A Review on Traditionally Used African Medicinal Plant Annickia chlorantha, Its Phytochemistry, and Anticancer Potential
Abstract
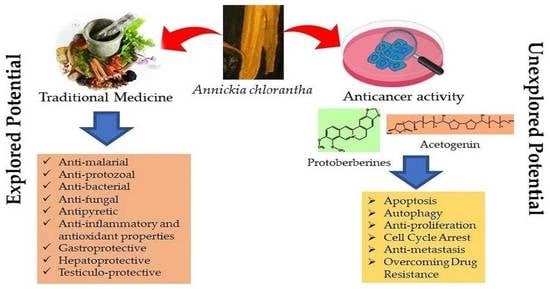
:1. Introduction
2. Taxonomy, Biodistribution, and Botanic Description
3. Phytochemical Composition and Toxicity
4. Traditional Uses and Biological Activities
5. Anti-Tumor Effects of A. chlorantha Extracts
6. Possible Anticancer Effects
6.1. Protoberberines
6.2. Acetogenins
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Soerjomataram, I. The Changing Global Burden of Cancer: Transitions in Human Development and Implications for Cancer Prevention and Control. In Disease Control Priorities, Third Edition (Volume 3): Cancer; The World Bank: Washington, DC, USA, 2015. [Google Scholar]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Kumar, A.; Kumar, P.; Ajeet, A. Anticancer Potential of Plants and Natural Products: A Review. Am. J. Pharmacol. Sci. 2013, 1, 104–115. [Google Scholar] [CrossRef]
- Hassan, B. Plants and Cancer Treatment. In Medicinal Plants—Use in Prevention and Treatment of Diseases; BoD–Books on Demand: Stockholm, Sweden, 2020. [Google Scholar]
- Mohan, L. Plant-Based Drugs as an Adjuvant to Cancer Chemotherapy. In Alternative Medicine—Update; IntechOpen: London, UK, 2021. [Google Scholar]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Barnum, C.R.; Endelman, B.J.; Shih, P.M. Utilizing Plant Synthetic Biology to Improve Human Health and Wellness. Front. Plant Sci. 2021, 12, 1824. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Plant-Derived Anticancer Compounds Used in Cancer Therapies. In Frontiers in Natural Product Chemistry; Bentham Science Publisher: Sharjah, United Arab Emirates, 2021. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Krishnamurti, C.; Chakra Rao, S.S.C. The isolation of morphine by Serturner. Indian J. Anaesth. 2016, 60, 861–862. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid. Based Complementary Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Efferth, T. African flora has the potential to fight multidrug resistance of cancer. BioMed Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef] [PubMed]
- Iwu, M.M. Handbook of African Medicinal Plants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lall, N. Underexplored Medicinal Plants from Sub-Saharan Africa: Plants with Therapeutic Potential for Human Health; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Olivier, D.K.; Van Vuuren, S.F.; Moteetee, A.N. Annickia affinis and A. chlorantha (Enantia chlorantha)—A review of two closely related medicinal plants from tropical Africa. J. Ethnopharmacol. 2015, 176, 438–462. [Google Scholar] [CrossRef]
- Davares, A.K.L.; Arsene, M.M.J.; Viktorovna, P.I.; Shommya, D. Enantia chlorantha and its Multiple Therapeutic Virtues: A Mini Review. J. Pharm. Res. Int. 2021, 33, 254–259. [Google Scholar] [CrossRef]
- Akinwale, S.G.; Chukwu, O.E.; Chioma, O.P.; Chukudi, A.J.; Olubunmi, A.G. Enantia chlorantha: A review. J. Pharmacogn. Phytochem. 2022, 11, 34–38. [Google Scholar] [CrossRef]
- Dawodu, A.O.; Moses, U.D.; Apena, A.; Adetoro, A.; Dairo, J.O. The Proximate Evaluation and Phytochemistry of Enantia chlorantha Stem Bark in Aqueous and Ethanolic Extract. Middle-East J. Sci. Res. 2014, 21, 2145–2148. [Google Scholar]
- Adesokan, A.A.; Yakubu, M.T.; Owoyele, B.V.; Akanji, M.A.; Soladoye, A.O.; Lawal, O.K. Effect of administration of aqueous and ethanolic extracts of Enantia chlorantha stem bark on brewer’s yeast-induced pyresis in rats. Afr. J. Biochem. Res. 2008, 2, 165–169. [Google Scholar]
- Olanlokun, J.O.; Akomolafe, S.F. Antioxidant potentials of various solvent extracts from stem bark of Enantia chlorantha. J. Biomed. Sci. Eng. 2013, 06, 877–884. [Google Scholar] [CrossRef]
- Odoh, U.; Okwor, I.; Ezejiofor, M. Phytochemical, trypanocidal and anti-microbial studies of Enantia chlorantha (Annonaceae) root. J. Pharm. Allied Sci. 2011, 7, 4. [Google Scholar] [CrossRef]
- Gill, L.S.; Akinwumi, C. Nigerian folk medicine: Practices and beliefs of the ondo people. J. Ethnopharmacol. 1986, 18, 257–266. [Google Scholar] [CrossRef]
- Menut, C.; Bessière, J.M.; Lamaty, G.; Zollo, P.H.A.; Fékam, F.B.; Chalchat, J.C.; Garry, R.P. Aromatic plants of tropical central africa. Part VII. A comparative study of the volatile constituents of the stem bark of Enantia chlorantha oliv. and Xylopia staudtii engl. & diels from cameroon. Flavour Fragr. J. 1992, 7, 259–261. [Google Scholar] [CrossRef]
- Nyegue, M.; Amvam-Zollo, P.H.; Etoa, F.X.; Agnaniet, H.; Chantal, M. Chemical and biological investigations of essential oils from stem barks of Enantia chlorantha Oliv. and Polyalthia suaveolens Engler. & Diels. from Cameroon. Nat. Prod. Commun. 2008, 3, 1934578X0800300711. [Google Scholar] [CrossRef]
- Boyom, F.F.; Kemgne, E.M.; Tepongning, R.; Ngouana, V.; Mbacham, W.F.; Tsamo, E.; Zollo, P.H.A.; Gut, J.; Rosenthal, P.J. Antiplasmodial activity of extracts from seven medicinal plants used in malaria treatment in Cameroon. J. Ethnopharmacol. 2009, 123, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Kemgne, E.A.M.; Mbacham, W.F.; Boyom, F.F.; Zollo, P.H.A.; Tsamo, E.; Rosenthal, P.J. In vitro sensitivity of Plasmodium falciparum field isolates to extracts from Cameroonian Annonaceae plants. Parasitol. Res. 2012, 110, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Agomo, P.U.; Idigo, J.C.; Afolabi, B.M. “Antimalarial” medicinal plants and their impact on cell populations in various organs of mice. Afr. J. Med. Med. Sci. 1992, 21, 39–46. [Google Scholar]
- Agbaje, E.O.; Onabanjo, A.O. Toxicological study of the extracts of anti-malarial medicinal plant Enantia chlorantha. Cent. Afr. J. Med. 1994, 40, 71–73. [Google Scholar]
- Tan, P.V.; Boda, M.; Enow-Orock, G.E.; Etoa, F.X.; Bitolog, P. Acute and sub-acute toxicity profile of the aqueous stem bark extract Enantia chlorantha Oliver (Annonaceae) in laboratory animals. Pharmacologyonline 2007, 1, 304–313. [Google Scholar]
- Adebiyi, O.E.; Abatan, M.O. Phytochemical and acute toxicity of ethanolic extract of Enantia chlorantha (oliv) stem bark in albino rats. Interdiscip. Toxicol. 2013, 6, 145–151. [Google Scholar] [CrossRef]
- Akintonwa, A.; Awodele, O.; Afolayan, G.; Coker, H.A.B. Mutagenic screening of some commonly used medicinal plants in Nigeria. J. Ethnopharmacol. 2009, 125, 461–470. [Google Scholar] [CrossRef]
- Mesmine, K.M.; George, E.-O.; Christophe, M.; Ernestine, N.T.E.Z.; Benjamin, N.; Siwe, G.; Paul, T. Evaluation of the toxicity of the aqueous stem bark extract of Enantia chlorantha on some reproductive and developmental parameters. J. Med. Plants Stud. 2020, 8, 86–94. [Google Scholar]
- Moody, J.O.; Ogundipe, O.D.; Akang, E.U.; Agbedana, E.O. Toxicological studies on the purified protoberberine alkaloidal fraction of Enantia chlorantha Oliv (ANNONACEAE). Afr. J. Med. Med. Sci. 2007, 36, 317–323. [Google Scholar] [PubMed]
- Kuete, V. Health Effects of Alkaloids from African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Tene Tcheghebe, O.; Ngouafong Tatong, F.; Jackson Seukep, A.; Author, C. Tene Tcheghebe O, Ngouafong Tatong F, Seukep AJ. Traditional uses, phytochemical and pharmacological profiles, and toxicity of Enantia chlorantha (Oliver): An overview. Edorium J. Med. 2016, 3, 12–18. [Google Scholar] [CrossRef]
- Wright, C.W.; Marshall, S.J.; Russell, P.F.; Anderson, M.M.; Phillipson, J.D.; Kirby, G.C.; Warhurst, D.C.; Schiff, J.L. In vitro antiplasmodial, antiamoebic, and cytotoxic activities of some monomeric isoquinoline alkaloids. J. Nat. Prod. 2000, 63, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
- Adesokan, A.A.; Akanji, M.A.; Yakubu, M.T. Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. Afr. J. Biotechnol. 2007, 6, 2502–2505. [Google Scholar] [CrossRef]
- Tan, P.V.; Boda, M.; Etoa, F.X. In vitro and in vivo anti-Helicobacter/Campylobacter activity of the aqueous extract of Enantia chlorantha. Pharm. Biol. 2010, 48, 349–356. [Google Scholar] [CrossRef]
- Donfack, V.D.; Roque, S.; Trigo, G.; Fokou, P.T.; Tchokouaha, L.Y.; Tsabang, N.; Zollo, P.A.; Correia-Neves, M.; Boyom, F.F. Antimycobacterial activity of selected medicinal plants extracts from Cameroon. Int. J. Biol. Chem. Sci. 2014, 8, 273. [Google Scholar] [CrossRef]
- Nyong, E.E.; Odeniyi, M.A.; Moody, J.O. In vitro and in vivo antimicrobial evaluation of alkaloidal extracts of Enantia chlorantha stem bark and their formulated ointments. Acta Pol. Pharm. Drug Res. 2015, 72, 14–52. [Google Scholar]
- Abike, T.O.; Osuntokun, O.T.; Modupe, A.O.; Adenike, A.F.; Atinuke, A.R. Antimicrobial Efficacy, Secondary Metabolite Constituents, Ligand Docking of Enantia chlorantha on Selected Multidrug Resistance Bacteria and Fungi. J. Adv. Biol. Biotechnol. 2020, 23, 17–32. [Google Scholar] [CrossRef]
- Agbaje, E.; Tijani, A.; Braimoh, O. Effects of Enantia chlorantha extracts in Laboratory-Induced Convulsion and Inflammation. Orient J. Med. 2004, 15, 68–71. [Google Scholar] [CrossRef]
- Njayou, F.; Moundipa, P.; Tchana, A.; Ngadjui, B.; Tchouanguep, F. Inhibition Of Microsomal Lipid Peroxidation And Protein Oxidation By Extracts From Plants Used In Bamun Folk Medicine (Cameroon) Against Hepatitis. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Muganza, D.M.; Fruth, B.; Lami, J.N.; Mesia, G.; Kambu, O.; Tona, G.; Kanyanga, R.C.; Cos, P.; Maes, L.; Apers, S.; et al. In vitro antiprotozoal and cytotoxic activity of 33 ethonopharmacologically selected medicinal plants from Democratic Republic of Congo. J. Ethnopharmacol. 2012, 141, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Vennerstrom, J.L.; Lovelace, J.K.; Waits, V.B.; Hanson, W.L.; Klayman, D.L. Berberine derivatives as antileishmanial drugs. Antimicrob. Agents Chemother. 1990, 34, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Nkwengoua, E.T.; Ngantchou, I.; Nyasse, B.; Denier, C.; Blonski, C.; Schneider, B. In vitro inhibitory effects of palmatine from Enantia chlorantha on Trypanosoma cruzi and Leishmania infantum. Nat. Prod. Res. 2009, 23, 1144–1150. [Google Scholar] [CrossRef]
- Agbaje, E.O.; Onabanjo, A.O. The effects of extracts of Enantia chlorantha in malaria. Ann. Trop. Med. Parasitol. 1991, 85, 585–590. [Google Scholar] [CrossRef]
- Kimbi, H.K.; Fagbenro-Beyioku, A.F. Efficacy of Cymbopogon giganteus and Enantia chrantha against chloroquine resistant Plasmodium yoelii nigeriensis. East Afr. Med. J. 1996, 73, 636–637. [Google Scholar] [PubMed]
- Kimbi, H.K.; Fagbenro-Beyioku, A.F.; Oyibo, W.A. Antimalarial herbs against chloroquine-resistant P. yoelii nigeriensis in mice. Indian J. Malariol. 1998, 35, 35–38. [Google Scholar] [PubMed]
- Adebajo, A.C.; Famuyiwa, F.G.; Aliyu, F.A. Properties for sourcing nigerian larvicidal plants. Molecules 2014, 19, 8363–8372. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Klayman, D.L. Protoberberine Alkaloids as Antimalarials. J. Med. Chem. 1988, 31, 1084–1087. [Google Scholar] [CrossRef]
- Nurain, I.; Ibitoye, O.; Bewaji, C.O. Molecular docking analysis of secondary metabolites of stem bark of Enantia chlorantha with human calcium pump. J. Proteins Proteom. 2018, 9, 1. [Google Scholar]
- Agbaje, E.; Onabanjo, A. Analgesic and Antipyretic Actions of Enantia chlorantha Extract in Some Laboratory Animals. Niger. J. Nat. Prod. Med. 1998, 2, 24–25. [Google Scholar] [CrossRef]
- Nyasse, B.; Nkwengoua, E.; Sondengam, B.; Denier, C.; Willson, M. Modified berberine and protoberberines from Enantia chlorantha as potential inhibitors of Trypanosoma brucei. Pharmazie 2002, 57, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Siminialayi, I.; Agbaje, E. Gastroprotective effects of the ethanolic extract of Enantia chlorantha in rats. West Afr. J. Pharmacol. Drug Res. 2005, 20, 35–38. [Google Scholar] [CrossRef]
- Tan, P.V.; Nyasse, B.; Enow-Orock, G.E.; Wafo, P.; Forcha, E.A. Prophylactic and healing properties of a new anti-ulcer compound from Enantia chlorantha in rats. Phytomedicine 2000, 7, 291–296. [Google Scholar] [CrossRef]
- Tan, P.V.; Nyasse, B.; Dimo, T.; Wafo, P.; Akahkuh, B.T. Synergistic and potentiating effects of ranitidine and two new anti-ulcer compounds from Enantia chlorantha and Voacanga africana in experimental animal models. Pharmazie 2002, 57, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Lassila, V.; Njimi, T.; Mengata, D.E. Natural protoberberine alkaloids from enantia chlorantha, palmatine, columbamine and jatrorrhizine for thioacetamide-traumatized rat liver. Cells Tissues Organs 1988, 131, 166–170. [Google Scholar] [CrossRef]
- Virtanen, P.; Lassila, V.; Njimi, T.; Mengata, D.E. Regeneration of D-Galactosamine-Traumatized Rat Liver with Natural Protoberberine Alkaloids from Enantia chlorantha. Cells Tissues Organs 1988, 132, 159–163. [Google Scholar] [CrossRef]
- Adebiyi, O.E.; Abatan, M.O. Protective Effects of Enantia chlorantha Stem Bark Extracts on Acetaminophen Induced Liver Damage in Rats. Jordan J. Biol. Sci. 2013, 6, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Salman, T.M.; Adesokan, A.A. Sperm quality of male rats treated with aqueous extract of Enantia chlorantha stem bark. Afr. J. Biotechnol. 2008, 7, 7. [Google Scholar]
- Saalu LC, O.A. Testiculo-Protective Effect of Stem Bark Extract of Enantia chlorantha on Lead Induced Toxicity in Adult Wistar Rat (Rattus norvergicus). Reprod. Syst. Sex. Disord. 2012, 1, 2. [Google Scholar] [CrossRef]
- Alford, C.M.; Onyejiaka, A.; Myles, E.L. Abstract 614: Cytotoxic activity of Enantia chlorantha, nauclea latifolia, and citrus medica extracts on carcinoma cells. Cancer Res. 2016, 76, 614. [Google Scholar] [CrossRef]
- Tshibangu, D.S.; Divakar, S.; Ramanathan, M.; Syamala, G.; Ngbolua, K.-T.; Mudogo, J.C.V.; Tshilanda, D.D.; Misengabu, N.M.; Mpiana, P.T. In Vitro Anticancer Assessment of Annickia chlorantha (Oliv.) Setten & Maas Stem (Annonaceae) Bark from Democratic Republic of Congo. J. Biosci. Med. 2016, 04, 23–29. [Google Scholar] [CrossRef]
- Kuete, V.; Fokou, F.W.; Karaosmanoğlu, O.; Beng, V.P.; Sivas, H. Cytotoxicity of the methanol extracts of Elephantopus mollis, Kalanchoe crenata and 4 other Cameroonian medicinal plants towards human carcinoma cells. BMC Complement. Altern. Med. 2017, 17, 280. [Google Scholar] [CrossRef]
- Virtanen, P.; Lassila, V.; Söderström, K.O. Protoberberine alkaloids from Enantia chlorantha therapy of allyl-alcohol- and D-galactosamine-traumatized rats. Pathobiology 1993, 61, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Huang, J.-L.; Yang, X.-Y.; Liu, J.-H.; Cao, H.-L.; Xiang, F.; Cheng, P.; Zeng, J.-G. Anticancer and Reversing Multidrug Resistance Activities of Natural Isoquinoline Alkaloids and their Structure-activity Relationship. Curr. Med. Chem. 2017, 25, 5088–5114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The anti-cancer mechanisms of berberine: A review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef]
- Kettmann, V.; Košt’álová, D.; Höltje, H.D. Human topoisomerase I poisoning: Docking protoberberines into a structure-based binding site model. J. Comput. Aided. Mol. Des. 2004, 18, 785–796. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.; Orellana, M.R. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106–131. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Xie, N.; Chai, Y.; Nie, Y.; Liu, K.; Liu, Y.; Yang, Y.; Su, J.; Zhang, C. Apoptosis Induction, a Sharp Edge of Berberine to Exert Anti-Cancer Effects, Focus on Breast, Lung, and Liver Cancer. Front. Pharmacol. 2022, 13, 803717. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Qureshi, M.Z.; Khalid, S.; Attar, R.; Martinelli, C.; Uteuliyev, Y.S.; Sadykov, B.N.; Taverna, S.; Poltronieri, P.; Xu, B. Regulation of cell signaling pathways by berberine in different cancers: Searching for missing pieces of an incomplete JIG-saw puzzle for an effective cancer therapy. Cancers 2019, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sheng, J.; Li, G.; Zhao, L.; Wang, Y.; Yang, W.; Yao, X.; Sun, L.; Zhang, Z.; Cui, R. Effects of berberine and its derivatives on cancer: A systems pharmacology review. Front. Pharmacol. 2020, 10, 1461. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine—A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef]
- Bao, M.; Cao, Z.; Yu, D.; Fu, S.; Zhang, G.; Yang, P.; Pan, Y.; Yang, B.; Han, H.; Zhou, Q. Columbamine suppresses the proliferation and neovascularization of metastatic osteosarcoma U2OS cells with low cytotoxicity. Toxicol. Lett. 2012, 215, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Cai, C.; Wu, X.; Fan, X.; Huang, W.; Zhou, J.; Wu, Q.; Huang, Y.; Zhao, W.; Zhang, F.; et al. An insight into the molecular mechanism of berberine towards multiple cancer types through systems pharmacology. Front. Pharmacol. 2019, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yao, Y.; Shen, B.; Liu, J.; Pan, Q.; Liu, N.; Li, L.; Huang, J.; Long, Z.; Shao, L. Columbamine suppresses the proliferation and malignization of colon cancer cells via abolishing Wnt/β-catenin signaling pathway. Cancer Manag. Res. 2019, 11, 8635–8645. [Google Scholar] [CrossRef]
- Tarabasz, D.; Kukula-Koch, W. Palmatine: A review of pharmacological properties and pharmacokinetics. Phyther. Res. 2020, 34, 33–50. [Google Scholar] [CrossRef]
- Yang, T.; Wei, L.; Ma, X.; Ke, H. Columbamine suppresses proliferation and invasion of melanoma cell A375 via HSP90-mediated STAT3 activation. J. Recept. Signal Transduct. 2020, 41, 99–104. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Bin Emran, T.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.M.; et al. Berberine as a potential anticancer agent: A comprehensive review. Molecules 2021, 26, 7368. [Google Scholar] [CrossRef]
- Zhong, F.; Chen, Y.; Chen, J.; Liao, H.; Li, Y.; Ma, Y. Jatrorrhizine: A Review of Sources, Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 2022, 12, 783127. [Google Scholar] [CrossRef]
- Alali, F.Q.; Liu, X.X.; McLaughlin, J.L. Annonaceous acetogenins: Recent progress. J. Nat. Prod. 1999, 62, 783127. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, A.; Figadère, B.; Zafra-Polo, M.C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 2005, 22, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Herrera, N.; Pérez-Plasencia, C.; Castro-Torres, V.A.; Martínez-Vázquez, M.; González-Esquinca, A.R.; Zentella-Dehesa, A. Selective Acetogenins and Their Potential as Anticancer Agents. Front. Pharmacol. 2019, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.N.d.C.; Faustino, D.C.; Allahdadi, K.J.; França, L.S.D.A.; Pinto, L.C. Acetogenins from Annonaceae plants: Potent antitumor and neurotoxic compounds. PharmaNutrition 2022, 20, 100295. [Google Scholar] [CrossRef]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing delivery of plant based anti-cancer drugs. Front. Pharmacol. 2018, 8, 1002. [Google Scholar] [CrossRef]
- More, M.P.; Pardeshi, S.R.; Pardeshi, C.V.; Sonawane, G.A.; Shinde, M.N.; Deshmukh, P.K.; Naik, J.B.; Kulkarni, A.D. Recent advances in phytochemical-based Nano-formulation for drug-resistant Cancer. Med. Drug Discov. 2021, 10, 100082. [Google Scholar] [CrossRef]



| Biological Activities | Plant Part/Sample/Isolated Phytochemical Used |
|---|---|
| Antiamoebic | Isolated Protoberberine alkaloids [41] |
| Antibacterial | Aqueous extract of stem bark [42,43] Isolated essential oils [29] Ethanol extract of root [26] Ethanol extracts of stem bark and stem and methanol fractions [44] Alkaloidal extracts of stem bark [45] Jartrorrhizine-1, canadine-1, argentine, jartrorrhizine and berberine (molecular docking analysis) [46] |
| Antifungal | Isolated essential oils [29] Ethanol extract of root [26] Alkaloidal extracts of stem bark [45] Argentinine-1, columbamine-1, jartrorrhizine-1, pseudocolumbamine-1 (molecular docking analysis) [46] |
| Anti-inflammatory and antioxidant properties | Boiled water bark extract [47] Isolated essential oils [29] Methanol-dichloromethane bark extracts [48] Methanol, n-hexane, chloroform, ethyl acetate and aqueous fractions of stem bark extracts [25] |
| Anti-leishmania | Aqueous extract stem bark [49] Isolated protoberberine alkaloids [50,51] |
| Antimalarial | Aqueous and ethanol extract of stem bark [49,52] Leaf and bark decoctions [32] Boiled water bark extracts [53,54] Methanol extract of stem bark [55] Aqueous, hexane, ethanol and methanol (acetogenin-rich) fractions of stem bark and stem [30,31] Isolated protoberberine alkaloids [41,56] 1,3-dibenzoyl-2-azepanone and 3,5-bis(1,1- dimethylethyl)-phenol (molecular docking analysis) [57] |
| Antipyretic properties | Aqueous extract of bark [58] Aqueous and ethanolic extracts of stem bark [24] |
| Anti-trypanosoma | Ethanol extract of root [26] Aqueous extract of stem bark [49] Isolated protoberberine alkaloids [51,59] |
| Gastroprotective | Ethanol extract of stem bark [60] Isolated Protoberberine alkaloids [61,62] |
| Haematological | Ethanol extract of stem bark [35] |
| Hepatoprotective | Hepasor (a protoberberine-containing extract) [63,64] Hexane, chloroform, ethyl acetate and methanol extracts of stem bark [65] |
| Testiculoprotective | Aqueous extract of stem bark [66,67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbadhikary, P.; George, B.P. A Review on Traditionally Used African Medicinal Plant Annickia chlorantha, Its Phytochemistry, and Anticancer Potential. Plants 2022, 11, 2293. https://doi.org/10.3390/plants11172293
Sarbadhikary P, George BP. A Review on Traditionally Used African Medicinal Plant Annickia chlorantha, Its Phytochemistry, and Anticancer Potential. Plants. 2022; 11(17):2293. https://doi.org/10.3390/plants11172293
Chicago/Turabian StyleSarbadhikary, Paromita, and Blassan P. George. 2022. "A Review on Traditionally Used African Medicinal Plant Annickia chlorantha, Its Phytochemistry, and Anticancer Potential" Plants 11, no. 17: 2293. https://doi.org/10.3390/plants11172293