Acquired Glucose-6-Phosphate Dehydrogenase Deficiency
Abstract
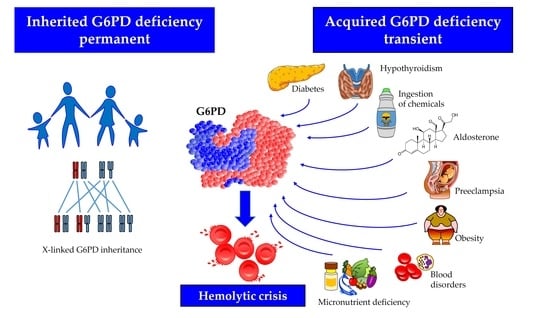
:1. Introduction
2. Factors Potentially Inducing Acquired G6PD Deficiency
2.1. Blood Disorders
2.2. Ingestion of Chemicals
2.3. Endocrine Disorders
2.3.1. Excess of Mineralocorticoids
2.3.2. Hypothyroidism
2.3.3. Diabetes
2.3.4. Obesity
2.4. Preeclampsia
2.5. Micronutrient Deficiency
3. Discussion
- G6PD is physiologically regulated by several hormones whose release may change in the course of certain diseases, such as primary hyperaldosteronism, diabetes, and hyperglucagonemia, responsible for the majority of acquired forms, producing a temporary post-transcriptional G6PD downregulation;
- Chemical substances may act directly as inhibitors of G6PD: accidental or voluntary ingestion of such substances can result in a hemolytic crisis undistinguishable from that occurring in inherited forms;
- In a number of hematological diseases, the presence of unknown circulating G6PD inhibitors (hormones? microRNAs?) has been postulated [46]. Experiments in which the plasma of these patients was incubated with red blood cells from healthy donors showed a significant decrease in the activity of many enzymes, therefore confirming the presence of an inhibitory agent [48]. However, this inactivating mechanism was demonstrable only in a limited percentage of cases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef]
- Beutler, E. G6PD deficiency. Blood 1994, 84, 3613–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzzatto, L.; Seneca, E. G6PD deficiency: A classic example of pharmacogenetics with on-going clinical implications. Br. J. Haematol. 2014, 164, 469–480. [Google Scholar] [CrossRef]
- Piomelli, S.; Corash, L.M.; Davenport, D.D.; Miraglia, J.; Amorosi, E.L. In vivo lability of glucose-6-phosphate dehydrogenase in GdA- and GdMediterranean deficiency. J. Clin. Investig. 1968, 47, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.; Han, D.; Sancheti, H.; Yap, L.P.; Kaplowitz, N.; Cadenas, E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J. Biol. Chem. 2010, 285, 39646–39654. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.A.; Mathews, I.I.; Horikoshi, N.; Matsui, T.; Kaur, M.; Wakatsuki, S.; Mochly-Rosen, D. Stabilization of glucose-6-phosphate dehydrogenase oligomers enhances catalytic activity and stability of clinical variants. J. Biol. Chem. 2022, 298, 101610. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshimoto, K.; Aoyama, K.; Ichihara, A. Hormonal regulations of glucose-6-phosphate dehydrogenase and lipogenesis in primary cultures of rat hepatocytes. J. Biochem. 1982, 91, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Manos, P.; Nakayama, R.; Holten, D. Regulation of glucose-6-phosphate dehydrogenase synthesis and mRNA abundance in cultured rat hepatocytes. Biochem. J. 1991, 276 Pt 1, 245–250. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Yang, J.; Ding, J.W.; Li, S.; Wu, H.; Xiong, Y.; Zhou, F.; Jiang, Y.R.; Teng, L.; Yang, J. Downregulation of glucose-6-phosphate dehydrogenase by microRNA-1 inhibits the growth of pituitary tumor cells. Oncol. Rep. 2018, 40, 3533–3542. [Google Scholar] [CrossRef] [Green Version]
- Barajas, J.M.; Reyes, R.; Guerrero, M.J.; Jacob, S.T.; Motiwala, T.; Ghoshal, K. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer. Sci. Rep. 2018, 8, 9105. [Google Scholar] [CrossRef]
- Pes, G.M.; Parodi, G.; Dore, M.P. Glucose-6-phosphate dehydrogenase deficiency and risk of cardiovascular disease: A propensity score-matched study. Atherosclerosis 2019, 282, 148–153. [Google Scholar] [CrossRef]
- Thomas, J.E.; Kang, S.; Wyatt, C.J.; Kim, F.S.; Mangelsdorff, A.D.; Weigel, F.K. Glucose-6-Phosphate Dehydrogenase Deficiency is Associated with Cardiovascular Disease in U.S. Military Centers. Tex. Heart Inst. J. 2018, 45, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Fois, A.; Dore, M.P.; Manca, A.; Scano, V.; Pirina, P.; Pes, G.M. Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Asthma. J. Clin. Med. 2021, 10, 5639. [Google Scholar] [CrossRef]
- Dore, M.P.; Errigo, A.; Bibbo, S.; Manca, A.; Pes, G.M. High Frequency of Glucose-6-Phosphate Dehydrogenase Deficiency in Patients Diagnosed with Celiac Disease. Nutrients 2022, 14, 1815. [Google Scholar] [CrossRef]
- Yen, W.C.; Wu, Y.H.; Wu, C.C.; Lin, H.R.; Stern, A.; Chen, S.H.; Shu, J.C.; Tsun-Yee Chiu, D. Impaired inflammasome activation and bacterial clearance in G6PD deficiency due to defective NOX/p38 MAPK/AP-1 redox signaling. Redox Biol. 2020, 28, 101363. [Google Scholar] [CrossRef]
- Crowell, S.B.; Crowell, E.B., Jr.; Mathew, M. Depression of erythrocyte glucose-6-phosphate dehydrogenase (G6PD) activity in enteric fever. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 183–186. [Google Scholar] [CrossRef]
- Hulshof, P.; Veenstra, J.; van Zwieten, R. Severe hemolytic anemia due to transient acquired G6PD deficiency after ingestion of sodium chlorite. Clin. Toxicol. 2019, 57, 65–66. [Google Scholar] [CrossRef]
- Naville, A.S.; Lazaro, E.; Boutin, J.; Prot-Leurent, C.; Mansier, O.; Richard, E.; Augis, V.; Weinmann, L.; Fuster, V.; Vial, J.P.; et al. Acquired glucose 6-phosphate dehydrogenase (G6PD) deficiency in a patient with Chronic Myelomonocytic Leukemia. Br. J. Haematol. 2022, 197, e45–e48. [Google Scholar] [CrossRef]
- Gheita, T.A.; Kenawy, S.A.; El Sisi, R.W.; Gheita, H.A.; Khalil, H. Subclinical reduced G6PD activity in rheumatoid arthritis and Sjogren’s Syndrome patients: Relation to clinical characteristics, disease activity and metabolic syndrome. Mod. Rheumatol. 2014, 24, 612–617. [Google Scholar] [CrossRef]
- Au, W.Y.; So, J.C.; Ma, S.K.; Lie, A.K. Glucose-6-phosphate-dehydrogenase deficiency and haematopoietic stem cell transplantation in Chinese patients. Hong Kong Med. J. 2009, 15, 35–38. [Google Scholar]
- Sagiv, E.; Fasano, R.M.; Luban, N.L.C.; Josephson, C.D.; Stowell, S.R.; Roback, J.D.; Francis, R.O.; Yee, M.E.M. Glucose-6-phosphate-dehydrogenase deficient red blood cell units are associated with decreased posttransfusion red blood cell survival in children with sickle cell disease. Am. J. Hematol. 2018, 93, 630–634. [Google Scholar] [CrossRef]
- Shalev, O.; Bogomolski-Yahalom, V.; Sharon, R. Hemolysis following transfusion of erythrocytes from a donor with G6PD deficiency and beta-thalassemia minor. Isr. J. Med. Sci. 1993, 29, 214–216. [Google Scholar]
- Khan, S.; Jamison, T.; Nguyen, C.; Namireddy, M.; Fisher, J. Berberine Use Unburies G6PD Deficiency. In Proceedings of the SHM Converge, Virtual, 3–7 May 2021. [Google Scholar]
- Marks, P.A.; Banks, J. Inhibition of Mammalian Glucose-6-Phosphate Dehydrogenase by Steroids. Proc. Natl. Acad. Sci. USA 1960, 46, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Dada, O.A.; Abugo, O.; Ogunmola, G.B. Thyroid hormones and the reactivities of genetic variants of human erythrocytic glucose-6-phosphate dehydrogenase. Enzyme 1983, 30, 217–222. [Google Scholar] [CrossRef]
- Czyzewska, U.; Tylicki, A.; Siemieniuk, M.; Strumilo, S. Changes of activity and kinetics of certain liver and heart enzymes of hypothyroid and T(3)-treated rats. J. Physiol. Biochem. 2012, 68, 345–351. [Google Scholar] [CrossRef]
- Fritz, R.S.; Kletzien, R.F. Regulation of glucose-6-phosphate dehydrogenase by diet and thyroid hormone. Mol. Cell. Endocrinol. 1987, 51, 13–17. [Google Scholar] [CrossRef]
- Lombardi, A.; Beneduce, L.; Moreno, M.; Diano, S.; Colantuoni, V.; Ursini, M.V.; Lanni, A.; Goglia, F. 3,5-diiodo-L-thyronine regulates glucose-6-phosphate dehydrogenase activity in the rat. Endocrinology 2000, 141, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Nehal, M.; Baquer, N.Z. Changes in hexokinase and glucose-6-phosphate dehydrogenase in red cells during hypo and hyperthyroidism. Biochem. Int. 1989, 19, 193–199. [Google Scholar]
- Zoller, L.C.; Axelson, J.F. A quantitative cytochemical analysis of large antral follicles in two types of rat polycystic ovaries. Anat. Rec. 1986, 215, 342–350. [Google Scholar] [CrossRef]
- Jain, S.K.; Parsanathan, R.; Levine, S.N.; Bocchini, J.A.; Holick, M.F.; Vanchiere, J.A. The potential link between inherited G6PD deficiency, oxidative stress, and vitamin D deficiency and the racial inequities in mortality associated with COVID-19. Free. Radic. Biol. Med. 2020, 161, 84–91. [Google Scholar] [CrossRef]
- Senmar, S. Congenital Hypothyroidism as the Main Cause of G6PD Deficiency Phenotype in Neonates. Available online: https://www.sid.ir/paper/790221/en (accessed on 27 September 2022).
- Agarwal, A.; Nayak, M.D.; Patil, A.; Manohar, C. Glucose 6 phosphate dehydrogenase deficiency unmasked by diabetic ketoacidosis: An underrated phenomenon. J. Clin. Diagn. Res. 2013, 7, 3012–3013. [Google Scholar] [CrossRef]
- ALjishi, F.; ALDarwish, M. Glucose-6-phosphate dehydrogenase deficiency induced haemolysis in a woman with newly diagnosed diabetes after normalisation of hyperglycaemia. Diabet. Med. 2017, 34, 1318–1321. [Google Scholar] [CrossRef]
- Carette, C.; Dubois-Laforgue, D.; Gautier, J.F.; Timsit, J. Diabetes mellitus and glucose-6-phosphate dehydrogenase deficiency: From one crisis to another. Diabetes Metab. 2011, 37, 79–82. [Google Scholar] [CrossRef]
- Choukem, S.P.; Sobngwi, E.; Garnier, J.P.; Letellier, S.; Mauvais-Jarvis, F.; Calvo, F.; Gautier, J.F. Hyperglycaemia per se does not affect erythrocyte glucose-6-phosphate dehydrogenase activity in ketosis-prone diabetes. Diabetes Metab. 2015, 41, 326–330. [Google Scholar] [CrossRef]
- Sobngwi, E.; Gautier, J.F.; Kevorkian, J.P.; Villette, J.M.; Riveline, J.P.; Zhang, S.; Vexiau, P.; Leal, S.M.; Vaisse, C.; Mauvais-Jarvis, F. High prevalence of glucose-6-phosphate dehydrogenase deficiency without gene mutation suggests a novel genetic mechanism predisposing to ketosis-prone diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 4446–4451. [Google Scholar] [CrossRef] [Green Version]
- McDermott, B.M.; Flatt, P.R.; Strain, J.J. Effects of copper deficiency and experimental diabetes on tissue antioxidant enzyme levels in rats. Ann. Nutr. Metab. 1994, 38, 263–269. [Google Scholar] [CrossRef]
- Afzal-Ahmed, I.; Mann, G.E.; Shennan, A.H.; Poston, L.; Naftalin, R.J. Preeclampsia inactivates glucose-6-phosphate dehydrogenase and impairs the redox status of erythrocytes and fetal endothelial cells. Free. Radic. Biol. Med. 2007, 42, 1781–1790. [Google Scholar] [CrossRef]
- Németh, I.; Orvos, H.; Boda, D. Blood glutathione redox status in gestational hypertension. Free. Radic. Biol. Med. 2001, 30, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Nasr, L.B.; Monet, J.D.; Lucas, P.; Bader, C.A. Vitamin D3 and glucose-6-phosphate dehydrogenase in rat duodenal epithelial cells. Am. J. Physiol. 1989, 257, G760–G765. [Google Scholar] [CrossRef]
- Bachelet, M.; Bourdeau, A.; Lair, M.; Bader, C.; Ben Nasr, L.; Thomas, M.; Ulmann, A. Effect of plasma levels of parathyroid hormone on NADPH pathways in kidney and liver. Kidney Int. 1985, 27, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, M.G.; Javier, L.M.; Litan, R.R.R.; Gonzaga, A.; Punzalan, J.M. Association of Sex and Zinc Deficiency with Glucose-6-Phosphate Dehydrogenase Deficiency in Filipino Children. Philipp. J. Sci. 2022, 151, 13–23. [Google Scholar] [CrossRef]
- Boivin, P.; Galand, C.; Hakim, J.; Kahn, A. Acquired erythroenzymopathies in blood disorders: Study of 200 cases. Br. J. Haematol. 1975, 31, 531–543. [Google Scholar] [CrossRef]
- Karafin, M.S.; Francis, R.O. Impact of G6PD status on red cell storage and transfusion outcomes. Blood Transfus. 2019, 17, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Arnold, H.; Blume, K.G.; Lohr, G.W. Mechanisms for acquired red cell enzyme defects. Blood 1977, 49, 1022–1023. [Google Scholar] [CrossRef]
- Renzaho, A.M.; Husser, E.; Polonsky, M. Should blood donors be routinely screened for glucose-6-phosphate dehydrogenase deficiency? A systematic review of clinical studies focusing on patients transfused with glucose-6-phosphate dehydrogenase-deficient red cells. Transfus. Med. Rev. 2014, 28, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Arnold, H.; Blume, K.G.; Lohr, G.W.; Boulard, M.; Najean, Y. “Acquired” red cell enzyme defects in hematological diseases. Clin. Chim. Acta 1974, 57, 187–189. [Google Scholar] [CrossRef]
- Tanphaichitr, V.S.; Suvatte, V.; Mahasandana, C.; Tuchinda, S. Transient, acquired glucose-6-phosphate dehydrogenase deficiency in Thai children with typhoid fever. Southeast Asian J. Trop. Med. Public Health 1982, 13, 105–109. [Google Scholar] [PubMed]
- Gordon, G.; Mackow, M.C.; Levy, H.R. On the mechanism of interaction of steroids with human glucose 6-phosphate dehydrogenase. Arch. Biochem. Biophys. 1995, 318, 25–29. [Google Scholar] [CrossRef]
- Leopold, J.A.; Dam, A.; Maron, B.A.; Scribner, A.W.; Liao, R.; Handy, D.E.; Stanton, R.C.; Pitt, B.; Loscalzo, J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat. Med. 2007, 13, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenezic, N.; Kostic, S.; Strac, D.S.; Grunauer, M.; Nenezic, D.; Radosavljevic, M.; Jancic, J.; Samardzic, J. Dehydroepiandrosterone (Dhea): Pharmacological Effects And Potential Therapeutic Application. Mini-Rev. Med. Chem. 2022. [Google Scholar] [CrossRef]
- Ennas, M.G.; Laconi, S.; Dessi, S.; Milia, G.; Murru, M.R.; Manconi, P.E. Influence of dehydroepiandrosterone on G-6-PD activity and 3H-thymidine uptake of human lymphocytes in vitro. Toxicol. Pathol. 1987, 15, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Ochi, R.; Chettimada, S.; Kizub, I.; Gupte, S.A. Dehydroepiandrosterone inhibits ICa,L and its window current in voltage-dependent and -independent mechanisms in arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1602–H1613. [Google Scholar] [CrossRef] [Green Version]
- Niort, G.; Boccuzzi, G.; Brignardello, E.; Bonino, L.; Bosia, A. Effect of dehydroepiandrosterone on human erythrocytes redox metabolism: Inhibition of glucose-6-phosphate dehydrogenase activity in vivo and in vitro. J. Steroid Biochem. 1985, 23, 657–661. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Jiang, C.; Fang, Z.; Feng, Y.; Jiang, W. The effect and mechanism of inhibiting glucose-6-phosphate dehydrogenase activity on the proliferation of Plasmodium falciparum. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 771–781. [Google Scholar] [CrossRef]
- Georgakouli, K.; Deli, C.K.; Zalavras, A.; Fatouros, I.G.; Kouretas, D.; Koutedakis, Y.; Jamurtas, A.Z. Alpha-lipoic acid supplementation up-regulates antioxidant capacity in adults with G6PD deficiency. Food Chem. Toxicol. 2013, 61, 69–73. [Google Scholar] [CrossRef]
- Kayser, L.; Thomsen, J. Glucose-6-phosphate dehydrogenase activity in monolayer cultures of thyroid epithelial cells: TSH and inhibition of nitrogen oxide synthase affect the enzyme activity and the oxygen sensitivity of the histochemical assay. Acta Histochem. 2005, 107, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ursini, M.V.; Parrella, A.; Rosa, G.; Salzano, S.; Martini, G. Enhanced expression of glucose-6-phosphate dehydrogenase in human cells sustaining oxidative stress. Biochem. J. 1997, 323 Pt 3, 801–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, D.S.; Kletzien, R.F. Ethanol modulation of the hormonal and nutritional regulation of glucose 6-phosphate dehydrogenase activity in primary cultures of rat hepatocytes. Biochem. J. 1984, 217, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumpo, D.J.; Kletzien, R.F. The effect of ethanol, alone and in combination with the glucocorticoids and insulin, on glucose-6-phosphate dehydrogenase synthesis and mRNA in primary cultures of hepatocytes. Biochem. J. 1985, 226, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsanathan, R.; Jain, S.K. Glucose-6-Phosphate Dehydrogenase Deficiency Activates Endothelial Cell and Leukocyte Adhesion Mediated via the TGFbeta/NADPH Oxidases/ROS Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 7474. [Google Scholar] [CrossRef] [PubMed]
- Heymann, A.D.; Cohen, Y.; Chodick, G. Glucose-6-phosphate dehydrogenase deficiency and type 2 diabetes. Diabetes Care 2012, 35, e58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.S.; Hsiao, L.Y.; Lin, C.Y.; Shih, M.C.; Hsieh, M.C.; Chang, J.G. Fasting glucose-to-HbA1c ratio is a good indicator of G6PD deficiency, but not thalassemia, in patients with type 2 diabetes mellitus. Clin. Chim. Acta 2020, 506, 9–15. [Google Scholar] [CrossRef]
- Govindarajan, S.; Zamir, I.; Bagewadi, S.; Moore, E. Manifestation of glucose-6-phosphate dehydrogenase deficiency in the wake of new-onset type 1 diabetes mellitus: A case report. J. Med. Case Rep. 2022, 16, 320. [Google Scholar] [CrossRef]
- Alzaki, A.A.; Alalawi, N.H. Diabetic Ketoacidosis Revealing Severe Glucose-6-Phosphate Dehydrogenase Deficiency (G6PD-D) Deficiency with Methemoglobinemia: A Case Report. Am. J. Case Rep. 2019, 20, 726–729. [Google Scholar] [CrossRef]
- Ansari, U.; Bhardwaj, P.; Quadri, H.; Barnes, M.; George, J. Diabetic Ketoacidosis Unmasking a Diagnosis of Glucose-6-Phosphate Dehydrogenase Deficiency: A Case Report and Literature Review. Cureus 2022, 14, e23842. [Google Scholar] [CrossRef]
- Shalev, O.; Wollner, A.; Menczel, J. Diabetic ketoacidosis does not precipitate haemolysis in patients with the Mediterranean variant of glucose-6-phosphate dehydrogenase deficiency. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 179–180. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Srivastav, V.; Bhate, P.; Gupta, V.; Nadkar, M.Y. Glucose-6-Phosphate Dehydrogenase Deficiency Unveiled by Diabetic Ketoacidosis: A Dual Dilemma. J. Assoc. Physicians India 2017, 65, 98–102. [Google Scholar]
- He, M.H.; Zhang, Y. Advanced glycation end products inhibit glucose-6-phosphate dehydrogenase activity and expression in human umbilical vein endothelial cells. Sheng Li Xue Bao 2012, 64, 646–650. [Google Scholar]
- Garcia, D.R.; Holten, D. Inhibition of rat liver glucose-6-phosphate dehydrogenase synthesis by glucagon. J. Biol. Chem. 1975, 250, 3960–3965. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxidative Med. Cell. Longev. 2017, 2017, 1930261. [Google Scholar] [CrossRef] [Green Version]
- Lebovitz, H.E.; Banerji, M.A. Ketosis-Prone Diabetes (Flatbush Diabetes): An Emerging Worldwide Clinically Important Entity. Curr. Diabetes Rep. 2018, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Rho, H.K.; Kim, K.H.; Choe, S.S.; Lee, Y.S.; Kim, J.B. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol. Cell. Biol. 2005, 25, 5146–5157. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.; Burdge, G.C.; Wootton, S.A.; Clark, M.L.; Frayn, K.N. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes 2002, 51, 2684–2690. [Google Scholar] [CrossRef] [Green Version]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Abdulhadi, N.H. Glucose 6 phosphate dehydrogenase (G6PD) deficiency is a possible risk factor for the development of preeclampsia. Med. Hypotheses 2004, 62, 780–782. [Google Scholar] [CrossRef]
- Miksicek, R.J.; Towle, H.C. Changes in the rates of synthesis and messenger RNA levels of hepatic glucose-6-phosphate and 6-phosphogluconate dehydrogenases following induction by diet or thyroid hormone. J. Biol. Chem. 1982, 257, 11829–11835. [Google Scholar] [CrossRef]
- Salati, L.M.; Amir-Ahmady, B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu. Rev. Nutr. 2001, 21, 121–140. [Google Scholar] [CrossRef]
- Timmers, K.I.; Knittle, J.L. Regulation of glucose-6-phosphate dehydrogenase activity during caloric restriction in human adipose tissue. Enzyme 1982, 28, 66–70. [Google Scholar] [CrossRef]
- Bao, B.Y.; Ting, H.J.; Hsu, J.W.; Lee, Y.F. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int. J. Cancer 2008, 122, 2699–2706. [Google Scholar] [CrossRef]
- Simmons, K.M.; Beaudin, S.G.; Narvaez, C.J.; Welsh, J. Gene Signatures of 1,25-Dihydroxyvitamin D3 Exposure in Normal and Transformed Mammary Cells. J. Cell. Biochem. 2015, 116, 1693–1711. [Google Scholar] [CrossRef]


| Clinical Condition | In Vitro Studies | In Vivo Studies | Studies in Humans |
|---|---|---|---|
| Blood disorders | – | – | Somatic mutation in bone marrow progenitor cells [18]; hematopoietic stem cell transplantation [20]; transfusion from deficient donors [21,22] |
| Ingestion of chemicals | – | – | Sodium chlorite [17] Herbal supplements [23] |
| Endocrine disorders | Excess of mineralocorticoids [24] Hypothyroidism [25] | Hypothyroid state by using drugs [26,27,28], Thyroidectomy [29] Polycystic ovary [30] | Excess of mineralocorticoids [31] Congenital hypothyroidism [32] Diabetes [33,34,35] Ketosis-prone diabetes [36,37] |
| Streptozotocin-induced diabetes [38] | Rheumatoid arthritis associated with metabolic syndrome [19] | ||
| Preeclampsia | – | – | Impaired redox status [39,40] |
| Micronutrient deficiency | – | Rat duodenal mucosa [41]; vitamin D deficiency [42] | Zinc deficiency [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pes, G.M.; Dore, M.P. Acquired Glucose-6-Phosphate Dehydrogenase Deficiency. J. Clin. Med. 2022, 11, 6689. https://doi.org/10.3390/jcm11226689
Pes GM, Dore MP. Acquired Glucose-6-Phosphate Dehydrogenase Deficiency. Journal of Clinical Medicine. 2022; 11(22):6689. https://doi.org/10.3390/jcm11226689
Chicago/Turabian StylePes, Giovanni Mario, and Maria Pina Dore. 2022. "Acquired Glucose-6-Phosphate Dehydrogenase Deficiency" Journal of Clinical Medicine 11, no. 22: 6689. https://doi.org/10.3390/jcm11226689