scholar] [CrossRef]
- Albanese, A.; Sorbo, F.D.; Comella, C.; Jinnah, H.A.; Mink, J.W.; Post, B.; Vidailhet, M.; Volkmann, J.; Warner, T.T.; Leentjens, A.F.G.; et al. Dystonia rating scales: Critique and recommendations. Mov. Disord. 2013, 28, 874–883. [Google Scholar] [CrossRef]
- Del Sorbo, F.; Albanese, A. Botulinum neurotoxins for the treatment of focal dystonias: Review of rating tools used in clinical trials. Toxicon 2015, 107, 89–97. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Tarsy, D. Impact on quality of life of botulinum toxin treatments for spasmodic dysphonia and oromandibular dystonia. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Charous, S.J.; Comella, C.L.; Fan, W. Jaw-opening dystonia: Quality of life after botulinum toxin injections. Ear Nose Throat J. 2011, 90, E9–E12. [Google Scholar] [CrossRef] [PubMed]
- Page, A.D.; Siegel, L.; Jog, M. Self-Rated communication-related quality of life of individuals with oromandibular dystonia receiving botulinum toxin injections. Am. J. Speech Lang. Pathol. 2017, 26, 674–681. [Google Scholar] [CrossRef]
- Page, A.D.; Elhayek, N.; Baylor, C.; Adams, S.; Jog, M.; Yorkston, K. Exploring the psychosocial impact of botulinum toxin type A injections for individuals with oromandibular dystonia: A qualitative study of patients’ experiences. Am. J. Speech Lang. Pathol. 2021, 30, 1314–1328. [Google Scholar] [CrossRef]
- Kuyper, D.J.; Parra, V.; Aerts, S.; Okun, M.S.; Kluger, B.M. The non-motor manifestations of dystonia: A systematic review. Mov. Disord. 2011, 26, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Klingelhoefer, L.; Kaiser, M.; Sauerbier, A.; Untucht, R.; Wienecke, M.; Mammadova, K.; Falkenburger, B.; Gregor, O.; Chaudhuri, K.R.; Reichmann, H. Emotional well-being and pain could be a greater determinant of quality of life compared to motor severity in cervical dystonia. J. Neural. Transm. 2021, 128, 305–314. [Google Scholar] [CrossRef]
- Ndukwe, I.; O’Riordan, S.; Walsh, C.B.; Hutchinson, M. Trust the patient not the doctor: The determinants of quality of life in cervical dystonia. Front. Neurol. 2020, 11, 991. [Google Scholar] [CrossRef]
- Junker, J.; Berman, B.D.; Hall, J.; Wahba, D.W.; Brandt, V.; Perlmutter, J.S.; Jankovic, J.; Malaty, I.A.; Shukla, A.W.; Reich, S.G.; et al. Quality of life in isolated dystonia: Non-motor manifestations matter. J. Neurol. Neurosurg. Psychiatry 2021, 92, 622–628. [Google Scholar] [CrossRef]
- Costanzo, M.; Belvisi, D.; Berardelli, I.; Maraone, A.; Baione, V.; Ferrazzano, G.; Cutrona, C.; Leodori, G.; Pasquini, M.; Conte, A.; et al. Effect of botulinum toxin on non-motor symptoms in cervical dystonia. Toxins 2021, 13, 647. [Google Scholar] [CrossRef]
- Nastasi, L.; Mostile, G.; Nicoletti, A.; Zappia, M.; Reggio, E.; Catania, S. Effect of botulinum toxin treatment on quality of life in patients with isolated lingual dystonia and oromandibular dystonia affecting the tongue. J. Neurol. 2016, 263, 1702–1708. [Google Scholar] [CrossRef]
- Scorr, L.M.; Silver, M.R.; Hanfelt, J.; Sperin, E.; Freeman, A.; Jinnah, H.A.; Factor, S.A. Pilot single-blind trial of AbobotulinumtoxinA in oromandibular dystonia. Neurotherapeutics 2018, 15, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrica 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Cano, S.J.; Warner, T.T.; Linacre, J.M.; Bhatia, K.P.; Thompson, A.J.; Fitzpatrick, R.; Hobart, J.C. Capturing the true burden of dystonia on patients: The Cervical Dystonia Impact Profile (CDIP-58). Neurology 2004, 63, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. How do I inject botulinum toxin into the lateral and medial pterygoid muscles? Mov. Disord. Clin. Pract. 2016, 4, 285. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Clinical characteristics of functional movement disorders in the stomatognathic system. Front. Neurol. 2020, 11, 23. [Google Scholar] [CrossRef]
- Yoshida, K. Effects of botulinum toxin type A on pain among trigeminal neuralgia, myofascial temporomandibular disorders, and oromandibular dystonia. Toxins 2021, 13, 605. [Google Scholar] [CrossRef]
- Berardelli, I.; Ferrazzano, G.; Pasquini, M.; Biondi, M.; Berardelli, A.; Fabbrini, G. Clinical course of psychiatric disorders in patients with cervical dystonia. Psychiatry Res. 2015, 229, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Neurophysiology of dystonia: The role of inhibition. Neurobiol. Dis. 2011, 42, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Stamelou, M.; Edwards, M.J.; Hallett, M.; Bhatia, K.P. The non-motor syndrome of primary dystonia: Clinical and pathophysio logical implications. Brain 2012, 135, 1668–1681. [Google Scholar] [CrossRef]
- Fabbrini, G.; Berardelli, I.; Moretti, G.; Pasquini, M.; Bloise, M.; Colosimo, C.; Biondi, M.; Berardelli, A. Psychiatric disorders in adult-onset focal dystonia: A case-control study. Mov. Disord. 2010, 25, 459–465. [Google Scholar] [CrossRef]
- Singer, C.; Papapetropoulos, S. A comparison of jaw-closing and jaw-opening idiopathic oromandibular dystonia. Parkinsonism Relat. Disord. 2006, 12, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Computer-aided design/computer-assisted manufacture-derived needle guide for injection of botulinum toxin into the lateral pterygoid muscle in patients with oromandibular dystonia. J. Oral Facial Pain Headache 2018, 32, e13–e21. [Google Scholar] [CrossRef]
- Yoshida, K. An electromyographic study on the superior head of the lateral pterygoid muscle during mastication from the standpoint of condylar movement. J. Jpn. Prosthodont. Soc. 1992, 36, 110–120. [Google Scholar]
- Yoshida, K. Eigenschaften der Kaumuskelaktivität während verschiedener Unterkieferbewegungen bei Patienten mit Diskusverlagerung ohne Reposition. Stomatologie 1999, 96, 107–121. [Google Scholar]
- Yoshida, K. Masticatory muscle responses associated with unloading of biting force during food crushing. J. Oral Rehabil. 1998, 25, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Usui, A.; Akita, K.; Yamaguchi, K. An anatomic study of the divisions of the lateral pterygoid muscle based on the findings of the origins and insertions. Surg. Radiol. Anat. 2008, 30, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Stöckle, M.; Fanghänel, J.; Knüttel, H.; Alamanos, C.; Behr, M. The morphological variations of the lateral pterygoid muscle: A systematic review. Ann. Anat. 2019, 222, 79–87. [Google Scholar] [CrossRef]
- Yoshida, K. Multilingual website and cyberconsultations for oromandibular dystonia. Neurol. Int. 2018, 10, 7536. [Google Scholar] [CrossRef]
- Logroscino, G.; Livrea, P.; Anaclerio, D.; Aniello, M.S.; Benedetto, G.; Cazzato, G.; Giampietro, L.; Manobianca, G.; Marra, M.; Martino, D.; et al. Agreement among neurologista on the clinical diagnosis of dystonia at different body sites. J. Neurol. Neurosurg. Psychiatry 2003, 74, 348–350. [Google Scholar] [CrossRef]
- Saraf, U.; Chandarana, M.; Divya, K.P.; Krishnan, S. Oromandibular dystonia—A systematic review. Ann. Indian Acad. Neurol. 2022, 25, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Involuntary Movements of the Stomatognathic Region. Available online: https://sites.google.com/site/oromandibulardystoniaenglish (accessed on 1 August 2022).
- Jankovic, J.; Orman, J. Botulinum a toxin for cranial-cervical dystonia: A double-blind, placebo-controlled study. Neurology 1987, 37, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.; Greene, P.E.; Fahn, S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann. Otol. Rhinol. Laryngol. 1989, 98, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, N.; Truong, D.D. Treatment of oromandibular dystonia with botulinum toxin. Laryngoscope 1991, 101, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Jankovic, J. Botulinum toxin A in patients with oromandibular dystonia: Long-term follow-up. Neurology 1999, 53, 2102–2107. [Google Scholar] [CrossRef]
- Rosales, R.L.; Ng, A.R.; Santos, M.M.D.-D.; Fernandez, H.H. The broadening application of chemodenervation in X-linked dystonia-parkinsonism (Part II): An open-label experience with botulinum toxin-A (Dysport®) injections for oromandibular, lingual, and truncal-axial dystonias. Int. J. Neurosci. 2011, 121 (Suppl. S1), 44–56. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Takagi, A.; Iizuka, T. Customized EMG needle insertion guide for the muscle afferent block of jaw-deviation and jaw-opening dystonias. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 664–669. [Google Scholar] [CrossRef]
- Yoshida, K. Sensory trick splint as a multimodal therapy for oromandibular dystonia. J. Prosthodont. Res. 2018, 62, 239–244. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, M.; Vereecke, L.; Bottenberg, P.; Jacquet, W.; Sims, A.B.; Santens, P. Oral appliances in the treatment of oromandibular dystonia: A systematic review. Acta Neurol. Belg. 2020, 120, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Coronoidotomy as treatment for trismus due to jaw-closing oromandibular dystonia. Mov. Disord. 2006, 21, 1028–1031. [Google Scholar] [CrossRef]
- Yoshida, K. Surgical intervention for oromandibular dystonia-related limited mouth opening: Long-term follow-up. J. Cranio-Maxillofac. Surg. 2017, 45, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Mouth opening retaining appliance after coronoidotomy for the treatment of trismus: Effects on pain during postoperative training and maximal extent of mouth opening. Clin. Surg. 2020, 5, 2737. [Google Scholar] [CrossRef]
- Ondo, W.G.; Simmons, J.H.; Shahid, M.H.; Hashem, V.; Hunter, C.; Jankovic, J. Onabotulinum toxin-A injections for sleep bruxism: A double-blind, placebo-controlled study. Neurology 2018, 90, e559–e564. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Canales, G.; Poluha, R.L.; Pinzón, N.A.; DaSilva, B.R.; Almeida, A.M.; Ernberg, M.; Manso, A.C.; Bonjardim, L.R.; Rizzatti-Barbosa, C.M. Efficacy of botulinum toxin type-A I in the improvement of mandibular motion and muscle sensibility in myofascial pain TMD Subjects: A randomized controlled trial. Toxins 2022, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Ammendolia, A.; Paduano, S.; Calafiore, D.; Marotta, N.; Migliario, M.; Fortunato, L.; Giudice, A.; Michelotti, A.; de Sire, A. Efficacy of rehabilitation on reducing pain in muscle-related temporomandibular disorders: A systematic review and meta-analysis of randomized controlled trials. J. Back Musculoskelet. Rehabil. 2022, 35, 921–936. [Google Scholar] [CrossRef] [PubMed]




| Subtypes | Jaw Closing | Tongue | Jaw Opening | Jaw Deviation | Jaw Protrusion | Lip | Total |
|---|---|---|---|---|---|---|---|
| No. of patients [N] | 223 | 86 | 50 | 23 | 13 | 13 | 408 |
| Age (years) [mean (SD)] (range) | 53.8 (15.6) (18–95) | 48.3 (14.3) (24–86) | 52.3 (18.1) (19–86) | 51.5 (15.7) (26–81) | 49.2 (11.4) (21–63) | 48.7 (11.8) (37–68) | 52.0 (15.6) (18–95) |
| Sex (women, men) [N (%)] | 155 (69.5), 68(30.5) | 51 (59.3), 35 (40.7) | 26 (51.0), 24 (47.1) | 14 (60.9), 9 (39.1) | 7 (53.8), 6 (46.2) | 10 (76.9), 3 (23.1) | 262 (64.2), 146 (35.8) |
| Duration (months) [mean (SD)] (range) | 51.5 (64.6) (1–276) | 39.0 (68.5) (1–180) | 48.7 (91.5) (1–180) | 41.0 (52.3) (2–228) | 26.0 (22.1) (3–60) | 56.8 (47.3) (4–156) | 47.3 (67.3) (1–276) |
| Tardive dystonia [N (%)] | 101 (45.3) | 27 (31.4) | 23 (45.1) | 7 (30.4) | 4 (28.6) | 6 (46.2) | 168 (41.2) |
| Other dystonia [N (%)] | 41 (18.4) | 7 (8.1) | 17 (34.0) | 4 (17.4) | 1 (7.7) | 3 (23.1) | 73 (17.9) |
| Cervical dystonia | 28 (12.6) | 1 (1.2) | 9 (18.0) | 2 (8.7) | 1 (7.7) | 2 (15.4) | 43 (10.5) |
| Blepharospasm | 16 (7.2) | 2 (2.3) | 4 (8.0) | 2 (8.7) | 0 | 1 (7.7) | 25 (6.1) |
| Writer’s cramp | 3 (1.3) | 2 (2.3) | 2 (4.0) | 0 | 0 | 0 | 7 (1.7) |
| Upper limb dystonia | 2 (0.9) | 1 (1.2) | 1 (2.0) | 1 (4.3) | 0 | 0 | 5 (1.2) |
| Lower limb dystonia | 2 (0.9) | 0 | 1 (2.0) | 0 | 0 | 0 | 3 (0.7) |
| Spasmodic dysphonia | 1 (0.4) | 0 | 1 (2.0) | 0 | 0 | 1 (7.7) | 3 (0.7) |
| Embouchure dystonia | 1 (0.4) | 1 (1.2) | 0 | 0 | 0 | 0 | 2 (0.5) |
| Jaw Closing | Tongue | Jaw Opening | Jaw Deviation | Jaw Protrusion | Lip | Total | |
|---|---|---|---|---|---|---|---|
| No. of patients [N] | 223 | 86 | 50 | 23 | 13 | 13 | 408 |
| No. of BoNT injection [mean (SD)], (range) | 4.7 (4.4) (2–22) | 6.4 (5.6) (3–27) | 6.1 (5.2) (2–25) | 6.4 (6.1) (3–25) | 4.2 (3.1) (2–9) | 5.8 (5.7) (2–17) | 5.4 (5.0) (2–27) |
| No. of injected muscles [mean (SD)], (range) | 3.9 (1.9) (1–12) | 3.1 (1.8) (2–12) | 3.8 (2.2) (2–10) | 3.8 (2.4) (1–6) | 3.3 (1.7) (2–6) | 4.4 (1.9) (1–8) | 3.7 (2.0) (1–12) |
| Target muscles [N (%)] | Masseter: 192 (86.1) Temporalis: 110 (49.3) Medial pterygoid: 39 (17.5) Lateral pterygoid: 26 (11.7) Posterior digastric: 10 (4.5) Orbicularis oris: 8 (3.6) Mentalis: 8 (3.6) Genioglossus: 8 (3.6) Sternocleidomastoid: 6 (2.7) Risorius: 5 (2.2) Zygomatic major 3 (1.3) Others: 7 (3.1) | Genioglossus: 86 (100) Lateral pterygoid: 19 (22.1) Masseter: 10 (11.6) Medial pterygoid: 3 (3.5) Orbicularis oris 3 (3.5) Temporalis: 2 (2.3) Posterior digastric: 2 (2.3) Others: 3 (3.5) | Lateral pterygoid: 48 (96) Anterior digastric: 10 (20) Posterior digastric: 8 (16) Genioglossus: 7 (14) Orbicularis oris 3 (6) Sternocleidomastoid: 3 (6) Platysma: 3 (6) Mentalis: 2 (4) Risorius: 1 (2) | Lateral pterygoid: 23 (100) Masseter: 7 (30.4)Temporalis: 4 (17.4) Risorius: 3 (13) Posterior digastric: 2 (9.5) Others: 4 (13) | Lateral pterygoid: 13 (100) Masseter: 3 (23.1) Temporalis: 3 (23.1) | Orbicularis oris: 9 (69.2) Risorius: 8 (61.5) Mentalis: 5 (38.5) Depressor labii inferioris: 3 (23.1) Masseter: 2 (15.4) Others: 3 (23.1) | Masseter: 210 (51.5) Lateral pterygoid: 129 (31.6) Temporalis: 119 (29.2) Genioglossus: 101 (24.8) Medical pterygoid: 42 (10.3) Orbicularis oris: 23 (5.6) Posterior digastric: 22 (5.4) Risorius: 17 (4.2) Mentalis: 15 (3.7)Anterior digastric: 10 (2.5) Sternocleidomastoid: 9 (2.2) Zygomatic major 3 (0.7) Platysma: 3 (0.7) Depressor labii inferioris: 3 (0.7) Others: 17 (4.2) |
| Improvement (%) [mean (SD)], (range) | 63.3 (16.2) 17.1–98.1 | 66.3 (20.8) 21.4–97.8 | 57.2 (23.1) 14.9–98.4 | 65.8 (19.2) 28.7–87.1 | 58.8 (17.5) 35.0–80.5 | 60.6 (15.1) 31.9–86.6 | 63.1 (18.6) 14.9–98.4 |
| Partial responder [N (%)] | 3 (1.3) | 4 (4.7) | 6 (12.0) | 1 (4.3) | 0 | 0 | 14 (3.4) |
| Follow-up (months) [mean (SD)], (range) | 27.8 (22.9) (12–98) | 37.2 (29.3) (12–87) | 35.6 (27.9) (12–74) | 38.4 (34.9) (12–78) | 23.6 (17.9) (12–32) | 38.1 (37.1) (12–62) | 31.8 (28.8) (12–87) |
| OMDRS Subscale (Range) | Baseline | Endpoint | p-Value |
|---|---|---|---|
| Examiner-rated scale [mean (SD)] | |||
| Severity (0–16) | 7.5 (2.9) | 3.3 (2.4) | p < 0.001 |
| Disability (0–30) | 9.1 (5.6) | 3.6 (3.9) | p < 0.001 |
| Pain (0–40) | 9.3 (10.6) | 3.2 (4.8) | p < 0.001 |
| Total examiner-rated scores (0–86) | 25.9 (14.1) | 10.0 (9.1) | p < 0.001 |
| Patient-rated questionnaire [mean (SD)] | |||
| General (0–20) | 14.2 (4.4) | 5.7 (3.7) | p < 0.001 |
| Eating (0–28) | 10.3 (7.7) | 4.4 (4.6) | p < 0.001 |
| Speech (0–16) | 8.0 (5.0) | 3.3 (3.1) | p < 0.001 |
| Cosmetic (0–28) | 12.8 (8.0) | 4.6 (4.6) | p < 0.001 |
| Social/family life (0–20) | 8.8 (5.7) | 4.3 (4.1) | p < 0.001 |
| Sleep (0–16) | 5.1 (5.3) | 2.4 (3.2) | p < 0.001 |
| Annoyance (0–32) | 16.2 (7.8) | 7.8 (6.6) | p < 0.001 |
| Mood (0–28) | 16.2 (7.8) | 8.3 (6.4) | p < 0.001 |
| Psychosocial functioning (0–40) | 15.0 (10.5) | 8.0 (7.5) | p < 0.001 |
| Total patient-rated scores (0–228) | 123.9 (53.2) | 48.2 (34.8) | p < 0.001 |
| OMDRS (0–314) | 148.9 (59.6) | 57.6 (40.6) | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K. Effects of Botulinum Toxin Therapy on Health-Related Quality of Life Evaluated by the Oromandibular Dystonia Rating Scale. Toxins 2022, 14, 656. https://doi.org/10.3390/toxins14100656
Yoshida K. Effects of Botulinum Toxin Therapy on Health-Related Quality of Life Evaluated by the Oromandibular Dystonia Rating Scale. Toxins. 2022; 14(10):656. https://doi.org/10.3390/toxins14100656
Chicago/Turabian StyleYoshida, Kazuya. 2022. "Effects of Botulinum Toxin Therapy on Health-Related Quality of Life Evaluated by the Oromandibular Dystonia Rating Scale" Toxins 14, no. 10: 656. https://doi.org/10.3390/toxins14100656