The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Rheumatoid Arthritis and Osteoarthritis
Abstract
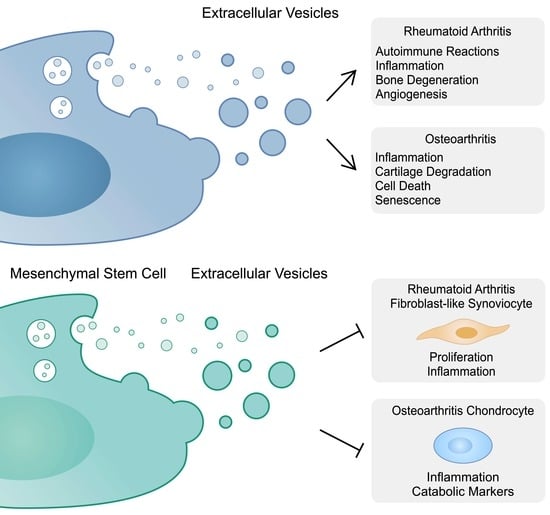
:1. Introduction
2. Extracellular Vesicles in the Pathogenesis of Rheumatoid Arthritis
2.1. Extracellular Vesicles and Rheumatoid Arthritis
2.2. Autoimmunity
2.3. Inflammation
2.4. Bone Degeneration
2.5. Angiogenesis
3. The Role of Extracellular Vesicles in Suppressing Rheumatoid Arthritis
3.1. Mesenchymal Stem Cells and Rheumatoid Arthritis
3.2. Mesenchymal Stem-Cell-Derived Extracellular Vesicles and Fibroblast-like Synoviocytes
3.3. Mesenchymal Stem-Cell-Derived Extracellular Vesicles and T Cells
3.4. Mesenchymal Stem-Cell-Derived Extracellular Vesicles and Macrophages
3.5. Extracellular Vesicles and Programmed Cell Death Pathways
4. The Role of Extracellular Vesicles in the Pathogenesis of Osteoarthritis
4.1. Osteoarthritis and Extracellular Vesicles
4.2. Cartilage Degeneration and Inflammation
4.3. Cartilage Calcification
4.4. Cell Death Pathways
4.5. Senescence
5. The Role of Extracellular Vesicles in Suppressing Osteoarthritis
5.1. Mesenchymal Stem Cells and Osteoarthritis
5.2. Mesenchymal Stem-Cell-Derived Extracellular Vesicles and Chondrocytes
5.3. Extracellular Vesicles Derived from Other Cells
5.4. Extracellular Vesicles Containing Mitochondria
5.5. Extracellular Vesicles and Osteoarthritis Pain
5.6. Boosting the Efficacy of Extracellular Vesicles
5.7. Apoptotic Bodies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, C.; Xiao, J.; Li, D.; Sun, Z.; Li, M. Endothelial extracellular vesicles modulate the macrophage phenotype: Potential implications in atherosclerosis. Scand. J. Immunol. 2018, 87, e12648. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A.; Tousif, S.; Wang, Y.; Hough, K.; Khan, S.; Strenkowski, J.; Chacko, B.K.; Darley-Usmar, V.M.; Deshane, J.S. Lung Tumor Cell-Derived Exosomes Promote M2 Macrophage Polarization. Cells 2020, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, Z.; Hou, Y.; Sun, W.; Zhang, R.; Zhao, L.; Wei, M.; Yang, G.; Yuan, L. Exosome-mediated delivery of inflammation-responsive. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Proulle, V.; Jüngel, A.; Ittah, M.; Miceli Richard, C.; Gottenberg, J.E.; Toti, F.; Benessiano, J.; Gay, S.; Freyssinet, J.M.; et al. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 2009, 11, R156. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liang, L.; Ji, Z.; Chen, S.; Liu, M.; Huang, Q.; Huang, Z.; Sun, S.; Ding, J.; Chen, J.; et al. Proteomics profiling of CD4 + T-cell-derived exosomes from patients with rheumatoid arthritis. Int. Immunopharmacol. 2023, 122, 110560. [Google Scholar] [CrossRef]
- Arntz, O.J.; Pieters, B.C.H.; Thurlings, R.M.; Wenink, M.H.; van Lent, P.L.E.M.; Koenders, M.I.; van den Hoogen, F.H.J.; van der Kraan, P.M.; van de Loo, F.A.J. Rheumatoid Arthritis Patients With Circulating Extracellular Vesicles Positive for IgM Rheumatoid Factor Have Higher Disease Activity. Front. Immunol. 2018, 9, 2388. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Turiák, L.; Wright, M.; Herczeg, P.; Lédeczi, Z.; Kittel, A.; Polgár, A.; Tóth, K.; Dérfalvi, B.; et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE 2012, 7, e49726. [Google Scholar] [CrossRef]
- Stojanovic, A.; Veselinovic, M.; Zong, Y.; Jakovljevic, V.; Pruner, I.; Antovic, A. Increased Expression of Extracellular Vesicles Is Associated With the Procoagulant State in Patients With Established Rheumatoid Arthritis. Front. Immunol. 2021, 12, 718845. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, Y.; Chu, Y.; Xie, J.; Ding, W.; Wang, F. Tissue factor expression in rheumatoid synovium: A potential role in pannus invasion of rheumatoid arthritis. Acta Histochem. 2013, 115, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Rydland, A.; Heinicke, F.; Flåm, S.T.; Mjaavatten, M.D.; Lie, B.A. Small extracellular vesicles have distinct CD81 and CD9 tetraspanin expression profiles in plasma from rheumatoid arthritis patients. Clin. Exp. Med. 2023, 23, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Arai, Y.; Mori, H.; Matsushita, Y.; Kubo, T.; Nakanishi, T. Small interfering RNA targeting CD81 ameliorated arthritis in rats. Biochem. Biophys. Res. Commun. 2009, 388, 467–472. [Google Scholar] [CrossRef]
- Fujii, Y.; Arai, Y.; Nakagawa, S.; Yamasaki, T.; Iijima, M.; Yamada, N.; Takahashi, K.; Nakanishi, M.; Nakanishi, T. CD81 inhibition with the cytoplasmic RNA vector producing anti-CD81 antibodies suppresses arthritis in a rat CIA model. Biochem. Biophys. Res. Commun. 2022, 604, 22–29. [Google Scholar] [CrossRef]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef]
- Karlsson, J.; Wetterö, J.; Potempa, L.A.; Fernandez-Botran, R.; O’Neill, Y.; Wirestam, L.; Mobarrez, F.; Sjöwall, C. Extracellular vesicles opsonized by monomeric C-reactive protein (CRP) are accessible as autoantigens in patients with systemic lupus erythematosus and associate with autoantibodies against CRP. J. Autoimmun. 2023, 139, 103073. [Google Scholar] [CrossRef]
- Hasilo, C.P.; Negi, S.; Allaeys, I.; Cloutier, N.; Rutman, A.K.; Gasparrini, M.; Bonneil, É.; Thibault, P.; Boilard, É.; Paraskevas, S. Presence of diabetes autoantigens in extracellular vesicles derived from human islets. Sci. Rep. 2017, 7, 5000. [Google Scholar] [CrossRef]
- Skriner, K.; Adolph, K.; Jungblut, P.R.; Burmester, G.R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006, 54, 3809–3814. [Google Scholar] [CrossRef] [PubMed]
- Ucci, F.M.; Recalchi, S.; Barbati, C.; Manganelli, V.; Capozzi, A.; Riitano, G.; Buoncuore, G.; Garofalo, T.; Ceccarelli, F.; Spinelli, F.R.; et al. Citrullinated and carbamylated proteins in extracellular microvesicles from plasma of patients with rheumatoid arthritis. Rheumatology 2023, 62, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Zhao, X.; Chandra, P.E.; Robinson, W.H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2011, 63, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; Albert, A.; Shnayder, R.; Gobezie, R.; Nigrovic, P.A.; et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2013, 5, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Villar-Vesga, J.; Grajales, C.; Burbano, C.; Vanegas-García, A.; Muñoz-Vahos, C.H.; Vásquez, G.; Rojas, M.; Castaño, D. Platelet-derived microparticles generated in vitro resemble circulating vesicles of patients with rheumatoid arthritis and activate monocytes. Cell Immunol. 2019, 336, 1–11. [Google Scholar] [CrossRef]
- Burbano, C.; Rojas, M.; Muñoz-Vahos, C.; Vanegas-García, A.; Correa, L.A.; Vásquez, G.; Castaño, D. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci. Rep. 2018, 8, 17917. [Google Scholar] [CrossRef]
- Atehortúa, L.; Rojas, M.; Vásquez, G.; Muñoz-Vahos, C.H.; Vanegas-García, A.; Posada-Duque, R.A.; Castaño, D. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 34. [Google Scholar] [CrossRef]
- Gao, W.; Liu, H.; Yuan, J.; Wu, C.; Huang, D.; Ma, Y.; Zhu, J.; Ma, L.; Guo, J.; Shi, H.; et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J. Cell. Mol. Med. 2016, 20, 2318–2327. [Google Scholar] [CrossRef]
- Barbati, C.; Vomero, M.; Colasanti, T.; Diociaiuti, M.; Ceccarelli, F.; Ferrigno, S.; Finucci, A.; Miranda, F.; Novelli, L.; Perricone, C.; et al. TNFα expressed on the surface of microparticles modulates endothelial cell fate in rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Zhang, H.G.; Liu, C.; Su, K.; Yu, S.; Zhang, L.; Zhang, S.; Wang, J.; Cao, X.; Grizzle, W.; Kimberly, R.P. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J. Immunol. 2006, 176, 7385–7393. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Aoki, W.; Satomura, A.; Shibasaki, S.; Ueda, M. Small RNAs detected in exosomes derived from the MH7A synovial fibroblast cell line with TNF-α stimulation. PLoS ONE 2018, 13, e0201851. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Gremese, E.; McSharry, C.; Tolusso, B.; Ferraccioli, G.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155-at the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2017, 8, 1932. [Google Scholar] [CrossRef]
- Oliveira, M.I.; Gonçalves, C.M.; Pinto, M.; Fabre, S.; Santos, A.M.; Lee, S.F.; Castro, M.A.; Nunes, R.J.; Barbosa, R.R.; Parnes, J.R.; et al. CD6 attenuates early and late signaling events, setting thresholds for T-cell activation. Eur. J. Immunol. 2012, 42, 195–205. [Google Scholar] [CrossRef]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef]
- Nakamachi, Y.; Uto, K.; Hayashi, S.; Okano, T.; Morinobu, A.; Kuroda, R.; Kawano, S.; Saegusa, J. Exosomes derived from synovial fibroblasts from patients with rheumatoid arthritis promote macrophage migration that can be suppressed by miR-124-3p. Heliyon 2023, 9, e14986. [Google Scholar] [CrossRef]
- Tekeoğlu, İ.; Harman, H.; Sağ, S.; Altındiş, M.; Kamanlı, A.; Nas, K. Levels of serum pentraxin 3, IL-6, fetuin A and insulin in patients with rheumatoid arthritis. Cytokine 2016, 83, 171–175. [Google Scholar] [CrossRef]
- Ekin, S.; Sivrikaya, A.; Akdağ, T.; Yilmaz, S.; Gülcemal, S. Elevated levels of neopterin and pentraxin 3 in patients with rheumatoid arthritis. Horm. Mol. Biol. Clin. Investig. 2021, 42, 419–423. [Google Scholar] [CrossRef]
- Asanuma, Y.F.; Aizaki, Y.; Noma, H.; Yokota, K.; Matsuda, M.; Kozu, N.; Takebayashi, Y.; Nakatani, H.; Hasunuma, T.; Kawai, S.; et al. Plasma pentraxin 3 is associated with progression of radiographic joint damage, but not carotid atherosclerosis, in female rheumatoid arthritis patients: 3-year prospective study. Mod. Rheumatol. 2020, 30, 959–966. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Wu, H.; Zhao, Q.; Wu, S. Exosomes derived from synovial fibroblasts under hypoxia aggravate rheumatoid arthritis by regulating Treg/Th17 balance. Exp. Biol. Med. 2020, 245, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Qian, F.Y.; Zhang, M.F.; Xu, A.L.; Wang, X.; Jiang, B.P.; Zhou, L.L. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J. Leukoc. Biol. 2019, 106, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kwon, J.E.; Lee, S.Y.; Lee, E.J.; Kim, D.S.; Moon, S.J.; Lee, J.; Kwok, S.K.; Park, S.H.; Cho, M.L. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017, 8, e2565. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Stavre, Z.; Gravallese, E.M. Bone Loss in Rheumatoid Arthritis: Basic Mechanisms and Clinical Implications. Calcif. Tissue Int. 2018, 102, 533–546. [Google Scholar] [CrossRef]
- Uenaka, M.; Yamashita, E.; Kikuta, J.; Morimoto, A.; Ao, T.; Mizuno, H.; Furuya, M.; Hasegawa, T.; Tsukazaki, H.; Sudo, T.; et al. Osteoblast-derived vesicles induce a switch from bone-formation to bone-resorption in vivo. Nat. Commun. 2022, 13, 1066. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Luo, X.; Peng, L.; Zhao, Z.; He, C.; He, Y. Exosomal miRNA-486-5p derived from rheumatoid arthritis fibroblast-like synoviocytes induces osteoblast differentiation through the Tob1/BMP/Smad pathway. Biomater. Sci. 2020, 8, 3430–3442. [Google Scholar] [CrossRef]
- Maeda, Y.; Farina, N.H.; Matzelle, M.M.; Fanning, P.J.; Lian, J.B.; Gravallese, E.M. Synovium-Derived MicroRNAs Regulate Bone Pathways in Rheumatoid Arthritis. J. Bone Miner. Res. 2017, 32, 461–472. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Liu, W.; Maye, P.; Zhang, J.; Zhang, Y.; Hurley, M.; Guo, C.; Boskey, A.; Sun, L.; et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005, 37, 945–952. [Google Scholar] [CrossRef]
- Zhou, B.; Peng, K.; Wang, G.; Chen, W.; Liu, P.; Chen, F.; Kang, Y. miR-483-3p promotes the osteogenesis of human osteoblasts by targeting Dikkopf 2 (DKK2) and the Wnt signaling pathway. Int. J. Mol. Med. 2020, 46, 1571–1581. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Bi, X.; Luo, X.; Hu, Z.; Liu, Y.; Shi, X.; Weng, W.; Mo, B.; Lu, Y.; et al. LncRNA NEAT1_1 suppresses tumor-like biologic behaviors of fibroblast-like synoviocytes by targeting the miR-221-3p/uPAR axis in rheumatoid arthritis. J. Leukoc. Biol. 2022, 111, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Damiani, A.; Russo, E.; Guiducci, S.; Li Gobbi, F.; Fusi, P.; Grossi, V.; Amedei, A.; Manfredi, M.; Infantino, M. The Association of uPA, uPAR, and suPAR System with Inflammation and Joint Damage in Rheumatoid Arthritis: suPAR as a Biomarker in the Light of a Personalized Medicine Perspective. J. Pers. Med. 2022, 12, 1984. [Google Scholar] [CrossRef] [PubMed]
- Quero, L.; Tiaden, A.N.; Hanser, E.; Roux, J.; Laski, A.; Hall, J.; Kyburz, D. miR-221-3p Drives the Shift of M2-Macrophages to a Pro-Inflammatory Function by Suppressing JAK3/STAT3 Activation. Front. Immunol. 2019, 10, 3087. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, A.B.; Breitwieser, K.; Ottinger, S.M.; Mobarrez, F.; Korotkova, M.; Rethi, B.; Jakobsson, P.J.; Catrina, A.I.; Wähämaa, H.; Saul, M.J. Extracellular miR-574-5p Induces Osteoclast Differentiation via TLR 7/8 in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 585282. [Google Scholar] [CrossRef] [PubMed]
- Donate, P.B.; Alves de Lima, K.; Peres, R.S.; Almeida, F.; Fukada, S.Y.; Silva, T.A.; Nascimento, D.C.; Cecilio, N.T.; Talbot, J.; Oliveira, R.D.; et al. Cigarette smoke induces miR-132 in Th17 cells that enhance osteoclastogenesis in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2017120118. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Majkowska, I.; Nagase, H.; Di Liegro, I.; Troeberg, L. Microvesicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol. 2012, 31, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef]
- Edhayan, G.; Ohara, R.A.; Stinson, W.A.; Amin, M.A.; Isozaki, T.; Ha, C.M.; Haines, G.K.; Morgan, R.; Campbell, P.L.; Arbab, A.S.; et al. Inflammatory properties of inhibitor of DNA binding 1 secreted by synovial fibroblasts in rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 87. [Google Scholar] [CrossRef]
- Ohara, R.A.; Edhayan, G.; Rasmussen, S.M.; Isozaki, T.; Remmer, H.A.; Lanigan, T.M.; Campbell, P.L.; Urquhart, A.G.; Lawton, J.N.; Chung, K.C.; et al. Citrullinated Inhibitor of DNA Binding 1 Is a Novel Autoantigen in Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 1241–1251. [Google Scholar] [CrossRef]
- Chen, Y.; Dang, J.; Lin, X.; Wang, M.; Liu, Y.; Chen, J.; Luo, X.; Hu, Z.; Weng, W.; Shi, X.; et al. RA Fibroblast-Like Synoviocytes Derived Extracellular Vesicles Promote Angiogenesis by miRNA-1972 Targeting p53/mTOR Signaling in Vascular Endotheliocyte. Front. Immunol. 2022, 13, 793855. [Google Scholar] [CrossRef]
- Maharajan, N.; Cho, G.W.; Jang, C.H. Therapeutic Application of Mesenchymal Stem Cells for Cochlear Regeneration. In Vivo 2021, 35, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Haikal, S.M.; Abdeltawab, N.F.; Rashed, L.A.; Abd El-Galil, T.I.; Elmalt, H.A.; Amin, M.A. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells 2019, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Jung, S.M.; Rim, Y.A.; Jung, H.; Lee, K.; Park, N.; Kim, J.; Jang, Y.; Park, Y.B.; Park, S.H.; et al. Intraperitoneal infusion of mesenchymal stem cell attenuates severity of collagen antibody induced arthritis. PLoS ONE 2018, 13, e0198740. [Google Scholar] [CrossRef]
- Vij, R.; Stebbings, K.A.; Kim, H.; Park, H.; Chang, D. Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: A phase I/IIa, open-label, non-randomized pilot trial. Stem Cell Res. Ther. 2022, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, S.; Li, S.; Li, M.; Shi, J.; Bai, W.; Wang, Q.; Zheng, L.; Liu, Y. Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A Prospective Phase I/II Study. Drug Des. Dev. Ther. 2019, 13, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Gomes, J.; Laranjeira, P.; Duarte, C.; Pedreiro, S.; Antunes, B.; Ribeiro, T.; Santos, F.; Martinho, A.; Fardilha, M.; et al. Immunomodulatory effect of human bone marrow-derived mesenchymal stromal/stem cells on peripheral blood T cells from rheumatoid arthritis patients. J. Tissue Eng. Regen. Med. 2020, 14, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Sun, F.; Ma, Y.; Li, F.; Li, C.; Yang, Z. Exosomes derived from berberine-treated bone marrow mesenchymal stem cells ameliorate inflammatory arthritis in rats with collagen-induced rheumatoid arthritis. Food Age Immunol. 2023, 34, 2220566. [Google Scholar] [CrossRef]
- You, S.; Koh, J.H.; Leng, L.; Kim, W.U.; Bucala, R. The Tumor-Like Phenotype of Rheumatoid Synovium: Molecular Profiling and Prospects for Precision Medicine. Arthritis Rheumatol. 2018, 70, 637–652. [Google Scholar] [CrossRef]
- Bruckner, S.; Capria, V.M.; Zeno, B.; Leblebicioglu, B.; Goyal, K.; Vasileff, W.K.; Awan, H.; Willis, W.L.; Ganesan, L.P.; Jarjour, W.N. The therapeutic effects of gingival mesenchymal stem cells and their exosomes in a chimeric model of rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 211. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.; Wang, X.; Cheng, W.; Hu, X.; Wang, Y.; Luo, B.; Huang, W.; Gu, J. miR-34a in extracellular vesicles from bone marrow mesenchymal stem cells reduces rheumatoid arthritis inflammation via the cyclin I/ATM/ATR/p53 axis. J. Cell. Mol. Med. 2021, 25, 1896–1910. [Google Scholar] [CrossRef]
- Meng, H.Y.; Chen, L.Q.; Chen, L.H. The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell. BMC Musculoskelet. Disord. 2020, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Nakamachi, Y. miR-124a as a key regulator of proliferation and MCP-1 secretion in synoviocytes from patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70 (Suppl. S1), i88–i91. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, Y.; Peng, A.; Wu, X. The diagnostic value of miR-124a expression in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Hum. Hered. 2023, 88, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tang, F.; Xiao, L.; Han, S.; Yao, X.; Zhang, Q.; Zhou, J.; Wang, Y. miR-205-5p in exosomes divided from chondrogenic mesenchymal stem cells alleviated rheumatoid arthritis via regulating MDM2 in fibroblast-like synoviocytes. J. Musculoskelet. Neuronal Interact. 2022, 22, 132–141. [Google Scholar] [PubMed]
- Lin, K.; Su, H.Y.; Jiang, L.F.; Chu, T.G.; Li, Z.; Chen, X.L.; Gao, W.Y. Influences of miR-320a on proliferation and apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis through targeting MAPK-ERK1/2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1907–1914. [Google Scholar] [CrossRef]
- Meng, Q.; Qiu, B. Exosomal MicroRNA-320a Derived From Mesenchymal Stem Cells Regulates Rheumatoid Arthritis Fibroblast-Like Synoviocyte Activation by Suppressing CXCL9 Expression. Front. Physiol. 2020, 11, 441. [Google Scholar] [CrossRef]
- Kuan, W.P.; Tam, L.S.; Wong, C.K.; Ko, F.W.; Li, T.; Zhu, T.; Li, E.K. CXCL 9 and CXCL 10 as Sensitive markers of disease activity in patients with rheumatoid arthritis. J. Rheumatol. 2010, 37, 257–264. [Google Scholar] [CrossRef]
- Shamsi, A.; Roghani, S.A.; Abdan, Z.; Soufivand, P.; Pournazari, M.; Bahrehmand, F.; Vafaei, A.; Salari, N.; Soroush, M.G.; Taghadosi, M. CXCL9 and its Receptor CXCR3, an Important Link Between Inflammation and Cardiovascular Risks in RA Patients. Inflammation 2023, 46, 2374–2385. [Google Scholar] [CrossRef]
- Qiu, M.; Mo, L.; Li, J.; Liang, H.; Zhu, W.; Zheng, X.; Duan, X.; Xu, W. Effects of miR-150-5p on the growth and SOCS1 expression of rheumatoid arthritis synovial fibroblasts. Clin. Rheumatol. 2020, 39, 909–917. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Xia, Y.; Yan, F.; Lu, Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J. Immunol. 2018, 201, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Zu, B.; Liu, L.; Wang, J.; Li, M.; Yang, J. MiR-140-3p inhibits the cell viability and promotes apoptosis of synovial fibroblasts in rheumatoid arthritis through targeting sirtuin 3. J. Orthop. Surg. Res. 2021, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, L.; Chen, D.; Fan, P.; Yu, H. Exosomal microRNA-140-3p from human umbilical cord mesenchymal stem cells attenuates joint injury of rats with rheumatoid arthritis by silencing SGK1. Mol. Med. 2022, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Q.; Yang, X.W.; Chen, Y.B.; Zhang, D.W.; Jiang, X.F.; Xue, P. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol. Cancer 2019, 18, 148. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, Y.Y.; Zhu, B.L.; Feng, F.Z.; Yan, H.; Zhang, H.Y.; Zhou, B. miR-21 regulates the proliferation and apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4149–4155. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Fang, Y.X.; Liu, Y.; Meng, F.R.; Wu, X.; Zhang, C.W.; Zhang, Y.; Liu, Y.Q.; Liu, D. MicroRNA-21 from bone marrow mesenchymal stem cell-derived extracellular vesicles targets TET1 to suppress KLF4 and alleviate rheumatoid arthritis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211007369. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, G.; Ferracin, M.; Saccenti, E.; Bagnari, V.; Farina, I.; Furini, F.; Galuppi, E.; Zagatti, B.; Trotta, F.; Negrini, M.; et al. Characterisation of peripheral blood mononuclear cell microRNA in early onset psoriatic arthritis. Clin. Exp. Rheumatol. 2017, 35, 113–121. [Google Scholar]
- Dong, L.; Wang, X.; Tan, J.; Li, H.; Qian, W.; Chen, J.; Chen, Q.; Wang, J.; Xu, W.; Tao, C.; et al. Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J. Cell. Mol. Med. 2014, 18, 2213–2224. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Pan, Z.; Zhang, Y. miR-143-3p regulates cell proliferation and apoptosis by targeting IGF1R and IGFBP5 and regulating the Ras/p38 MAPK signaling pathway in rheumatoid arthritis. Exp. Ther. Med. 2018, 15, 3781–3790. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Ma, C.; Guan, C.; Ma, X.; Meng, S. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-κB pathway. J. Orthop. Surg. Res. 2021, 16, 116. [Google Scholar] [CrossRef]
- Shen, M.Y.; Jiang, B.P.; Zhang, M.F.; Wang, X.; Zhu, H.; Gu, Z.N.; Zhou, X.P.; Lu, Y.; Zhou, L.L. MicroRNA-143-3p ameliorates collagen-induced arthritis by polarizing naive CD4. J. Clin. Lab. Anal. 2023, 37, e24845. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Amirsaadat, S.; Jalil, A.T.; Kadhim, M.M.; Abasi, M.; Pilehvar, Y. Curcumin-Loaded Mesenchymal Stem Cell-Derived Exosomes Efficiently Attenuate Proliferation and Inflammatory Response in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. Appl. Biochem. Biotechnol. 2023, 195, 51–67. [Google Scholar] [CrossRef]
- Pratt, A.G.; Siebert, S.; Cole, M.; Stocken, D.D.; Yap, C.; Kelly, S.; Shaikh, M.; Cranston, A.; Morton, M.; Walker, J.; et al. Targeting synovial fibroblast proliferation in rheumatoid arthritis (TRAFIC): An open-label, dose-finding, phase 1b trial. Lancet Rheumatol. 2021, 3, e337–e346. [Google Scholar] [CrossRef] [PubMed]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons From Rheumatoid Arthritis. Front. Immunol. 2019, 10, 353. [Google Scholar] [CrossRef]
- Lawson, C.A.; Brown, A.K.; Bejarano, V.; Douglas, S.H.; Burgoyne, C.H.; Greenstein, A.S.; Boylston, A.W.; Emery, P.; Ponchel, F.; Isaacs, J.D. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology 2006, 45, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Gorycka, A.; Wajda, A.; Romanowska-Próchnicka, K.; Walczuk, E.; Kuca-Warnawin, E.; Kmiolek, T.; Stypinska, B.; Rzeszotarska, E.; Majewski, D.; Jagodzinski, P.P.; et al. Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 572858. [Google Scholar] [CrossRef] [PubMed]
- Campe, J.; Ullrich, E. T Helper Cell Lineage-Defining Transcription Factors: Potent Targets for Specific GVHD Therapy? Front. Immunol. 2021, 12, 806529. [Google Scholar] [CrossRef] [PubMed]
- Bolandi, Z.; Hashemi, S.M.; Abasi, M.; Musavi, M.; Aghamiri, S.; Miyanmahaleh, N.; Ghanbarian, H. In vitro naive CD4. Mol. Biol. Rep. 2023, 50, 9037–9046. [Google Scholar] [CrossRef]
- Xu, K.; Ma, D.; Zhang, G.; Gao, J.; Su, Y.; Liu, S.; Liu, Y.; Han, J.; Tian, M.; Wei, C.; et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles ameliorate collagen-induced arthritis via immunomodulatory T lymphocytes. Mol. Immunol. 2021, 135, 36–44. [Google Scholar] [CrossRef]
- Tian, X.; Wei, W.; Cao, Y.; Ao, T.; Huang, F.; Javed, R.; Wang, X.; Fan, J.; Zhang, Y.; Liu, Y.; et al. Gingival mesenchymal stem cell-derived exosomes are immunosuppressive in preventing collagen-induced arthritis. J. Cell. Mol. Med. 2022, 26, 693–708. [Google Scholar] [CrossRef]
- Hyland, M.; Mennan, C.; Davies, R.; Wilson, E.; Tonge, D.P.; Clayton, A.; Kehoe, O. Extracellular vesicles derived from umbilical cord mesenchymal stromal cells show enhanced anti-inflammatory properties via upregulation of miRNAs after pro-inflammatory priming. Stem Cell Rev. Rep. 2023, 19, 2391–2406. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, R.; He, X.; Bian, J.; Zhao, W.; Shi, W.; Ruan, Q. MicroRNA-21 Regulates Diametrically Opposed Biological Functions of Regulatory T Cells. Front. Immunol. 2021, 12, 766757. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chen, H.; Li, Y.; Zhong, H.; Sun, W.; Wang, J.; Zhang, T.; Ma, J.; Yan, S.; Zhang, J.; et al. Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann. Rheum. Dis. 2018, 77, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.X.; Chou, K.Y.; Hwang, J.J.; Wang, H.S. The effects of IL-1β stimulated human umbilical cord mesenchymal stem cells on polarization and apoptosis of macrophages in rheumatoid arthritis. Sci. Rep. 2023, 13, 10612. [Google Scholar] [CrossRef]
- Choi, E.W.; Lim, I.R.; Park, J.H.; Song, J.; Choi, B.; Kim, S. Exosomes derived from mesenchymal stem cells primed with disease-condition-serum improved therapeutic efficacy in a mouse rheumatoid arthritis model via enhanced TGF-β1 production. Stem Cell Res. Ther. 2023, 14, 283. [Google Scholar] [CrossRef]
- Takenaka, M.; Yabuta, A.; Takahashi, Y.; Takakura, Y. Interleukin-4-carrying small extracellular vesicles with a high potential as anti-inflammatory therapeutics based on modulation of macrophage function. Biomaterials 2021, 278, 121160. [Google Scholar] [CrossRef]
- Canavan, M.; Floudas, A.; Veale, D.J.; Fearon, U. The PD-1:PD-L1 axis in Inflammatory Arthritis. BMC Rheumatol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Li, W.; Sun, J.; Feng, S.L.; Wang, F.; Miao, M.Z.; Wu, E.Y.; Wallet, S.; Loeser, R.; Li, C. Intra-articular delivery of AAV vectors encoding PD-L1 attenuates joint inflammation and tissue damage in a mouse model of rheumatoid arthritis. Front. Immunol. 2023, 14, 1116084. [Google Scholar] [CrossRef]
- Xu, F.; Fei, Z.; Dai, H.; Xu, J.; Fan, Q.; Shen, S.; Zhang, Y.; Ma, Q.; Chu, J.; Peng, F.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles with High PD-L1 Expression for Autoimmune Diseases Treatment. Adv. Mater. 2022, 34, e2106265. [Google Scholar] [CrossRef]
- He, H.; Chen, Q.; Fan, H.; Leng, X.Y.; Zhu, F.; Gao, F.; Zhou, Q.; Dong, Y.; Yang, J. Extracellular vesicles produced by bone marrow mesenchymal stem cells overexpressing programmed death-ligand 1 ameliorate dextran sodium sulfate-induced ulcerative colitis in rats by regulating Th17/Treg cell balance through PTEN/PI3K/AKT/mTOR axis. J. Gastroenterol. Hepatol. 2022, 37, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhu, W.; Li, H.; Ma, D.; Liu, W.; Yu, W.; Wang, L.; Cao, Y.; Jiang, Y. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 2020, 30, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, S.; Naz, S.I.; Jain, V.; Soderblom, E.J.; Aliferis, C.; Kraus, V.B. Comprehensive characterization of pathogenic synovial fluid extracellular vesicles from knee osteoarthritis. Clin. Immunol. 2023, 257, 109812. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Kato, T.; Miyaki, S.; Ishitobi, H.; Nakamura, Y.; Nakasa, T.; Lotz, M.K.; Ochi, M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014, 16, R163. [Google Scholar] [CrossRef]
- Zeng, G.; Deng, G.; Xiao, S.; Li, F. Fibroblast-like Synoviocytes-derived Exosomal PCGEM1 Accelerates IL-1β-induced Apoptosis and Cartilage Matrix Degradation by miR-142-5p/RUNX2 in Chondrocytes. Immunol. Investig. 2022, 51, 1284–1301. [Google Scholar] [CrossRef]
- Ni, Z.; Kuang, L.; Chen, H.; Xie, Y.; Zhang, B.; Ouyang, J.; Wu, J.; Zhou, S.; Chen, L.; Su, N.; et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019, 10, 522. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Zhao, F.; Liu, M.; Wang, F.; Kang, M.; He, W.; Lv, Z. Exosomal circ-BRWD1 contributes to osteoarthritis development through the modulation of miR-1277/TRAF6 axis. Arthritis Res. Ther. 2021, 23, 159. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, K.; Zhou, W.; Wu, H.; Lu, Y. Exosome-mediated circ_0001846 participates in IL-1β-induced chondrocyte cell damage by miR-149-5p-dependent regulation of WNT5B. Clin. Immunol. 2021, 232, 108856. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Rasheed, N.; Abdulmonem, W.A.; Khan, M.I. MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes. Sci. Rep. 2019, 9, 6882. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, X.; Lu, J.; Huang, G.; Dang, L.; Zhang, H.; Zhong, C.; Zhang, Z.; Li, D.; Li, F.; et al. Exosomal transfer of osteoclast-derived miRNAs to chondrocytes contributes to osteoarthritis progression. Nat. Aging 2021, 1, 368–384. [Google Scholar] [CrossRef]
- Wu, X.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Osteoarthritic Subchondral Bone Release Exosomes That Promote Cartilage Degeneration. Cells 2021, 10, 251. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, J.; Zhang, L.; Li, J.; Yan, T.; Zhou, P.; Zhang, S. Knockdown of circ-PRKCH alleviates IL-1β-treated chondrocyte cell phenotypic changes through modulating miR-502-5p/ADAMTS5 axis. Autoimmunity 2022, 55, 179–191. [Google Scholar] [CrossRef]
- Meng, Y.; Qiu, S.; Sun, L.; Zuo, J. Knockdown of exosome-mediated lnc-PVT1 alleviates lipopolysaccharide-induced osteoarthritis progression by mediating the HMGB1/TLR4/NF-κB pathway via miR-93-5p. Mol. Med. Rep. 2020, 22, 5313–5325. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Zhang, R.; Fan, Z.; Li, W.; Mao, R.; Du, Z.; Yao, X.; Ma, Y.; Yan, Y.; et al. Stage-specific and location-specific cartilage calcification in osteoarthritis development. Ann. Rheum. Dis. 2023, 82, 393–402. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, R.; Hou, S.; He, F.; Ma, Y.; Ye, T.; Yu, S.; Chen, H.; Wang, H.; Zhang, M. Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis. Arthritis Res. Ther. 2022, 24, 44. [Google Scholar] [CrossRef]
- Jeon, H.; Im, G.I. Autophagy in osteoarthritis. Connect. Tissue Res. 2017, 58, 497–508. [Google Scholar] [CrossRef]
- Yan, J.; Shen, M.; Sui, B.; Lu, W.; Han, X.; Wan, Q.; Liu, Y.; Kang, J.; Qin, W.; Zhang, Z.; et al. Autophagic LC3. Sci. Adv. 2022, 8, eabn1556. [Google Scholar] [CrossRef]
- Cheng, N.T.; Meng, H.; Ma, L.F.; Zhang, L.; Yu, H.M.; Wang, Z.Z.; Guo, A. Role of autophagy in the progression of osteoarthritis: The autophagy inhibitor, 3-methyladenine, aggravates the severity of experimental osteoarthritis. Int. J. Mol. Med. 2017, 39, 1224–1232. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Y.; Xue, F.; Liu, K.; Zhu, B.; Gao, J.; Yin, J.; Zhang, C.; Li, G. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine 2022, 76, 103847. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sun, K.; Yu, S.; Luo, J.; Guo, J.; Lin, J.; Wang, G.; Guo, Z.; Ye, Y.; Guo, F. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Transl. 2021, 27, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Ji, L.; Pang, Y.; Zhao, D.; Gao, J. Exosomes from osteoarthritic fibroblast-like synoviocytes promote cartilage ferroptosis and damage via delivering microRNA-19b-3p to target SLC7A11 in osteoarthritis. Front. Immunol. 2023, 14, 1181156. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Hu, H.; Li, Y.; Hu, Y. Pyroptosis Plays a Role in Osteoarthritis. Aging Dis. 2020, 11, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Terkawi, M.A.; Kitahara, K.; Yokota, S.; Shiota, J.; Nishida, Y.; Matsumae, G.; Alhasan, H.; Hamasaki, M.; Hontani, K.; et al. Noncanonical Pyroptosis Triggered by Macrophage-Derived Extracellular Vesicles in Chondrocytes Leading to Cartilage Catabolism in Osteoarthritis. Arthritis Rheumatol. 2023, 75, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Jeon, O.H.; Wilson, D.R.; Clement, C.C.; Rathod, S.; Cherry, C.; Powell, B.; Lee, Z.; Khalil, A.M.; Green, J.J.; Campisi, J.; et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019, 4, e125019. [Google Scholar] [CrossRef]
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallaee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a-5p Promotes Joint Destruction During Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439. [Google Scholar] [CrossRef]
- Varela-Eirín, M.; Varela-Vázquez, A.; Guitián-Caamaño, A.; Paíno, C.L.; Mato, V.; Largo, R.; Aasen, T.; Tabernero, A.; Fonseca, E.; Kandouz, M.; et al. Targeting of chondrocyte plasticity via connexin43 modulation attenuates cellular senescence and fosters a pro-regenerative environment in osteoarthritis. Cell Death Dis. 2018, 9, 1166. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girão, H. Role of connexin 43 in different forms of intercellular communication-gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef]
- Varela-Eirín, M.; Carpintero-Fernández, P.; Guitián-Caamaño, A.; Varela-Vázquez, A.; García-Yuste, A.; Sánchez-Temprano, A.; Bravo-López, S.B.; Yañez-Cabanas, J.; Fonseca, E.; Largo, R.; et al. Extracellular vesicles enriched in connexin 43 promote a senescent phenotype in bone and synovial cells contributing to osteoarthritis progression. Cell Death Dis. 2022, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Kim, K.I.; Lee, M.C.; Lee, J.H.; Moon, Y.W.; Lee, W.S.; Lee, H.J.; Hwang, S.C.; In, Y.; Shon, O.J.; Bae, K.C.; et al. Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Sports Med. 2023, 51, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell. Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hu, N.; Zhang, Y.; Chen, Y.; Su, C.; Lv, Y.; Shen, Y. MEG3 promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-361-5p/FOXO1 axis. BMC Med. Genom. 2019, 12, 201. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.Y.; Peng, W.M.; Yuan, B.; Bi, Q.; Xu, Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Pu, G.; Wu, J.; Qin, F. Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 567. [Google Scholar] [CrossRef]
- Chen, D.; Kim, D.J.; Shen, J.; Zou, Z.; O’Keefe, R.J. Runx2 plays a central role in Osteoarthritis development. J. Orthop. Transl. 2020, 23, 132–139. [Google Scholar] [CrossRef]
- Nagata, K.; Hojo, H.; Chang, S.H.; Okada, H.; Yano, F.; Chijimatsu, R.; Omata, Y.; Mori, D.; Makii, Y.; Kawata, M.; et al. Runx2 and Runx3 differentially regulate articular chondrocytes during surgically induced osteoarthritis development. Nat. Commun. 2022, 13, 6187. [Google Scholar] [CrossRef] [PubMed]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.I.; Tofiño-Vian, M.; Silvestre, A.; Castejón, M.A.; Alcaraz, M.J. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Transl. 2021, 30, 61–69. [Google Scholar] [CrossRef]
- Duan, A.; Shen, K.; Li, B.; Li, C.; Zhou, H.; Kong, R.; Shao, Y.; Qin, J.; Yuan, T.; Ji, J.; et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res. Ther. 2021, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, X.; Qu, Z.; Hao, J.; Zhang, W. Hypoxia-Preconditioned Extracellular Vesicles from Mesenchymal Stem Cells Improve Cartilage Repair in Osteoarthritis. Membranes 2022, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Stöckl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; König, M.A.; Johnstone, B.; Grässel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef]
- Whitty, C.; Pernstich, C.; Marris, C.; McCaskie, A.; Jones, M.; Henson, F. Sustained delivery of the bone morphogenetic proteins BMP-2 and BMP-7 for cartilage repair and regeneration in osteoarthritis. Osteoarthr. Cartil. Open 2022, 4, 100240. [Google Scholar] [CrossRef]
- Sun, W.; Qu, S.; Ji, M.; Sun, Y.; Hu, B. BMP-7 modified exosomes derived from synovial mesenchymal stem cells attenuate osteoarthritis by M2 polarization of macrophages. Heliyon 2023, 9, e19934. [Google Scholar] [CrossRef]
- Muttigi, M.S.; Han, I.; Park, H.K.; Park, H.; Lee, S.H. Matrilin-3 Role in Cartilage Development and Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 590. [Google Scholar] [CrossRef]
- Long, L.; Zou, G.; Cheng, Y.; Li, F.; Wu, H.; Shen, Y. MATN3 delivered by exosome from synovial mesenchymal stem cells relieves knee osteoarthritis: Evidence from. J. Orthop. Transl. 2023, 41, 20–32. [Google Scholar] [CrossRef]
- Xu, C.; Mi, Z.; Dong, Z.; Chen, X.; Ji, G.; Kang, H.; Li, K.; Zhao, B.; Wang, F. Platelet-Derived Exosomes Alleviate Knee Osteoarthritis by Attenuating Cartilage Degeneration and Subchondral Bone Loss. Am. J. Sports Med. 2023, 51, 2975–2985. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Chen, Z.; Zhang, Y.; Li, J.; Liu, S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Wang, D.; Yuan, Z. The Fibroblast-Like Synoviocyte Derived Exosomal Long Non-coding RNA H19 Alleviates Osteoarthritis Progression Through the miR-106b-5p/TIMP2 Axis. Inflammation 2020, 43, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Mi, M.; Shi, S.; Li, T.; Holz, J.; Lee, Y.J.; Sheu, T.J.; Liao, Q.; Xiao, T. TIMP2 deficient mice develop accelerated osteoarthritis via promotion of angiogenesis upon destabilization of the medial meniscus. Biochem. Biophys. Res. Commun. 2012, 423, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yao, Y.; Zhao, D.; Zou, H.; Lai, C.; Xiang, G.; Wang, G.; Luo, L.; Shi, Y.; Li, Y.; et al. LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am. J. Transl. Res. 2021, 13, 1245–1256. [Google Scholar]
- Wang, H.; Shu, J.; Zhang, C.; Wang, Y.; Shi, R.; Yang, F.; Tang, X. Extracellular Vesicle-Mediated miR-150-3p Delivery in Joint Homeostasis: A Potential Treatment for Osteoarthritis? Cells 2022, 11, 2766. [Google Scholar] [CrossRef]
- Wang, J.; Sun, T. Mir-25-3p in extracellular vesicles from fibroblast-like synoviocytes alleviates pyroptosis of chondrocytes in knee osteoarthritis. J. Bioenerg. Biomembr. 2023, 55, 365–380. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wang, W.; Tu, B.; Zhu, Y.; Fan, C.; Li, Y. Overexpression of SOX9 alleviates the progression of human osteoarthritis in vitro and in vivo. Drug Des. Dev. Ther. 2019, 13, 2833–2842. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Zheng, X.; Huang, M.; Cheng, W.; Shan, H.; Gao, X.; Zhang, M.; Sheng, L.; Dai, J.; et al. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020, 11, 763. [Google Scholar] [CrossRef]
- Kan, S.; Duan, M.; Liu, Y.; Wang, C.; Xie, J. Role of Mitochondria in Physiology of Chondrocytes and Diseases of Osteoarthritis and Rheumatoid Arthritis. Cartilage 2021, 13, 1102S–1121S. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, S.; Lu, Y.; Wan, M.; Cheng, J.; Liu, J. MitoEVs: A new player in multiple disease pathology and treatment. J. Extracell. Vesicles 2023, 12, e12320. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Fahey, M.J.; Pugliese, B.R.; Irwin, R.M.; Antonyak, M.A.; Delco, M.L. Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front. Bioeng. Biotechnol. 2022, 10, 870193. [Google Scholar] [CrossRef]
- Yu, M.; Wang, D.; Chen, X.; Zhong, D.; Luo, J. BMSCs-derived Mitochondria Improve Osteoarthritis by Ameliorating Mitochondrial Dysfunction and Promoting Mitochondrial Biogenesis in Chondrocytes. Stem Cell Rev. Rep. 2022, 18, 3092–3111. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, J.; Fan, Y.; Li, J.; You, T.; He, S.; Xiao, G.; Chen, D. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann. Rheum. Dis. 2019, 78, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Hotham, W.E.; Pattison, L.A.; Ma, Q.; Henson, F.M.D.; Smith, E.S.J. Role of Human Mesenchymal Stem Cells and Derived Extracellular Vesicles in Reducing Sensory Neuron Hyperexcitability and Pain Behaviors in Murine Osteoarthritis. Arthritis Rheumatol. 2023, 75, 352–363. [Google Scholar] [CrossRef]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain. 2017, 18, 34. [Google Scholar] [CrossRef]
- Lu, K.; Wang, Q.; Hao, L.; Wei, G.; Wang, T.; Lu, W.W.; Xiao, G.; Tong, L.; Zhao, X.; Chen, D. miR-204 ameliorates osteoarthritis pain by inhibiting SP1-LRP1 signaling and blocking neuro-cartilage interaction. Bioact. Mater. 2023, 26, 425–436. [Google Scholar] [CrossRef]
- Liu, W.; Liu, A.; Li, X.; Sun, Z.; Liu, Y.; Wang, G.; Huang, D.; Xiong, H.; Yu, S.; Zhang, X.; et al. Dual-engineered cartilage-targeting extracellular vesicles derived from mesenchymal stem cells enhance osteoarthritis treatment via miR-223/NLRP3/pyroptosis axis: Toward a precision therapy. Bioact. Mater. 2023, 30, 169–183. [Google Scholar] [CrossRef]
- Zhao, S.; Xiu, G.; Wang, J.; Wen, Y.; Lu, J.; Wu, B.; Wang, G.; Yang, D.; Ling, B.; Du, D.; et al. Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J. Nanobiotechnol. 2023, 21, 341. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Xie, X.; Yuan, J.; Gong, L.; Zhu, Z.; Zhang, J.; Li, H.; Yang, Y.; Wang, Y. Reversing the surface charge of MSC-derived small extracellular vesicles by εPL-PEG-DSPE for enhanced osteoarthritis treatment. J. Extracell. Vesicles 2021, 10, e12160. [Google Scholar] [CrossRef]
- Tang, H.; Luo, H.; Zhang, Z.; Yang, D. Mesenchymal Stem Cell-Derived Apoptotic Bodies: Biological Functions and Therapeutic Potential. Cells 2022, 11, 3879. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yang, J.; Su, X.; Li, X.; Lei, Y.; Dong, L.; Chen, H.; Chen, C.; Zhao, C.; Zhang, H.; et al. The miR-21-5p enriched in the apoptotic bodies of M2 macrophage-derived extracellular vesicles alleviates osteoarthritis by changing macrophage phenotype. Genes. Dis. 2023, 10, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J.; et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res. Ther. 2020, 11, 507. [Google Scholar] [CrossRef]
- Li, J.; Wei, C.; Yang, Y.; Gao, Z.; Guo, Z.; Qi, F. Apoptotic bodies extracted from adipose mesenchymal stem cells carry microRNA-21-5p to induce M2 polarization of macrophages and augment skin wound healing by targeting KLF6. Burns 2022, 48, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Qiu, X.; Yang, X.; Bao, L.; Pu, F.; Liu, X.; Li, C.; Xuan, K.; Zhou, J.; et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy 2020, 16, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Maunder, D.; Brown, P.M.; Barron-Millar, B.; Lendrem, D.W.; Naamane, N.; Macdonald, J.; Wang, X.N.; Isaacs, J.D.; Anderson, A.E.; Morgan, A.W.; et al. Micro-RNA content of circulating extracellular vesicles in early rheumatoid arthritis as biomarkers and mediators of methotrexate efficacy. Rheumatology 2023, kead569. [Google Scholar] [CrossRef]
- Yan, F.; Zhong, Z.; Wang, Y.; Feng, Y.; Mei, Z.; Li, H.; Chen, X.; Cai, L.; Li, C. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J. Nanobiotechnol. 2020, 18, 115. [Google Scholar] [CrossRef]
- Shi, M.M.; Yang, Q.Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef]
- Zarrabi, M.; Shahrbaf, M.A.; Nouri, M.; Shekari, F.; Hosseini, S.E.; Hashemian, S.R.; Aliannejad, R.; Jamaati, H.; Khavandgar, N.; Alemi, H.; et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: A randomized controlled trial. Stem Cell Res. Ther. 2023, 14, 169. [Google Scholar] [CrossRef] [PubMed]


| Mechanism Involved in the Pathogenesis of RA | Role of EVs in RA Progression | Reference |
|---|---|---|
| Autoimmunity | EVs are associated with the presence of citrullinated proteins | [21,22,26,27,28] |
| Immune complexes formed with EVs induce pro-inflammatory responses | ||
| Inflammation | EVs contain TNF-α which can stimulate NF-κB pathway | [33,34,37,38,42] |
| Inflammatory environment stimulates the secretion of EVs with ncRNA that further modulate inflammation | ||
| Microparticles derived from synovial fluid promote inflammatory responses in FLSs | ||
| RA-FLS-derived exosomes promote macrophage migration through pentraxin 3 | ||
| Exosomes derived from synovial fibroblasts containing miR-424 promote Th17 T cell differentiation | ||
| Bone degradation | Exosomes derived from RA-FLSs suppress osteoblast differentiation | [47,48,55] |
| TNF-stimulated FLS contain miR-221-3p which inhibits osteoblast differentiation | ||
| Th17 cells stimulated with cigarette smoke-enriched medium or aryl hydrocarbon receptor agonist promote the secretion of EVs containing miR-132 that stimulates osteoclastogenesis | ||
| Extracellular matrix degradation | RA-FLSs secrete microvesicles with proteases that degrade aggrecan | [56] |
| Angiogenesis | RA-FLSs secrete exosomes containing inhibitor of DNA binding 1, which has pro-angiogenic features | [58,60] |
| RA-FLS-derived EVs can promote angiogenesis by secreting miR-1972 that regulates p53/mTOR |
| Mechanism Involved in the Pathogenesis of OA | Role of EVs in OA Progression | Reference |
|---|---|---|
| Inflammation | Exosomes derived from IL-1β-stimulated synovial fibroblasts promoted TNFα expression in chondrocytes. | [117,119,120,126] |
| EVs derived from IL-1β-stimulated chondrocytes further promoted IL-1β production in macrophages. | ||
| Silencing circ-BRWD1, which can be secreted in exosomes by chondrocytes, reduced the expression of IL-6 and IL-8. | ||
| Chondrocyte stimulation with LPS promotes the secretion of exosomal lncRNA PVT1, which plays a role in inflammatory responses. | ||
| Cartilage Degradation | Exosomes derived from IL-1β-stimulated synovial fibroblasts promoted MMP-13 and suppressed ACAN expression. | [117,118,120,121,123,125,126] |
| LncRNA PCGEM1 present in exosomes derived from OA-FLSs promoted chondrocyte apoptosis. Furthermore, it promoted MMP-13 and inhibited COL2A1 and Aggrecan expression in chondrocytes. | ||
| IL-1β-stimulated chondrocytes secrete exosomes with elevated circ-BRWD1 and circ_0001846 expression, which take part in cartilage degradation. | ||
| Osteoclast secrete exosomes containing miRNA that can suppress tissue inhibitors of metalloproteinase (TIMPs) in chondrocytes. | ||
| IL-1β-stimulated chondrocytes can transfer circ-PRKCH in exosomes, which can modulate ADAMTS5 expression. | ||
| Chondrocyte stimulation with LPS promotes the secretion of exosomal lncRNA PVT1, PVT1 knockdown reduced MMP-13 and promoted aggrecan. | ||
| Cartilage Calcification | EVs mediate the process of cartilage calcification, observed in the pathogenesis of OA. | [128,130] |
| Cell death | Exosomes derived from OA-FLSs promote ferroptosis in IL-1β-stimulated chondrocytes. | [134,136] |
| EVs derived from LPS-stimulated macrophages promote pyroptosis in chondrocytes. | ||
| Senescence | EVs derived from senescent cells induce senescence in chondrocytes. | [138,142] |
| EVs enriched with Cx43 promote senescent state in other cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakinowska, E.; Kiełbowski, K.; Pawlik, A. The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Rheumatoid Arthritis and Osteoarthritis. Cells 2023, 12, 2716. https://doi.org/10.3390/cells12232716
Bakinowska E, Kiełbowski K, Pawlik A. The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Rheumatoid Arthritis and Osteoarthritis. Cells. 2023; 12(23):2716. https://doi.org/10.3390/cells12232716
Chicago/Turabian StyleBakinowska, Estera, Kajetan Kiełbowski, and Andrzej Pawlik. 2023. "The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Rheumatoid Arthritis and Osteoarthritis" Cells 12, no. 23: 2716. https://doi.org/10.3390/cells12232716