Gaidropsarus gallaeciae (Gadiformes: Gaidropsaridae), a New Northeast Atlantic Rockling Fish, with Commentary on the Taxonomy of the Genus †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
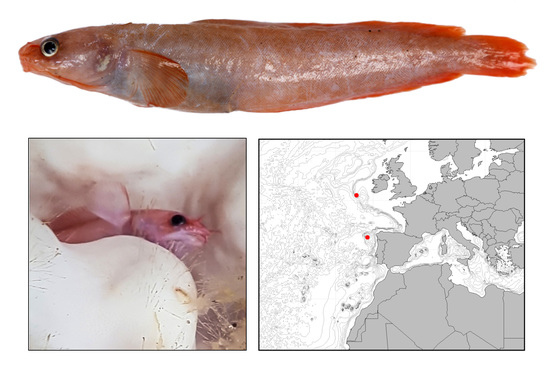
3.1. Holotype
3.2. Paratypes
3.3. Comparative Material Examined
3.4. Diagnosis
3.5. Differential Diagnosis
3.6. Etymology
3.7. Description
3.8. Molecular Taxonomic Remarks
3.9. Habitat and Distribution
3.10. Accompanying Fauna
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balushkin, A.V. On the first occurrence of the rockling Gaidropsarus pakhorukovi Shcherbachev (Gairdropsarini, Lotidae, Gadidae) and on species diagnostics of G. pakhorukovi and G. parini Svetovidov. J. Ichthyol. 2009, 49, 723–729. [Google Scholar] [CrossRef]
- Barros-García, D.; Bañón, R.; Arronte, J.C.; Fernández-Peralta, L.; García, R.; Iglésias, S.P.; Sellos, D.Y.; Pedro-Barreiros, J.; Sebastián-Comesaña, A.; de Carlos, A. New insights into the systematics of North Atlantic Gaidropsarus (Gadiformes, Gadidae): Flagging synonymies and hidden diversity. Mar. Biol. Res. 2018, 14, 17–29. [Google Scholar] [CrossRef]
- Barros-García, D.; Sebastián-Comesaña, A.; Bañón, R.; Baldó, F.; Arronte, J.C.; Froufe, E.; de Carlos, A. Multilocus species delimitation analyses show junior synonyms and deep-sea unknown species of genus Gaidropsarus (Teleostei: Gadiformes) in the North Atlantic/Mediterranean Sea area. Mar. Biol. 2022, submitted. [CrossRef]
- Biscoito, M.; Saldanha, L. Gaidropsarus mauli a new species of three-bearded rockling (Gadiformes, Gadidae) from the Lucky Strike hydrothermal vent field (Mid-Atlantic Ridge) and the Biscay Slope (Northeastern Atlantic). Zootaxa 2018, 4459, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Roa-Varón, A.; Dikow, R.B.; Carnevale, G.; Tornabene, L.; Baldwin, C.C.; Li, C.; Hilton, E.J. Confronting sources of systematic error to resolve historically contentious relationships: A case study using Gadiform fishes (Teleostei, Paracanthopterygii, Gadiformes). Syst. Biol. 2021, 70, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.M.; Inada, T.; Iwamoto, T.; Scialabba, N. FAO species catalogue. Gadiform fishes of the world (Order Gadiformes). An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date. FAO Fish. Synop. 1990, 10, 1–442. [Google Scholar]
- Francisco, S.M.; Robalo, J.I.; Stefanni, S.; Levy, A.; Almada, V.C. Gaidropsarus (Gadidae, Teleostei) of the North Atlantic Ocean: A brief phylogenetic review. J. Fish Biol. 2014, 85, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Bañón, R.; Arronte, J.C.; Rodríguez-Cabello, C.; Piñeiro, C.G.; Punzón, A.; Serrano, A. Commented checklist of marine fishes from the Galicia Bank seamount (NW Spain). Zootaxa 2016, 4067, 293–333. [Google Scholar] [CrossRef] [Green Version]
- Bañón, R.; Ruiz-Pico, S.; Baldó, F.; de Carlos, A. Unexpected deep-sea fish species on the Porcupine Bank (NE Atlantic): Biogeographical implications. J. Fish Biol. 2020, 97, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Morato, T.; Hoyle, S.D.; Allain, V.; Nicol, S.J. Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl. Acad. Sci. USA 2010, 107, 9707–9711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeda, K.; Takashima, S.; Yamakita, T.; Tsuchida, S.; Fujiwara, Y. Deep-sea fish fauna on the seamounts of southern Japan with taxonomic notes on the observed species. J. Mar. Sci. Eng. 2021, 9, 1294. [Google Scholar] [CrossRef]
- Linley, T.D.; Lavaleye, M.; Maiorano, P.; Bergman, M.; Capezzuto, F.; Cousins, N.J.; Priede, I.G. Effects of cold-water corals on fish diversity and density (European continental margin: Arctic, NE Atlantic and Mediterranean Sea): Data from three baited lander systems. Deep-Sea Res. II Top. Stud. Oceanogr. 2017, 145, 8–21. [Google Scholar] [CrossRef]
- Iwamoto, T.; Okamoto, M. A new grenadier fish of the genus Lucigadus (Macrouridae, Gadiformes, Teleostei) from the Emperor Seamounts, Northwestern Pacific. Proc. Calif. Acad. Sci. 2015, 62, 369–380. [Google Scholar]
- Møller, P.R.; Schwarzhans, W.W.; Lauridsen, H.; Nielsen, J.G. Bidenichthys okamotoi, a new species of the Bythitidae (Ophidiiformes, Teleostei) from the Koko Seamount, Central North Pacific. J. Mar. Sci. Eng. 2021, 9, 1399. [Google Scholar] [CrossRef]
- Cuvelier, D.; Sarrazin, J.; Colaco, A.; Copley, J.; Desbruyeres, D.; Glover, A.G.; Tyler, P.; Santos, R. Distribution and spatial variation of hydrothermal faunal assemblages at Lucky Strike (Mid-Atlantic Ridge) revealed by high-resolution video image analysis. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 2026–2040. [Google Scholar] [CrossRef] [Green Version]
- Söffker, M.; Sloman, K.A.; Hall-Spencer, J.M. In situ observations of fish associated with coral reefs off Ireland. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 818–825. [Google Scholar] [CrossRef] [Green Version]
- Wienberg, C.; Beuck, L.; Heidkamp, S.; Hebbeln, D.; Freiwald, A.; Pfannkuche, O.; Monteys, X. Franken Mound: Facies and biocoenoses on a newly-discovered “carbonate mound” on the western Rockall Bank, NE Atlantic. Facies 2008, 54, 1–24. [Google Scholar] [CrossRef]
- Morato, T.; Kvile, K.Ø.; Taranto, G.H.; Tempera, F.; Narayanaswamy, B.E.; Hebbeln, D.; Menezes, G.; Wienberg, C.; Santos, R.S.; Pitcher, T.J. Seamount physiography and biology in North-East Atlantic and Mediterranean Sea. Biogeosciences 2013, 10, 3039–3054. [Google Scholar] [CrossRef] [Green Version]
- Bañón, R.; de Carlos, A.; Farias, C.; Vilas-Arrondo, N.; Baldó, F. Exploring deep-sea biodiversity in the Porcupine Bank (NE Atlantic) through fish integrative taxonomy. J. Mar. Sci. Eng. 2021, 9, 1075. [Google Scholar] [CrossRef]
- Johnston, G.; O’Hea, B.; Dransfeld, L. Fish species recorded during deepwater trawl surveys on the continental shelf and the Porcupine Bank, 2006–2008. Ir. Nat. J. 2010, 31, 130–134. [Google Scholar]
- O’Riordan, C.E. Some interesting fishes and other marine fauna from the Porcupine Bank. Ir. Nat. J. 1984, 21, 321–323. [Google Scholar]
- Serrano, A.; González-Irusta, J.M.; Punzón, A.; García-Alegre, A.; Lourido, A.; Ríos, P.; Blanco, M.; Gómez-Ballesteros, M.; Druet, M.; Cristobo, J.; et al. Deep-sea benthic habitats modeling and mapping in a NE Atlantic seamount (Galicia Bank). Deep-Sea Res. Part I Oceanogr. Res. Pap. 2017, 126, 115–127. [Google Scholar] [CrossRef]
- Svetovidov, A.N. Review of three-bearded rocklings of the genus Gaidropsarus Rafinesque, 1810 (Gadidae) with description of a new species. J. Ichthyol. 1986, 62, 115–135. [Google Scholar]
- Svetovidov, A.N. Gadidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 2, pp. 680–710. [Google Scholar]
- Saitou, N.; Nei, M. The neighbour-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Cohen, D.M. Gaidropsaridae. In The Living Marine Resources of the Eastern Central Atlantic. Bony fishes. Part 1. Elopiformes to Scorpaeniformes; Carpenter, K.E., De Angelis, N., Eds.; FAO: Rome, Italy, 2016; Volume 3, pp. 2015–2022. [Google Scholar]
- Ward, R.D.; Hanner, R.; Hebert, P.D.N. The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 2009, 74, 329–356. [Google Scholar] [CrossRef] [PubMed]
- Bañón, R.; Arronte, J.C.; Vázquez-Dorado, S.; del Río, J.L.; de Carlos, A. DNA barcoding of the genus Lepidion (Gadiformes: Moridae) with recognition of Lepidion eques as a junior synonym of Lepidion lepidion. Mol. Ecol. Resour. 2013, 13, 189–199. [Google Scholar] [CrossRef]
- Collett, R. Diagnoses de poissons nouveaux provenant des campagnes de “L’Hirondelle.” V. Descriptions de deux espèces nouvelles de genre Onus Risso. Bull. Soc. Zool. Fr. 1890, 15, 105–109. [Google Scholar]
- Andrew, T.G.; Hecht, T.; Heemstra, P.C.; Lutjeharms, J.R.E. Fishes of the Tristan da Cunha Group and Gough Island, South Atlantic Ocean. Ichthyol. Bull. 1995, 63, 1–43. [Google Scholar]
- Orsi Relini, L.; Relini, G. Gaidropsarus granti from a Ligurian seamont: A Mediterranean native species? Mar. Ecol. 2014, 35, 35–40. [Google Scholar] [CrossRef]
- Fernández, A.; Pereiro, F.X.; Iglesias, S.; Porteiro, C.; Pallarés, P. La Pesquería demersal gallega, estrategias de pesca para su regulación racional en base a la Merluza. Bol. Inst. Esp. Oceanogr. 1978, 4, 69–109. [Google Scholar]
- Roy, D.; Hurlbut, T.R.; Ruzzante, D.E. Biocomplexity in a demersal exploited fish, white hake (Urophycis tenuis): Depth-related structure and inadequacy of current management approaches. Can. J. Fish. Aquat. Sci. 2012, 69, 415–429. [Google Scholar] [CrossRef]
- Ingram, T. Speciation along a depth gradient in a marine adaptive radiation. Proc. R. Soc. B Biol. Sci. 2011, 278, 613–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendry, A.P. Ecological speciation! Or the lack thereof? Can. J. Fish. Aquat. Sci. 2009, 66, 1383–1398. [Google Scholar] [CrossRef] [Green Version]
- González-Irusta, J.M.; De la Torriente, A.; Punzón, A.; Blanco, M.; Arronte, J.C.; Bañón, R.; Cartes, J.; Serrano, A. Living at the top. Connectivity limitations and summit depth drive fish diversity patterns in an isolated seamount. Mar. Ecol. Prog. Ser. 2021, 670, 121–137. [Google Scholar] [CrossRef]
- Milligan, R.J.; Spence, G.; Murray Roberts, J.; Bailey, D.M. Fish communities associated with cold-water corals vary with depth and substratum type. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2016, 114, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Quattrini, A.M.; Nizinski, M.S.; Chaytor, J.D.; Demopoulos, A.W.; Roark, E.B.; France, S.C.; Moore, J.A.; Heyl, T.; Auster, P.J.; Kinlan, B.; et al. Exploration of the canyon-incised continental margin of the Northeastern United States reveals dynamic habitats and diverse communities. PLoS ONE 2015, 10, e0139904. [Google Scholar] [CrossRef] [PubMed]
- Freiwald, A.; (Executive Director Senckenberg am Meer, Wilhelmshaven, Hamburg, Germany). Personal communication, 2022.
- Paulus, E. Shedding light on deep-sea biodiversity—A highly vulnerable habitat in the face of anthropogenic change. Front. Mar. Sci. 2021, 8, 667048. [Google Scholar] [CrossRef]
- Daly-Engel, T.S.; Baremore, I.E.; Grubbs, R.D.; Gulak, S.J.; Graham, R.T.; Enzenauer, M.P. Resurrection of the sixgill shark Hexanchus vitulus Springer & Waller, 1969 (Hexanchiformes, Hexanchidae), with comments on its distribution in the northwest Atlantic Ocean. Mar. Biodivers. 2019, 49, 759–768. [Google Scholar] [CrossRef]
- Renner, S.S. A Return to Linnaeus’s Focus on Diagnosis, Not Description: The Use of DNA Characters in the Formal Naming of Species. Syst. Biol. 2016, 65, 1085–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, A.C.; Marinoni, L. DNA barcoding and traditional taxonomy unified through integrative taxonomy: A view that challenges the debate questioning both methodologies. Biota Neotrop. 2010, 10, 339–346. [Google Scholar] [CrossRef] [Green Version]





| Holotype | Paratypes | Mean ± SE | |||||
|---|---|---|---|---|---|---|---|
| TL | 111.4 | 88.2 | 101.6 | 105.5 | 74.3 | 65.8 | 91.1 ± 7.4 |
| SL | 98.5 | 76.4 | 89.2 | 95.3 | 65.1 | 57.4 | 80.3 ± 6.8 |
| As % SL | |||||||
| Head length | 22.4 | 21.1 | 25.2 | 22.9 | 24 | 23.9 | 23.3 ± 0.6 |
| 1st Predorsal length | 21.8 | 27.7 | 22.6 | 22.4 | 23 | 24.7 | 23.7 ± 0.9 |
| 3rd Predorsal length | 37.9 | 34.4 | 36.4 | 33.7 | 40.4 | 38 | 36.8 ± 1 |
| 2nd dorsal base fin length | 11.7 | 9.8 | 11.3 | 10.3 | 11.4 | 11.7 | 11 ± 0.3 |
| 3rd dorsal base fin length | 56.4 | 59.7 | 64.4 | 59 | 60.1 | 55.9 | 59.3 ± 1.2 |
| Anal base fin length | 46.4 | 48 | 47.2 | 46.6 | 39.6 | 45.6 | 45.6 ± 1.2 |
| Pectoral fin length | 15.6 | 16.1 | 17.5 | 16.1 | 16.9 | 15.3 | 16.3 ± 0.3 |
| Pelvic fin length | 16.2 | 18.8 | 17.8 | 16.4 | 18 | 19 | 17.7 ± 0.5 |
| Preanal length | 48.9 | 45.9 | 48.4 | 43.4 | 47.2 | 49.1 | 47.2 ± 0.9 |
| Body depth | 19.9 | 15.7 | 19.4 | 21.6 | 19.7 | 19.5 | 19.3 ± 0.8 |
| Prepectoral length | 22.2 | 22 | 22.8 | 22 | 24.1 | 27.5 | 23.4 ± 0.9 |
| Prepelvic length | 17.9 | 18.6 | 19.3 | 16.8 | 20.4 | 21.3 | 19.1 ± 0.7 |
| Caudal peduncle height | 6.3 | 6.2 | 7.4 | 6.9 | 7.2 | 7.7 | 7 ± 0.2 |
| As % HL | |||||||
| Snout length | 24 | 21.1 | 20.9 | 24.3 | 25 | 22.6 | 23 ± 0.7 |
| Eye diameter | 15.8 | 20.5 | 19.6 | 19.7 | 18.6 | 17.5 | 18.6 ± 0.7 |
| Postorbital length | 64.3 | 58.4 | 54.7 | 56 | 56.4 | 60.6 | 58.4 ± 1.5 |
| Interorbital space | 24.4 | 21.7 | 23.1 | 28 | 25 | 26.3 | 24.8 ± 0.9 |
| Upper jaw length | 37.9 | 44.7 | 39.6 | 47.2 | 41 | 43.1 | 42.3 ± 1.4 |
| Lower jaw length | 34.4 | 37.3 | 32 | 36.7 | 36.5 | 40.1 | 36.2 ± 1.1 |
| Chin barbel length | 16.7 | 16.8 | 18.6 | 21.1 | 21.8 | 22.6 | 19.6 ± 1.1 |
| 1st dorsal fin ray length | 15.8 | 19.3 | 24 | 22 | 26.3 | 27 | 22.4 ± 1.8 |
| Meristic | |||||||
| 3rd dorsal fin rays | 60 | 57 | 57 | 58 | 54 | 58 | 57.3 ± 0.8 |
| Anal fin rays | 44 | 52 | 46 | 49 | 50 | 50 | 48.5 ± 1.2 |
| Pectoral fin rays | 22 | 23 | 21 | 22 | 23 | 22 | 22.2 ± 0.3 |
| Ventral fin rays | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Gill rakers (inner) | 1 + 8 | 1 + 5 | 1 + 6 | 1 + 6 | 1 + 8 | 1 + 6 | 7.5 ± 0.5 |
| Gill rakers (outer) | 1 + 8 | 1 + 7 | 1 + 7 | 1 + 8 | 1 + 6 | 1 + 7 | 8.2 ± 0.3 |
| Vertebrae | 44 | 43 | – | – | – | – | 43.5 ± 0.3 |
| Species | GGAL | GMAU | GARG | GENS | GMED |
|---|---|---|---|---|---|
| As % SL | |||||
| Head length | 21.1–25.2 | 23.4–25.4 | 19.7–25.1 | 19–22 | 18.8–24 |
| 1st Predorsal length | 21.8–27.7 | 22.8–23.9 | 20.7–22.6 | 18.7–20.2 | 18.6–18.9 |
| 3rd Predorsal length | 33.7–40.4 | 36–36.3 | 31.7–36.8 | 29.1–32.3 | 20.1–38.5 |
| 2nd dorsal base fin length | 9.8–11.7 | — | 8.6–11.4 | 8–11.3 | 13.2–18.4 |
| 3rd dorsal base fin length | 55.9–64.4 | — | 57.1–62.4 | 59.3–64.4 | 54.1–60.9 |
| Anal base fin length | 39.6–48 | — | 38.7–39.8 | 39.9–46.3 | 45–52.2 |
| Pectoral fin length | 15.3–17.5 | 17.8–19.4 | 16.1–18.9 | 17–20 | 12.3–14.6 |
| Pelvic fin length | 16.2–19 | 27.5–33.3 | 18.1–21.5 | 17–26.3 | 13–15.5 |
| Preanal length | 43.4–49.1 | 50.8–53.1 | 51.4–53.4 | 48–50 | 44.1–51.1 |
| Body depth | 15.7–21.6 | 15.2–21.9 | 15.6–23.5 | 16.7–25.2 | 14–19.3 |
| Prepectoral length | 22–27.5 | — | 20.7–28 | 17.8–21.3 | 20.5–22.7 |
| Prepelvic length | 16.8–21.3 | — | 16.9–19.9 | 12.7–16 | 15–17.2 |
| Caudal peduncle height | 6.2–7.7 | 5.6–6.8 | 5.5–7.4 | 5.1–7.2 | 4.5–6.3 |
| As% HL | |||||
| Snout length | 20.9–25 | 25.4–26.7 | 25.2–27 | 23.6–27.9 | 18.8–30.4 |
| Eye diameter | 15.8–20.5 | 10.4–12 | 14.8–21.8 | 17.3–24.5 | 13.3–22.5 |
| Postorbital length | 54.7–64.3 | — | 57.3–58.4 | 54.1–59.1 | 60.63.5 |
| Interorbital space | 21.7–28 | 20.9–21.3 | 13.1–23.1 | 14.4–25.1 | 9.1–25.7 |
| Upper jaw length | 37.9–47.2 | — | 44.7–47.7 | 45.3–64.8 | 42.9–45.6 |
| Lower jaw length | 32–40.1 | — | 36.6–41.1 | 36.1–60.3 | 38.9–40.2 |
| Chin barbel length | 16.7–22.6 | 26.9 | 19.8–23.8 | 15.1–20.8 | 15.3–18.5 |
| 1st dorsal fin ray length | 15.8–27 | 21.3–25.4 | 24.1–43 | 82.1–145.5 | 14.9–42 |
| Meristic | |||||
| 3rd dorsal fin rays | 54–60 | 57–58 | 52–65 | 52–64 | 48–63 |
| Anal fin rays | 44–52 | 46–47 | 43–51 | 40–48 | 41–53 |
| Pectoral fin rays | 21–23 | 25–26 | 22–25 | 20–27 | 15–19 |
| Ventral fin rays | 7 | 9 | 7–8 | 6–7 | 5–8 |
| Gill rakers (inner) | 6–9 | 9 | 10–11 | 12–14 | 9–11 |
| Gill rakers (outer) | 7–9 | 7–8 | 8–11 | 11–13 | 7–10 |
| Vertebrae | 43–44 | 47–48 | 49–53 | 50–54 | 46–50 |
| Species | GVUL | GGRA | GMAC | GINS | GNOV |
| As % SL | |||||
| Head length | 23.6–25.9 | 20.9–25.5 | 19.3–23.2 | 18.7–21.5 | 17.9–20.7 |
| 1st Predorsal length | 22.1–24 | — | 20.5–22.9 | — | — |
| 3rd Predorsal length | 36.4–38.1 | 21.1–37.9 | 33.3–38.3 | — | — |
| 2nd dorsal base fin length | 11.3–13.9 | 10.7 | 8.6–11.7 | 8.5–9.8 | — |
| 3rd dorsal base fin length | 54.9–61.1 | 54.4–59.7 | 55.6–66.3 | 65.1–67.5 | 58.5–65.3 |
| Anal base fin length | 40.5–45.3 | 43.6–45.6 | 48.5–50 | 46.4–49.6 | 48.2–51.5 |
| Pectoral fin length | 14.1–15.4 | 13.8–15.4 | 14.7–15.5 | — | — |
| Pelvic fin length | 17.4–20.3 | 15.5–23.1 | 9.6–16.1 | — | — |
| Preanal length | 48.9–54.8 | 48.7–54.8 | 44–47.6 | — | — |
| Body depth | 14.8–20.4 | 13.1–14 | 14.2–19.5 | — | — |
| Prepectoral length | 23.5–25.4 | — | 19.3–25 | — | — |
| Prepelvic length | 18.6–20.7 | — | 16.3–20.2 | — | — |
| Caudal peduncle height | 7–8.5 | 5.6–6.9 | 4.8–7.1 | 6.8–8.5 | 6.3–8.1 |
| As % HL | |||||
| Snout length | 21.2–26.6 | 19.3–29.2 | 21–26 | 27.6 | — |
| Eye diameter | 10.5–16.7 | 13.7–18.8 | 16–23.7 | — | 15.2–19 |
| postorbital length | 55.3–65.2 | 59.6 | 54.3–59.1 | — | |
| Interorbital space | 14.4–19.5 | 10.5–17.6 | 12.5–26.5 | 16.7–19.4 | 15.2–18.7 |
| Upper jaw length | 42.3–49.3 | 42.9 | 46.2–52.9 | 59.2–61.3 | — |
| Lower jaw length | 36.8–40 | 41.7 | 36.6–44 | 48.4–55.1 | — |
| Barbel length | 19.6–24.2 | — | 14–22.2 | — | — |
| 1st dorsal ray length | 9.5–16.9 | 12.7–14.9 | 10.1–25.1 | 11.2–25 | 20–27.9 |
| Meristic | |||||
| 3rd dorsal fin rays | 56–64 | 55–60 | 48–59 | 66–70 | 56–69 |
| Anal fin rays | 46–54 | 45–52 | 40–50 | 50–57 | 50–59 |
| Pectoral fin rays | 20–24 | 20–22 | 17–22 | 19–22 | 20–21 |
| Ventral fin rays | 6–7 | 7–8 | 6–7 | — | 7–8 (5) |
| Gillraker (inner) | 10–11 | — | 8–11 | 9 | 9–10 |
| Gillraker (outer) | 7–9 | 10 | 6–9 | 7 | 6–8 |
| Vertebrae | 46–49 | 44–47 | 43–47 | 47–49 | 46–49 |
| Species | GCAP | GPAK | GPAR | ||
| As % SL | |||||
| Head length | 19.4–22.5 | 23.7–24.7 | 22.1–22.8 | ||
| 1st Predorsal length | — | 24.4–25.3 | 17.8–18.5 | ||
| 3rd Predorsal length | — | — | — | ||
| 2nd dorsal base fin length | 12.2–13.2 | 12.8–15.5 | 10.4–11.6 | ||
| 3rd dorsal base fin length | — | 55.3 | 56–58.1 | ||
| Anal base fin length | 48.4–49 | 41.7 | 43.8–48.6 | ||
| Pectoral fin length | — | 17.7–19.2 | 17.3–17.8 | ||
| Pelvic fin length | — | — | 19.9–20.7 | ||
| Preanal length | — | — | 45.2–48.5 | ||
| Body depth | 16.5–17.3 | — | — | ||
| Prepectoral length | — | — | — | ||
| Prepelvic length | — | 22.4 | — | ||
| Caudal peduncle height | 7–8.1 | 6.5 | 6.7–7.1 | ||
| As % HL | |||||
| Snout length | 28.2–33.2 | — | — | ||
| Eye diameter | 16.1–20.9 | 17.2–19.8 | 13.9–16.4 | ||
| postorbital length | — | — | |||
| Interorbital space | 13.5–19.5 | 16 | — | ||
| Upper jaw length | 48.8–52.1 | — | — | ||
| Lower jaw length | — | — | — | ||
| Barbel length | — | — | — | ||
| 1st dorsal ray length | 19.5–32.5 | 12–15.1 | 26.7–28 | ||
| Meristic | |||||
| 3rd dorsal fin rays | 43–52 | 60–62 | 60–64 | ||
| Anal fin rays | 37–43 | 50–51 | 52–53 | ||
| Pectoral fin rays | 18–21 | 22–26 | 23–25 | ||
| Ventral fin rays | 6–7 | 7–8 | 7–8 | ||
| Gillraker (inner) | 8–9 | 9 | 10 | ||
| Gillraker (outer) | 4–9 | 9 | 7 | ||
| Vertebrae | 41–43 | 46–47 | 47–48 | ||
| Species | Coloration | Distribution |
|---|---|---|
| Gaidropsarus gallaeciae sp. nov. | Pinkish overall, greyish on the abdominal region | NE Atlantic: Galicia Bank and Porcupine Bank, 751–788 m depth |
| Gaidropsarus mauli | Pinkish overall, less intense on the abdominal region, varying from more or less uniform to a mottled pattern | Atlantic Ocean: Azores and Bay of Biscay; 850–1700 m depth |
| Gaidropsarus argentatus | Uniform reddish-brown to light brick red; pink ventrally | Arctic and Atlantic Oceans, from west Spitzbergen, Norwegian Sea, Iceland, south Greenland and off Labrador; 150–2260 m depth |
| Gaidropsarus ensis | Light brick red, belly tinted red with blue-grey tinge | N Atlantic: Off Newfoundland and Labrador and west of British Isles; 600–1500 m depth |
| Gaidropsarus mediterraneus | Varied, back and upper flank brown, sometimes reddish brown, grading to pale brown-white on the ventral, with pale spots along the sides; blackish with white blotches mainly in the Macaronesian specimens | NE Atlantic, from Norway and British Isles south to Morocco, including Canaries, Azores and Madeira, and Mediterranean; 0–40 m depth |
| Gaidropsarus vulgaris | Pale cream to pink or reddish with chocolate brown spots on head and body | NE Atlantic, from Norway and Iceland south to Gibraltar, including Madeira and Mediterranean; 10–120 m depth |
| Gaidropsarus granti | Back brown, with irregular brown creamy blotches and spots and a whitish longitudinal sinuous band along upper flank | NE Atlantic, in Porcupine Bank (southwest of Ireland); Galicia Bank off Spain; Azores, Madeira and Canary Islands and Mediterranean; 20–823 m depth |
| Gaidropsarus macrophthalmus | Back mottled deep brown, flanks reddish, belly pink | NE Atlantic, from Faroe Islands and British Isles to south of the Azores and Mediterranean; 150–600 m depth |
| Gaidropsarus insularum | Chocolate-brown | SE Atlantic: southern tip of Africa, Tristan da Cunha and Gough islands; SW Indic: St. Paul and Amsterdam Islands; littoral |
| Gaidropsarus novaezealandiae | Head, body and fins dark reddish brown, purplish grey ventrally | SW Pacific: New Zealand and south of Tasmania; 0–50 m, but two specimens collected at 300–500 m |
| Gaidropsarus capensis | Unknown in live fish | SE Atlantic and SW Indian Oceans: southern Africa; to 45 m depth |
| Gaidropsarus pakhorukovi | Brownish-grey, darker dorsally | SW Atlantic: Rio Grande Seamount; 670–1190 m depth |
| Gaidropsarus parini | Chocolate-brown to grey | SE Pacific: Nazca Ridge; 310–680 m depth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañón, R.; Baldó, F.; Serrano, A.; Barros-García, D.; de Carlos, A. Gaidropsarus gallaeciae (Gadiformes: Gaidropsaridae), a New Northeast Atlantic Rockling Fish, with Commentary on the Taxonomy of the Genus. Biology 2022, 11, 860. https://doi.org/10.3390/biology11060860
Bañón R, Baldó F, Serrano A, Barros-García D, de Carlos A. Gaidropsarus gallaeciae (Gadiformes: Gaidropsaridae), a New Northeast Atlantic Rockling Fish, with Commentary on the Taxonomy of the Genus. Biology. 2022; 11(6):860. https://doi.org/10.3390/biology11060860
Chicago/Turabian StyleBañón, Rafael, Francisco Baldó, Alberto Serrano, David Barros-García, and Alejandro de Carlos. 2022. "Gaidropsarus gallaeciae (Gadiformes: Gaidropsaridae), a New Northeast Atlantic Rockling Fish, with Commentary on the Taxonomy of the Genus" Biology 11, no. 6: 860. https://doi.org/10.3390/biology11060860