Superior Antioxidant Capacity of Berberis iliensis—HPLC-Q-TOF-MS Based Phytochemical Studies and Spectrophotometric Determinations
Abstract
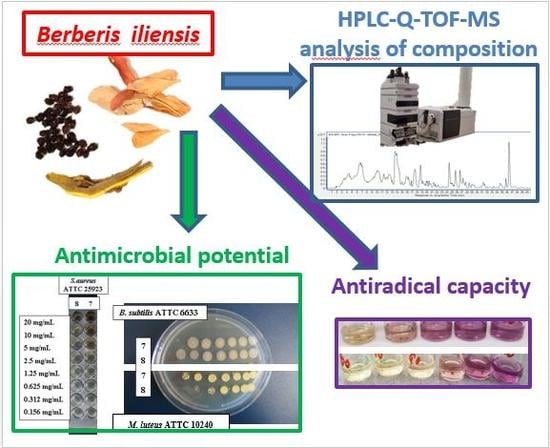
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction
2.4. The Conditions of Qualitative and Quantitative HPLC-ESI-Q-TOF-MS Analyses
2.5. The Determination of the Antioxidant Potential of the Obtained Extracts by Spectrophotometric Methods
2.5.1. 2,2-Diphenyl-1-Picrylhydrazyl Assay
2.5.2. The Determination of the Total Phenolic Content (TPC)
2.6. In Vitro Anitimicrobial Assay
3. Results and Discussion
3.1. The Extracts Profiling by HPLC-ESI-Q-TOF-MS
3.2. Quantitative Analysis of Alkaloids and Phenolics in the Extracts
3.3. The Determination of the Antioxidant Potential of Various Organs and Extracts from Berberis iliensis
3.4. The Antimicrobial Activity Assessment of Berberis iliensis Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rashmi, A.; Rajasekaran, A.; Pant, J. The genus Berberis Linn.: A review. Pharmacogn. Rev. 2008, 2, 369–385. [Google Scholar]
- Belov, N.V. Calendula, Marshmallow, Celandine and Other Folk Medicinal Plants in the Great Encyclopedia of Herbal Medicine; Mn. AST: Moscow, Russia, 2005; pp. 31–33. ISBN 5-17-031-498-1. [Google Scholar]
- Grinkevich, N.I.; Balandina, I.A.; Ermakova, V.A. Medicinal Plants: Reference Guide; High School: Moscow, Russia, 1991. [Google Scholar]
- Srivastava, S.; Srivastava, M.; Misra, A.; Pandey, G.; Rawat, A.K.S. A review on biological and chemical diversity in Berberis (Berberidaceae). EXCLI J. 2015, 14, 247–267. [Google Scholar] [PubMed]
- Baratova, M.R.; Saribaeva, N.N.; Rakhimov, A.D.; Karimova, M.K.; Mirkhomidova, N.A. Medicinal properties of barberry. Mod Trends Dev Sci Technol. 2015, 8, 80–81. [Google Scholar]
- Popov, A.P. Forest Medicinal Plants, 2nd ed.; Ekologiya: Moscow, Russia, 1992; ISBN 5-7120-0702-9. [Google Scholar]
- Pozharskiy, A.S.; Chekalin, S.V. Molecular study of Berberis iliensis M. Pop. and Berberis sphaerocarpa Kar. et Kir. wild populations in South-East Kazakhstan using ISSR markers. Int. J. Biol. Chem. 2015, 8, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Kukula-Koch, W. The Elevation of LC-ESI-Q-TOF-MS Response in the Analysis of Isoquinoline Alkaloids from Some Papaveraceae and Berberidaceae Representatives. J. Anal. Meth. Chem. 2017, 2017, 8384107. [Google Scholar] [CrossRef] [Green Version]
- Kukula-Koch, W.; Aligiannis, N.; Halabalaki, M.; Skaltsounis, A.L.; Glowniak, K.; Kalpoutzakis, E. Influence of extraction procedures on phenolic content and antioxidant activity of Cretan barberry herb. Food Chem. 2013, 138, 406–413. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Koch, W.; Angelis, A.; Halabalaki, M.; Aligiannis, N. Application of pH-zone refining hydrostatic countercurrent chromatography (hCCC) for the recovery of antioxidant phenolics and the isolation of alkaloids from Siberian barberry herb. Food Chem. 2016, 203, 394–401. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent Author links open overlay panel. Met. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Popiołek, Ł.; Biernasiuk, A.; Malm, A. Synthesis and antimicrobial activity of new 1,3-thiazolidin-4-one derivatives obtained from carboxylic acid hydrazides. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 251–260. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Olech, M.; Pietrzak, W.; Nowak, R. Characterization of free and bound phenolic acids and flavonoid aglycones in Rosa rugosa Thunb. leaves and achenes using LC-ESI-MS/MS-MRM methods. Molecules 2020, 25, 1804. [Google Scholar] [CrossRef] [Green Version]
- Mohamed Isa, S.S.P.; Ablat, A.; Mohamad, J. The Antioxidant and Xanthine Oxidase Inhibitory Activity of Plumeria rubra Flowers. Molecules 2018, 23, 400. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix tetrasperma Impair Virulence and Inhibit Quorum Sensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garran, T.A.; Ji, R.; Chen, J.L.; Xie, D.; Guo, L.; Huang, L.Q.; Lai, C. Elucidation of metabolite isomers of Leonurus japonicus and Leonurus cardiaca using discriminating metabolite isomerism strategy based on ultra-high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1598, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol Composition and (Bio)Activity of Berberis Species and Wild Strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zunjar, V.; Mammen, D.; Trivedi, B.M. Antioxidant activities and phenolics profiling of different parts of Carica papaya by LCMS-MS. Nat. Prod. Res. 2015, 29, 2097–2099. [Google Scholar] [CrossRef]
- Beelders, T.; de Beer, D.; Stander, M.; Joubert, E. Comprehensive Phenolic Profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and -MS/MS Reveals Novel Xanthone and Benzophenone Constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Serra, I.; Ubiali, D.; Cecchini, D.A.; Calleri, E.; Albertini, A.M.; Terreni, M.; Temporini, C. Assessment of immobilized PGA orientation via the LC-MS analysis of tryptic digests of the wild type and its 3K-PGA mutant assists in the rational design of a high-performance biocatalyst. Anal. Bioanal. Chem. 2012, 405, 745–753. [Google Scholar] [CrossRef]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MSnof Four New Classes of p-Coumaric Acid-Containing Diacyl Chlorogenic Acids in Green Coffee Beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, Z.; Lin, Y.; Chen, M.; Pan, G.; Huang, C. Study on the PK profiles of magnoflorine and its potential interaction in Cortex phellodendri decoction by LC-MS/MS. Anal. Bioanal. Chem. 2013, 406, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Karimov, A.; Shakirov, R. Berberis alkaloids. XX. Investigation of the alkaloids of Berberis iliensis. Khimiya Prir. Soedin. 1993, 1, 83–84. [Google Scholar] [CrossRef]
- Hostalkova, A.; Marikova, J.; Opletal, J.; Hulcova, K.; Kunes, J.; Novakova, L. Isoquinoline Alkaloids from Berberis vulgaris as Potential Lead Compounds for the Treatment of Alzheimer’s Disease. J. Nat. Prod. 2019, 82, 239–248. [Google Scholar] [CrossRef]
- He, J.; Feng, Y.; Ouyang, H.; Yu, B.; Chang, Y.; Pan, G.; Gao, X. A sensitive LC–MS/MS method for simultaneous determination of six flavonoids in rat plasma: Application to a pharmacokinetic study of total flavonoids from mulberry leaves. J. Pharm. Biomed. Anal. 2013, 84, 189–195. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Shen, A.Z. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Bajpai, V.; Singh, A.; Arya, K.R.; Srivastava, M.; Kumar, B. Rapid screening for the adulterants of Berberis aristatausing direct analysis in real-time mass spectrometry and principal component analysis for discrimination. Food Addit. Contam. Part A 2015, 32, 799–807. [Google Scholar] [CrossRef]
- Plazas, E.; Casoti, R.; Murillo, M.A.; Da Costa, F.B.; Cuca, L.E. Metabolomic profiling of Zanthoxylum species: Identification of anti-cholinesterase alkaloids candidates. Phytochemistry 2019, 168, 112128. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, N.; Fei, F.; Sun, R.; Feng, S.; He, J.; Wang, G. Sensitive Analysis and Pharmacokinetic Study of Berberrubine Using LC-MS/MS. Chin. Herb. Med. 2017, 9, 236–249. [Google Scholar] [CrossRef]
- Tešević, V.; Aljančić, I.; Vajs, V.; Živković, M.; Nikićević, N.; Urošević, I.; Vujisić, L. Development and validation of an LC- MS/MS method with a multiple reactions monitoring mode for the quantification of vanillin and syringaldehyde in plum brandies. J. Serb. Chem. Soc. 2014, 79, 1537–1543. [Google Scholar] [CrossRef]
- Lee, J.-T.; Pao, L.-H.; Hsieh, C.-D.; Huang, P.-W.; Hu, O.Y.P. Development and validation of an LC-MS/MS method for simultaneous quantification of hesperidin and hesperetin in rat plasma for pharmacokinetic studies. Anal. Meth. 2017, 9, 3329–3337. [Google Scholar] [CrossRef]
- Yang, Y.; Ying, S.; Li, T.; Zhen, J.; Chen, D.; Wang, J. A sensitive LC-MS/MS-based bioanalytical method for quantification of salviaflaside and rosmarinic acid in rat plasma and its application in a pharmacokinetic study. Biomed. Chromatogr. 2018, 32, 4259. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Wray, V. (E)-0-p-coumaroyl-, (E)-0-feuloyl-derivtives of glutaric acid in Citrus beate Risch. Phytochem 1988, 27, 3327–3329. [Google Scholar]
- Żółtaszek, R.; Hanausek, M.; Kiliańska, Z.M.; Walaszek, Z. Biologiczna rola kwasu D-glukarowego i jego pochodnych; potencjalne zastosowanie w medycynie [The biological role of D-glucaric acid and its derivatives: Potential use in medicine]. Postepy Hig. Med. Dosw. 2008, 62, 451–462. [Google Scholar]
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-Gutiérrez, I.; von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatogr. A 2013, 1281, 38–45. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Arya, K.-R.; Sharma, K.-R.; Kumar, B. Quantitative determination of isoquinolinealkaloids and chlorogenic acid in Berberisspecies using ultra high performance liquidchromatography with hybrid triplequadrupole linear ion trap massspectrometry. J. Sep. Sci. 2015, 38, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough study of reactivity of various compound classes towards the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Szwajgier, D.; Gustaw, W. The addition of malt to milk-based desserts: Influence on rheological properties and phenolic acid content. LWT Food Sci. Technol. 2015, 62, 400–407. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols—Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Özgen, M.; Saraçoglu, O.; Geçer, E.N. Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L.) fruits. Hort. Environ. Biotechnol. 2012, 53, 447–451. [Google Scholar] [CrossRef]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Marzec, Z.; Szwerc, W.; Głowniak, K. Green tea quality evaluation based on its catechins and metals composition in combination with chemometric analysis. Molecules 2018, 23, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł. Black tea samples origin discrimination using analytical investigation of secondary metabolites, antiradical scavenging activity and chemometric approach. Molecules 2018, 23, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapata, I.C.; Alzate, A.F.; Zapata, K.; Arias, J.P.; Puertas, M.A.; Rojano, B. E ect of pH, temperature and time of extraction on the antioxidant properties of Vaccinium meridionale Swartz. J. Berry Res. 2019, 9, 39–49. [Google Scholar] [CrossRef]
- Bastias-Montesa, J.M.; Vidal-San Martin, C.; Munoz-Farina, O.; Petzold-Maldonadoa, G.; Quevedo-Léonc, R.; Wang, H.; Yi, Y.; Cespedes-Acuna, C.L. Cryoconcentration procedure for aqueous extracts of maqui fruits prepared by centrifugation and filtration from fruits harvested in different years from the same localities. J. Berry Res. 2019, 9, 377–394. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Stajčić, S.; Ćetković, G.; Čanadanović, V.; Ćebović, T.; Vulić, J. Polyphenolic composition, antiradical and hepatoprotective activities of bilberry and blackberry pomace extracts. J. Berry Res. 2019, 9, 349–362. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of phenolic acid antimicrobial and antioxidant structure-property relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Benbettaieb, N.; Nyagaya, J.; Seuvre, A.M.; Debeaufort, F. Antioxidant activity and release kinetics of caffeic and p-coumaric acids from hydrocolloid-based active films for healthy packaged food. J. Agric. Food Chem. 2018, 66, 6906–6916. [Google Scholar] [CrossRef]
- Kumari, D.-S.; Ramakrishnan, E.; Devi, R. Phytochemical Screening, Antioxidant, Antityrosinase and Antigenotoxic Potential of Amaranthus viridis Extract. Ind. J. Pharmacol. 2018, 50, 3. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH· assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Olas, B.; Saluk-Juszczak, J.; Wachowicz, B. D-glucaro 1,4-lactone and resveratrol as antioxidants in blood platelets. Cell. Biol. Toxicol. 2008, 24, 189–199. [Google Scholar] [CrossRef]
- El Khalki, L.; Tilaoui, M.; Jaafari, A.; Ait Mouse, H.; Zyad, A. Studies on the dual cytotoxicity and antioxidant properties of Berberis vulgaris extracts and its main constituent Berberine. Adv. Pharmacol. Sci. 2018, 2018, 3018498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirwaikar, A.; Shirwaikar, A.; Rajendran, K.; Punitha, I.S. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006, 29, 1906–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Srivastava, S.; Rawat, A.K.S. Antimicrobial activities of Indian Berberis species. Fitoterapia 2007, 78, 574–576. [Google Scholar] [CrossRef]
- Jatinder Pal Singh, J.P.; Kaur, A.; Singh, N.; Nim, L.; Shevkani, K.; Kaur, H.; Arora, D.S. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci. Technol. 2016, 65, 1025–1030. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Bowles, B.L.; Miller, A.J. Caffeic acid activity against Clostridium botulinum spores. J. Food Sci. 1994, 59, 905–908. [Google Scholar] [CrossRef]
- Antonio, A.G.; Moraes, R.S.; Perrone, D.; Maja, L.C.; Santos, K.R.N.; Jório, N.L.P.; Farah, A. Species, roasting degree and decaffeination influence the antibacterial activity of coffee against Streptococcus mutans. Food Chem. 2010, 118, 782–788. [Google Scholar] [CrossRef]
- Rocha, J.E.; Coutinho, H.D.M.; Saraiva, C.R.N.; Lucas dos Santos, A.T.; Machado, A.J.T.; Calixto, J.T.; Menezes, I.R.A.; Martins da Costa, J.G.; Ribeiro-Filho, J.; Colares, A.V. HPLC-DAD analysis and antifungal effect of Hyptis martiusii Benth (Lamiaceae) against Candida strains. Asian Pac. J. Trop. Biomed. 2019, 9, 123–128. [Google Scholar]
- Wen, A.; Delaquis, P.; Stanich, K.; Toivonen, P. Antilisterial activity of selected phenolic acids. Food Microbiol. 2003, 20, 305–311. [Google Scholar] [CrossRef]
- Martinez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; De La Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef]
- Peng, L.; Kang, S.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Li, Z.; Zou, Y.; Liang, X.; Li, L.; et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015, 8, 5217–5223. [Google Scholar] [PubMed]
- Kong, W.; Li, Z.; Xiao, X.; Zhao, Y.; Zhang, P. Activity of berberine on Shigella dysenteriae investigated by microcalorimetry and multivariate analysis. J. Therm. Anal. Calorim. 2010, 102, 331–336. [Google Scholar] [CrossRef]
- Sahibzada, M.U.K.; Sadig, A.; Faidah, H.S.; Khurram, M.; Amin, M.U.; Hasseb, A.; Kakar, M. Berberine nanoparticles with enhanced in vitro bioavailability: Characterization and antimicrobial activity. Drug Des. Dev. Ther. 2018, 12, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.R.; Batista de Andrade Neto, J.; da Silva, C.R.; de Sousa Campos, R.; Silva, R.A.C.; Freitas, D.D.; Aires do Nascimento, F.B.S.; Dantas de Andrade, L.N.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: Action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghayan, S.S.; Mogadam, H.K.; Fazli, M.; Darban-Sarokhalil, D.; Khoramrooz, S.S.; Jabalameli, F.; Yaslianifard, S.; Mirzaii, M. The effects of berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn infections. Avicenna J. Med. Biotechnol. 2017, 9, 2–7. [Google Scholar] [PubMed]
- Taechowisan, T. Antibacterial and cytotoxicity activities of major compounds from Tinospora cordifolia Willd. Growing on Mangifera indica L. Int. J. Nutr. 2019, 3, 32–42. [Google Scholar]
- Kim, J.; Ha Quang Bao, T.; Shin, Y.; Kim, K.-Y. Antifungal activity of magnoflorine against Candida strains. World J. Microbiol. Biotechnol. 2018, 34, 167. [Google Scholar] [CrossRef]
- Wang, T.; Shao, J.; Da, W.; Li, Q.; Shi, G.; Wu, D.; Wang, C. Strong synergism of palmatine and fluconazole/itraconazole against planktonic and biofilm cells of Candida species and efflux-associated antifungal mechanism. Front. Microbiol. 2018, 9, 2892. [Google Scholar] [CrossRef]
- Campos, R.D.; Silva, C.R.; Andrade Neto, J.B.; Sampaio, L.S.; Nascimento, F.B.; Moraes Filho, M.O.; Cavalcanti, B.C.; Magalhães, H.I.; Gomes, A.O.; Lobo, M.D.; et al. Antifungal activity of palmatine against strains of Candida spp. resistant to azoles in planktonic cells and biofilm. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3657–3669. [Google Scholar] [CrossRef] [Green Version]
- Volleková, A.; Košt’álová, D.; Kettmann, V.; Tóth, J. Antifungal activity of Mahonia aquifolium extract and its major protoberberine alkaloids. Phytother. Res. 2003, 17, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| No. | Ion.(+/−) | Rt. | Molecular Formula | m/z Calculated | m/z Experimental | Delta | RDB | MS/MS Fragments | Proposed Compound | Ref. | Fr. | Rx. | Fol. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − | 2.69 | C6H8O7 | 191.0197 | 191.0185 | 6.39 | 3 | 129, 111 | Citric acid | [17] | + | + | − |
| 2 | − | 3.1 | C13H16O10 | 331.0671 | 331.0671 | −0.09 | 6 | 241, 169, 125 | Galloyl-glucose | [18] | + | − | − |
| 3 | − | 4.2 | C15H16O11 | 371.0620 | 371.0598 | 5.87 | 8 | 209, 129 | 3-Caffeoylglucaric acid | [19,20] | + | − | + |
| 4 | − | 4.6 | C7H12O6 | 191.0561 | 191.0568 | −3.58 | 3.5 | 111 | Quinic acid | [9,21] | − | + | + |
| 5 | − | 6.6 | C15H16O11 | 371.0620 | 371.0601 | 5.07 | 8 | 209 | 4-Caffeoylglucaric acid | [19] | + | − | + |
| 6 | − | 9.8 | C15H16O11 | 371.0620 | 371.0596 | 6.41 | 8 | 209, 129 | 2-Caffeoylglucaric acid | [19] | + | − | + |
| 7 | − | 12.1 | C9H10O5 | 197.0455 | 197.0459 | −1.78 | 5 | − | Syringic acid | [21] | + | − | − |
| 8 | − | 12.5 | C15H16O11 | 371.0620 | 371.0603 | 4.53 | 8 | 209, 112 | 5-Caffeoylglucaric acid | [19] | + | − | + |
| 9 | − | 12.9 | C7H6O3 | 137.0244 | 137.0255 | −7.84 | 5 | − | Hydroxybenzoic acid isomers | [22] | + | + | − |
| 10 | − | 14.4 | C16H18O9 | 353.0878 | 353.0897 | −5.35 | 8 | 191, 129 | Chlorogenic acid | [23] | + | + | + |
| 11 | − | 14.7 | C8H8O3 | 151.0401 | 151.0398 | 1.76 | 5 | 107 | Mandelic acid | [24] | + | − | − |
| 12 | − | 15.3 | C9H8O4 | 179.035 | 179.0346 | 2.12 | 6 | 135, 120 | Caffeic acid | [21] | + | − | − |
| 13 | − | 15.4 | C16H18O8 | 337.0929 | 337.0900 | 8.55 | 8 | 191, 173, 163 | Coumaroyl-quinic acid isomers | [25] | + | − | + |
| 14 | + | 15.6 | C20H24O4N | 342.1700 | 342.1680 | −1.23 | 7 | 256, 192, 104 | Magnoflorine | [26] | + | + | + |
| 15 | − | 16.0 | C16H18O9 | 353.0878 | 353.0895 | −4.78 | 8 | 191, 129 | Neochlorogenic acid | [23] | + | + | + |
| 16 | + | 17.3 | C18H21NO3 | 299.1521 | 299.1513 | 0.99 | 9.5 | 284, 252, 237 | N-methylcoclaurine | [27] | + | + | + |
| 17 | + | 17.8 | C37H40N2O6 | 609.2959 | 609.2950 | 1.5 | 19 | 566, 381 | Berbamine | [28] | tr | + | tr |
| 18 | + | 18.0 | C37H40N2O6 | 609.2959 | 609.2939 | 3.31 | 19 | 578, 566, 381 | Oxyacanthine | [27] | + | + | + |
| 19 | − | 18.4 | C27H30O16 | 609.1461 | 609.1443 | 2.96 | 13 | 518, 300, 169 | Rutin | [17] | + | − | − |
| 20 | − | 18.7 | C16H18O9 | 353.0878 | 353.0843 | 9.9 | 8 | 291,173 | (Z)-chlorogenic acid | [23] | + | + | + |
| 21 | − | 19.1 | C21H20O12 | 463.0882 | 463.0843 | 8.4 | 12 | 300, 151 | Isoquercetin | [29] | + | − | + |
| 22 | + | 19.2 | C19H18NO4 | 324.1230 | 324.1219 | 3.5 | 12 | 206, 121 | Dementhyleneberberine | [30] | + | + | + |
| 23a,b | + | 19.3/19.6 | C38H42N2O6 | 623.3116 | 623.3092 | 3.8 | 19 | 400, 268, 174 | Obaberine/berbamunine | [27,31] | + | + | + |
| 24 | − | 20.3 | C21H20O11 | 447.0933 | 447.0897 | 8 | 12 | 301 | Quercetin glucoside | [17] | + | − | + |
| 25 | + | 20.5 | C20H20O4N | 338.1385 | 338.1379 | 2.33 | 12 | 126 | Jatrorrhizine | [32] | + | + | + |
| 26 | + | 21.6 | C19H15NO4 | 322.1074 | 322.1075 | −0.36 | 13 | − | Berberrubine | [33] | + | + | + |
| 27 | − | 21.9 | C9H10O4 | 181.0506 | 181.0531 | −13.6 | 5 | 108 | Syringaldehyde | [34] | + | − | − |
| 28 | + | 22.3 | C21H22O4N | 352.1543 | 352.1517 | 7.5 | 12 | 292, 155 | Palmatine | [26] | + | + | + |
| 29 | + | 22.8 | C18H22NO4 | 336.1230 | 336.1214 | 4.88 | 13 | 231, 110 | Berberine | [32] | + | + | + |
| 30 | − | 24.4 | C20H18O11 | 433.0776 | 433.0797 | −4.76 | 12 | 300 | Guaiaverin | [17] | − | + | − |
| 31 | − | 24.5 | C16H14O6 | 301.0718 | 301.0719 | −0.46 | 10 | 240, 159 | Hesperetin | [35] | − | + | − |
| 32 | − | 24.6 | C15H10O7 | 301.0354 | 301.0385 | −10.34 | 11 | 229, 151 | Quercetin | [17] | + | + | + |
| 33 | − | 28.1 | C18H16O8 | 359.0772 | 359.0762 | 2.89 | 11 | 228, 197 | Rosmarinic acid | [36] | + | + | + |
| Extract | DPPH [IC50 µg/mL] | F-C [mg GA/L] |
|---|---|---|
| Fruits | ||
| Ethanol | 1820 ± 150 | 32.4 ± 3.5 |
| Ethanol-water (1:1 v/v) | 620 ± 58.1 | 112 ± 10.1 |
| Ethanol-water (7:3 v/v) | 760 ± 55 | 55.6 ± 4.5 |
| Water | 1590 ± 128 | 38.7 ± 2.2 |
| Roots | ||
| Ethanol | 4900 ± 342 | 21.1 ± 1.95 |
| Ethanol-water (1:1 v/v) | 3700 ± 250 | 26.5 ± 2.11 |
| Ethanol-water (7:3 v/v) | 3400 ± 230 | 24.3 ± 2.24 |
| Water | 5600 ± 420 | 17.6 ± 1.55 |
| Leaves | ||
| Ethanol | 1590 ± 138 | 68.7 ± 5.52 |
| Ethanol-water (1:1 v/v) | 80 ± 6.36 | 440 ± 17.1 |
| Ethanol-water (7:3 v/v) | 604 ± 54.5 | 118 ± 10.9 |
| Water | 110 ± 10.5 | 372 ± 12.5 |
| Gallic acid (0.01 mg/10 mL) | 25 ± 1.02 | NT |
| Species of Microorganisms | MIC (MBC or MFC) [mg/mL] and {MBC/MIC or MFC/MIC} of the Studied Extracts and Controls (µg/mL) | ||||
|---|---|---|---|---|---|
| Fruits (50:50) | Leaves (50:50) | Roots (50:50) | CIP or NY * | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC 25923 | 10, (20), {2} | 2.5, (10), {4} | 1.25, (5), {4} | 0.48, (0.48), {1} |
| Staphylococcus aureus ATCC 6538 | 5, (20), {4} | 2.5, (>20), {>8} | 1.25, (5), {4} | 0.24, (0.24), {1} | |
| Staphylococcus aureus ATCC 43300 | 5, (20), {4} | 2.5, (20), {8} | 2.5, (5), {2} | 0.24, (0.24), {1} | |
| Staphylococcus epidermidis ATCC 12228 | 10, (20), {2} | 10, (10), {1} | 0.31, (0.62), {2} | 0.12, (0.12), {1} | |
| Micrococcus luteus ATCC 10240 | 5, (10), {2} | 2.5, (2.5), {1} | 0.02, (0.02), {1} | 0.98, (1.96), {2} | |
| Bacillus subtilis ATCC 6633 | 10, (10), {1} | 20, (20), {1} | 2.5, (2.5), {1} | 0.03, (0.03), {1} | |
| Bacillus cereus ATCC 10876 | 10, (10), {1} | 1.25, (2.5), {2} | 1.25, (5), {4} | 0.06, (0.12), {2} | |
| Gram-negative bacteria | Bordetella bronchiseptica ATCC 4617 | 5, (10), {2} | 2.5, (10), {4} | 5, (10), {2} | 0.98, (0.98), {1} |
| Klebsiellapneumoniae ATCC 13883 | 5, (20), {4} | 20, (20), {1} | 20, (20), {1} | 0.12, (0.12), {1} | |
| Salmonella typhimurium ATCC 14028 | 10, (>20), {>2} | 20, (>20), {>1} | 10, (20), {2} | 0.06, (0.06), {1} | |
| Escherichiacoli ATCC 25922 | 10, (20), {2} | 10, (20), {2} | 10, (20), {2} | 0.004, (0.004), {1} | |
| Pseudomonas aeruginosa ATCC 9027 | 10, (20), {2} | 20, (20), {1} | 10, (20), {2} | 0.48, (0.98), {2} | |
| Fungi | Candida albicans ATCC 10231 | 5, (10), {2} | 5, (10), {2} | 0.62, (5), {8} | 0.48 *, (0.48), {1} |
| Candida parapsilosis ATCC 22019 | 5, (10), {2} | 5, (10), {2} | 0.62, (1.25), {2} | 0.24 *, (0.48), {2} | |
| Candida glabrata ATCC 90030 | 10, (20), {2} | 10, (10), {1} | 0.62, (10), {16} | 0.24 *, (0.48), {2} | |
| Candida krusei ATCC 14243 | 5, (10), {2} | 5, (10), {2} | 0.31, (2.5), {8} | 0.24 *, (0.24), {1} | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdykerimova, S.; Sakipova, Z.; Nakonieczna, S.; Koch, W.; Biernasiuk, A.; Grabarska, A.; Malm, A.; Kozhanova, K.; Kukula-Koch, W. Superior Antioxidant Capacity of Berberis iliensis—HPLC-Q-TOF-MS Based Phytochemical Studies and Spectrophotometric Determinations. Antioxidants 2020, 9, 504. https://doi.org/10.3390/antiox9060504
Abdykerimova S, Sakipova Z, Nakonieczna S, Koch W, Biernasiuk A, Grabarska A, Malm A, Kozhanova K, Kukula-Koch W. Superior Antioxidant Capacity of Berberis iliensis—HPLC-Q-TOF-MS Based Phytochemical Studies and Spectrophotometric Determinations. Antioxidants. 2020; 9(6):504. https://doi.org/10.3390/antiox9060504
Chicago/Turabian StyleAbdykerimova, Saniia, Zuriyadda Sakipova, Sylwia Nakonieczna, Wojciech Koch, Anna Biernasiuk, Aneta Grabarska, Anna Malm, Kaldanay Kozhanova, and Wirginia Kukula-Koch. 2020. "Superior Antioxidant Capacity of Berberis iliensis—HPLC-Q-TOF-MS Based Phytochemical Studies and Spectrophotometric Determinations" Antioxidants 9, no. 6: 504. https://doi.org/10.3390/antiox9060504