Hemiurid Trematodes (Digenea: Hemiuridae) from Marine Fishes off the Coast of Rio de Janeiro, Brazil, with Novel Molecular Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host and Parasite Collection and Morphological Evaluation
2.2. DNA Sequence Generation and Phylogenetic Analyses
3. Results
3.1. Morphological Evaluation
- Aphanurinae Skrjabin and Guschanskaja, 1954
- Myosaccium Montgomery, 1957
- Myosaccium ecaude Montgomery, 1957
- Host: Sardinella brasiliensis (Steindachner) (Clupeidae).
- Infection rates: two out of two; three–four specimens per fish; seven specimens in total.
- Representatives DNA sequences: OP918123 (28S); OP948303 (cox1).
- Voucher material: CHIOC 39908 a–e.
| Species | Myosaccium ecaude Montgomery, 1957 | Myosaccium opisthonemae (Siddiqi & Cable, 1960) | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | Present study | Montgomery [38] | León-Règagnon et al. [39] | Overstreet [46] | Siddiqi and Cable [47] | Kohn and Buhruheim [48] | ||
| Locality | Atlantic Ocean, Rio de Janeiro, Brazil | Pacific Ocean, California, USA | Pacific Ocean, La Chamela Bay, Mexico | Atlantic Ocean, Florida, USA | Atlantic Ocean, Playa Mani, Puerto Rico | Atlantic Ocean, Rio de Janeiro, Brazil | ||
| Host | Sardinella brasiliensis | Sardinops sagax | Opisthonema libertae | Sardinella aurita | Opisthonema oglinum | Sardinella aurita | ||
| Range (n = 4) | Mean | Range (n = 10) | Range (n = 30) | Mean | Range (n = 12) | Range (n = 40) | Range (n = NP) | |
| Body length | 924–1009 | 906 | 1380–1530 | 920–1120 | 1020 | 440–900 | 534–827 | 650–1040 |
| Body width | 221–248 | 232 | 360–400 | 280–350 | 320 | – | 153–193 | 120–200 |
| Forebody length | 182−224 | 204 | – | – | – | – | – | – |
| Hindbody length | 449−629 | 538 | – | – | – | – | – | – |
| Pre-oral lobe length | 17−26 | 21 | – | – | – | – | – | – |
| Oral sucker length | 71−88 | 79 | 90–100 | 60–80 | 70 | – | 52–69 | 40–80 |
| Oral sucker width | 78−102 | 88 | 110–120 | 80–140 | 90 | – | 68–79 | 50–90 |
| Pharynx length | 40−51 | 47 | 60–70 | – | – | – | 31–37 | 30–50 |
| Pharynx width | 47−54 | 50 | 60–70 | – | – | – | – | 40–60 |
| Ventral sucker length | 126−169 | 143 | – | 150–190 | 170 | – | 94–120 | 170–170 |
| Ventral sucker width | 140−169 | 154 | – | 140–210 | 180 | – | – | 70–170 |
| DIBAE | 118−168 | 139 | – | – | – | – | – | – |
| DTVS | 44−106 | 62 | – | – | – | – | – | – |
| Anterior testis length | 29−53 | 40 | – | – | – | – | 29–69 | 30–80 |
| Anterior testis width | 47−51 | 49 | – | – | – | – | – | 20–70 |
| Posterior testis length | 31–50 | 40 | – | – | – | – | – | 30–90 |
| Posterior testis width | 51–67 | 62 | – | – | – | – | – | 30–70 |
| Post-testicular region length | 322–410 | 381 | – | – | – | – | – | – |
| Seminal vesicle length | 99−152 (n = 3) | 131 | – | – | – | – | – | 30–180 |
| Seminal vesicle width | 57−74 (n = 3) | 63 | – | – | – | – | – | 30–60 |
| Sinus-sac length | 73–105 | 90 | 130–170 | – | – | – | – | 60 |
| Sinus-sac width | 21–26 | 24 | 30–40 | – | – | – | – | – |
| Ovary length | 43−66 | 57 | – | – | – | – | 39–69 | 30–50 |
| Ovary width | 64−80 | 69 | – | – | – | – | – | 20–50 |
| Vitellarium length | 81–117 | 107 | – | – | – | – | – | – |
| Vitellarium width | 85–111 | 101 | – | – | – | – | – | – |
| Egg length | 23–30 (n = 10) * | 28 | 15–18 | 26–29 | 28 | 21–26 | 29–32 | 30–41 |
| Egg width | 8–13 (n = 10)* | 9 | 9 | 10–13 | 11 | 9–11 | 12–15 | 9–13 |
| Body length/body width | 1:3.35–4.19 | 1:3.90 | – | – | – | – | – | – |
| Oral/ventral sucker width | 1:1.66−1.79 | 1:1.75 | 1:1.57–1.75 | 1:2.30 | 1:230 | 1:1.60–1.80 | 1:160 | 1:1.45–2.32 |
| Forebody/body length, % | 21−24 | 23 | – | – | – | – | – | – |
| Post-testicular region/body length, % | 39–44 | 42 | – | – | – | – | – | – |
- Dinurinae Looss, 1907
- Ectenurus Looss, 1907
- Ectenurus virgula Linton, 1910
- Hosts: Anisotremus virginicus (Linnaeus) (Haemulidae), Decapterus punctatus (Cuvier) (Carangidae), Prionotus punctatus (Bloch) (Triglidae).
- Infection rates: A. virginicus, two out of five; one–five specimens per fish; six specimens in total; D. punctatus, one out of one; two specimens in total; P. punctatus 1 out of 23; two specimens in total.
- Representatives DNA sequences: OP918121, OP918122, OP918126 (28); OP918136 (ITS1-5.8S-ITS2); OP948304 (cox1).
- Voucher material: CHIOC 39798; CHIOC 39799 a–e; CHIOC 39800 a,b.
| Source | Present Study | León-Règagnon et al. [39] | Linton [49] | ||
|---|---|---|---|---|---|
| Locality | Atlantic Ocean, Rio de Janeiro, Brazil | Pacific Ocean, Jalisco, Mexico | Atlantic Ocean, Florida, USA | ||
| Host | Anisotremus virginicus, Prionotus punctatus | Caranx hippos, Ophioscion scierus, Trachinotus rhodopus | Sardinella aurita | ||
| Range (n = 5) | Mean | Range (n = 9) | Mean | Range (n = 1) | |
| Body length | 1061–1278 | 1157 | 1450–1600 | 1520 | 3000 |
| Body width | 198–310 | 253 | 440–510 | 470 | 500 |
| Ecsoma length | 252 (n = 1) | 252 | 560–880 | 720 | – |
| Total length | 1342 (n = 1) | 1342 | – | – | – |
| Forebody length | 163−239 | 189 | – | – | – |
| Hindbody length | 684−818 | 743 | – | – | – |
| Pre-oral lobe length | 13−19 | 17 | – | – | – |
| Oral sucker length | 74−94 | 82 | 150 | – | 150 |
| Oral sucker width | 73−95 | 82 | – | – | – |
| Pharynx length | 43−56 | 49 | – | – | 80 |
| Pharynx width | 42−54 | 50 | – | – | – |
| Oesophagus length | 20–41 | 32 | – | – | – |
| Ventral sucker length | 186−276 | 212 | 320 | – | 380 |
| Ventral sucker width | 172−281 | 225 | – | – | – |
| DIBAE | 112−179 | 156 | – | – | – |
| DTVS | 32−118 | 66 | – | – | – |
| Anterior testis length | 96−171 | 121 | – | – | – |
| Anterior testis width | 91−114 | 102 | – | – | – |
| Posterior testis length | 95–162 | 114 | – | – | – |
| Posterior testis width | 77–120 | 102 | – | – | – |
| Post-testicular region | 412–547 | 488 | – | – | – |
| Seminal vesicle length | 85−183 | 126 | – | – | – |
| Seminal vesicle width | 29−88 | 53 | – | – | – |
| Sinus-sac length | 125–194 | 154 | – | – | 180–260* |
| Sinus-sac width | 20–32 | 27 | – | – | – |
| Ovary length | 61−92 | 79 | – | – | – |
| Ovary width | 99−125 | 111 | – | – | – |
| Vitellarium length | 99–151 | 123 | – | – | – |
| Vitellarium width | 149–242 | 191 | – | – | – |
| Egg length | 16–19 | 17 | – | – | 17 |
| Egg width | 8–12 | 10 | – | – | 8 |
| Body length/body width | 1:4.61–5.51 | 1:4.66 | – | – | – |
| Oral/ventral sucker width | 1:2.26−3.22 | 1:2.75 | 1:2.3 | – | – |
| Ecsoma/body length, % | 23 (n = 1) | 23 | – | – | – |
| Forebody/body length, % | 15−20 | 16 | – | – | – |
| Post-testicular region/body length, % | 39–45 | 42 | – | – | – |
- Hemiurinae Looss, 1899
- Parahemiurus Vaz and Pereira, 1930
- Parahemiurus merus (Linton, 1910)
- Hosts: Harengula clupeola (Cuvier), Sardinella brasiliensis (Steindachner) (Clupeidae).
- Infection rates: H. clupeola, two out of three; two–six specimens per fish; eight in total; S. brasiliensis, one out of two; two specimens in total.
- Representatives DNA sequences: OP918124, OP918125 (28S).
- Voucher material: CHIOC 39909; CHIOC 39910 a–e.
- Lecithochirinae Lühe, 1901
- Lecithochirium floridense (Manter, 1934)
- Host: Percophis brasiliensis Quoy and Gaimard (Percophidae).
- Infection rates: one out of five; five specimens in total.
- Representatives DNA sequences: OP918131 (28S); OP918025 (cox1).
- Voucher material: CHIOC 39902 a–c.
- Lecithochirium microstomum Chandler, 1935
- Hosts: Prionotus punctatus (Bloch) (Triglidae), Trichiurus lepturus (Linnaeus) (Trichiuridae).
- Infection rates: Pr. punctatus, 1 out of 23; three specimens in total; T. lepturus, one out of one; 10 specimens in total.
- Representative DNA sequences: L. microstomum: OP918119, OP918120, OP918127 (28S); OP918137 (ITS2); OP918021, OP948302, OP918022 (cox1); T. lepturus: OP905634 (cox1).
- Voucher material: CHIOC 39903 a,b; CHIOC 39904 a–g.
- Lecithochirium cf. muraenae Manter, 1940
- Host: Gymnothorax vicinus (Castelnau) (Muraenidae).
- Infection rates: one out of two; four specimens in total.
- Representative DNA sequences: OP918128 (28S); OP918138 (ITS2); OP918023, OP948305 (cox1).
- Voucher material: CHIOC 39901 a–d.
- Lecithochirium synodi Manter, 1931
- Hosts: Anisotremus virginicus (Linnaeus) (Haemulidae), Pseudopercis numida Miranda Ribeiro (Pinguipedidae).
- Infection rates: A. virginicus, one out of five; four specimens in total; Ps. numida, one out of one; three specimens in total.
- Representative DNA sequences: OP918129, OP918130, OP918132 (28S); OP918139 (ITS2); OP918024, OP918026 (cox1); Ps. numida: OP925860 (cox1).
- Voucher material: CHIOC 39906 a,b; CHIOC 39907 a,b.
- Lecithochirium sp.
- Host: Trichiurus lepturus (Linnaeus) (Trichiuridae).
- Infection rates: one out of one; three specimens in total.
- Representative DNA sequences: not available.
- Voucher material: CHIOC 39905.
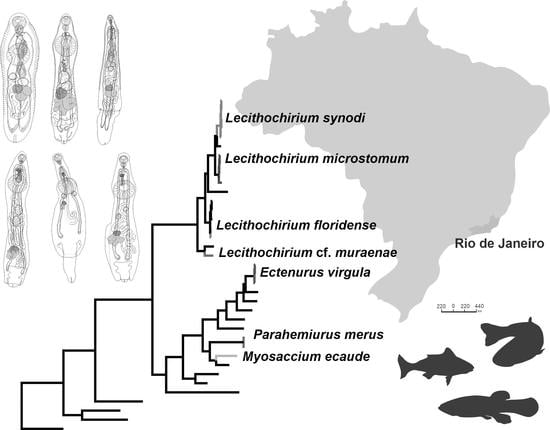
3.2. Molecular Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miloslavich, P.; Klein, E.; Díaz, J.M.; Hernández, C.E.; Bigatti, G.; Campos, L.; Artigas, F.; Castillo, J.; Penchaszadeh, P.; Neill, P.E.; et al. Marine Biodiversity in the Atlantic and Pacific Coasts of South America: Knowledge and Gaps. PLoS ONE 2011, 6, e14631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2022. Available online: https://www.fishbase.org, version (02/2022) (accessed on 20 October 2022).
- Krasnov, B.R.; Poulin, R. Relationships between Parasite Diversity and Host Diversity. In Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics; Morand, S., Krasnov, B.R., Littlewood, D.T.J., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 27–38. [Google Scholar]
- Kohn, A.; Fernandes, B.M.; Cohen, S.C. South American Trematodes Parasites of Fishes; Fiocruz, Instituto Oswaldo Cruz: Rio de Janeiro, Brazil, 2007; p. 318. [Google Scholar]
- Luque, J.; Pereira, F.; Alves, P.; Oliva, M.; Timi, J. Helminth parasites of South American fishes: Current status and characterization as a model for studies of biodiversity. J. Helminthol. 2017, 91, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.A.; Diaz, P.E.; Cribb, T.H. Knowledge of marine fish trematodes of Atlantic and Eastern Pacific Oceans. Syst. Parasitol. 2016, 93, 223–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoja, C.; Telles, B.; Paschoal, F.; Luque, J.L.; Kudlai, O. Digenean trematodes infecting the frigate tuna Auxis thazard (Scombriformes, Scombridae) off the Rio de Janeiro coast, Brazil, including molecular data. Parasite 2022, 29, 44. [Google Scholar] [CrossRef]
- Gibson, D.I. Family Hemiuridae Looss, 1899. In Keys to the Trematoda; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CAB International and the Natural History Museum: London, UK, 2002; Volume 1, pp. 305–340. [Google Scholar]
- Eiras, J.C.; Velloso, A.L.; Pereira, J. Parasitos de Peixes Marinhos da América do Sul; FURG: Rio Grande, Brazil, 2016. [Google Scholar]
- Blair, D.; Bray, R.A.; Barker, S.C. Molecules and morphology in phylogenetic studies of the Hemiuroidea (Digenea: Trematoda: Platyhelminthes). Mol. Phylogenetics Evol. 1998, 9, 15–25. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Beloded, A.Y.; Ngo, H.D.; Ha, N.V.; Tang, N.V. Phylogenetic relationships of Hemiuridae (Digenea: Hemiuroidea) with new morphometric and molecular data of Aphanurus mugilis Tang, 1981 (Aphanurinae) from mullet fish of Vietnam. Parasitol. Int. 2017, 66, 824–830. [Google Scholar] [CrossRef]
- Sokolov, S.G.; Atopkin, D.M.; Urabe, M.; Gordeev, I.I. Phylogenetic analysis of the superfamily Hemiuroidea (Platyhelminthes, Neodermata: Trematoda) based on partial 28S rDNA sequences. Parasitology 2019, 146, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Louvard, C.; Cutmore, S.C.; Yong, R.Q.Y.; Dang, C.; Cribb, T.H. First elucidation of a didymozoid life cycle: Saccularina magnacetabula n. gen. n. sp. infecting an arcid bivalve. Int. J. Parasitol. 2022, 52, 407–425. [Google Scholar] [CrossRef]
- Almeida, F.D.M.; Barquete, V.; Pereira, J., Jr. Progenetic metacercariae of Parahemiurus merus (Platyhelminthes, Digenea, Hemiurudae) infecting Parasagitta friderici (Chaetognatha) from southern coast Brazil. Atlântica 2009, 31, 35–38. [Google Scholar] [CrossRef]
- França, L.F.; Knoff, M.; Fonseca, M.C.D.; Gomes, D.C.; Ferreira, M.S.; Felizardo, N.N.; São Clemente, S.C.; Mattos, D.P.D. Lecithochirium monticellii digenetic trematode parasites of Trichiurus lepturus (Actinopterygii) from the state of Rio de Janeiro, Brazil, with notes on its taxonomy. An. Da Acad. Bras. De Ciências 2020, 92, e20190161. [Google Scholar] [CrossRef]
- Chaves, L.; Paschoal, P. Community ecology of the metazoan parasites of the Atlantic thread herring, Opisthonema oglinum (Lesueur, 1818) (Actinopterygii: Clupeidae) from the Sepetiba Bay, Rio de Janeiro, Brazil. Braz. J. Biol. 2021, 81, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Pleijel, F.; Jondelius, U.; Norlinder, E.; Nygren, A.; Oxelman, B.; Schander, C.; Sundberg, P.; Thollesson, M. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol. Phylogenetics Evol. 2008, 48, 369–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faltýnková, A.; Pantoja, C.; Skírnisson, K.; Kudlai, O. Unexpected diversity in northern Europe: Trematodes from salmonid fishes in Iceland with two new species of Crepidostomum Braun, 1900. Parasitol. Res. 2020, 119, 2439–2462. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, S.; Soldánová, M.; Pérez-Del-Olmo, A.; Dangel, D.R.; Sitko, J.; Sures, B.; Kostadinova, A. Molecular prospecting for European Diplostomum (Digenea: Diplostomidae) reveals cryptic diversity. Int. J. Parasitol. 2013, 43, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Blair, D.; McManus, D. A Molecular Phylogeny of the Human Schistosomes. Mol. Phylogenetics Evol. 1995, 4, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Springer, Y.P.; Keeney, D.B.; Poulin, R. Intra- and interclonal phenotypic and genetic variability of the trematode Maritrema novaezealandensis. Biol. J. Linn. Soc. 2011, 103, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Wee, N.Q.-X.; Cribb, T.H.; Bray, R.A.; Cutmore, S.C. Two known and one new species of Proctoeces from Australian teleosts: Variable host-specificity for closely related species identified through multi-locus molecular data. Parasitol. Int. 2017, 66, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Snyder, S.D.; Tkach, V.V. Phylogenetic and biogeographical relationships among some holarctic frog lung flukes (Digenea: Haematoloechidae). J. Parasitol. 2001, 87, 1433–1440. [Google Scholar] [CrossRef]
- Galazzo, D.E.; Dayanandan, S.; Marcogliese, D.J.; McLaughlin, J.D. Molecular systematics of some North American species of Diplostomum (Digenea) based on rDNA-sequence data and comparisons with European congeners. Can. J. Zool. 2002, 80, 2207–2217. [Google Scholar] [CrossRef] [Green Version]
- Tkach, V.V.; Littlewood, D.T.; Olson, P.D.; Kinsella, J.M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Adlard, R.D.; Bray, R.A. A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). Int. J. Parasitol. 1998, 28, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Cutmore, S.C.; Miller, T.L.; Curran, S.S.; Bennett, M.B.; Cribb, T.H. Phylogenetic relationships of the Gorgoderidae (Platyhelminthes: Trematoda), including the proposal of a new subfamily (Degeneriinae n. subfam.). Parasitol. Res. 2013, 112, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, D.T.; Curini-Galletti, M.; Herniou, E.A. The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol. Phylogenetics Evol. 2000, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, D.T.; Rohde, K.; Clough, K.A. Parasite speciation within or between host species?—Phylogenetic evidence from site-specific polystome monogeneans. Int. J. Parasitol. 1997, 27, 1289–1297. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science 1321 Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE); Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2010; pp. 1–8. [Google Scholar]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Menezes, N.A.; Figueiredo, J.L. Manual de Peixes Marinhos do Sudeste do Brasil v Teleostei (4); Museu de Zoologia USP: São Paulo, Brasil, 1985; 105p. [Google Scholar]
- Montgomery, W.R. Studies on Digenetic Trematodes from Marine Fishes of La Jolla, California. Trans. Am. Microsc. Soc. 1957, 76, 13–36. [Google Scholar] [CrossRef]
- León-Règagnon, V.; Pérez-Ponce de León, G.; Lamothe-Argumedo, R. Hemiuriformes de peces marinos de la Bahía de Chamela, México, con la descripción de una nueva especie del género Hysterolecitha (Digenea: Hemiuridae: Lecithasterinae). Ann. Inst. Biol. Zool. Ser. 1997, 68, 1–34. [Google Scholar]
- Pérez-Ponce de León, G.; García Prieto, L.; Rosas Villa, C. Helmintofauna de Opisthonema libertate y Harengula thrissina (Osteichthyes: Clupeidae) de la bahía de Chamela, Jalisco, México. Rev. De Biol. Trop. 2000, 48, 759–763. [Google Scholar]
- Sánchez-Serrano, S.; Cáceres-Martínez, J. Primer registro helmintológico de la sardina monterrey Sardinops sagax en Baja California, México, durante dos estaciones del año. Hidrobiológica 2017, 27, 1–11. [Google Scholar]
- Jacobson, K.; Baldwin, R.; Banks, M.; Emmett, R. Use of parasites to clarify residency and migration patterns of Pacific sardine (Sardinops sagax) in the California Current. Fish. Bull. 2019, 117, 196–210. [Google Scholar] [CrossRef]
- Del-Río-Zaragoza, O.B.; Hernández-Rodríguez, M.; Vivanco-Aranda, M.; Zavala-Hamz, V.A. Blood parameters and parasitic load in Sardinops sagax (Jenyns, 1842) from Todos Santos Bay, Baja California, Mexico. Lat. Am. J. Aquat. Res. 2018, 46, 1110–1115. [Google Scholar] [CrossRef]
- Luque, J.L.; Vinas, R.A.; Paraguassú, A.R.; Alves, D.R. Metazoários Parasitos das sardinhas Sardinella brasiliensis e Harengula clupeola (Osteichthyes, Clupeidae) do litoral do Estado do Rio de Janeiro, Brasil. Rev. Univ. Rural. Ser. Ciências Da Vida 2000, 22, 71–76. [Google Scholar]
- Moreira, J.; Paschoal, F.; Cezar, A.; Luque, J. Community ecology of the metazoan parasites of Brazilian sardinella, Sardinella brasiliensis (Steindachner, 1879) (Actinopterygii: Clupeidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Braz. J. Biol. 2015, 75, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Overstreet, R.M. Digenetic Trematodes of Marine Teleost Fishes from Biscayne Bay, Florida; University of Miami: Coral Gables, FL, USA, 1969. [Google Scholar]
- Siddiqi, A.H.; Cable, R.M. Digenetic trematodes of marine fishes of Puerto Rico. Sci. Surv. Porto Rico Virgin Isl. 1960, 17, 257–369. [Google Scholar]
- Kohn, A.; Buhrnheim, P.F. A new host and new geographic distribution for Myosaccium ecaude Montgomery, 1957 (Trematoda, Hemiuridae). Atas Soc. Biol. 1964, 8, 50–52. [Google Scholar]
- Linton, E. Helminth Fauna of the Dry Tortugas II. Trematodes. In Papers from the Tortugas Laboratory of the Carnegie Institute of Washington; General Books LLC: Memphis, TN, USA, 1910; Volume 4, pp. 11–98. [Google Scholar]
- Manter, H.W. The digenetic trematodes of marine fishes of Tortugas, Florida. Am. Midl. Nat. 1947, 38, 257–416. [Google Scholar] [CrossRef]
- Sparks, A.K. Some digenetic trematodes of marine fishes of the Bahama Islands. Bull. Mar. Sci. Gulf Caribb. 1957, 7, 255–265. [Google Scholar]
- Szidat, L. Versuch Einer Zoogeographie des Sud-Atlantik Mit Hilfe von Leitparasiten der Meeresfische; Parasitologische Schriftenreihe; Fischer: New York, NY, USA, 1961; Volume 13, pp. 1–98. [Google Scholar]
- Nahhas, F.M.; Cable, R.M. Digenetic and aspidogastrid trematodes from marine fishes of Curaçao and Jamaica. Tulane Stud. Zool. 1964, 11, 169–228. [Google Scholar] [CrossRef]
- Rees, G. Some helminth parasites of fishes of Bermuda and an account of the attachment organ of Alcicornis carangis MacCallum,1917 (Digenea: Bucephalidae). Parasitology 1970, 60, 195–221. [Google Scholar] [CrossRef]
- Fischthal, J.H.; Thomas, J.D. Some hemiurid trematodes of marine fishes from Ghana. Helminthol. Soc. Wash. 1971, 38, 181–189. [Google Scholar]
- Fischthal, J.H.; Thomas, J.D. Digenetic trematodes of fish from the Volta River drainage system in Ghana prior to the construction of the Volta Dam at Akosombo in May 1964. J. Helminthol. 1972, 46, 91–105. [Google Scholar] [CrossRef]
- Parukhin, A.M. Parasitic Worms of Food Fishes from the Southern Seas; Akademiya Nauk Ukrainskoi SSR, Ordena Trudovogo Krasnogo Zhameni Institut Biologii Yuzhnykh Morei; Kobalevskogo, A.O., Ed.; Izdatelstvo Naukova Dumka: Kiev, Ukraine, 1976; p. 182. (In Russian) [Google Scholar]
- Braicovich, P.E.; Pantoja, C.; Pereira, A.N.; Luque, J.L.; Timi, J.T. Parasites of the Brazilian flathead Percophis brasiliensis reflect West Atlantic biogeographic regions. Parasitology 2017, 144, 169–178. [Google Scholar] [CrossRef]
- Travassos, L.; Freitas, J.F.T.; Btihrnheim, P.F. Relatório da excursão do Instituto Oswaldo Cruz ao estado do Espírito Santo em novembro de 1964. Bol. Do Mus. De Biol. Mello Leitão 1967, 31, 1–5. [Google Scholar]
- Gibson, D.I.; Bray, R.A. Hemiuridae (Digenea) of fishes from the north-east Atlantic. British Museum (Natural History). Bull. Br. Mus. Zool. Ser. 1986, 51, 1–125. [Google Scholar]
- Pereira, A.N.; Pantoja, C.; Luque, J.L.; Timi, J.T. Parasites of Urophycis brasiliensis (Gadiformes: Phycidae) as indicators of marine ecoregions in coastal areas of the South American Atlantic. Parasitol. Res. 2014, 113, 4281–4292. [Google Scholar] [CrossRef]
- Bray, R.A. Hemiuridae (Digenea) from marine fishes of the southern Indian Ocean: Dinurinae, Elytrophallinae, Glomericirrinae and Plerurinae. Syst. Parasitol. 1990, 17, 183–217. [Google Scholar] [CrossRef]
- Vaz, Z.; Pereira, C. Nouvel hemiuride parasite de Sardinella aurita Cuv. et al., Parahemiurus n.g. Compte Rendu Des Séances De La Société De Biol. 1930, 103, 1315–1317. [Google Scholar]
- Timi, J.T.; Martorelli, S.R.; Sardella, N.H. Digenetic trematodes parasitic on Engraulis anchoita (Pisces: Engraulidae) from Argentina and Uruguay. Folia Parasitol. 1999, 46, 132–138. [Google Scholar]
- Bray, R.A. A review of the genus Parahemiurus Vaz & Pereira, 1930 (Digenea: Hemiuridae). Syst. Parasitol. 1990, 15, 1–21. [Google Scholar]
- Wallet, M.; Kohn, A. Trématodes parasites de poissons marins du littoral de Rio de Janeiro, Brésil. Memórias Do Inst. Oswaldo Cruz 1987, 82, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Benicio, L.; Moreira, J.; Paschoal, F. Community ecology of the metazoan parasites of the Atlantic anchoveta, Cetengraulis edentulus (Actinopterygii: Engraulidae) from the Sepetiba Bay, Rio de Janeiro, Brazil. Zoologia 2022, 39, e21034. [Google Scholar] [CrossRef]
- Bray, R.A. Hemiuridae (Digenea) from marine fishes of the southern Indian Ocean: Genus Lecithochirium Lühe, 1901 (Lecithochiriinae). Syst. Parasitol. 1991, 18, 193–219. [Google Scholar] [CrossRef]
- Teixeira de Freitas, J.F.; Gomes, D.C. Sobre uma nova espécie do gênero Lecithochirium Luehe, 1901, (Trematoda, Hemiuroidea). Memórias Do Inst. Oswaldo Cruz 1971, 69, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Chandler, A.C. Parasites of fishes in Galveston Bay. Proc. United States Natl. Mus. 1935, 83, 123–157. [Google Scholar] [CrossRef]
- Teixeira de Freitas, J.F.; Kohn, A. Nova espécie do gênero Glomericirrus Yamaguti, 1937: (Trematoda, Hemiuridae). Memórias Do Inst. Oswaldo Cruz 1965, 63, 229–235. [Google Scholar] [CrossRef]
- Fernandes, B.M.; Arci, A.D.; Cohen, S.C. New data on some species of Monogenea and Digenea parasites of marine fish from the coast of the State of Rio de Janeiro, Brazil. Rev. Bras. De Parasitol. Veterinária 2009, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-F.; Peng, W.-F.; Gao, P.; Fu, M.-J.; Wu, H.-Z.; Lu, M.-K.; Gao, J.-Q.; Xiao, J. Digenean parasites of Chinese marine fishes: A list of species, hosts and geographical distribution. Syst. Parasitol. 2010, 75, 1–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-García, M.A.; García-Prieto, L.; Garrido-Olvera, L. Description of a new species of Pseudopecoelus (Trematoda: Opecoelidae) with new records of trematodes of marine fishes from the Pacific coast of Mexico. Rev. Mex. Biodivers. 2018, 89, 22–28. [Google Scholar] [CrossRef]
- Razarihelisoa, M. Sur quelques trématodes digènes de poissons de Nossibé (Madagascar). Bull. De La Société Zool. De Fr. 1959, 84, 421–434. [Google Scholar]
- Kardousha, M.M. Redescription of ten species of digenetic trematodes from Marine fishes from the Emirati Coasts of the Arabian Gulf. Arab. Gulf J. Sci. Res. 2003, 21, 217–226. [Google Scholar]
- Silva, L.O.; Luque, J.L.; Alves, D.R. Metazoan parasites of the Atlantic cutlassfish, Trichiurus lepturus (Osteichthyes: Trichiuridae) from coastal zone of the State of Rio de Janeiro, Brazil. Parasitol. Al Dia 2000, 24, 97–101. [Google Scholar]
- Silva, L.O.; Luque, J.L.; Alves, D.R.; Paraguassu, A.R. Ecologia da comunidadeparasitaria do peixe-espada Trichiurus lepturus (Osteichthyes: Trichiuridae) do litoral do estado do Rio de Janeiro, Brasil. Rev. Bras. De Zoociências 2000, 2, 115–133. [Google Scholar]
- Carvalho, A.R.; Luque, J.L. Seasonal variation in metazoan parasites of Trichiurus lepturus (Perciformes: Trichiuridae) of Rio de Janeiro, Brazil. Braz. J. Biol. 2011, 71, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Manter, H.W. Digenetic Trematodes of Fishes from the Galapagos Islands and the Neighboring Pacific; Allan Hancock Pacific Expeditions; The University of Southern California Publications: Los Angeles, CA, USA, 1940; Volume 2, pp. 325–497. [Google Scholar]
- Tantaleán, M.V.; Sarmiento, L.B.; Huiza, A.F. Digeneos (Trematoda) del Peru. Bol. De Lima 1992, 14, 47–84. [Google Scholar]
- Linton, E. Notes on trematode parasites of fishes. Proc. United States Natl. Mus. Wash. 1898, 20, 507–548. [Google Scholar] [CrossRef]
- Gu, C.D.; Shen, J.W. Digenetic trematodes of ribbonfish, Trichiurus haumela (Forskal) and their distribution in the fishing grounds of China Seas. Acta Zool. Sin. 1981, 27, 53–63. [Google Scholar]
- Krupenko, D.Y.; Gonchar, A.G.; Kremnev, G.A.; Uryadova, A.A. On the life cycle of Hemiurus levinseni Odhner, 1905 (Digenea: Hemiuridae). Invertebr. Zoöl. 2020, 17, 205–218. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. 2022. Available online: https://www.marinespecies.org (accessed on 15 October 2022).
- Bullard, S.A.; Barse, A.M.; Curran, S.S.; Morris, J.A., Jr. First record of a digenean from invasive Lionfish, Pterois cf. volitans, (Scorpaeniformes: Scorpaenidae) in the Northwestern Atlantic Ocean. J. Parasitol. 2011, 97, 833–837. [Google Scholar] [PubMed]
- Pérez-Ponce de León, G.; León-Règagnon, V.; Scott, M. Theletrum lamothei sp. nov. (Digenea), parasite of Echidna nocturna from Cuajiniquil, Guanacaste, and other digenes of marine fishes from Costa Rica. Rev. De Biol. Trop. 1998, 46, 345–354. [Google Scholar]
- Calhoun, D.M.; Curran, S.S.; Pulis, E.E.; Provaznik, J.M.; Franks, J.S. Hirudinella ventricosa (Pallas, 1774) Baird, 1853 represents a species complex based on ribosomal DNA. Syst. Parasitol. 2013, 86, 197–208. [Google Scholar] [CrossRef]
- Pérez-Ponce de León, G.; Hernández-Mena, D.I. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J. Helminthol. 2019, 93, 260–276. [Google Scholar] [CrossRef] [Green Version]
- Chan-Martin, A.D.J.; Castellanos-Martínez, S.; Aguirre-Macedo, M.; Martínez-Aquino, A. Immature trematodes of Lecithochirium sp. (Digenea: Hemiuridae) in the California two-spot octopus (Octopus bimaculatus) from Mexico. Parasitol. Res. 2022, 121, 2651–2660. [Google Scholar] [CrossRef]
- Madhavi, R.; Bray, R.A. Digenetic Trematodes of Indian Marine Fishes; Springer: Heidelberg, Germany, 2018. [Google Scholar]
- Faltýnková, A.; Georgieva, S.; Kostadinova, A.; Bray, R.A. Biodiversity and evolution of digeneans of fishes in the Southern Ocean. In Biodiversity and Evolution of Parasitic Life in the Southern Ocean; Parasitology Research Monographs; Klimpel, S., Kuhn, T., Mehlhorn, H., Eds.; Springer: Cham, Switzerland, 2017; Volume 9, pp. 49–75. [Google Scholar]
- Luque, J.L.; Poulin, R. Metazoan parasite species richness in Neotropical fishes: Hotspots and the geography of biodiversity. Parasitology 2007, 134, 865–878. [Google Scholar] [CrossRef]




| Source | Present Study | León-Règagnon et al. [39] | Linton [49] | Vaz and Pereira [63] | Timi et al. [64] | Bray [65] | |||
|---|---|---|---|---|---|---|---|---|---|
| Locality | Atlantic Ocean, Rio de Janeiro, Brazil | Pacific Ocean, Jalisco, Mexico | Atlantic Ocean, Florida, USA | Atlantic Ocean, São Paulo, Brazil | Atlantic Ocean, Argentinean and Uruguayan coasts | Atlantic, Indian, and Pacific oceans | |||
| Host | Harengula clupeola, Sardinella brasiliensis | Balsitidae, Clupeidae, Engraulidae, Haemulidae | Sardinella aurita | Sardinella aurita | Eugraulis anchoita | Carangidae, Clupeidae, Haemulidae, Merlucciidae, Pomatomidae, Salmonidae, Scorpaenidae, Sparidae | |||
| Range (n = 4) | Mean | Range (n = 4) | Mean | Range (n = 1) | Range (n = NP) | Range (n = 20) | Mean | Range (n = NP) | |
| Body length | 704–1619 | 1067 | 890–1580 | 1100 | 2940 | 1510–1930 | 720–1441 | 987 | 800–2990 |
| Body width | 146–407 | 260 | 210–480 | 290 | 500 | 360–400 | 120–247 | 171 | 210–570 |
| Ecsoma length | 257−612 | 435 | 180–680 | 420 | – | 480–500 | – | – | Extend to 1020 |
| Total length | 1061–2231 | 1646 | – | – | – | – | – | – | – |
| Forebody length | 118−360 | 207 | – | – | – | – | – | – | – |
| Hindbody length | 479−2062 | 1083 | – | – | – | – | – | – | – |
| Pre-oral lobe length | 7−22 | 12 | – | – | – | – | 8–36 | 15 | 8–50 |
| Oral sucker length | 53−83 | 65 | 40–60 | 50 | 90 | 80 | 50–80 | 63 | 36–83 |
| Oral sucker width | 53−86 | 68 | 60–70 | 60 | – | 80 | 53–80 | 66 | 51–82 |
| Pharynx length | 39−57 | 47 | – | – | 70 | 40 | 33–50 | 41 | 32–64 |
| Pharynx width | 37−58 | 44 | – | – | – | 40 | 30–50 | 40 | 32–51 |
| Oesophagus length | 10–37 | 26 | – | – | – | – | – | – | – |
| Ventral sucker length | 101−182 | 133 | 120–150 | 140 | 210 | 180–200 | 106–165 | 133 | 101–170 |
| Ventral sucker width | 103−186 | 139 | 100–190 | 130 | – | – | 99–200 | 137 | 124–170 |
| DIBAE | 122−187 | 147 | – | – | – | – | – | – | – |
| DTVS | 114–377 | 203 | – | – | – | – | – | – | – |
| Anterior testis length | 45−82 | 70 | – | – | – | 60–120 | 40–83 | 64 | 40–152 |
| Anterior testis width | 34−89 | 61 | – | – | – | – | 50–132 | 78 | 68–145 |
| Posterior testis length | 39–87 | 69 | – | – | – | – | 36–90 | 74 | 48–170 |
| Posterior testis width | 42–74 | 61 | – | – | – | – | 46–125 | 88 | 64–170 |
| Post-testicular region length | 300–886 | 516 | – | – | – | – | – | – | – |
| Seminal vesicle length | 58−177 | 107 | – | – | – | – | 50–96 | 73 | 48–278 |
| Seminal vesicle width | 27−105 | 58 | – | – | – | – | 36–63 | 46 | 41–130 |
| Sinus-sac length | 128–256 | 176 | – | – | – | 120–180 | – | – | 95–220 |
| Sinus-sac width | 14–30 | 19 | – | – | – | – | – | – | – |
| Ovary length | 32−93 | 65 | – | – | – | 80–160 | 40–102 | 73 | 47–234 |
| Ovary width | 40−129 | 87 | – | – | – | – | 76–149 | 104 | 80–233 |
| Vitellarium length (dextral) | 42–131 | 75 | – | – | – | 80–180 | 56–132 | 97 | – |
| Vitellarium width (dextral) | 43–93 | 66 | – | – | – | – | 43–116 | 80 | – |
| Vitellarium length (sinistral) | 38–129 | 72 | – | – | – | – | 59–178 | 118 | – |
| Vitellarium width (sinistral) | 46–119 | 77 | – | – | – | – | 50–142 | 99 | – |
| DBOT | 114–377 | 203 | – | – | – | – | – | – | – |
| Egg length | 22–27 | 25 | 20–25 | 22 | 27 | 24 | 24–32 | 28 | 20–32 |
| Egg width | 7–11 | 9 | 9–13 | 11 | 10 | 10–14 | 8–12 | 10 | – |
| Body length/body width | 1:2.83–5.65 | 1:4.32 | – | – | – | – | – | – | – |
| Oral/ventral sucker width | 1:1.94−2.16 | 1:2.03 | 1:2.90 | – | – | – | 1:1.71–2.5 | 1:1.58 | 1:1.76–2.58 |
| Ecsoma/body length, % | 32−38 | 35 | – | – | – | – | – | – | – |
| Forebody/body length, % | 14−32 | 19 | – | – | – | – | – | – | 6–19 |
| Post-testicular region/body length, % | 37–55 | 46 | – | – | – | – | – | – | – |
| Source | Present Study | León-Règagnon et al. [39] | Timi et al. [64] | Wallet and Kohn [66] | Chandler [70] | Teixeira de Freitas and Kohn [71] | |||
|---|---|---|---|---|---|---|---|---|---|
| Locality | Atlantic Ocean, Rio de Janeiro, Brazil | Atlantic Ocean, Chamela Bay, Mexico | Atlantic Ocean, Argentinean and Uruguayan coasts | Atlantic Ocean, Rio de Janeiro, Brazil | Atlantic Ocean, Texas, USA | Atlantic Ocean, Rio de Janeiro, Brazil | |||
| Host | Prionotus punctatus, Trichiurus lepturus | Carangidae, Engraulidae, Fistularidae, Lutjanidae, Scombridae | Engraulis anchoita | Trichiurus lepturus | Trichiurus lepturus | Trichiurus lepturus | |||
| Range (n = 6) | Mean | Range (n = 10) | Mean | Range (n = 20) | Mean | Range (n = 3) | Range (n = NP) | Range (n = NP) | |
| Body length | 3104–4292 | 3734 | 1830−3920 | 2940 | 744−1592 | 1034 | 3640–4270 | 2750–4800 | 3330−5170 |
| Body width | 641–769 | 690 | 450–810 | 600 | 224−496 | 323 | 570−840 | 875–1000 | 750−1170 |
| Ecsoma length | 974–1351 (n = 4) | 1135 | − | − | 112−384 | 219 | − | 1000 | − |
| Total length | 4078–6418 (n = 4) | 5084 | − | − | − | − | − | 3760 (n = 1) | − |
| Forebody length | 520−732 | 635 | − | − | − | − | − | − | − |
| Hindbody length | 2215−3119 | 2616 | − | − | − | − | − | − | − |
| Pre-oral lobe length | 33−73 | 51 | − | − | 11−40 | 21 | − | − | − |
| Oral sucker length | 170−198 | 184 | 90−180 | 130 | 88−145 | 120 | 140−150 | 140–200 | 170−220 |
| Oral sucker width | 164−202 | 180 | 110−180 | 150 | 97−160 | 121 | 180−190 | − | 200−250 |
| Pharynx length | 95−106 | 99 | − | − | 53−78 | 62 | 190−210 | 70–110 | 70−120 |
| Pharynx width | 93−111 | 99 | − | − | 46−82 | 65 | − | − | 100–120 |
| Oesophagus length | 38–84 | 56 | − | − | − | − | − | − | − |
| Ventral sucker length | 413−555 | 485 | 280−540 | 450 | 155−309 | 208 | 470−480 | 365−540 | 330−610 |
| Ventral sucker width | 450−536 | 502 | 310−490 | 440 | 153−296 | 208 | 510−610 | − | 400−640 |
| DIBAE | 296−350 | 334 | − | − | − | − | − | − | − |
| DTVS | 209−409 | 304 | − | − | − | − | − | − | − |
| Anterior testis length | 244−302 | 274 | − | − | 23−118 | 55 | 180−230 | − | 200−370 |
| Anterior testis width | 157−243 | 194 | − | − | 25−141 | 62 | 200−280 | − | 180−420 |
| Posterior testis length | 270–305 | 288 | − | − | − | − | − | − | 230−380 |
| Posterior testis width | 187–242 | 212 | − | − | − | − | − | − | 250−450 |
| Post-testicular region | 1295–2335 | 1711 | − | − | − | − | − | − | − |
| Seminal vesicle length | 188−462 | 360 | − | − | 65−153 | 91 | − | − | 670−900 |
| Seminal vesicle width | 65−236 | 164 | − | − | 25−74 | 43 | − | − | 170−230 |
| Sinus-sac length | 139–221 | 172 | − | − | − | − | − | − | − |
| Sinus-sac width | 103–154 | 120 | − | − | − | − | − | − | − |
| Ovary length | 184−271 | 232 | − | − | 32−99 | 53 | 150−180 | − | 220−400 |
| Ovary width | 239−292 | 260 | − | − | 40−147 | 71 | 200−280 | − | 330−430 |
| Vitellarium length | 246–357 | 314 | − | − | − | − | − | − | − |
| Vitellarium width | 211–442 | 285 | − | − | − | − | − | − | − |
| DBOT | 467–600 | 532 | − | − | − | ||||
| Egg length | 18–26 | 21 | 15–21 | 18 | 15–19 | 16 | 19−23 | 16 | 15−22 |
| Egg width | 10–15 | 11 | 9−13 | 11 | 8–11 | 9 | 9−14 | 12 | 11−13 |
| Body length/body width | 1:4.84–5.93 | 1:5.41 | − | − | − | − | − | − | − |
| Oral/ventral sucker width | 1:2.53−3.12 | 1:2.82 | 1:2.9−4.6 | 1:3.5 | − | − | 1:3.0−3.30 | 1:2.50–2.80 | 1:2.12−2.97 |
| Ecsoma/body length, % | 29−31 (n = 4) | 30 | − | − | − | − | − | − | − |
| Forebody/body length, % | 16−20 | 17 | − | − | − | − | − | − | − |
| Post-testicular region/body length, % | 39–54 | 45 | − | − | − | − | − | − | − |
| Species | Lecithochirium cf. muraenae | Lecithochirium muraenae Manter, 1940 | Lecithochirium sp. | Lecithochirium monticellii Linton (1898) | Lecithochirium monticellii | |||
|---|---|---|---|---|---|---|---|---|
| Source | Present study | Manter [80] | Present study | França et al. [15] | França et al. [15] | |||
| Locality | Atlantic Ocean, Rio de Janeiro, Brazil | Pacific Ocean, Cape Elena, Ecuador | Atlantic Ocean, Rio de Janeiro, Brazil | Atlantic Ocean, Rio de Janeiro, Brazil | Atlantic Ocean, Rio de Janeiro, Brazil | |||
| Host | Gymnothorax vicinus | Muraena clepsydra | Trichiurus lepturus | Trichiurus lepturus | Trichiurus lepturus | |||
| Range (n = 3) | Mean | (n = 3) | (n = 1) | Range (n = 10) | Range (n = 10) | Mean | ||
| Body length | 2206–2415 | 2276 | 3684–5359 | 2607 | 2020–4020 | 2840 | 1020–1900 | 1400 |
| Body width | 610–889 | 742 | 958–1120 | 694 | 270–520 | 400 | 220–500 | 350 |
| Ecsoma length | 520 (n = 1) | 520 | – | – | 150–1520 | 580 | 170–500 | 250 |
| Total length | 2726 (n = 1) | 2726 | – | – | – | – | – | – |
| Forebody length | 455−530 | 499 | 756–1188 | 552 | – | – | – | – |
| Hindbody length | 1353−1466 | 1394 | 1608 | – | – | – | – | |
| Pre-oral lobe length | 12−33 | 22 | 60 | – | – | – | – | |
| Oral sucker length | 135−163 | 149 | 300–375 | 173 | 60–140 | 100 | 70–130 | 100 |
| Oral sucker width | 154−197 | 169 | – | 182 | – | – | – | – |
| Pharynx length | 85−95 | 90 | 120–170 | 111 | – | – | – | – |
| Pharynx width | 83−112 | 94 | 75–120 | 92 | – | – | – | – |
| Oesophagus length | 19–42 | 30 | – | 108 | – | – | – | – |
| Ventral sucker length | 366−454 | 398 | 715–883 | 424 | 210–410 | 230 | 150–310 | 260 |
| Ventral sucker width | 358−465 | 403 | – | 447 | – | – | – | – |
| DIBAE | 272−317 | 287 | – | 393 | – | – | – | – |
| DTVS | − | − | − | 204 | − | − | − | − |
| Anterior testis length | 116−120 | 114 | – | 91 | – | – | – | – |
| Anterior testis width | 98−113 | 108 | – | 72 | 50–200 | 120 | 50–130 | 80 |
| Posterior testis length | 96–103 | 101 | – | 117 | 50–190 | 90 | 50–110 | 90 |
| Posterior testis width | 111–128 | 119 | – | 100 | 60–210 | 140 | 60–150 | 80 |
| Post-testicular region | 1251–1383 | 1304 | – | 1273 | 60–170 | 110 | 60–130 | 80 |
| Seminal vesicle length | 267−321 | 287 | – | 217 | 110–340 | 200 | 50–170 | 100 |
| Seminal vesicle width | 109−131 | 118 | – | 87 | – | – | – | – |
| Sinus-sac length | 116–129 | 123 | – | 179 | 50–100 | 70 | 20–100 | – |
| Sinus-sac width | 45–46 | 46 | – | 46 | – | – | – | 60 |
| Ovary length | 75−89 | 81 | – | 100 | 50–220 | 110 | 50–130 | 70 |
| Ovary width | 99−162 | 127 | – | 158 | 60–200 | 130 | 60–150 | 100 |
| Vitellarium length | 78–130 | 101 | – | 344 | – | – | – | – |
| Vitellarium width | 98–161 | 124 | – | 230 | – | – | – | – |
| DBOT | 48–72 | 63 | – | 291 | – | – | – | – |
| Egg length | 10–14 | 12 | 15–19 | 11–15 | 23–28 | 24 | 15–28 | 18 |
| Egg width | 9–13 | 11 | 10–12 | 9–11 | 10–13 | 11 | 10–13 | 10 |
| Body length/body width | 1:2.72–3.62 | 1:3.12 | – | 1:3.76 | – | – | – | – |
| Oral/ventral sucker width | 1:2.32−2.47 | 1:2.38 | 1:3.70 | 1:2.46 | 1:1.95–2.41 | – | 1:1.73–2.10 | – |
| Ecsoma/body length, % | 24 (n = 1) | 24 | – | – | – | – | – | – |
| Forebody/body length, % | 21−24 | 22 | – | 21 | – | – | – | – |
| Post-testicular region/body length, % | 57–58 | 57 | – | 49 | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantoja, C.; Kudlai, O. Hemiurid Trematodes (Digenea: Hemiuridae) from Marine Fishes off the Coast of Rio de Janeiro, Brazil, with Novel Molecular Data. Animals 2022, 12, 3355. https://doi.org/10.3390/ani12233355
Pantoja C, Kudlai O. Hemiurid Trematodes (Digenea: Hemiuridae) from Marine Fishes off the Coast of Rio de Janeiro, Brazil, with Novel Molecular Data. Animals. 2022; 12(23):3355. https://doi.org/10.3390/ani12233355
Chicago/Turabian StylePantoja, Camila, and Olena Kudlai. 2022. "Hemiurid Trematodes (Digenea: Hemiuridae) from Marine Fishes off the Coast of Rio de Janeiro, Brazil, with Novel Molecular Data" Animals 12, no. 23: 3355. https://doi.org/10.3390/ani12233355