Coronary Revascularization and Long-Term Survivorship in Chronic Coronary Syndrome
Abstract
:1. Chronic Coronary Syndrome: Definition and Perspective
2. Surgical Revascularization: “The Great Hope”
3. Percutaneous Revascularization: “The Unfulfilled Dream”
4. The Ischemia Trial: “The Déjà-Vu Reality”
5. Real-Life Registries: “The Uncomfortable Truth”
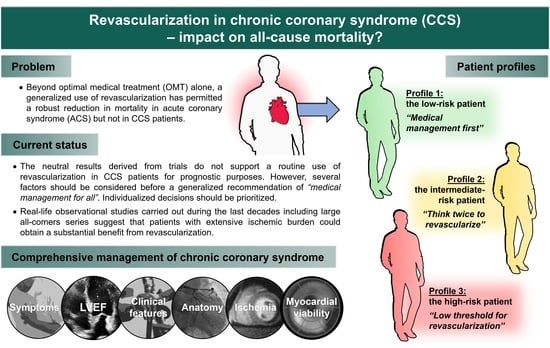
6. And Now What?
6.1. Initial Assessment of CCS
6.2. Left Ventricular Ejection Fraction
6.3. The Need for OMT
6.4. Revascularization and Patient Profiles
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.G.; Mark, D.B.; Gersh, B.J. Revascularization in Stable Coronary Disease: Evidence and Uncertainties. Nat. Rev. Cardiol. 2018, 15, 408–419. [Google Scholar] [CrossRef]
- Carrel, A. On the Experimental Surgery of the Thoracic Aorta and Heart. Ann. Surg. 1910, 52, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Vineberg, A.; Miller, G. Internal Mammary Coronary Anastomosis in the Surgical Treatment of Coronary Artery Insufficiency. Can. Med. Assoc. J. 1951, 64, 204–210. [Google Scholar] [PubMed]
- Goetz, R.H.; Rohman, M.; Haller, J.D.; Dee, R.; Rosenak, S.S. Internal Mammary-Coronary Artery Anastomosis. A Nonsuture Method Employing Tantalum Rings. J. Thorac. Cardiovasc. Surg. 1961, 41, 378–386. [Google Scholar] [CrossRef]
- Sabiston, D.C., Jr.; Rienhoff, W.F., Jr. Lecture. The Coronary Circulation. Johns. Hopkins Med. J. 1974, 134, 314–329. [Google Scholar] [PubMed]
- Kolessov, V.I. Mammary Artery-Coronary Artery Anastomosis as Method of Treatment for Angina Pectoris. J. Thorac. Cardiovasc. Surg. 1967, 54, 535–544. [Google Scholar] [CrossRef]
- Melly, L.; Torregrossa, G.; Lee, T.; Jansens, J.-L.; Puskas, J.D. Fifty Years of Coronary Artery Bypass Grafting. J. Thorac. Dis. 2018, 10, 1960–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favaloro, R.G. Saphenous Vein Autograft Replacement of Severe Segmental Coronary Artery Occlusion. Ann. Thorac. Surg. 1968, 5, 334–339. [Google Scholar] [CrossRef]
- VA Coronary Artery Bypass Surgery Cooperative Study Group. Eighteen-Year Follow-up in the Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery for Stable Angina. The VA Coronary Artery Bypass Surgery Cooperative Study Group. Circulation 1992, 86, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Varnauskas, E.; The European Coronary Surgery Study Group. Twelve-Year Follow-up of Survival in the Randomized European Coronary Surgery Study. N. Engl. J. Med. 1988, 319, 332–337. [Google Scholar] [CrossRef]
- Alderman, E.L.; Bourassa, M.G.; Cohen, L.S.; Davis, K.B.; Kaiser, G.G.; Killip, T.; Mock, M.B.; Pettinger, M.; Robertson, T.L. Ten-Year Follow-up of Survival and Myocardial Infarction in the Randomized Coronary Artery Surgery Study. Circulation 1990, 82, 1629–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, V.S.; Guinn, G.A. Prospective Randomized Study of the Surgical Therapy of Stable Angina. Cardiovasc. Clin. 1977, 8, 131–144. [Google Scholar]
- Kloster, F.E.; Kremkau, E.L.; Ritzmann, L.W.; Rahimtoola, S.H.; Rösch, J.; Kanarek, P.H. Coronary Bypass for Stable Angina: A Prospective Randomized Study. N. Engl. J. Med. 1979, 300, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.J.; Coggin, C.J.; Gersh, B.J.; Fisher, L.D.; Myers, W.O.; Oberman, A.; Sheffield, L.T. Ten-Year Follow-up of Quality of Life in Patients Randomized to Receive Medical Therapy or Coronary Artery Bypass Graft Surgery. The Coronary Artery Surgery Study (CASS). Circulation 1990, 82, 1647–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group Eleven-Year Survival in the Veterans Administration Randomized Trial of Coronary Bypass Surgery for Stable Angina. N. Engl. J. Med. 1984, 311, 1333–1339. [CrossRef] [PubMed]
- Yusuf, S.; Zucker, D.; Passamani, E.; Peduzzi, P.; Takaro, T.; Fisher, L.D.; Kennedy, J.W.; Davis, K.; Killip, T.; Norris, R.; et al. Effect of Coronary Artery Bypass Graft Surgery on Survival: Overview of 10-Year Results from Randomised Trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994, 344, 563–570. [Google Scholar] [CrossRef]
- Meyer, J.A. Werner Forssmann and Catheterization of the Heart, 1929. Ann. Thorac. Surg. 1990, 49, 497–499. [Google Scholar] [CrossRef]
- Sones, F.M.; Shirey, E.K. Cine Coronary Arteriography. Mod. Concepts Cardiovasc. Dis. 1962, 31, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.E. The Development of Coronary Artery Surgery: Personal Recollections. Tex. Heart Inst. J. 2002, 29, 10–14. [Google Scholar] [PubMed]
- Judkins, M.P. Selective Coronary Arteriography: Part I: A Percutaneous Transfemoral Technic. Radiology 1967, 89, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Grüntzig, A. Transluminal Dilatation of Coronary-Artery Stenosis. Lancet 1978, 311, 263. [Google Scholar] [CrossRef]
- Dotter, C.T.; Judkins, M.P. Transluminal Treatment of Arteriosclerotic Obstruction: Description of a New Technic and a Preliminary Report of Its Application. Circulation 1964, 30, 654–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, A.F.; Folland, E.D.; Hartigan, P. A Comparison of Angioplasty with Medical Therapy in the Treatment of Single-Vessel Coronary Artery Disease. N. Engl. J. Med. 1992, 326, 10–16. [Google Scholar] [CrossRef]
- Folland, E.D.; Hartigan, P.M.; Parisi, A.F. Percutaneous Transluminal Coronary Angioplasty versus Medical Therapy for Stable Angina Pectoris. J. Am. Coll. Cardiol. 1997, 29, 1505–1511. [Google Scholar] [CrossRef]
- Coronary Angioplasty versus Medical Therapy for Angina: The Second Randomised Intervention Treatment of Angina (RITA-2) Trial. RITA-2 Trial Participants. Lancet 1997, 350, 461–468. [CrossRef]
- Pitt, B.; Waters, D.; Brown, W.V.; van Boven, A.J.; Schwartz, L.; Title, L.M.; Eisenberg, D.; Shurzinske, L.; McCormick, L.S. Aggressive Lipid-Lowering Therapy Compared with Angioplasty in Stable Coronary Artery Disease. N. Engl. J. Med. 1999, 341, 70–76. [Google Scholar] [CrossRef]
- Leimgruber, P.P.; Roubin, G.S.; Hollman, J.; Cotsonis, G.A.; Meier, B.; Douglas, J.S.; King, S.B.; Gruentzig, A.R. Restenosis after Successful Coronary Angioplasty in Patients with Single-Vessel Disease. Circulation 1986, 73, 710–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular Stents to Prevent Occlusion and Re-Stenosis after Transluminal Angioplasty. N. Engl. J. Med. 1987, 316, 701–706. [Google Scholar] [CrossRef]
- Serruys, P.W.; Strauss, B.H.; Beatt, K.J.; Bertrand, M.E.; Puel, J.; Rickards, A.F.; Meier, B.; Goy, J.-J.; Vogt, P.; Kappenberger, L.; et al. Angiographic Follow-up after Placement of a Self-Expanding Coronary-Artery Stent. N. Engl. J. Med. 1991, 324, 13–17. [Google Scholar] [CrossRef]
- Morice, M.-C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. A Randomized Comparison of a Sirolimus-Eluting Stent with a Standard Stent for Coronary Revascularization. N. Engl. J. Med. 2002, 346, 1773–1780. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.; Drzewiecki, J.; Banning, A.; Grube, E.; Hauptmann, K.; Silber, S.; Dudek, D.; Fort, S.; Schiele, F.; Zmudka, K.; et al. Randomized Study to Assess the Effectiveness of Slow- and Moderate-Release Polymer-Based Paclitaxel-Eluting Stents for Coronary Artery Lesions. Circulation 2003, 108, 788–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesaro, A.; Moscarella, E.; Gragnano, F.; Perrotta, R.; Diana, V.; Pariggiano, I.; Concilio, C.; Alfieri, A.; Cesaro, F.; Mercone, G.; et al. Transradial Access versus Transfemoral Access: A Comparison of Outcomes and Efficacy in Reducing Hemorrhagic Events. Expert Rev. Cardiovasc. Ther. 2019, 17, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weintraub, W.S.; Spertus, J.A.; Kolm, P.; Maron, D.J.; Zhang, Z.; Jurkovitz, C.; Zhang, W.; Hartigan, P.M.; Lewis, C.; Veledar, E.; et al. Effect of PCI on Quality of Life in Patients with Stable Coronary Disease. N. Engl. J. Med. 2008, 359, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaitman, B.R.; Hardison, R.M.; Adler, D.; Gebhart, S.; Grogan, M.; Ocampo, S.; Sopko, G.; Ramires, J.A.; Schneider, D.; Frye, R.L.; et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes Randomized Trial of Different Treatment Strategies in Type 2 Diabetes Mellitus with Stable Ischemic Heart Disease: Impact of Treatment Strategy on Cardiac Mortality and Myocardial Infarction. Circulation 2009, 120, 2529–2540. [Google Scholar] [CrossRef]
- Brooks, M.M.; Chung, S.-C.; Helmy, T.; Hillegass, W.B.; Escobedo, J.; Melsop, K.A.; Massaro, E.M.; McBane, R.D.; Hyde, P.; Hlatky, M.A.; et al. Health Status After Treatment for Coronary Artery Disease and Type 2 Diabetes Mellitus in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial. Circulation 2010, 122, 1690–1699. [Google Scholar] [CrossRef] [Green Version]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.J.; Fearon, W.F.; Barbato, E.; Tonino, P.A.L.; Engstrøm, T.; Kääb, S.; Dambrink, J.-H.; Rioufol, G.; et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.A.; Jones, P.G.; Maron, D.J.; O’Brien, S.M.; Reynolds, H.R.; Rosenberg, Y.; Stone, G.W.; Harrell, F.E.; Boden, W.E.; Weintraub, W.S.; et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N. Engl. J. Med. 2020, 382, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- BARI 2D Study Group; Frye, R.L.; August, P.; Brooks, M.M.; Hardison, R.M.; Kelsey, S.F.; MacGregor, J.M.; Orchard, T.J.; Chaitman, B.R.; Genuth, S.M.; et al. A Randomized Trial of Therapies for Type 2 Diabetes and Coronary Artery Disease. N. Engl. J. Med. 2009, 360, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Pijls, N.H.J.; Kalesan, B.; Barbato, E.; Tonino, P.A.L.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Mobius-Winckler, S.; Rioufol, G.; et al. Fractional Flow Reserve-Guided PCI versus Medical Therapy in Stable Coronary Disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [Green Version]
- Katritsis, D.G.; Ioannidis, J.P.A. Percutaneous Coronary Intervention Versus Conservative Therapy in Nonacute Coronary Artery Disease: A Meta-Analysis. Circulation 2005, 111, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.J.; Hayes, S.W.; Hartigan, P.M.; Weintraub, W.S.; O’Rourke, R.A.; Dada, M.; Spertus, J.A.; et al. Optimal Medical Therapy with or without Percutaneous Coronary Intervention to Reduce Ischemic Burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial Nuclear Substudy. Circulation 2008, 117, 1283–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonino, P.A.L.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U.; Ikeno, F.; vant Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijls, N.H.J.; Fearon, W.F.; Tonino, P.A.L.; Siebert, U.; Ikeno, F.; Bornschein, B.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention in Patients with Multivessel Coronary Artery Disease. J. Am. Coll. Cardiol. 2010, 56, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Nunen, L.X.; Zimmermann, F.M.; Tonino, P.A.L.; Barbato, E.; Baumbach, A.; Engstrøm, T.; Klauss, V.; MacCarthy, P.A.; Manoharan, G.; Oldroyd, K.G.; et al. Fractional Flow Reserve versus Angiography for Guidance of PCI in Patients with Multivessel Coronary Artery Disease (FAME): 5-Year Follow-up of a Randomised Controlled Trial. Lancet 2015, 386, 1853–1860. [Google Scholar] [CrossRef]
- Chacko, L.; Howard, J.P.; Rajkumar, C.; Nowbar, A.N.; Kane, C.; Mahdi, D.; Foley, M.; Shun-Shin, M.; Cole, G.; Sen, S.; et al. Effects of Percutaneous Coronary Intervention on Death and Myocardial Infarction Stratified by Stable and Unstable Coronary Artery Disease: A Meta-Analysis of Randomized Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e00636. [Google Scholar] [CrossRef]
- Bravata, D.M.; Gienger, A.L.; McDonald, K.M.; Sundaram, V.; Perez, M.V.; Varghese, R.; Kapoor, J.R.; Ardehali, R.; Owens, D.K.; Hlatky, M.A. Systematic Review: The Comparative Effectiveness of Percutaneous Coronary Interventions and Coronary Artery Bypass Graft Surgery. Ann. Intern. Med. 2007, 147, 703. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K. Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting in the Management of Chronic Stable Angina: A Critical Appraisal. J. Cardiovasc. Dis. Res. 2010, 1, 54–58. [Google Scholar] [CrossRef] [Green Version]
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators Comparison of Coronary Bypass Surgery with Angioplasty in Patients with Multivessel Disease. N. Engl. J. Med. 1996, 335, 217–225. [CrossRef] [PubMed]
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; Mogensen, L.J.H.; et al. Percutaneous Coronary Angioplasty versus Coronary Artery Bypass Grafting in the Treatment of Unprotected Left Main Stenosis: Updated 5-Year Outcomes from the Randomised, Non-Inferiority NOBLE Trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef]
- Stone, G.W.; Hochman, J.S.; Williams, D.O.; Boden, W.E.; Ferguson, T.B.; Harrington, R.A.; Maron, D.J. Medical Therapy with versus without Revascularization in Stable Patients with Moderate and Severe Ischemia. J. Am. Coll. Cardiol. 2016, 67, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Instituto Nacional de Estadística INEbase/Demografía y Población. Available online: https://www.ine.es/dyngs/INEbase/es/categoria.htm?c=Estadistica_P&cid=1254734710984 (accessed on 16 May 2020).
- Maron, D.J.; Hochman, J.S.; O’Brien, S.M.; Reynolds, H.R.; Boden, W.E.; Stone, G.W.; Bangalore, S.; Spertus, J.A.; Mark, D.B.; Alexander, K.P.; et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) Trial: Rationale and Design. Am. Heart J. 2018, 201, 124–135. [Google Scholar] [CrossRef]
- Nallamothu, B.K. The ISCHEMIA Trial Meets the Rashomon Effect: Lessons for Clinical Practice. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006527. [Google Scholar] [CrossRef]
- Shaw, L.J.; Berman, D.S.; Picard, M.H.; Friedrich, M.G.; Kwong, R.Y.; Stone, G.W.; Senior, R.; Min, J.K.; Hachamovitch, R.; Scherrer-Crosbie, M.; et al. Comparative Definitions for Moderate-Severe Ischemia in Stress Nuclear, Echocardiography, and Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2014, 7, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, D.J.; Harrington, R.A.; Hochman, J.S. Planning and Conducting the ISCHEMIA Trial: Setting the Record Straight. Circulation 2018, 138, 1384–1386. [Google Scholar] [CrossRef]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The Performance of Non-Invasive Tests to Rule-in and Rule-out Significant Coronary Artery Stenosis in Patients with Stable Angina: A Meta-Analysis Focused on Post-Test Disease Probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef]
- Marcos-Garces, V.; Gavara, J.; Monmeneu, J.V.; Lopez-Lereu, M.P.; Bosch, M.J.; Merlos, P.; Perez, N.; Rios-Navarro, C.; De Dios, E.; Bonanad, C.; et al. Vasodilator Stress CMR and All-Cause Mortality in Stable Ischemic Heart Disease. JACC Cardiovasc. Imaging 2020, 13, 1674–1686. [Google Scholar] [CrossRef]
- Sheridan, D.J.; Julian, D.G. Achievements and Limitations of Evidence-Based Medicine. J. Am. Coll. Cardiol. 2016, 68, 204–213. [Google Scholar] [CrossRef]
- Herwig, A.; Weltermann, B. Study Protocol for a Matter of Heart: A Qualitative Study of Patient Factors Driving Overuse of Cardiac Catheterisation. BMJ Open 2017, 7, e017629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boiten, H.J.; Ekmen, H.; Zijlstra, F.; van Domburg, R.T.; Schinkel, A.F.L. Impact of Early Coronary Revascularization on Long-Term Outcomes in Patients with Myocardial Ischemia on Dobutamine Stress Echocardiography. Am. J. Cardiol. 2016, 118, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.-S.; Bangalore, S.; Chaudhry, F.A. Prognostic Implications of Stress Echocardiography and Impact on Patient Outcomes: An Effective Gatekeeper for Coronary Angiography and Revascularization. J. Am. Soc. Echocardiogr. 2010, 23, 832–839. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Porter, T.; Lorenzoni, V.; Pontone, G.; De Santis, D.; De Rosa, A.; Guaricci, A.I. Effect of Coronary Revascularization on the Prognostic Value of Stress Myocardial Contrast Wall Motion and Perfusion Imaging. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the Short-Term Survival Benefit Associated with Revascularization Compared with Medical Therapy in Patients with No Prior Coronary Artery Disease Undergoing Stress Myocardial Perfusion Single Photon Emission Computed Tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef] [Green Version]
- Hachamovitch, R.; Rozanski, A.; Shaw, L.J.; Stone, G.W.; Thomson, L.E.J.; Friedman, J.D.; Hayes, S.W.; Cohen, I.; Germano, G.; Berman, D.S. Impact of Ischaemia and Scar on the Therapeutic Benefit Derived from Myocardial Revascularization vs. Medical Therapy among Patients Undergoing Stress-Rest Myocardial Perfusion Scintigraphy. Eur. Heart J. 2011, 32, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Spertus, J.A.; Chan, P.S.; Sperry, B.W.; Thompson, R.C.; Al Badarin, F.; Kennedy, K.F.; Case, J.A.; Courter, S.; Saeed, I.M.; et al. Extent of Myocardial Ischemia on Positron Emission Tomography and Survival Benefit with Early Revascularization. J. Am. Coll. Cardiol. 2019, 74, 1645–1654. [Google Scholar] [CrossRef]
- Gargiulo, P.; Dellegrottaglie, S.; Bruzzese, D.; Savarese, G.; Scala, O.; Ruggiero, D.; D’Amore, C.; Paolillo, S.; Agostoni, P.; Bossone, E.; et al. The Prognostic Value of Normal Stress Cardiac Magnetic Resonance in Patients with Known or Suspected Coronary Artery Disease: A Meta-Analysis. Circ. Cardiovasc. Imaging 2013, 6, 574–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, M.J.; McVey, C.M.; Berger, J.S.; Kramer, C.M.; Salerno, M. Prognostic Value of Stress Cardiac Magnetic Resonance Imaging in Patients with Known or Suspected Coronary Artery Disease. J. Am. Coll. Cardiol. 2013, 62, 826–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodi, V.; Sanchis, J.; Lopez-Lereu, M.P.; Nunez, J.; Mainar, L.; Monmeneu, J.V.; Husser, O.; Dominguez, E.; Chorro, F.J.; Llacer, A. Prognostic Value of Dipyridamole Stress Cardiovascular Magnetic Resonance Imaging in Patients with Known or Suspected Coronary Artery Disease. J. Am. Coll. Cardiol. 2007, 50, 1174–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodi, V.; Sanchis, J.; Lopez-Lereu, M.P.; Nunez, J.; Mainar, L.; Monmeneu, J.V.; Ruiz, V.; Rumiz, E.; Husser, O.; Moratal, D.; et al. Prognostic and Therapeutic Implications of Dipyridamole Stress Cardiovascular Magnetic Resonance on the Basis of the Ischaemic Cascade. Heart 2008, 95, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Bodi, V.; Husser, O.; Sanchis, J.; Núñez, J.; Monmeneu, J.V.; López-Lereu, M.P.; Bosch, M.J.; Rumiz, E.; Miñana, G.; García, C.; et al. Prognostic Implications of Dipyridamole Cardiac MR Imaging: A Prospective Multicenter Registry. Radiology 2012, 262, 91–100. [Google Scholar] [CrossRef]
- Vincenti, G.; Masci, P.G.; Monney, P.; Rutz, T.; Hugelshofer, S.; Gaxherri, M.; Muller, O.; Iglesias, J.F.; Eeckhout, E.; Lorenzoni, V.; et al. Stress Perfusion CMR in Patients with Known and Suspected CAD. JACC Cardiovasc. Imaging 2017, 10, 526–537. [Google Scholar] [CrossRef]
- Husser, O.; Bodí, V.; Sanchís, J.; Mainar, L.; Núñez, J.; López-Lereu, M.P.; Monmeneu, J.V.; Ruiz, V.; Rumiz, E.; Moratal, D.; et al. Additional Diagnostic Value of Systolic Dysfunction Induced by Dipyridamole Stress Cardiac Magnetic Resonance Used in Detecting Coronary Artery Disease. Rev. Esp. Cardiol. 2009, 62, 383–391. [Google Scholar] [CrossRef]
- Marcos-Garces, V.; Gavara, J.; Monmeneu, J.V.; Lopez-Lereu, M.P.; Perez, N.; Rios-Navarro, C.; De Dios, E.; Moratal, D.; Miñana, G.; Nuñez, J.; et al. A Novel Clinical and Stress Cardiac Magnetic Resonance (C-CMR-10) Score to Predict Long-Term All-Cause Mortality in Patients with Known or Suspected Chronic Coronary Syndrome. JCM 2020, 9, 1957. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.Y.; Ge, Y.; Steel, K.; Bingham, S.; Abdullah, S.; Fujikura, K.; Wang, W.; Pandya, A.; Chen, Y.-Y.; Mikolich, J.R.; et al. Cardiac Magnetic Resonance Stress Perfusion Imaging for Evaluation of Patients with Chest Pain. J. Am. Coll. Cardiol. 2019, 74, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Hausleiter, J.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.-J.; Kim, Y.-J.; et al. Coronary Computed Tomographic Angiography as a Gatekeeper to Invasive Diagnostic and Surgical Procedures: Results from the Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) Registry. J. Am. Coll. Cardiol. 2012, 60, 2103–2114. [Google Scholar] [CrossRef] [Green Version]
- Völz, S.; Dworeck, C.; Redfors, B.; Pétursson, P.; Angerås, O.; Gan, L.-M.; Götberg, M.; Sarno, G.; Venetsanos, D.; Grimfärd, P.; et al. Survival of Patients with Angina Pectoris Undergoing Percutaneous Coronary Intervention with Intracoronary Pressure Wire Guidance. J. Am. Coll. Cardiol. 2020, 75, 2785–2799. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.-M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous Coronary Intervention in Stable Angina (ORBITA): A Double-Blind, Randomised Controlled Trial. Lancet 2018, 391, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.J.; Phillips, L.M.; Nagel, E.; Newby, D.E.; Narula, J.; Douglas, P.S. Comparative Effectiveness Trials of Imaging-Guided Strategies in Stable Ischemic Heart Disease. JACC Cardiovasc. Imaging 2017, 10, 321–334. [Google Scholar] [CrossRef]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N. Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Raharja, A.; Archbold, R.A.; Mathur, A. Validity of Inducible Ischaemia as a Surrogate for Adverse Outcomes in Stable Coronary Artery Disease. Heart 2018, 104, 1733–1738. [Google Scholar] [CrossRef] [Green Version]
- Cesaro, A.; Gragnano, F.; Di Girolamo, D.; Moscarella, E.; Diana, V.; Pariggiano, I.; Alfieri, A.; Perrotta, R.; Golino, P.; Cesaro, F.; et al. Functional Assessment of Coronary Stenosis: An Overview of Available Techniques. Is Quantitative Flow Ratio a Step to the Future? Expert Rev. Cardiovasc. Ther. 2018, 16, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Gaudino, M.; Chen, R.J.; Bader Eddeen, A.; Ruel, M. Long-Term Outcomes in Patients with Severely Reduced Left Ventricular Ejection Fraction Undergoing Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting. JAMA Cardiol. 2020. [Google Scholar] [CrossRef]
- Orlandini, A.; Castellana, N.; Pascual, A.; Botto, F.; Cecilia Bahit, M.; Chacon, C.; Luz Diaz, M.; Diaz, R. Myocardial Viability for Decision-Making Concerning Revascularization in Patients with Left Ventricular Dysfunction and Coronary Artery Disease: A Meta-Analysis of Non-Randomized and Randomized Studies. Int. J. Cardiol. 2015, 182, 494–499. [Google Scholar] [CrossRef]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Metra, M.; Morais, J.; Atar, D. The Myth of ‘Stable’ Coronary Artery Disease. Nat. Rev. Cardiol. 2020, 17, 9–21. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; Taglialatela, V.; Gragnano, F.; Moscarella, E.; Fimiani, F.; Conte, M.; Barletta, V.; Monda, E.; Limongelli, G.; Severino, S.; et al. Low-Dose Ticagrelor in Patients With High Ischemic Risk and Prior Myocardial Infarction: A Multicenter Prospective Real-World Observational Study. J. Cardiovasc. Pharmacol. 2020, 76, 173–180. [Google Scholar] [CrossRef]
- Kotseva, K.; De Backer, G.; De Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Lifestyle and Impact on Cardiovascular Risk Factor Control in Coronary Patients across 27 Countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V Registry. Eur. J. Prev. Cardiol. 2019, 26, 824–835. [Google Scholar] [CrossRef]
- Ferrari, R.; Camici, P.G.; Crea, F.; Danchin, N.; Fox, K.; Maggioni, A.P.; Manolis, A.J.; Marzilli, M.; Rosano, G.M.C.; Lopez-Sendon, J.L. Expert Consensus Document: A “diamond” Approach to Personalized Treatment of Angina. Nat. Rev. Cardiol. 2018, 15, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Verheye, S.; Jolicœur, E.M.; Behan, M.W.; Pettersson, T.; Sainsbury, P.; Hill, J.; Vrolix, M.; Agostoni, P.; Engstrom, T.; Labinaz, M.; et al. Efficacy of a Device to Narrow the Coronary Sinus in Refractory Angina. N. Engl. J. Med. 2015, 372, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Zaman, A. ORBITA: What Goes Around, Comes Around… Or Does It? Interventional Cardiology Review. Interv. Cardiol. 2018, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Gersh, B.J.; Alexander, K.P.; Granger, C.B.; Stone, G.W. Coronary Artery Disease in Patients ≥80 Years of Age. J. Am. Coll. Cardiol. 2018, 71, 2015–2040. [Google Scholar] [CrossRef] [PubMed]




| Trials | No. of Patients | Follow-Up (Years) * | Annualized All-Cause Mortality Rate (%/Year) | ||||
|---|---|---|---|---|---|---|---|
| CABG | OMT | Total | CABG | OMT | p | ||
| VA [10] | 332 | 354 | 686 | 18 | 3.9% | 3.7% | 0.6 |
| ECSS [11] | 394 | 373 | 767 | 12 | 1.9% | 2.4% | 0.04 |
| CASS [12,15] | 390 | 390 | 780 | 10 | 1.8% | 2.1% | 0.25 |
| Trials | No. of Patients | Follow-Up (Years) * | Annualized All-Cause Mortality Rate (%/Year) | ||||
|---|---|---|---|---|---|---|---|
| Rev | OMT | Total | Rev | OMT | p | ||
| COURAGE [34,35] | 1149 | 1138 | 2287 | 4.6 | 1.6% | 1.8% | 0.38 |
| BARI 2D [36,37] | 1176 | 1192 | 2368 | 5.3 | 2.2% | 2.3% | 0.97 |
| FAME 2 [38] | 447 | 441 | 888 | 5 | 1% | 1% | NS |
| ISCHEMIA [39,40] | 2588 | 2591 | 5179 | 3.2 | 2.8% | 2.6% | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabaldon-Perez, A.; Marcos-Garces, V.; Gavara, J.; Rios-Navarro, C.; Miñana, G.; Bayes-Genis, A.; Husser, O.; Sanchis, J.; Nunez, J.; Chorro, F.J.; et al. Coronary Revascularization and Long-Term Survivorship in Chronic Coronary Syndrome. J. Clin. Med. 2021, 10, 610. https://doi.org/10.3390/jcm10040610
Gabaldon-Perez A, Marcos-Garces V, Gavara J, Rios-Navarro C, Miñana G, Bayes-Genis A, Husser O, Sanchis J, Nunez J, Chorro FJ, et al. Coronary Revascularization and Long-Term Survivorship in Chronic Coronary Syndrome. Journal of Clinical Medicine. 2021; 10(4):610. https://doi.org/10.3390/jcm10040610
Chicago/Turabian StyleGabaldon-Perez, Ana, Victor Marcos-Garces, Jose Gavara, Cesar Rios-Navarro, Gema Miñana, Antoni Bayes-Genis, Oliver Husser, Juan Sanchis, Julio Nunez, Francisco Javier Chorro, and et al. 2021. "Coronary Revascularization and Long-Term Survivorship in Chronic Coronary Syndrome" Journal of Clinical Medicine 10, no. 4: 610. https://doi.org/10.3390/jcm10040610