The Management of Brain Metastases—Systematic Review of Neurosurgical Aspects
Abstract
:Simple Summary
Abstract
1. Epidemiology of Brain Metastases
2. The Incidence of Brain Metastases
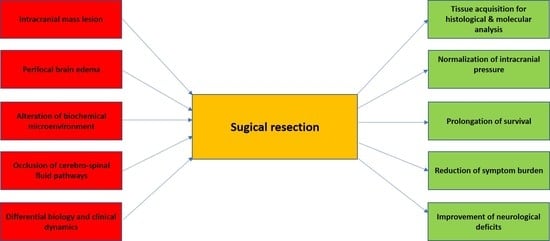
3. The Role of Surgical Resection in the Management of BM Patients
- (1)
- The primary cause of CNS failure-related death in BM patients is the intracranial mass lesion that results in elevated intracranial pressure and increasing brain stem compression [45]. The resection of the intraaxial lesion, accompanied by the reduction of perifocal edema based on removing the leaky peritumoral vasculature, results in an improvement of intracranial compliance, reduced intracranial pressure, and improved overall survival. This aspect is highlighted in the two landmark studies prospectively demonstrating the prolonged median overall survival in BM patients receiving microsurgical resection plus whole-brain radiation therapy (WBRT) versus WBRT only [46,47]. In more recent, mostly retrospective studies, a significant effect of surgical resection on the functional condition and overall survival was demonstrated [48,49,50].
- (2)
- The brain as a host organ is highly susceptible to functional impairment due to local pressure and changed local biochemical environment in the context of metastatic tumor growth [51]. Consequently, the surgical evacuation of a metastatic tumor, mainly if located in an eloquent area of the brain, will frequently lead to reduced symptom burden and the improvement of focal neurological deficits [52,53,54,55]. In a recent publication reporting functional improvement rates in BM patients, it was demonstrated that more than 20% of all BM patients suffer from hemiparesis, 11.3% display speech disturbances, and 23.2% show signs of cerebellar dysfunction. That portfolio of focal neurological deficits has led to a reduced functional independency in most of the affected patients, which was significantly improved after surgical resection [55]. Concordantly, a recent report highlighted the importance of neurological deficits on the overall prognosis in patients with BM [3]. Consequently, the impact of surgical resection on neurological function not only enhances the potential quality of life in these patients, but it also leads to an improved postsurgical Karnofsky Score (KPI) and the recursive partitioning (RPA) score, which is an important parameter to tailor adjuvant treatment structure [56]. This effect is even more pronounced in elderly patients with symptomatic BM undergoing surgical resection [57]. An improvement of KPI and RPA score in this prognostically poor patient subgroup was associated with a much higher likeliness to receive adjuvant local and systemic treatment, including molecular targeted therapy, resulting in more prolonged overall survival [57].
- (3)
- As shown in Patchell`s landmark paper, even in patients that were diagnosed with metastatic cancer, an intraaxial lesion is not a metastatic tumor in 11% of the affected patients [47]. One might hypothesize that the application of modern imaging technologies might have improved the diagnostic sensitivity and specificity of the current diagnostic platforms [58,59,60]. However, even high-end imaging approaches, such as amide proton transfer-weighted imaging, molecular MRI [61,62,63], or positron emission tomography [64], do not allow for the definitive diagnosis of an intraaxial lesion, in a patient with metastatic cancer. That indicates the pivotal need for histological confirmation of suspicious lesions, which is well reflected by the clinical experience of treating neurooncologists [65].
- (4)
- Finally, increasing evidence has indicated significant differences in the biology of primary cancers and the corresponding BMs, possibly resulting in additional therapeutic options [66]. In a practice-changing study, Brastianos et al. have demonstrated that more than 50% of all analyzed BMs show treatable molecular alterations that were not detectable in the primary tumor [67]. The potential reason for this observation might be the brain’s specific microenvironment, which induces profound changes in the biology of those cancer cells, which managed to home in the CNS. The extracellular matrix of the brain and the specific metabolic conditions of the CNS may prompt the cancer cells to acquire a more brain-specific phenotype [68,69]. For example, a recent study has demonstrated a significant induction of HER-2 protein expression in the BM tissue of metastatic breast cancer patients as compared to the primary tumor, potentially leading to a successful treatment strategy with anti HER-2 substances [70]. Consequently, a microsurgical resection may serve the purpose of tissue acquisition for molecular analysis, leading to so far undetected targets for systemic treatment, improving the prognosis of the affected patient population.
4. Surgical Morbidity and Mortality in the Resection of Brain Metastases
5. Resection of Multiple Brain Metastases
6. The Role of Surgery for Recurrent Brain Metastases
7. Evolution of the Surgical Techniques
8. Local Therapeutic Approaches Alternative to Surgery
8.1. Laser Interstitial Thermal Therapy (LITT)
8.2. Stereotactic Radiosurgery (SRS)
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 11 January 2021).
- Rouse, C.; Gittleman, H.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S. Years of potential life lost for brain and CNS tumors relative to other cancesrs in adults in the United States, 2010. Neuro Oncol. 2016, 18, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steindl, A.; Yadavalli, S.; Gruber, K.A.; Seiwald, M.; Gatterbauer, B.; Dieckmann, K.; Frischer, J.M.; Klikovits, T.; Zöchbauer-Müller, S.; Grisold, A.; et al. Neurological symptom burden impacts survival prognosis in patients with newly diagnosed non-small cell lung cancer brain metastases. Cancer 2020, 126, 4341–43181. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M. Brain Tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Cha, S. Imaging diagnosis of brain metastasis. Prog. Neurol. Surg. 2012, 25, 55–73. [Google Scholar] [CrossRef]
- Cha, S. Neuroimaging in neuro-oncology. Neurotherapeutics 2009, 6, 465–477. [Google Scholar] [CrossRef]
- Moravan, M.J.; Fecci, P.E.; Anders, C.K.; Clarke, J.M.; Salama, A.K.S.; Adamson, J.D.; Floyd, S.R.; Torok, J.A.; Salama, J.K.; Sampson, J.H.; et al. Current multidisciplinary management of brain metastases. Cancer 2020, 126, 1390–1406. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [Green Version]
- Long, D.M. Capillary ultrastructure in human metastatic brain tumors. J. Neurosurg. 1979, 51, 53–58. [Google Scholar] [CrossRef]
- Pachter, J.S.; de Vries, H.E.; Fabry, Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 2003, 62, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Salamero-Boix, A.; Niesel, K.; Alekseeva, T.; Sevenich, L. Microenvironmental Regulation of Tumor Progression and Therapeutic Response in Brain Metastasis. Front. Immunol. 2019, 10, 1713. [Google Scholar] [CrossRef]
- Beasley, K.D.; Toms, S.A. The molecular pathobiology of metastasis to the brain: A review. Neurosurg. Clin. N. Am. 2011, 22, 7–14. [Google Scholar] [CrossRef]
- Fidler, I.J. The role of the organ microenvironment in brain metastasis. Semin. Cancer Biol. 2011, 21, 107–112. [Google Scholar] [CrossRef]
- Nolan, C.; Deangelis, L.M. Overview of metastatic disease of the central nervous system. Handb. Clin. Neurol. 2018, 149, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.B.; Chernik, N.L. Intracranial metastases from systemic cancer. Adv. Neurol. 1978, 19, 579–592. [Google Scholar] [PubMed]
- Tsukada, Y.; Fouad, A.; Pickren, J.W.; Lane, W.W. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 1983, 52, 2349–2354. [Google Scholar] [CrossRef]
- Erickson, A.W.; Das, S. The Impact of Targeted Therapy on Intracranial Metastatic Disease Incidence and Survival. Front. Oncol. 2019, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Nahed, B.V.; Alvarez-Breckenridge, C.; Brastianos, P.K.; Shih, H.; Sloan, A.; Ammirati, M.; Kuo, J.S.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Surgery in the Management of Adults With Metastatic Brain Tumors. Neurosurgery 2019, 84, E152–E155. [Google Scholar] [CrossRef] [Green Version]
- Ascha, M.S.; Ostrom, Q.T.; Wright, J.; Kumthekar, P.; Bordeaux, J.S.; Sloan, A.E.; Schumacher, F.R.; Kruchko, C.; Barnholtz-Sloan, J.S. Lifetime Occurrence of Brain Metastases Arising from Lung, Breast, and Skin Cancers in the Elderly: A SEER-Medicare Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 917–925. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Alexandru, D.; Bota, D.A.; Linskey, M.E. Epidemiology of central nervous system metastases. Prog. Neurol. Surg. 2012, 25, 13–29. [Google Scholar] [CrossRef]
- Wang, B.X.; Ou, W.; Mao, X.Y.; Liu, Z.; Wu, H.Q.; Wang, S.Y. Impacts of EGFR mutation and EGFR-TKIs on incidence of brain metastases in advanced non-squamous NSCLC. Clin. Neurol. Neurosurg. 2017, 160, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, P.H.; Peterson, S.L.; Vigneau, F.D.; Shore, R.D.; Quarshie, W.O.; Islam, K.; Schwartz, A.G.; Wozniak, A.J.; Gadgeel, S.M. Risk of brain metastases in patients with nonmetastatic lung cancer: Analysis of the Metropolitan Detroit Surveillance, Epidemiology, and End Results (SEER) data. Cancer 2016, 122, 1921–1927. [Google Scholar] [CrossRef]
- Pelletier, E.M.; Shim, B.; Goodman, S.; Amonkar, M.M. Epidemiology and economic burden of brain metastases among patients with primary breast cancer: Results from a US claims data analysis. Breast Cancer Res. Treat. 2008, 108, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, J.S.; Kim, I.A. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J. Cancer Res. Clin. Oncol. 2018, 144, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Samlowski, W.E.; Moon, J.; Witter, M.; Atkins, M.B.; Kirkwood, J.M.; Othus, M.; Ribas, A.; Sondak, V.K.; Flaherty, L.E. High frequency of brain metastases after adjuvant therapy for high-risk melanoma. Cancer Med. 2017, 6, 2576–2585. [Google Scholar] [CrossRef]
- Sampson, J.H.; Carter, J.H., Jr.; Friedman, A.H.; Seigler, H.F. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998, 88, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Loh, D.; Hogg, F.; Edwards, P.; MacColl, J.; Brogna, C.; Bhangoo, R.; Ashkan, K.; Vergani, F. Two-year experience of multi-disciplinary team (MDT) outcomes for brain metastases in a tertiary neuro-oncology centre. Br. J. Neurosurg. 2018, 32, 53–60. [Google Scholar] [CrossRef]
- Bartelt, S.; Lutterbach, J. Brain metastases in patients with cancer of unknown primary. J. Neurooncol. 2003, 64, 249–253. [Google Scholar] [CrossRef]
- Graf, A.H.; Buchberger, W.; Langmayr, H.; Schmid, K.W. Site preference of metastatic tumours of the brain. Virchows Arch. A Pathol. Anat. Histopathol. 1988, 412, 493–498. [Google Scholar] [CrossRef]
- Cacho-Díaz, B.; Lorenzana-Mendoza, N.A.; Chávez-Hernandez, J.D.; González-Aguilar, A.; Reyes-Soto, G.; Herrera-Gómez, Á. Clinical manifestations and location of brain metastases as prognostic markers. Curr. Probl. Cancer 2019, 43, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Mongan, J.P.; Fadul, C.E.; Cole, B.F.; Zaki, B.I.; Suriawinata, A.A.; Ripple, G.H.; Tosteson, T.D.; Pipas, J.M. Brain metastases from colorectal cancer: Risk factors, incidence, and the possible role of chemokines. Clin. Colorectal Cancer 2009, 8, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyeong, S.; Cha, Y.J.; Ahn, S.G.; Suh, S.H.; Son, E.J.; Ahn, S.J. Subtypes of breast cancer show different spatial distributions of brain metastases. PLoS ONE 2017, 12, e0188542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, E.F.; Slater, J. Metastatic brain tumors. Results of surgical and nonsurgical treatment. Surg. Clin. N. Am. 1964, 44, 865–872. [Google Scholar] [CrossRef]
- Zimm, S.; Wampler, G.L.; Stablein, D.; Hazra, T.; Young, H.F. Intracerebral metastases in solid-tumor patients: Natural history and results of treatment. Cancer 1981, 48, 384–394. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Mehta, M.P.; Patchell, R.A.; Burri, S.H.; Robinson, P.D.; Morris, R.E.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Cobbs, C.S.; et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2009, 96, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Vecht, C.J.; Hovestadt, A.; Verbiest, H.B.; van Vliet, J.J.; van Putten, W.L. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: A randomized study of doses of 4, 8, and 16 mg per day. Neurology 1994, 44, 675–680. [Google Scholar] [CrossRef]
- Mikkelsen, T.; Paleologos, N.A.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; Kondziolka, D.; et al. The role of prophylactic anticonvulsants in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010, 96, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Antuna, A.R.; Vega, M.A.; Sanchez, C.R.; Fernandez, V.M. Brain Metastases of Non-Small Cell Lung Cancer: Prognostic Factors in Patients with Surgical Resection. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2018, 79, 101–107. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Jiang, W.; Brown, P.D.; Braunstein, S.; Sneed, P.; Wattson, D.A.; Shih, H.A.; Bangdiwala, A.; Shanley, R.; Lockney, N.A.; et al. The Prognostic Value of BRAF, C-KIT, and NRAS Mutations in Melanoma Patients with Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1069–1077. [Google Scholar] [CrossRef]
- Le Scodan, R.; Jouanneau, L.; Massard, C.; Gutierrez, M.; Kirova, Y.; Cherel, P.; Gachet, J.; Labib, A.; Mouret-Fourme, E. Brain metastases from breast cancer: Prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer 2011, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.D.; Wagner, K.M.; Prabhu, S.S.; McAleer, M.F.; McCutcheon, I.E.; Sawaya, R. Neurosurgical management of brain metastases. Clin. Exp. Metastasis 2017, 34, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann. Neurol. 1993, 33, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Bindal, A.K.; Bindal, R.K.; Hess, K.R.; Shiu, A.; Hassenbusch, S.J.; Shi, W.M.; Sawaya, R. Surgery versus radiosurgery in the treatment of brain metastasis. J. Neurosurg. 1996, 84, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Cagney, D.N.; Brigell, R.H.; Martin, A.M.; Besse, L.A.; Catalano, P.J.; Phillips, J.G.; Pashtan, I.M.; Bi, W.L.; Claus, E.B.; et al. Neurosurgical Resection and Stereotactic Radiation Versus Stereotactic Radiation Alone in Patients with a Single or Solitary Brain Metastasis. World Neurosurg. 2019, 122, e1557–e1561. [Google Scholar] [CrossRef]
- Paek, S.H.; Audu, P.B.; Sperling, M.R.; Cho, J.; Andrews, D.W. Reevaluation of surgery for the treatment of brain metastases: Review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery 2005, 56, 1021–1034. [Google Scholar]
- Lin, N.U.; Wefel, J.S.; Lee, E.Q.; Schiff, D.; van den Bent, M.J.; Soffietti, R.; Suh, J.H.; Vogelbaum, M.A.; Mehta, M.P.; Dancey, J.; et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: Neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013, 14, e407–e416. [Google Scholar] [CrossRef]
- Pintea, B.; Baumert, B.; Kinfe, T.M.; Gousias, K.; Parpaley, Y.; Bostrom, J.P. Early motor function after local treatment of brain metastases in the motor cortex region with stereotactic radiotherapy/radiosurgery or microsurgical resection: A retrospective study of two consecutive cohorts. Radiat. Oncol. 2017, 12, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetto, M.; Ciccarino, P.; Lombardi, G.; Rolma, G.; Cecchin, D.; Della Puppa, A. Surgery on motor area metastasis. Neurosurg. Rev. 2016, 39, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Schodel, P.; Schebesch, K.M.; Brawanski, A.; Proescholdt, M.A. Surgical resection of brain metastases-impact on neurological outcome. Int. J. Mol. Sci. 2013, 14, 8708–8718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schödel, P.; Jünger, S.T.; Wittersheim, M.; Reinhardt, H.C.; Schmidt, N.O.; Goldbrunner, R.; Proescholdt, M.; Grau, S. Surgical resection of symptomatic brain metastases improves the clinical status and facilitates further treatment. Cancer Med. 2020, 9, 7503–7510. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Nestle, U.; Motaref, B.; Walter, K.; Niewald, M.; Schnabel, K. Prognostic factors in brain metastases: Should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 297–302. [Google Scholar] [CrossRef]
- Proescholdt, M.; Jünger, S.T.; Schödel, P.; Schebesch, K.M.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Kocher, M.; Schulz, H.; et al. Brain Metastases in Elderly Patients-The Role of Surgery in the Context of Systemic Treatment. Brain Sci. 2021, 11, 123. [Google Scholar] [CrossRef]
- Bendini, M.; Marton, E.; Feletti, A.; Rossi, S.; Curtolo, S.; Inches, I.; Ronzon, M.; Longatti, P.; Di Paola, F. Primary and metastatic intraaxial brain tumors: Prospective comparison of multivoxel 2D chemical-shift imaging (CSI) proton MR spectroscopy, perfusion MRI, and histopathological findings in a group of 159 patients. Acta Neurochir. 2011, 153, 403–412. [Google Scholar] [CrossRef]
- Sternberg, E.J.; Lipton, M.L.; Burns, J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. AJNR Am. J. Neuroradiol. 2014, 35, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Law, M.; Cha, S.; Knopp, E.A.; Johnson, G.; Arnett, J.; Litt, A.W. High-grade gliomas and solitary metastases: Differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002, 222, 715–721. [Google Scholar] [CrossRef]
- Serres, S.; Soto, M.S.; Hamilton, A.; McAteer, M.A.; Carbonell, W.S.; Robson, M.D.; Ansorge, O.; Khrapitchev, A.; Bristow, C.; Balathasan, L.; et al. Molecular MRI enables early and sensitive detection of brain metastases. Proc. Natl. Acad. Sci. USA 2012, 109, 6674–6679. [Google Scholar] [CrossRef] [Green Version]
- Mehrabian, H.; Desmond, K.L.; Soliman, H.; Sahgal, A.; Stanisz, G.J. Differentiation between Radiation Necrosis and Tumor Progression Using Chemical Exchange Saturation Transfer. Clin. Cancer Res. 2017, 23, 3667–3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamimura, K.; Nakajo, M.; Yoneyama, T.; Fukukura, Y.; Hirano, H.; Goto, Y.; Sasaki, M.; Akamine, Y.; Keupp, J.; Yoshiura, T. Histogram analysis of amide proton transfer-weighted imaging: Comparison of glioblastoma and solitary brain metastasis in enhancing tumors and peritumoral regions. Eur. Radiol. 2019, 29, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Langen, K.J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 2019, 21, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, K.A.; Nair, M.N. Surgery for brain metastases. Surg. Neurol. Int. 2013, 4, S203–S208. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.D.; Zheng, S.; Xiu, J.; Zhou, S.; Khasraw, M.; Brastianos, P.K.; Kesari, S.; Hu, J.; Rudnick, J.; Salacz, M.E.; et al. Profiles of brain metastases: Prioritization of therapeutic targets. Int. J. Cancer 2018, 143, 3019–3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef] [Green Version]
- Neman, J.; Termini, J.; Wilczynski, S.; Vaidehi, N.; Choy, C.; Kowolik, C.M.; Li, H.; Hambrecht, A.C.; Roberts, E.; Jandial, R. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl. Acad. Sci. USA 2014, 111, 984–989. [Google Scholar] [CrossRef] [Green Version]
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef]
- Duchnowska, R.; Sperinde, J.; Chenna, A.; Huang, W.; Weidler, J.M.; Winslow, J.; Haddad, M.; Paquet, A.; Lie, Y.; Trojanowski, T.; et al. Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases. Neuro Oncol. 2015, 17, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Pessina, F.; Navarria, P.; Cozzi, L.; Ascolese, A.M.; Maggi, G.; Rossi, M.; Riva, M.; Scorsetti, M.; Bello, L. Role of Surgical Resection in Patients with Single Large Brain Metastases: Feasibility, Morbidity, and Local Control Evaluation. World Neurosurg. 2016, 94, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Haar, F.; Patterson, R.H., Jr. Surgery for metastatic intracranial neoplasm. Cancer 1972, 30, 1241–1245. [Google Scholar] [CrossRef]
- Sundaresan, N.; Galicich, J.H. Surgical treatment of brain metastases. Clinical and computerized tomography evaluation of the results of treatment. Cancer 1985, 55, 1382–1388. [Google Scholar] [CrossRef]
- Bindal, R.K.; Sawaya, R.; Leavens, M.E.; Lee, J.J. Surgical treatment of multiple brain metastases. J. Neurosurg. 1993, 79, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Wronski, M.; Arbit, E.; Burt, M.; Galicich, J.H. Survival after surgical treatment of brain metastases from lung cancer: A follow-up study of 231 patients treated between 1976 and 1991. J. Neurosurg. 1995, 83, 605–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Kim, D.G.; Kim, J.W.; Han, J.H.; Kim, Y.H.; Park, C.K.; Kim, C.Y.; Paek, S.H.; Jung, H.W. The role of surgical resection in the management of brain metastasis: A 17-year longitudinal study. Acta Neurochir. 2013, 155, 389–397. [Google Scholar] [CrossRef]
- Johans, S.J.; Garst, J.R.; Burkett, D.J.; Grahnke, K.; Martin, B.; Ibrahim, T.F.; Anderson, D.E.; Prabhu, V.C. Identification of Preoperative and Intraoperative Risk Factors for Complications in the Elderly Undergoing Elective Craniotomy. World Neurosurg. 2017, 107, 216–225. [Google Scholar] [CrossRef]
- Noordijk, E.M.; Vecht, C.J.; Haaxma-Reiche, H.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 711–717. [Google Scholar] [CrossRef]
- Chen, R.C.; Royce, T.J.; Extermann, M.; Reeve, B.B. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Semin. Radiat. Oncol. 2012, 22, 265–271. [Google Scholar] [CrossRef]
- Brusselaers, N.; Lagergren, J. The Charlson Comorbidity Index in Registry-based Research. Methods Inf. Med. 2017, 56, 401–406. [Google Scholar] [CrossRef]
- Stark, A.M.; Stohring, C.; Hedderich, J.; Held-Feindt, J.; Mehdorn, H.M. Surgical treatment for brain metastases: Prognostic factors and survival in 309 patients with regard to patient age. J. Clin. Neurosci. 2011, 18, 34–38. [Google Scholar] [CrossRef]
- Patel, C.K.; Vemaraju, R.; Glasbey, J.; Shires, J.; Northmore, T.; Zaben, M.; Hayhurst, C. Trends in peri-operative performance status following resection of high grade glioma and brain metastases: The impact on survival. Clin. Neurol. Neurosurg. 2018, 164, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Kim, P.; Truwit, C.L. Functional magnetic resonance imaging-guided brain tumor resection. Top. Magn. Reson. Imaging 2009, 19, 205–212. [Google Scholar] [CrossRef]
- Dubey, A.; Kataria, R.; Sinha, V.D. Role of Diffusion Tensor Imaging in Brain Tumor Surgery. Asian J. Neurosurg. 2018, 13, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Picht, T.; Mularski, S.; Kuehn, B.; Vajkoczy, P.; Kombos, T.; Suess, O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery 2009, 65, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.H.; See, A.A.Q.; Ang, B.T.; King, N.K.K. Awake Craniotomy for Resection of Brain Metastases: A Systematic Review. World Neurosurg. 2018, 120, e1128–e1135. [Google Scholar] [CrossRef]
- Patel, A.J.; Suki, D.; Hatiboglu, M.A.; Rao, V.Y.; Fox, B.D.; Sawaya, R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J. Neurosurg. 2015, 122, 1132–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogne, S.G.; Ronning, P.; Helseth, E.; Johannesen, T.B.; Langberg, C.W.; Lote, K.; Scheie, D.; Meling, T.R. Craniotomy for brain metastases: A consecutive series of 316 patients. Acta Neurol. Scand. 2012, 126, 23–31. [Google Scholar] [CrossRef]
- Pan, P.C.; Donovan, L.E.; Magge, R.S. Supportive Medical Management of Brain Metastases Patients Including Treatment Complications; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 31–51. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Akdur, K.; Sawaya, R. Neurosurgical management of patients with brain metastasis. Neurosurg. Rev. 2020, 43, 483–495. [Google Scholar] [CrossRef]
- Gavrilovic, I.T.; Posner, J.B. Brain metastases: Epidemiology and pathophysiology. J. Neurooncol. 2005, 75, 5–14. [Google Scholar] [CrossRef]
- Bochev, P.; Klisarova, A.; Kaprelyan, A.; Chaushev, B.; Dancheva, Z. Brain metastases detectability of routine whole body (18)F-FDG PET and low dose CT scanning in 2502 asymptomatic patients with solid extracranial tumors. Hell. J. Nucl. Med. 2012, 15, 125–129. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahgal, A.; Aoyama, H.; Kocher, M.; Neupane, B.; Collette, S.; Tago, M.; Shaw, P.; Beyene, J.; Chang, E.L. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: Individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Hirshman, B.R.; Wilson, B.; Carroll, K.T.; Proudfoot, J.A.; Goetsch, S.J.; Alksne, J.F.; Ott, K.; Aiyama, H.; Nagano, O.; et al. Survival Patterns of 5750 Stereotactic Radiosurgery-Treated Patients with Brain Metastasis as a Function of the Number of Lesions. World Neurosurg. 2017, 107, 944–951.e941. [Google Scholar] [CrossRef] [Green Version]
- Hazuka, M.B.; Burleson, W.D.; Stroud, D.N.; Leonard, C.E.; Lillehei, K.O.; Kinzie, J.J. Multiple brain metastases are associated with poor survival in patients treated with surgery and radiotherapy. J. Clin. Oncol. 1993, 11, 369–373. [Google Scholar] [CrossRef]
- Iwadate, Y.; Namba, H.; Yamaura, A. Significance of surgical resection for the treatment of multiple brain metastases. Anticancer Res. 2000, 20, 573–577. [Google Scholar] [PubMed]
- Salvati, M.; Tropeano, M.P.; Maiola, V.; Lavalle, L.; Brogna, C.; Colonnese, C.; Frati, A.; D’Elia, A. Multiple brain metastases: A surgical series and neurosurgical perspective. Neurol. Sci. 2018, 39, 671–677. [Google Scholar] [CrossRef]
- Hong, N.; Yoo, H.; Gwak, H.S.; Shin, S.H.; Lee, S.H. Outcome of surgical resection of symptomatic cerebral lesions in non-small cell lung cancer patients with multiple brain metastases. Brain Tumor Res. Treat. 2013, 1, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Schackert, G.; Steinmetz, A.; Meier, U.; Sobottka, S.B. Surgical management of single and multiple brain metastases: Results of a retrospective study. Onkologie 2001, 24, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, B.E.; Brown, P.D.; Foote, R.L.; Stafford, S.L.; Schomberg, P.J. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J. Neurooncol. 2003, 61, 73–80. [Google Scholar] [CrossRef]
- Wesseling, P.; von Deimling, A.; Aldape, K. Metastatic Tumours of the CNS, 4th ed.; IARC Press: Lyon, France, 2007. [Google Scholar]
- Berghoff, A.S.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.A.; Goodman, S.L.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro Oncol. 2013, 15, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Neves, S.; Mazal, P.R.; Wanschitz, J.; Rudnay, A.C.; Drlicek, M.; Czech, T.; Wustinger, C.; Budka, H. Pseudogliomatous growth pattern of anaplastic small cell carcinomas metastatic to the brain. Clin. Neuropathol. 2001, 20, 38–42. [Google Scholar]
- Varlotto, J.M.; Flickinger, J.C.; Niranjan, A.; Bhatnagar, A.K.; Kondziolka, D.; Lunsford, L.D. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 452–464. [Google Scholar] [CrossRef]
- Mitsuya, K.; Nakasu, Y.; Hayashi, N.; Deguchi, S.; Oishi, T.; Sugino, T.; Yasui, K.; Ogawa, H.; Onoe, T.; Asakura, H.; et al. Retrospective analysis of salvage surgery for local progression of brain metastasis previously treated with stereotactic irradiation: Diagnostic contribution, functional outcome, and prognostic factors. BMC Cancer 2020, 20, 331. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, M.; Cobbs, C.S.; Linskey, M.E.; Paleologos, N.A.; Ryken, T.C.; Burri, S.H.; Asher, A.L.; Loeffler, J.S.; Robinson, P.D.; Andrews, D.W.; et al. The role of retreatment in the management of recurrent/progressive brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010, 96, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Alexander, E., 3rd; Loeffler, J.S. Recurrent brain metastases. Neurosurg. Clin. N. Am. 1996, 7, 517–526. [Google Scholar] [CrossRef]
- Al-Zabin, M.; Ullrich, W.O.; Brawanski, A.; Proescholdt, M.A. Recurrent brain metastases from lung cancer: The impact of reoperation. Acta Neurochir. 2010, 152, 1887–1892. [Google Scholar] [CrossRef]
- Arbit, E.; Wronski, M.; Burt, M.; Galicich, J.H. The treatment of patients with recurrent brain metastases. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer 1995, 76, 765–773. [Google Scholar] [CrossRef]
- Bindal, R.K.; Sawaya, R.; Leavens, M.E.; Hess, K.R.; Taylor, S.H. Reoperation for recurrent metastatic brain tumors. J. Neurosurg. 1995, 83, 600–604. [Google Scholar] [CrossRef]
- Kamp, M.A.; Fischer, I.; Dibué-Adjei, M.; Munoz-Bendix, C.; Cornelius, J.F.; Steiger, H.J.; Slotty, P.J.; Turowski, B.; Rapp, M.; Sabel, M. Predictors for a further local in-brain progression after re-craniotomy of locally recurrent cerebral metastases. Neurosurg. Rev. 2018, 41, 813–823. [Google Scholar] [CrossRef]
- Kennion, O.; Holliman, D. Outcome after craniotomy for recurrent cranial metastases. Br. J. Neurosurg. 2017, 1–5. [Google Scholar] [CrossRef]
- Schackert, G.; Schmiedel, K.; Lindner, C.; Leimert, M.; Kirsch, M. Surgery of recurrent brain metastases: Retrospective analysis of 67 patients. Acta Neurochir. 2013, 155, 1823–1832. [Google Scholar] [CrossRef]
- Sundaresan, N.; Sachdev, V.P.; DiGiacinto, G.V.; Hughes, J.E. Reoperation for brain metastases. J. Clin. Oncol. 1988, 6, 1625–1629. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Zorro, O.; Lobato-Polo, J.; Flickinger, J.C.; Lunsford, L.D. The results of resection after stereotactic radiosurgery for brain metastases. J. Neurosurg. 2009, 111, 825–831. [Google Scholar] [CrossRef]
- Truong, M.T.; St Clair, E.G.; Donahue, B.R.; Rush, S.C.; Miller, D.C.; Formenti, S.C.; Knopp, E.A.; Han, K.; Golfinos, J.G. Results of surgical resection for progression of brain metastases previously treated by gamma knife radiosurgery. Neurosurgery 2006, 59, 86–97. [Google Scholar] [CrossRef]
- Vecil, G.G.; Suki, D.; Maldaun, M.V.; Lang, F.F.; Sawaya, R. Resection of brain metastases previously treated with stereotactic radiosurgery. J. Neurosurg. 2005, 102, 209–215. [Google Scholar] [CrossRef]
- Jagannathan, J.; Bourne, T.D.; Schlesinger, D.; Yen, C.P.; Shaffrey, M.E.; Laws, E.R., Jr.; Sheehan, J.P. Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery 2010, 66, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schebesch, K.M.; Rosengarth, K.; Brawanski, A.; Proescholdt, M.; Wendl, C.; Höhne, J.; Ott, C.; Lamecker, H.; Doenitz, C. Clinical Benefits of Combining Different Visualization Modalities in Neurosurgery. Front. Surg. 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Schaffner, M.; Shiban, E.; Droese, D.; Obermuller, T.; Gempt, J.; Meyer, B.; Ringel, F. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: Clinical article. J. Neurosurg. 2013, 118, 1269–1278. [Google Scholar] [CrossRef]
- Kamp, M.A.; Rapp, M.; Buhner, J.; Slotty, P.J.; Reichelt, D.; Sadat, H.; Dibue-Adjei, M.; Steiger, H.J.; Turowski, B.; Sabel, M. Early postoperative magnet resonance tomography after resection of cerebral metastases. Acta Neurochir. 2015, 157, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, R.J.; Ferraro, N.; Tsimpas, A. Yield and utility of routine postoperative imaging after resection of brain metastases. J. Neurooncol. 2014, 118, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Ulrich, C.T.; Seifert, V.; Gasser, T. Intraoperative magnetic resonance imaging in the surgical treatment of cerebral metastases. J. Surg. Oncol. 2010, 101, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Colditz, M.J.; Leyen, K.; Jeffree, R.L. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 2: Theoretical, biochemical and practical aspects. J. Clin. Neurosci. 2012, 19, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; Ciccarino, P.; Lombardi, G.; Rolma, G.; Cecchin, D.; Rossetto, M. 5-Aminolevulinic acid fluorescence in high grade glioma surgery: Surgical outcome, intraoperative findings, and fluorescence patterns. Biomed. Res. Int. 2014, 2014, 232561. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Fischer, I.; Buhner, J.; Turowski, B.; Cornelius, J.F.; Steiger, H.J.; Rapp, M.; Slotty, P.J.; Sabel, M. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget 2016, 7, 66776–66789. [Google Scholar] [CrossRef] [Green Version]
- Kamp, M.A.; Grosser, P.; Felsberg, J.; Slotty, P.J.; Steiger, H.J.; Reifenberger, G.; Sabel, M. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: A retrospective study. Acta Neurochir. 2012, 154, 223–228, discussion 228. [Google Scholar] [CrossRef]
- Hohne, J.; Hohenberger, C.; Proescholdt, M.; Riemenschneider, M.J.; Wendl, C.; Brawanski, A.; Schebesch, K.M. Fluorescein sodium-guided resection of cerebral metastases-an update. Acta Neurochir. 2017, 159, 363–367. [Google Scholar] [CrossRef]
- Okuda, T.; Kataoka, K.; Taneda, M. Metastatic brain tumor surgery using fluorescein sodium: Technical note. Minim. Invasive Neurosurg. 2007, 50, 382–384. [Google Scholar] [CrossRef]
- MacDonald, T.J.; Tabrizi, P.; Shimada, H.; Zlokovic, B.V.; Laug, W.E. Detection of brain tumor invasion and micrometastasis in vivo by expression of enhanced green fluorescent protein. Neurosurgery 1998, 43, 1437–1442, discussion 1442–1433. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Zhang, J.; Zhu, Z.Q.; Li, Y.P.; Zhong, W.Y.; Chen, J.B.; Pan, Z.Y.; Xia, H.C. Application of fluorescein sodium in breast cancer brain-metastasis surgery. Cancer Manag. Res. 2018, 10, 4325–4331. [Google Scholar] [CrossRef] [Green Version]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated with a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.J.; Suki, D.; Hatiboglu, M.A.; Abouassi, H.; Shi, W.; Wildrick, D.M.; Lang, F.F.; Sawaya, R. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J. Neurosurg. 2010, 113, 181–189. [Google Scholar] [CrossRef]
- Suki, D.; Hatiboglu, M.A.; Patel, A.J.; Weinberg, J.S.; Groves, M.D.; Mahajan, A.; Sawaya, R. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 2009, 64, 664–674. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Astner, S.T.; Grosu, A.L.; Andratschke, N.H.; Molls, M. The role of postoperative radiotherapy after resection of a single brain metastasis. Combined analysis of 643 patients. Strahlenther. Onkol. 2007, 183, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.G.; Rutten, I.; Dehing-Oberije, C.; Twijnstra, A.; Dirx, M.J.; Debougnoux-Huppertz, R.M.; Lambin, P.; Kubat, B. A pathology-based substrate for target definition in radiosurgery of brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Raore, B.; Schniederjan, M.; Prabhu, R.; Brat, D.J.; Shu, H.K.; Olson, J.J. Metastasis infiltration: An investigation of the postoperative brain-tumor interface. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1075–1080. [Google Scholar] [CrossRef]
- Siam, L.; Bleckmann, A.; Chaung, H.N.; Mohr, A.; Klemm, F.; Barrantes-Freer, A.; Blazquez, R.; Wolff, H.A.; Luke, F.; Rohde, V.; et al. The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget 2015, 6, 29254–29267. [Google Scholar] [CrossRef] [Green Version]
- Kamp, M.A.; Slotty, P.J.; Cornelius, J.F.; Steiger, H.J.; Rapp, M.; Sabel, M. The impact of cerebral metastases growth pattern on neurosurgical treatment. Neurosurg. Rev. 2018, 41, 77–86. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.Z.; Nam, B.H.; Shin, S.H.; Yang, H.S.; Lee, J.S.; Zo, J.I.; Lee, S.H. Reduced local recurrence of a single brain metastasis through microscopic total resection. J. Neurosurg. 2009, 110, 730–736. [Google Scholar] [CrossRef]
- Kamp, M.A.; Dibue, M.; Santacroce, A.; Zella, S.M.; Niemann, L.; Steiger, H.J.; Rapp, M.; Sabel, M. The tumour is not enough or is it? Problems and new concepts in the surgery of cerebral metastases. Ecancermedicalscience 2013, 7, 306. [Google Scholar]
- Korinth, M.C.; Delonge, C.; Hutter, B.O.; Gilsbach, J.M. Prognostic factors for patients with microsurgically resected brain metastases. Onkologie 2002, 25, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Dibue, M.; Niemann, L.; Reichelt, D.C.; Felsberg, J.; Steiger, H.J.; Szelenyi, A.; Rapp, M.; Sabel, M. Proof of principle: Supramarginal resection of cerebral metastases in eloquent brain areas. Acta Neurochir. 2012, 154, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Breckwoldt, M.O.; Bode, J.; Sahm, F.; Kruwel, T.; Solecki, G.; Hahn, A.; Wirthschaft, P.; Berghoff, A.S.; Haas, M.; Venkataramani, V.; et al. Correlated MRI and Ultramicroscopy (MR-UM) of Brain Tumors Reveals Vast Heterogeneity of Tumor Infiltration and Neoangiogenesis in Preclinical Models and Human Disease. Front. Neurosci. 2018, 12, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orringer, D.A.; Golby, A.; Jolesz, F. Neuronavigation in the surgical management of brain tumors: Current and future trends. Expert Rev. Med. Devices 2012, 9, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Eschbacher, J.; Hattendorf, G.; Coons, S.W.; Preul, M.C.; Smith, K.A.; Nakaji, P.; Spetzler, R.F. Intraoperative confocal microscopy for brain tumors: A feasibility analysis in humans. Neurosurgery 2011, 68, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman spectroscopy in cancer diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, M.; Schackert, G.; Salzer, R.; Krafft, C. Raman spectroscopic imaging for in vivo detection of cerebral brain metastases. Anal. Bioanal. Chem. 2010, 398, 1707–1713. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.S.; Kundishora, A.J.; Elsamadicy, A.A.; Chiang, V.L. Laser interstitial thermal therapy in neuro-oncology applications. Surg. Neurol. Int. 2020, 11, 231. [Google Scholar] [CrossRef]
- Holste, K.G.; Orringer, D.A. Laser interstitial thermal therapy. Neuro-Oncol. Adv. 2020, 2, vdz035. [Google Scholar] [CrossRef] [Green Version]
- Salehi, A.; Kamath, A.A.; Leuthardt, E.C.; Kim, A.H. Management of Intracranial Metastatic Disease with Laser Interstitial Thermal Therapy. Front. Oncol. 2018, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Rammo, R.; Asmaro, K.; Schultz, L.; Scarpace, L.; Siddiqui, S.; Walbert, T.; Kalkanis, S.; Lee, I. The safety of magnetic resonance imaging-guided laser interstitial thermal therapy for cerebral radiation necrosis. J. Neurooncol. 2018, 138, 609–617. [Google Scholar] [CrossRef]
- Medvid, R.; Ruiz, A.; Komotar, R.J.; Jagid, J.R.; Ivan, M.E.; Quencer, R.M.; Desai, M.B. Current Applications of MRI-Guided Laser Interstitial Thermal Therapy in the Treatment of Brain Neoplasms and Epilepsy: A Radiologic and Neurosurgical Overview. AJNR Am. J. Neuroradiol. 2015, 36, 1998–2006. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, K.; Sakai, T.; Fujishima, I.; Ryu, H.; Uemura, K.; Yokoyama, T. Stereotactic interstitial laser-hyperthermia using Nd-YAG laser. Stereotact. Funct. Neurosurg. 1990, 54–55, 501–505. [Google Scholar] [CrossRef]
- Menovsky, T.; Beek, J.F.; van Gemert, M.J.; Roux, F.X.; Bown, S.G. Interstitial laser thermotherapy in neurosurgery: A review. Acta Neurochir. 1996, 138, 1019–1026. [Google Scholar] [CrossRef]
- Missios, S.; Bekelis, K.; Barnett, G.H. Renaissance of laser interstitial thermal ablation. Neurosurg. Focus 2015, 38, E13. [Google Scholar] [CrossRef]
- Kaye, J.; Patel, N.V.; Danish, S.F. Laser interstitial thermal therapy for in-field recurrence of brain metastasis after stereotactic radiosurgery: Does treatment with LITT prevent a neurologic death? Clin. Exp. Metastasis 2020, 37, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, D.G.; Menaker, S.A.; Jermakowicz, W.J.; Shah, A.H.; Luther, E.M.; Jamshidi, A.M.; Semonche, A.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Multiple Iterations of Magnetic Resonance-Guided Laser Interstitial Thermal Ablation of Brain Metastases: Single Surgeon’s Experience and Review of the Literature. Oper. Neurosurg. 2020, 19, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rennert, R.C.; Khan, U.; Tatter, S.B.; Field, M.; Toyota, B.; Fecci, P.E.; Judy, K.; Mohammadi, A.M.; Landazuri, P.; Sloan, A.; et al. Patterns of Clinical Use of Stereotactic Laser Ablation: Analysis of a Multicenter Prospective Registry. World Neurosurg. 2018, 116, e566–e570. [Google Scholar] [CrossRef] [PubMed]
- Alattar, A.A.; Bartek, J., Jr.; Chiang, V.L.; Mohammadi, A.M.; Barnett, G.H.; Sloan, A.; Chen, C.C. Stereotactic Laser Ablation as Treatment of Brain Metastases Recurring after Stereotactic Radiosurgery: A Systematic Literature Review. World Neurosurg. 2019, 128, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Balasubramanian, S.; Silva, D.; Barnett, G.H.; Mohammadi, A.M. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: An overview. Expert Rev. Neurother. 2016, 16, 223–232. [Google Scholar] [CrossRef]
- Ahluwalia, M.; Barnett, G.H.; Deng, D.; Tatter, S.B.; Laxton, A.W.; Mohammadi, A.M.; Leuthardt, E.; Chamoun, R.; Judy, K.; Asher, A.; et al. Laser ablation after stereotactic radiosurgery: A multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J. Neurosurg. 2018, 130, 804–811. [Google Scholar] [CrossRef]
- Bastos, D.C.A.; Rao, G.; Oliva, I.C.G.; Loree, J.M.; Fuentes, D.T.; Stafford, R.J.; Beechar, V.B.; Weinberg, J.S.; Shah, K.; Kumar, V.A.; et al. Predictors of Local Control of Brain Metastasis Treated With Laser Interstitial Thermal Therapy. Neurosurgery 2020, 87, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Deng, D.; Vera, A.; Chiang, V.L. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J. Neurooncol. 2019, 142, 309–317. [Google Scholar] [CrossRef]
- Sharma, M.; Habboub, G.; Behbahani, M.; Silva, D.; Barnett, G.H.; Mohammadi, A.M. Thermal injury to corticospinal tracts and postoperative motor deficits after laser interstitial thermal therapy. Neurosurg. Focus 2016, 41, E6. [Google Scholar] [CrossRef] [Green Version]
- Leksell, L. The stereotaxic method and radiosurgery of the brain. Acta Chir. Scand. 1951, 102, 316–319. [Google Scholar]
- Nieder, C.; Grosu, A.L.; Gaspar, L.E. Stereotactic radiosurgery (SRS) for brain metastases: A systematic review. Radiat. Oncol. 2014, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, H.; Das, S.; Larson, D.A.; Sahgal, A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget 2016, 7, 12318–12330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougie, E.; Masson-Cote, L.; Mathieu, D. Comparison between surgical resection and stereotactic radiosurgery in patients with a single brain metastasis from non-small cell lung cancer. World Neurosurg. 2015. [Google Scholar] [CrossRef]
- Rades, D.; Bohlen, G.; Pluemer, A.; Veninga, T.; Hanssens, P.; Dunst, J.; Schild, S.E. Stereotactic radiosurgery alone versus resection plus whole-brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer 2007, 109, 2515–2521. [Google Scholar] [CrossRef]
- Churilla, T.M.; Chowdhury, I.H.; Handorf, E.; Collette, L.; Collette, S.; Dong, Y.; Alexander, B.M.; Kocher, M.; Soffietti, R.; Claus, E.B.; et al. Comparison of Local Control of Brain Metastases With Stereotactic Radiosurgery vs Surgical Resection: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2019, 5, 243–247. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.P.; Iturria, N.J.; Link, M.J.; Pollock, B.E.; Ballman, K.V.; O’Fallon, J.R. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1169–1176. [Google Scholar] [CrossRef]
- Rades, D.; Veninga, T.; Hornung, D.; Wittkugel, O.; Schild, S.E.; Gliemroth, J. Single brain metastasis: Whole-brain irradiation plus either radiosurgery or neurosurgical resection. Cancer 2012, 118, 1138–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöggl, A.; Kitz, K.; Reddy, M.; Wolfsberger, S.; Schneider, B.; Dieckmann, K.; Ungersböck, K. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir. 2000, 142, 621–626. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Press, R.H.; Patel, K.R.; Boselli, D.M.; Symanowski, J.T.; Lankford, S.P.; McCammon, R.J.; Moeller, B.J.; Heinzerling, J.H.; Fasola, C.E.; et al. Single-Fraction Stereotactic Radiosurgery (SRS) Alone Versus Surgical Resection and SRS for Large Brain Metastases: A Multi-institutional Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 459–467. [Google Scholar] [CrossRef]
- Muacevic, A.; Wowra, B.; Siefert, A.; Tonn, J.C.; Steiger, H.J.; Kreth, F.W. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: A randomized controlled multicentre phase III trial. J. Neurooncol. 2008, 87, 299–307. [Google Scholar] [CrossRef]
- O’Beirn, M.; Benghiat, H.; Meade, S.; Heyes, G.; Sawlani, V.; Kong, A.; Hartley, A.; Sanghera, P. The Expanding Role of Radiosurgery for Brain Metastases. Medicines 2018, 5, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graber, J.J.; Cobbs, C.S.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Use of Stereotactic Radiosurgery in the Treatment of Adults with Metastatic Brain Tumors. Neurosurgery 2019, 84, E168–E170. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proescholdt, M.A.; Schödel, P.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Schebesch, K.-M. The Management of Brain Metastases—Systematic Review of Neurosurgical Aspects. Cancers 2021, 13, 1616. https://doi.org/10.3390/cancers13071616
Proescholdt MA, Schödel P, Doenitz C, Pukrop T, Höhne J, Schmidt NO, Schebesch K-M. The Management of Brain Metastases—Systematic Review of Neurosurgical Aspects. Cancers. 2021; 13(7):1616. https://doi.org/10.3390/cancers13071616
Chicago/Turabian StyleProescholdt, Martin A., Petra Schödel, Christian Doenitz, Tobias Pukrop, Julius Höhne, Nils Ole Schmidt, and Karl-Michael Schebesch. 2021. "The Management of Brain Metastases—Systematic Review of Neurosurgical Aspects" Cancers 13, no. 7: 1616. https://doi.org/10.3390/cancers13071616