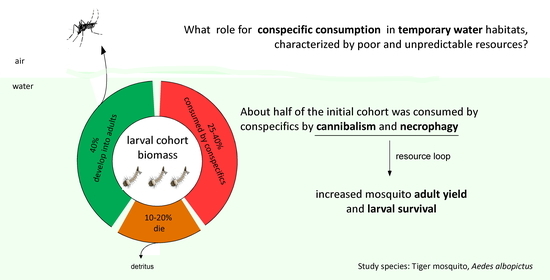
Cannibalism and Necrophagy Promote a Resource Loop and Benefit Larval Development in Insects of Temporary Waters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Experiment 1: The Consumption Rate of Conspecific Individuals
2.3. Experiment 2: The Effects of Conspecific Consumption
3. Results
3.1. Experiment 1: The Consumption Rate of Conspecific Individuals
3.2. Experiment 2: The Effects of Conspecific Consumption
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, D.D. The Biology of Temporary Waters; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Carpenter, S.R. Resource limitation of larval tree hole mosquitoes subsisting on beech detritus. Ecology 1983, 64, 219–223. [Google Scholar] [CrossRef]
- Kitching, R.L. Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Ann. Rev. Entomol. 2001, 46, 729–760. [Google Scholar] [CrossRef]
- Yee, D.A.; Juliano, S.A. Consequences of detritus type in an aquatic microsystem: Effects on water quality, micro-organisms and performance of the dominant consumer. Freshw. Biol. 2006, 51, 448–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juliano, S.A. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Ann. Rev. Entomol. 2009, 54, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Yee, D.A.; Kaufman, M.G.; Ezeakacha, N.F. How diverse detrital environments influence nutrient stoichiometry between males and females of the co-occurring container mosquitoes Aedes albopictus, Ae. aegypti, and Culex quinquefasciatus. PLoS ONE 2015, 10, e0133734. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A. Thirty Years of Aedes albopictus (Diptera: Culicidae) in America: An introduction to current perspectives and future challenges. J. Med. Entomol. 2016, 53, 989–991. [Google Scholar] [CrossRef]
- David, J.P.; Rey, D.; Pautou, M.P.; Meyran, J.C. Differential toxicity of leaf litter to dipteran larvae of mosquito developmental sites. J. Invertebr. Pathol. 2000, 75, 9–18. [Google Scholar] [CrossRef]
- David, J.P.; Tilquin, M.; Rey, D.; Ravanel, P.; Meyran, J.C. Mosquito larval consumption of toxic arborescent leaf-litter, and its biocontrol potential. Med. Vet. Entomol. 2003, 17, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Muturi, E.J. Relationship between leaf litter identity, expression of cytochrome P450 genes and life history traits of Aedes aegypti and Aedes albopictus. Acta Trop. 2012, 122, 94–100. [Google Scholar] [CrossRef]
- Lancaster, J.; Downes, B.J. Aquatic Entomology; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Kaspari, M.; Powers, J.S. Biogeochemistry and geographical ecology: Embracing all twenty-five elements required to build organisms. Am. Nat. 2016, 188, S62–S73. [Google Scholar] [CrossRef]
- Denno, R.F.; Fagan, W.F. Might nitrogen limitation promote omnivory among carnivorous arthropods? Ecology 2002, 84, 2522–2531. [Google Scholar] [CrossRef]
- Kohl, K.D.; Coogan, S.C.P.; Raubenheimer, D. Do wild carnivores forage for prey or for nutrients? BioEssays 2015, 37, 701–709. [Google Scholar] [CrossRef]
- Fox, L.R. Cannibalism in natural populations. Annu. Rev. Ecol. Evol. Syst. 1975, 6, 87–106. [Google Scholar] [CrossRef]
- Polis, G.A. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Systemat. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Elgar, M.A.; Crespi, B.J. Cannibalism: Ecology and Evolution among Diverse Taxa; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Richardson, M.L.; Mitchell, R.F.; Reagel, P.F.; Hanks, L.M. Causes and consequences of cannibalism in non-carnivorous insects. Annu. Rev. Entomol. 2010, 55, 39–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, A.F.K.; Roselino, A.C.; Cappelari, A.F.; Zucoloto, F.S. Cannibalism in insects. In Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, A.R., Parra, J.L.P., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 177–194. [Google Scholar]
- Via, S. Cannibalism facilitates the use of a novel environment in the flour beetle, Tribolium castaneum. Heredity 1999, 82, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayeh, A. Cannibalism in invasive, native and biocontrol populations of the harlequin ladybird. BMC Evol. Biol. 2014, 14, 15. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.W.; Norris, M.E. Survivorship advantage of conspecific necrophagy in overwintering boxelder bugs (Heteroptera: Rhophalidae). Ann. Entomol. Soc. Am. 2004, 97, 500–503. [Google Scholar] [CrossRef]
- Bara, J.J.; Clark, T.M.; Remold, S.K. Utilization of larval and pupal detritus by Aedes aegypti and Aedes albopictus. J. Vector Ecol. 2014, 39, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Maák, I.; Tóth, E.; Lenda, M.; Lőrinczi, G.; Kiss, A.; Juhász, O.; Czechowski, W.; Torma, A. Behaviours indicating cannibalistic necrophagy in ants are modulated by the perception of pathogen infection level. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Clements, A.N. Behaviour and aspects of the biology of larvae. In The Biology of Mosquitoes: Sensory Reception and Behaviour; CABI Publishing: Wallingford, UK, 1999; pp. 135–205. [Google Scholar]
- Silberbush, A.; Tsurim, I.; Rosen, R.; Margalith, Y.; Ovadia, O. Species-specific non-physical interference competition among mosquito larvae. PLoS ONE 2014, 9, e88650. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Porretta, D.; Bellini, R.; Nascetti, G.; Urbanelli, S. Molecular systematics and origin of the Mediterranean Sea rock-pool mosquitoes of the Aedes mariae complex. Ann. Entomol. Soc. Am. 2015, 108, 593–599. [Google Scholar] [CrossRef]
- Urbanelli, S.; Porretta, D.; Mastrantonio, V.; Bellini, R.; Pieraccini, G.; Romoli, R.; Crasta, G.; Nascetti, G. Hybridization, natural selection, and evolution of reproductive isolation: A 25-years survey of an artificial sympatric area between two mosquito sibling species of the Aedes mariae complex. Evolution 2014, 68, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Emory, R.W. Cannibalism in Anopheles stephensi Liston. Mosq. News 1976, 36, 198–200. [Google Scholar]
- Koenekoop, R.; Livdahl, T. Cannibalism among Aedes triseriatus larvae. Ecol. Entomol. 1986, 11, 111–114. [Google Scholar] [CrossRef]
- Annis, B.; Umi, T.I.; Sarojo, B.; Hamzah, N.; Trenggono, B. Laboratory studies of larval cannibalism in Toxorhynchites amboinensis (Diptera:Culicidae). J. Med. Entomol. 1990, 27, 777–783. [Google Scholar] [CrossRef]
- Rajavel, A.R. Cannibalistic behavior in Armigeres subalbatus (Diptera: Culicidae). SE Asian J. Trop. Med. Public Health 1992, 23, 453–457. [Google Scholar]
- Lounibos, L.P.; Escher, R.L.; Duzak, D.; Martin, E.A. Body size, sexual receptivity and larval cannibalism in relation to protandry among Toxorhynchites mosquitoes. Oikos 1996, 77, 309–316. [Google Scholar] [CrossRef]
- Koenraadt, C.J.M.; Takken, W. Cannibalism and predation among larvae of the Anopheles gambiae complex. Med. Vet. Entomol. 2003, 17, 61–67. [Google Scholar] [CrossRef]
- Koenraadt, C.J.M.; Majambere, S.; Hemerik, L.; Takken, W. The effects of food and space on the occurrence of cannibalism and predation among larvae of Anopheles gambiae sl. Entomol. Exp. Appl. 2004, 112, 125–134. [Google Scholar] [CrossRef]
- Porretta, D.; Mastrantonio, V.; Crasta, G.; Bellini, R.; Comandatore, F.; Rossi, P.; Favia, G.; Bandi, C.; Urbanelli, S. Intra-instar larval cannibalism in Anopheles gambiae (s.s.) and Anopheles stephensi (Diptera: Culicidae). Parasites Vectors 2016, 9, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Mastrantonio, V.; Crasta, G.; Puggioli, A.; Bellini, R.; Urbanelli, S.; Porretta, D. Cannibalism in temporary waters: Simulations and laboratory experiments revealed the role of spatial shape in the mosquito Aedes albopictus. PLoS ONE 2018, 13, e0198194. [Google Scholar] [CrossRef] [Green Version]
- Porretta, D.; Mastrantonio, V.; Bellini, R.; Somboon, P.; Urbanelli, S. Glacial History of a Modern Invader: Phylogeography and Species Distribution Modelling of the Asian Tiger Mosquito Aedes albopictus. PLoS ONE 2012, 7, e44515. [Google Scholar] [CrossRef]
- Kotsakiozi, P.; Richardson, J.B.; Pichler, V.; Favia, G.; Martins, A.J.; Urbanelli, S.; Armbruster, P.A.; Caccone, A. Population genomics of the Asian tiger mosquito, Aedes albopictus: Insights into the recent worldwide invasion. Ecol. Evol. 2017, 7, 10143–10157. [Google Scholar] [CrossRef] [Green Version]
- Sherpa, S.; Blum, M.G.B.; Capblancq, T.; Cumer, T.; Rioux, D.; Després, L. Unravelling the invasion history of the Asian tiger mosquito in Europe. Mol. Ecol. 2019, 28, 2360–2377. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J. The role of Aedes albopictus as an arbovirus vector. Parassitologia 1995, 37, 109–113. [Google Scholar] [PubMed]
- Wong, P.S.; Li, M.Z.; Chong, C.S.; Ng, L.C.; Tan, C.H. Aedes (Stegomyia) albopictus (Skuse): A potential vector of Zika virus in Singapore. PloS Negl. Trop. Dis. 2013, 7, e2348. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rúa, A.; Marconcini, M.; Madec, Y.; Manni, M.; Carraretto, D.; Gomulski, L.M.; Gasperi, G.; Failloux, A.B.; Malacrida, A.R. Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Commun Biol. 2020, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W.A. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 1988, 1, 1–39. [Google Scholar]
- Schaffner, F. The Mosquitoes of Europe/Les Moustiques d’Europe. Logiciel D’identification et D’enseignement (CD-Rom); IRD Editions & EID Mediterranèe: Montpellier, France, 2001. [Google Scholar]
- Carrieri, M.; Bacchi, M.; Bellini, R.; Maini, S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ. Entomol. 2003, 32, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Walsh, R.K.; Bradley, C.; Apperson, C.S.; Gould, F. An experimental field study of delayed density dependence in natural populations of Aedes albopictus. PLoS ONE 2012, 7, e35959. [Google Scholar] [CrossRef] [Green Version]
- Osius, G.; Rojek, D. Normal goodness-of-fit tests for multinomial models with large degrees-of-freedom. J. Am. Stat. Ass. 1992, 87, 1145–1152. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 5 July 2021).
- Kaplan, E.; Meier, P. Nonparametric estimation from incomplete observations. J. Amer. Statist. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P. Drawing Survival Curves using ‘ggplot2’, version 0.4.6; Survminer R package. 2019. Available online: https://CRAN.R-project.org/package=survminer (accessed on 5 July 2021).
- Daugherty, M.P.; Alto, B.W.; Juliano, S.A. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2000, 37, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Vijendravarma, R.K.; Narasimha, S.; Kawecki, T.J. Predatory cannibalism in Drosophila melanogaster larvae. Nat. Commun. 2013, 4, 1879. [Google Scholar] [CrossRef] [Green Version]
- Snyder, W.E.; Joseph, S.B.R.; Preziosi, F.; Moore, A.J. Nutritional benefits of cannibalism for the lady beetle Harmonia axyridis (Coleoptera: Coccinellidae) when prey quality is poor. Environ. Entomol. 2000, 29, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Barros-Bellanda, H.C.H.; Zucoloto, F.S. Egg cannibalism in Ascia monuste in the field; opportunistic, preferential and very frequent. J. Ethol. 2005, 23, 133–138. [Google Scholar] [CrossRef]
- Church, S.C.; Sherratt, T.N. The selective advantages of cannibalism in a Neotropical mosquito. Behav. Ecol. Sociobiol. 1996, 39, 117–123. [Google Scholar] [CrossRef]
- Phuc, H.K.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Fu, G.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variable | Df | Deviance Resid. | Df Resid. | Dev | Pr(>Chi) |
|---|---|---|---|---|---|
| Laboratory conditions | |||||
| Cannibalism rate | |||||
| Null | 86 | 611.00 | |||
| stage | 1 | 515.43 | 88 | 97.57 | 2 × 10−16 *** |
| Density | 1 | 0.38 | 87 | 95.19 | 0.537 |
| Stage × Density | 1 | 0.00 | 86 | 95.19 | 0.999 |
| Necrophagy rate | |||||
| Null | 89 | 168.11 | |||
| stage | 1 | 71.79 | 88 | 96.32 | 2 × 10−16 *** |
| Density | 1 | 0.30 | 87 | 96.02 | 0.581 |
| Stage × Density | 1 | 1.42 | 86 | 94.60 | 0.233 |
| Adult yield | |||||
| Null | 44 | 137.40 | |||
| Density | 1 | 0.930 | 43 | 136.47 | 0.335 |
| Semi-field conditions | |||||
| Cannibalism rate | |||||
| Null | 89 | 317.05 | |||
| stage | 1 | 279.203 | 88 | 37.85 | 2 × 10−16 *** |
| Density | 1 | 2.599 | 87 | 35.25 | 0.1070 |
| Stage × Density | 1 | 0.000 | 86 | 35.25 | 0.9999 |
| Necrophagy rate | |||||
| Null | 89 | 266.596 | |||
| stage | 1 | 187.488 | 88 | 79.108 | 2 × 10−16 *** |
| Density | 1 | 0.708 | 87 | 78.400 | 0.40008 |
| Stage × Density | 1 | 3.947 | 86 | 74.453 | 0.05051 |
| Adult yield | |||||
| Null | 44 | 51.545 | |||
| Density | 1 | 2.334 | 43 | 49.212 | 0.1266 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrantonio, V.; Crasta, G.; Urbanelli, S.; Porretta, D. Cannibalism and Necrophagy Promote a Resource Loop and Benefit Larval Development in Insects of Temporary Waters. Insects 2021, 12, 657. https://doi.org/10.3390/insects12070657
Mastrantonio V, Crasta G, Urbanelli S, Porretta D. Cannibalism and Necrophagy Promote a Resource Loop and Benefit Larval Development in Insects of Temporary Waters. Insects. 2021; 12(7):657. https://doi.org/10.3390/insects12070657
Chicago/Turabian StyleMastrantonio, Valentina, Graziano Crasta, Sandra Urbanelli, and Daniele Porretta. 2021. "Cannibalism and Necrophagy Promote a Resource Loop and Benefit Larval Development in Insects of Temporary Waters" Insects 12, no. 7: 657. https://doi.org/10.3390/insects12070657