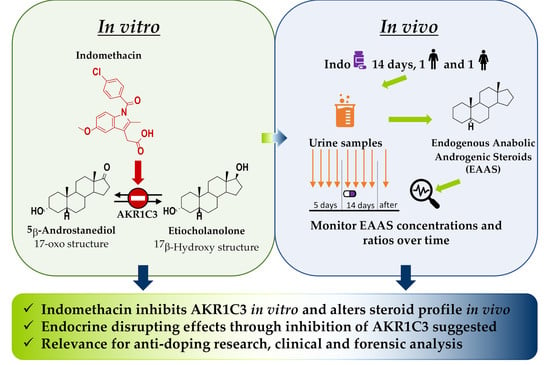
Influence of Indomethacin on Steroid Metabolism: Endocrine Disruption and Confounding Effects in Urinary Steroid Profiling of Anti-Doping Analyses
Abstract
:1. Introduction
2. Results
2.1. Qualitative Incubation In Vitro
2.2. Km Determination In Vitro
2.3. IC50 Determination In Vitro
2.4. In Vivo Administration Trial
3. Discussion
3.1. In Vitro Overview Incubation
- The substrate is an endogenous steroid, which plays a role in the steroid module of the Athlete Biological Passport of the World Anti-Doping Agency;
- No side reaction is taking place besides the formation of the desired product;
- The initial velocity is fast enough to be monitored spectro-fluorometrically.
3.2. Kinetic Measurements In Vitro
3.3. In Vivo Administration Trial
4. Materials and Methods
4.1. Materials
4.2. In Vitro Qualitative Incubation
4.3. In Vitro Kinetic Assay
4.4. Administration Study
4.5. Sample Preparation before Chromatographic Analysis
4.6. Fluorometric Analysis
4.7. GC-MS Analysis
4.8. GC-QToF Analysis
4.9. GC-MS/MS Analysis
4.10. Data Analysis of Kinetic Values
4.11. Data Analysis of Steroid Profiling and Indomethacin Detection in Urine Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Anti-Doping Agency. 2018 Anti-Doping Testing Figures. Available online: https://www.wada-ama.org/en/resources/laboratories/anti-doping-testing-figures-report (accessed on 1 July 2020).
- World Anti-Doping Agency. The World Anti-Doping Code. 2020 Prohibited List. Available online: https://www.wada-ama.org/en/resources/science-medicine/prohibited-list-documents (accessed on 1 July 2020).
- World Anti-Doping Agency. WADA Technical Document—TD2018EAAS. Endogenous Anabolic Androgenic Steroids Measurement and Reporting. Available online: https://www.wada-ama.org/en/resources/science-medicine/td2018eaas-0 (accessed on 27 July 2020).
- Martinez-Brito, D.; Correa Vidal, M.T.; De la Torre, X.; Garcia-Mir, V.; Ledea Lozano, O.; Granda Fraga, M. Reference ranges for the urinary steroid profile in a Latin-American population. Drug Test. Anal. 2013, 5, 619–626. [Google Scholar] [CrossRef]
- Mareck-Engelke, U.; Geyer, H.; Donike, M. Stability of steroid profiles (2): Excretion rates from morning urines. In Recent Advances in Doping Analysis; Donike, M., Geyer, H., Gotzmann, U., Mareck-Engelke, U., Rauth, S., Eds.; Sport und Buch Strauß: Köln, Germany, 1993; pp. 85–89. [Google Scholar]
- Mareck-Engelke, U.; Geyer, H.; Donike, M. Stability of steroid profiles (3): Ratios and excretion rates of endogenous steroids in male urines collected over 24 h. In Recent Advances in Doping Analysis (2); Donike, M., Geyer, H., Gotzmann, U., Mareck-Engelke, U., Eds.; Sport und Buch Strauß: Köln, Germany, 1995; pp. 121–133. [Google Scholar]
- Sottas, P.E.; Saugy, M.; Saudan, C. Endogenous steroid profiling in the athlete biological passport. Endocrinol. Metab. Clin. North Am. 2010, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, M.; De la Torre, X.; Fiacco, I.; Palermo, A.; Botrè, F. Drug-drug interaction and doping, part 1: An in vitro study on the effect of non-prohibited drugs on the phase I metabolic profile of toremifene. Drug Test. Anal. 2014, 6, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, M.; De la Torre, X.; Fiacco, I.; Botrè, F. Drug-drug interaction and doping, part 2: An in vitro study on the effect of non-prohibited drugs on the phase I metabolic profile of stanozolol. Drug Test. Anal. 2014, 6, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Mareck, U.; Geyer, H.; Opfermann, G.; Thevis, M.; Schänzer, W. Factors influencing the steroid profile in doping control analysis. J. Mass Spectrom. 2008, 43, 877–891. [Google Scholar] [CrossRef]
- Penning, T.M. The aldo-keto reductases (AKRs): Overview. Chem. Biol. Interact. 2015, 234, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Byrns, M.C.; Steckelbroeck, S.; Penning, T.M. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem. Pharmacol. 2008, 75, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Gobec, S.; Brozic, P.; Rizner, T.L. Nonsteroidal anti-inflammatory drugs and their analogues as inhibitors of aldo-keto reductase AKR1C3: New lead compounds for the development of anticancer agents. Bioorg. Med. Chem. Lett. 2005, 15, 5170–5175. [Google Scholar] [CrossRef]
- Gorski, T.; Cadore, E.L.; Pinto, S.S.; Da Silva, E.M.; Correa, C.S.; Beltrami, F.G.; Kruel, L.F. Use of NSAIDs in triathletes: Prevalence, level of awareness and reasons for use. Br. J. Sports Med. 2011, 45, 85–90. [Google Scholar] [CrossRef]
- Tscholl, P.; Alonso, J.M.; Dolle, G.; Junge, A.; Dvorak, J. The use of drugs and nutritional supplements in top-level track and field athletes. Am. J. Sports Med. 2010, 38, 133–140. [Google Scholar] [CrossRef]
- Penning, T.M.; Burczynski, M.E.; Jez, J.M.; Hung, C.-F.; Lin, H.-K.; Ma, H.; Moore, M.; Palackal, N.; Ratnam, K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1–AKR1C4) of the aldo-keto reductase superfamily: Functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000, 351, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Beranič, N.; Stefane, B.; Brus, B.; Gobec, S.; Rižner, T.L. New enzymatic assay for the AKR1C enzymes. Chem. Biol. Interact. 2013, 202, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Cer, R.Z.; Mudunuri, U.; Stephens, R.; Lebeda, F.J. IC50 to Ki: A web-based tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res. 2009, 37, W441–W445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrns, M.C.; Penning, T.M. Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase (AKR1C3): Role in breast cancer and inhibition by non-steroidal anti-inflammatory drug analogs. Chem. Biol. Interact. 2009, 178, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovering, A.L.; Ride, J.P.; Bunce, C.M.; Desmond, J.C.; Cummings, S.M.; White, S.A. Crystal structures of prostaglandin D2 11-Ketoreductase (AKR1C3) in complex with the nonsteroidal anti-inflammatory drugs flufenamic acid and indomethacin. Cancer Res. 2004, 64, 1802–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, M.; Schmoldt, A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie 2003, 58, 447–474. [Google Scholar] [PubMed]
- Stoll, A.; Iannone, M.; De Gregorio, G.; Molaioni, F.; De la Torre, X.; Botrè, F.; Parr, M.K. Influence of pain killers on the urinary anabolic steroid profile. J. Anal. Toxicol. 2020, in press. [Google Scholar]
- Mareck-Engelke, U.; Geyer, H.; Schänzer, W. The interpretation of female steroid profiles. In Recent Advances in Doping Analysis (5); Schänzer, W., Geyer, H., Gotzmann, U., Mareck-Engelke, U., Eds.; Sport und Buch Strauß: Köln, Germany, 1998; Volume 5, pp. 51–70. [Google Scholar]
- Mullen, J.E.; Thorngren, J.O.; Schulze, J.J.; Ericsson, M.; Garevik, N.; Lehtihet, M.; Ekstrom, L. Urinary steroid profile in females—The impact of menstrual cycle and emergency contraceptives. Drug Test. Anal. 2017, 9, 1034–1042. [Google Scholar] [CrossRef]
- Mareck-Engelke, U.; Geyer, H.; Donike, M. Stability of steroid profiles (4): The circadian rhythm of urinary ratios and excretion rates of endogenous steroids in female and its menstrual dependency. In Recent Advances in Doping Analysis (2); Donike, M., Geyer, H., Gotzmann, A., Mareck-Engelke, U., Eds.; Sport und Buch Strauss: Köln, Germany, 1995; pp. 135–155. [Google Scholar]
- Kristensen, D.M.; Skalkam, M.L.; Audouze, K.; Lesne, L.; Desdoits-Lethimonier, C.; Frederiksen, H.; Brunak, S.; Skakkebaek, N.E.; Jegou, B.; Hansen, J.B.; et al. Many putative endocrine disruptors inhibit prostaglandin synthesis. Environ. Health. Perspect. 2011, 119, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Albert, O.; Desdoits-Lethimonier, C.; Lesne, L.; Legrand, A.; Guille, F.; Bensalah, K.; Dejucq-Rainsford, N.; Jegou, B. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum. Reprod. 2013, 28, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, D.M.; Lesne, L.; Le Fol, V.; Desdoits-Lethimonier, C.; Dejucq-Rainsford, N.; Leffers, H.; Jegou, B. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. Int. J. Androl. 2012, 35, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S.; Lau, I.; Bartke, A.; Chang, M. Effect of indomethacin on blood plasma levels of LH and testosterone in male rats. J. Reprod. Fert. 1975, 42, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Barkay, J.; Harpaz-Kerpel, S.; Ben-Ezra, S.; Gordon, S.; Zuckerman, H. The prostaglandin inhibitor effect of antiinflammatory drugs in the therapy of male infertility. Fertil. Steril. 1984, 42, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Knuth, U.; Kühne, J.; Crosby, J.; Bals-Pratsch, M.; Kelly, R.; Nieschlag, E. Indomethacin and oxaprozin lower seminal prostaglandin levels but do not influence sperm motion characteristics and serum hormones of young healthy men in a placebo-controlled double-blind trial. J. Androl. 1989, 10, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Mazaud-Guittot, S.; Nicolas Nicolaz, C.; Desdoits-Lethimonier, C.; Coiffec, I.; Ben Maamar, M.; Balaguer, P.; Kristensen, D.M.; Chevrier, C.; Lavoue, V.; Poulain, P.; et al. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. J. Clin. Endocrinol. Metab. 2013, 98, E1757–E1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penning, T.M. AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase): Roles in malignancy and endocrine disorders. Mol. Cell. Endocrinol. 2019, 489, 82–91. [Google Scholar] [CrossRef]
- Mazzarino, M.; Abate, M.G.; Alocci, R.; Rossi, F.; Stinchelli, R.; Molaioni, F.; De la Torre, X.; Botrè, F. Urine stability and steroid profile: Towards a screening index of urine sample degradation for anti-doping purpose. Anal. Chim. Acta 2011, 683, 221–226. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, X.; Colamonici, C.; Curcio, D.; Jardines, D.; Molaioni, F.; Parr, M.K.; Botrè, F. Detection of formestane abuse by mass spectrometric techniques. Drug Test. Anal. 2014, 6, 1133–1140. [Google Scholar] [CrossRef]
- Iannone, M.; Palermo, A.; De la Torre, X.; Romanelli, F.; Sansone, A.; Sansone, M.; Lenzi, A.; Botrè, F. Effects of transdermal administration of testosterone gel on the urinary steroid profile in hypogonadal men: Implications in antidoping analysis. Steroids 2019, 152, 108491. [Google Scholar] [CrossRef]
- Leinonen, A.; Kuuranne, T.; Moisander, T.; Rautava, K. Artificial urine as sample matrix for calibrators and quality controls in determination of testosterone to epitestosterone ratio. In Recent Advances in Doping Analysis; Schänzer, W., Geyer, H., Gotzmann, U., Mareck, U., Eds.; Sportverlag Strauß: Köln, Germany, 2007; Volume 15, pp. 401–404. [Google Scholar]
- Hamada, C. Statistical analysis for toxicity studies. J. Toxicol. Pathol. 2018, 31, 15–22. [Google Scholar] [CrossRef] [Green Version]







| Male Volunteer | Female Volunteer | |
|---|---|---|
| Age | 29 | 30 |
| Weight | 63 kg | 60 kg |
| Hight | 172 cm | 178 cm |
| BMI | 21.3 kg/m2 | 18.9 kg/m2 |
| Dietary habits | Normal diet (no restriction) | Normal diet (no restriction) |
| Training | Three times/week (swim and run) | No |
| Oral contraceptive | Ø | No |
| Analyte | Precursor Ion [m/z] | Product Ion [m/z] | Collision Energy [eV] |
|---|---|---|---|
| Indomethacin (mono-TMS) | 429 | 139; 246; 312 | 30; 30; 30 |
| O-desmethylindomethacin (bis-TMS) | 487; 304 | 139; 216; 232 | 30; 30; 30 |
| N-deschlorobenzoylindomethacin (bis-TMS) | 363; 348; 246 | 246; 320; 174 | 30; 10; 30 |
| Probenecid (mono-TMS) | 178; 342 | 104; 268 | 20; 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoll, A.; Iannone, M.; De Gregorio, G.; Molaioni, F.; de la Torre, X.; Botrè, F.; Parr, M.K. Influence of Indomethacin on Steroid Metabolism: Endocrine Disruption and Confounding Effects in Urinary Steroid Profiling of Anti-Doping Analyses. Metabolites 2020, 10, 463. https://doi.org/10.3390/metabo10110463
Stoll A, Iannone M, De Gregorio G, Molaioni F, de la Torre X, Botrè F, Parr MK. Influence of Indomethacin on Steroid Metabolism: Endocrine Disruption and Confounding Effects in Urinary Steroid Profiling of Anti-Doping Analyses. Metabolites. 2020; 10(11):463. https://doi.org/10.3390/metabo10110463
Chicago/Turabian StyleStoll, Anna, Michele Iannone, Giuseppina De Gregorio, Francesco Molaioni, Xavier de la Torre, Francesco Botrè, and Maria Kristina Parr. 2020. "Influence of Indomethacin on Steroid Metabolism: Endocrine Disruption and Confounding Effects in Urinary Steroid Profiling of Anti-Doping Analyses" Metabolites 10, no. 11: 463. https://doi.org/10.3390/metabo10110463