Genus Salsola: Chemistry, Biological Activities and Future Prospective—A Review
Abstract
:1. Introduction
2. Taxonomy and Distribution
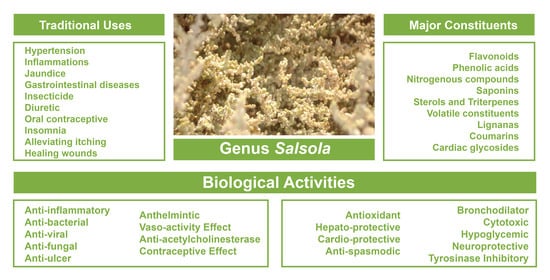
3. Traditional Uses of Genus Salsola
4. Chemistry of Salsola
4.1. General Procedures for Isolation of Bioactive Compounds from the Genus
4.2. S. baryosma (Schult.) Dandy (Caroxylon imbricata var. imbricatum)
4.3. S. collina Pall.
4.4. S. cyclophylla (Baker) (Synonyme of Caroxylon cyclophyllum (Baker) Akhani and Roalson)
4.5. S. foetida Vest ex Schult. (Synonyme of Suaeda foetida (Vest ex Schult.) Moq.)
4.6. S. grandis Freitag, Vural and Adigüzel
4.7. S. imbricata Forssk. Moq. (Synonyme of Caroxylon imbricatum (Forssk.) Moq.)
4.8. S. inermis Forssk. (Synonyme of Caroxylon inerme (Forssk.) Akhani and Roalson)
4.9. S. kali L. (S. spinosa Lam.)
4.10. S. komarovii Iljin
4.11. S. laricifolia Litv. ex Drobow
4.12. S. longifolia Forssk.
4.13. S. micranthera Botsch. (Synonym of Caroxylon micrantherum (Botsch.) Sukhor.)
4.14. S. oppositifolia Pall.
4.15. S. soda L. (Synonym of Soda inermis Fourr.)
4.16. S. somalensis N.E.Br.
4.17. S. tetragona Delile (Synonym of Caroxylon tetragonum (Delile) Moq.)
4.18. S. tetrandra Forssk. (Synonym of Caroxylon tetrandrum (Forssk.) Akhani and Roalson)
4.19. S. tomentosa (Moq.) Spach
4.20. S. vermiculata L. (Synonym of Caroxylon vermiculatum (L.) Akhani and Roalson)
4.21. S. villosa Schult. (Synonym of Caroxylon villosum (Schult.) Akhani and Roalson)
4.22. S. volkensii Schweinf. and Asch.
5. Overview of the Benefits, Uses and Medicinal Properties of Salsola Genus
5.1. Anti-Inflammatory, Analgesic and Anti-Nociceptive Activity
5.2. Antibacterial Activity
5.3. Anti-Viral Activity
5.4. Anti-Fungal Activity
5.5. Anti-Oxidant, Hepato-Protective and Cardio-Protective Activity
5.6. Contraceptive Effect
5.7. Anti-Spasmodic and Bronchodilator Activity
5.8. Anti-Ulcer Activity
5.9. Anthelmintic Activity
5.10. Cytotoxic Activity
5.11. Vaso-Activity Effect
5.12. Hypoglycemic Effect
5.13. Anti-Acetylcholinesterase and Anti-Butyrylcholinesterase Activity
5.14. Neuroprotective Activity
5.15. Tyrosinase Inhibitory Activity
5.16. Other Activities
5.17. As a Fodder
6. Conclusions and Future Prospective
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botschantzev, V. A synopsis of Salsola (Chenopodiaceae) from south and south-west Africa. Kew Bull. 1974, 29, 597–614. [Google Scholar] [CrossRef]
- Mabberley, D.J. The Plant-Book: A Portable Dictionary of the Vascular Plants; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Pyankov, V.I.; Voznesenskaya, E.V.; Kuz’min, A.N.; Ku, M.S.; Ganko, E.; Franceschi, V.R.; Black, C.C.; Edwards, G.E. Occurrence of C 3 and C 4 photosynthesis in cotyledons and leaves of Salsola species (Chenopodiaceae). Photosynth. Res. 2000, 63, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S.; Tanveer, A.; Rasool, G.; Hanif, Z.; Ali, H.H. Genus Salsola: Its benefits, uses, environmental perspectives and future aspects—A review. J. Rangel. Sci. 2018, 8, 315–328. [Google Scholar]
- Reimann, C.; Breckle, S.W. Salt tolerance and ion relations of Salsola kali L.: Differences between ssp. tragus (L.) Nyman and ssp. ruthenica (Iljin) Soó. New Phytol. 1995, 130, 37–45. [Google Scholar] [CrossRef]
- Idzikowska, K. Morphological and anatomical structure of generative organs of Salsola kali ssp. ruthenica [Iljin] Soo at the SEM level. Acta Soc. Bot. Pol. 2005, 74, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Pyankov, V.; Ziegler, H.; Kuz’min, A.; Edwards, G. Origin and evolution of C 4 photosynthesis in the tribe Salsoleae (Chenopodiaceae) based on anatomical and biochemical types in leaves and cotyledons. Plant Syst. Evol. 2001, 230, 43–74. [Google Scholar] [CrossRef]
- Toderich, K.; Shuyskaya, E.; Taha, F.; Ismail, S.; Gismatullina, L.; Li, E. Adaptive fruit structural mechanisms of Asiatic Salsola species and its germplasm conservation and utilization. J. Arid Land Stud. 2012, 22, 73–76. [Google Scholar]
- Mosyakin, S.L. A taxonomic synopsis of the genus Salsola (Chenopodiaceae) in North America. Ann. Mo. Bot. Gard. 1996, 387–395. [Google Scholar] [CrossRef]
- Farmer, D.A.; Fowler, J.L.; Hageman, J.H. Evaluation of Protein and Nutritive Fiber Content of Cultivated Russian-Thistle 1. J. Agron. 1976, 68, 691–692. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Seed germination in the Great Basin halophyte Salsola iberica. Can. J. Bot. 2002, 80, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Sokolowska-Krzaczek, A.; Skalicka-Wozniak, K.; Czubkowska, K. Variation of phenolic acids from herb and roots of Salsola kali L. Acta Soc. Bot. Pol. 2009, 78, 197–201. [Google Scholar]
- Lodhi, M. Allelopathic potential of Salsola kali L. and its possible role in rapid disappearance of weedy stage during revegetation. J. Chem. Ecol. 1979, 5, 429–437. [Google Scholar] [CrossRef]
- Boulos, L. The Identity, Typification and Distribution of Salsola imbricata Forsskål: Studies in the Chenopodiaceae of Arabia 1. Kew Bull. 1991, 137–140. [Google Scholar] [CrossRef]
- Toderich, K.N.; Shuyskaya, E.V.; Ozturk, M.; Juylova, A.; Gismatulina, L. Pollen morphology of some Asiatic species of genus Salsola (Chenopodiaceae) and its taxonomic relationships. Pak. J. Bot. 2010, 42, 155–174. [Google Scholar]
- Akhani, H.; Edwards, G.; Roalson, E.H. Diversification of the old world Salsoleae sl (Chenopodiaceae): Molecular phylogenetic analysis of nuclear and chloroplast data sets and a revised classification. Int. J. Plant Sci. 2007, 168, 931–956. [Google Scholar] [CrossRef]
- Turki, Z. Chemotaxonomical studies of the genus Salsola (Chenopodiaceae) in Egypt. Feddes Repert. 1999, 110, 81–87. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanical Gardens, Kew. Published on the Internet. 2022. Available online: http://plantsoftheworldonline.org/ (accessed on 2 February 2022).
- Jahromi, N.S.M.; Jonoubi, P.; Majd, A.; Dehghani, M. Investigating the anatomy of the halophyte Salsola crassa and the impact of industrial wastewater on its vegetative and generative structures. Turk. J. Bot. 2019, 43, 785–797. [Google Scholar] [CrossRef]
- Klopper, R.; Van Wyk, A. The genus Salsola (Chenopodiaceae) in southern Africa: Systematic significance of leaf anatomy. S. Afr. J. Bot. 2001, 67, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Bercu, R.; Bavaru, E. Anatomical aspects of Salsola kali subsp. ruthenica (Chenopodiaceae). Phytol. Balc. 2004, 10, 227–232. [Google Scholar]
- Carolin, R.; SWL, J.; Vesk, M. Leaf structure in Chenopodiaceae. Aust. J. Bot. 1982, 30, 387–392. [Google Scholar] [CrossRef]
- Kühn, U.; Bittrich, V.; Carolin, R.; Freitag, H.; Hedge, I.; Uotila, P.; Wilson, P. Chenopodiaceae. In Flowering Plants· Dicotyledons; Springer: Berlin/Heidelberg, Germany, 1993; pp. 253–281. [Google Scholar]
- Woldu, Y.; Abegaz, B. Isoflavonoids from Salsola somalensis. Phytochemistry 1990, 29, 2013–2015. [Google Scholar] [CrossRef]
- Abegaz, B.M.; Woldu, Y. Isoflavonoids from the roots of Salsola somalensis. Phytochemistry 1991, 30, 1281–1284. [Google Scholar] [CrossRef]
- Rasheed, D.M.; El Zalabani, S.M.; Koheil, M.A.; El-Hefnawy, H.M.; Farag, M.A. Metabolite profiling driven analysis of Salsola species and their anti-acetylcholinesterase potential. Nat. Prod. Res. 2013, 27, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, B.; Dagne, E. Comparative bioassay studies of some traditional anthelmintic plants, plant extracts and modern drugs. Sinet: Ethiop. J. Sci. 1978, 1, 117–122. [Google Scholar]
- Chaachouay, N.; Douira, A.; Zidane, L. Herbal Medicine Used in the Treatment of Human Diseases in the Rif, Northern Morocco. Arab. J. Sci. Eng. 2022, 47, 131–153. [Google Scholar] [CrossRef]
- Narantuyaa, S.; Batsuren, D.; Batirov, É.K.; Malikov, V. Chemical study of plants of the Mongolian flora coumarins of Salsola laricifolia. Chem. Nat. Compd. 1986, 22, 228–229. [Google Scholar] [CrossRef]
- Samdan, N.; Batsukh, O. Medicinal Plants of Mongolia: Mongolia. In Natural Products of Silk Road Plants; CRC Press: Boca Raton, FL, USA, 2020; pp. 7–47. [Google Scholar]
- Basson, P.; Morgenthal, J.; Bilbrough, R.; Marais, J.L.; Kruger, S.; Van Der Merwe, J. “Grootlamsiekte”, a specific syndrome of prolonged gestation in sheep caused by a shrub Salsola tuberculata (Fenzl ex Moq) Schinz var. tomentosa CA Smith ex Aellen. Onderstepoort J. Vet. Res. 1969, 36, 59–104. [Google Scholar]
- Swart, P.; Swart, A.C.; Louw, A.; van der Merwe, K.J. Biological activities of the shrub Salsola tuberculatiformis Botsch.: Contraceptive or stress alleviator? Bioessays 2003, 25, 612–619. [Google Scholar] [CrossRef]
- Al-Saleh, F.; Ali, H.; Mirza, M. Chemical constituents of some medicinal plants growing in Bahrain. Fitoterapia 1993, 64, 251. [Google Scholar]
- Ajaib, M.; Farooq, S.; Khan, K.M.; Perveen, S.; Shah, S. Phytochemical Analysis and Anthelmintic Activity of Salsola imbricata. J. Chem. Soc. Pak. 2019, 41, 198. [Google Scholar]
- Taia, W.K.; Shiha, M.A.; Al-Kogali, A.A.; Abd-Almaged, A.M. Anatomical and chemo-taxonomical investigations within some Salsola L. species grown in the western costal region of Egypt. Int. J. Sci. Res. Manag. 2018, 6, B-2018. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.; Alhowail, A.H.; Eldeeb, H.M.; Sajid, M.S.; Abd-Elmoniem, E.M.; Alghulayqeh, O.A.; Kandil, Y.I.; Khan, R.A. Phytochemical Analysis, Pharmacological and Safety Evaluations of Halophytic Plant, Salsola cyclophylla. Molecules 2021, 26, 2384. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, H.-A.; Lee, Y.-s.; Kim, D.-W.; Oh, G.-W.; Woo, J.; Cho, Y.; Jeong, J.-H.; Kim, O. Protective effect of halophyte Salsola komarovi Iljin against gastric ulcer Induced by alcohol treatment in rats. J. Biomed. Res. 2014, 15, 170–175. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Statti, G.; Menichini, F. Inhibitory effects on the digestive enzyme α-amylase of three Salsola species (Chenopodiaceae) in vitro. Pharmazie 2007, 62, 473–475. [Google Scholar] [PubMed]
- Mohammed, H.A.; Al-Omar, M.S.; Aly, M.S.; Hegazy, M.M. Essential oil constituents and biological activities of the halophytic plants, Suaeda vermiculata Forssk and Salsola cyclophylla Bakera growing in Saudi Arabia. J. Essent. Oil Bear. Plants 2019, 22, 82–93. [Google Scholar] [CrossRef]
- Al-Jaloud, A.A.; Chaudhary, S.A.; Bashour, I.I.; Qureshit, S.; Al-Shanghitti, A. Nutrient evaluation of some arid range plants in Saudi Arabia. J. Arid Environ. 1994, 28, 299–311. [Google Scholar] [CrossRef]
- Cho, H.K.; Suh, W.S.; Kim, K.H.; Kim, S.Y.; Lee, K.R. Phytochemical constituents of Salsola komarovii and their effects on NGF induction. Nat. Prod. Sci. 2014, 20, 95–101. [Google Scholar]
- Beyaoui, A.; Chaari, A.; Ghouila, H.; Ali Hamza, M.h.; Ben Jannet, H. New antioxidant bibenzyl derivative and isoflavonoid from the Tunisian Salsola tetrandra Folsk. Nat. Prod. Res. 2012, 26, 235–242. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mehmood, S.; Ifzal, R.; Malik, A.; Afza, N.; Rashid, F.; Mahmood, A.; Iqbal, L. Butyrylcholinesterase inhibitory triterpenes from Salsola baryosma. Pol. J. Chem. 2007, 81, 1427–1432. [Google Scholar]
- Ahmad, Z.; Mehmood, S.; Fatima, I.; Malik, A.; Ifzal, R.; Afza, N.; Iqbal, L.; Latif, M.; Nizami, T.A. Structural determination of salsolins A and B, new antioxidant polyoxygenated triterpenes from Salsola baryosma, by 1D and 2D NMR spectroscopy. Magn. Reson. Chem. 2008, 46, 94–98. [Google Scholar] [CrossRef]
- Alturkistani, T.; Bin Afif, M.; Alzahrani, R.; Alnouno, R.; Badr, J.M. Phytochemical investigation of Salsola kali extract. RPBS 2017, 1, 70–72. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Maharvi, G.; Ashraf, M.; Riaz, N.; Afza, N.; Khan, K.; Khan, M.; Jabbar, A.; Janbaz, K. Phytochemical studies on Salsola baryosma. J. Chem. Soc. Pak. 2006, 28, 176–178. [Google Scholar]
- Jin, Y.-S.; Du, J.-L.; Yang, Y.; Jin, L.; Song, Y.; Zhang, W.; Chen, H.-S. Chemical and biologically active constituents of Salsola collina. Chem. Nat. Compd. 2011, 47, 257–260. [Google Scholar] [CrossRef]
- Ahmed, S.; Ashraf, M.; Jabbar, A.; Janbaz, K.; Khan, M.; Gilani, A.; Choudhary, M. Pharmacological screening of Salsola baryosma. J. Chem. Soc. Pak. 2006, 28, 82. [Google Scholar]
- Küçükboyacı, N.; Küpeli Akkol, E.; Suntarİhsan Çalış, İ.; Çalış, İ. In vivo Anti-Inflammatory and Antinociceptive Activities of the Extracts and Chemical Constituents of an Endemic Turkish Plant, Salsola grandis. Rec. Nat. Prod. 2016, 10, 369–379. [Google Scholar]
- Orhan, I.E.; Kucukboyaci, N.; Calis, I.; Cerón-Carrasco, J.P.; den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H. Acetylcholinesterase inhibitory assessment of isolated constituents from Salsola grandis Freitag, Vural & Adıgüzel and molecular modeling studies on N-acetyltryptophan. Phytochem. Lett. 2017, 20, 373–378. [Google Scholar]
- Hamed, A.I.; Masullo, M.; Sheded, M.G.; Mahalel, U.A.; Tawfik, M.M.; Perrone, A.; Piacente, S. Triterpene saponins from Salsola imbricata. Phytochem. Lett. 2011, 4, 353–356. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Y.-B.; Zhang, J.; Li, P.; Yao, Y.-Z. Studies on chemical constituents of Salsola collina. China J. Chin. Mater. Med. 2007, 32, 409–413. [Google Scholar]
- Zhao, Y.; Ding, X. Studies on the alkaloids from Salsola collina Pall. Acta Pharm. Sin. 2004, 39, 598–600. [Google Scholar]
- Ghorab, H.; Khettaf, A.; Lehbili, M.; Kabouche, A.; Magid, A.A.; Harakat, D.; Voutquenne-Nazabadioko, L.; Kabouche, Z. A new cardenolide and other compounds from Salsola tetragona. Nat. Prod. Commun. 2017, 12, 1934578X1701200102. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.M.; Maharvi, G.M.; Abbaskhan, A.; Hayat, S.; Khan, M.T.H.; Makhmoor, T.; Choudhary, M.I.; Shaheen, F. Three tyrosinase inhibitors and antioxidant compounds from Salsola foetida. Helv. Chim. Acta 2003, 86, 457–464. [Google Scholar] [CrossRef]
- Ahmad, S.; Ashraf, M.; Riaz, N.; Choudhary, M.I.; Maharvi, G.M.; Afza, N.; Jabbar, A.; Janbaz, K.H.; Khan, M.S. Salsolide, a new p-hydroxyphenylglycol derivative from Salsola baryosma. J. Chem. Soc. Pak. 2008, 30, 110–112. [Google Scholar]
- Kaur, P.; Bains, N. Extraction of flavonoids from in vitro and in vivo tissue culture of some important halophytes of western Rajasthan. Int. J. Pharm. Tech. Res. 2012, 4, 1167–1171. [Google Scholar]
- Glushchenko, A.; Georgiyants, V.; Bevz, N. Development and Estimation of Validation Characteristics for The Quantitative Determination of Glycoalkaloid in Salsola collina L. Extracts. Acta Chim. Pharm. Indica 2015, 5, 47–54. [Google Scholar]
- Khurelbat, D.; Purevkhuu, M.; Luvsansharav, B.; Bandi, S.; Tseveen, D.; Sanjjav, T.; Dorjbal, E.; Miegombo, A. The hepatoprotective activity of the herbal preparation Salivin against carbon tetrachloride (CCl4) induced hepatotoxicity in rabbits. Curr. Issues Pharm. Med. Sci. 2014, 27, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Syrchina, A.; Vereshchagin, A.; Larin, M.; Semenov, A. Flavonoids of Salsola collina. Chem. Nat. Compd. 1989, 25, 619–620. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Y.-B.; Zhang, J.; Li, P.; Yao, Y.-Z. A new alkaloid from Salsola collina. Acta Pharm. Sin. 2007, 42, 618–620. [Google Scholar]
- Syrchina, A.; Gorshkov, A.; Shcherbakov, V.; Zinchenko, S.; Vereshchagin, A.; Zaikov, K.; Semenov, A. Flavonolignans of Salsola collina. Chem. Nat. Compd. 1992, 28, 155–158. [Google Scholar] [CrossRef]
- Zaikov, K.; Syrchina, A.; Vereshchagin, A.; Chernousova, A.; Semenov, A. An investigation of the chemical composition of the seeds of Salsola collina. Chem. Nat. Compd. 1992, 28, 627–628. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Yuan, W.; Li, Z.-Z.; Jin, H.; Gong, Y.-L. Salsola collina ethyl acetate extract alleviates diabetic gastroparesis possibly through oxidative stress inhibition. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012021. [Google Scholar]
- Syrchina, A.; Chernykh, E.; Rafeichikova, I.; Zaikov, K.; Vereshchagin, A. Carbohydrates, carbohydrate ethers, and alcohols of Salsola collina. Chem. Nat. Compd. 1991, 27, 364. [Google Scholar] [CrossRef]
- Munir, U.; Perveen, A.; Qamarunnisa, S. Comparative Pharmacognostic evaluation of some species of the genera Suaeda and Salsola leaf (Chenopodiaceae). Pak. J. Pharm. Sci. 2014, 27, 1309–1315. [Google Scholar] [PubMed]
- Shehab, N.G.; Abu-Gharbieh, E. Phenolic profiling and evaluation of contraceptive effect of the ethanolic extract of Salsola imbricata Forssk. in male albino rats. Evid. Based Complement. Alternat. Med. 2014, 2014, 695291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, O.; Morita, T.; Kasai, R.; Kinouchi, J.; Sanada, S.; Ida, Y.; Shoji, J. Study on saponins of rhizomes of Panax pseudo-ginseng subsp. himalaicus collected at Tzatogang and Pari-la, Bhutan-Himalaya. Chem. Pharm. Bull. 1985, 33, 2323–2330. [Google Scholar] [CrossRef] [Green Version]
- Espada, A.; Rodriguez, J.; Villaverde, M.C.; Riguera, R. Hypoglucaemic triterpenoid saponins from Boussingaultia baselloides. Can. J. Chem. 1990, 68, 2039–2044. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Ben Jannet, H.; Mighri, Z.; Chriaa, J.; Abreu, P.M. Phytochemical constituents from Salsola tetrandra. J. Nat. Prod. 2006, 69, 1366–1369. [Google Scholar] [CrossRef]
- Osman, S.M.; El Kashak, W.A.; Wink, M.; El Raey, M.A. New isorhamnetin derivatives from Salsola imbricata Forssk. leaves with distinct anti-inflammatory activity. Pharmacogn. Mag. 2016, 12, S47. [Google Scholar]
- Oueslati, M.H.; Bouajila, J.; Jannet, H.B. Two New Bioactive Biphenylpropanoidsfrom the Roots of Salsola imbricata (Chenopodiaceae) Growing in Saudi Arabia. Orient. J. Chem. 2017, 33, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Akhter, N.; Shaiq Ali, M.; Nazir, M.; Riaz, N.; Moazzam, M.; Arshad, M.; Jabbar, A. Structure determination of salisomide and salisoflavan, two new secondary metabolites from Salsola imbricata, by 1D and 2D NMR spectroscopy. Magn. Reson. Chem. 2009, 47, 263–265. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Statti, G.A.; Passalacqua, N.G.; Peruzzi, L.; Menichini, F. In vitro angiotensin converting enzyme inhibiting activity of Salsola oppositifolia Desf., Salsola soda L. and Salsola tragus L. Nat. Prod. Res. 2007, 21, 846–851. [Google Scholar] [CrossRef]
- Karawya, M.; Wassel, G.; Baghdadi, H.; Ahmed, Z. Phytochemical study of certain Salsola species. Planta Med. 1972, 21, 173–176. [Google Scholar] [CrossRef]
- Salt, T.A.; Adler, J.H. Diversity of sterol composition in the family Chenopodiaceae. Lipids 1985, 20, 594–601. [Google Scholar] [CrossRef]
- Lee, H.J.; Pan, C.-H.; Kim, E.-S.; Kim, C.Y. Online high-performance liquid chromatography (HPLC)-ABTS+ based assay and HPLC-electrospray ionization mass spectrometry analysis of antioxidant phenolic compounds in Salsola komarovii. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 317–321. [Google Scholar] [CrossRef]
- Annaev, C.; Isamukhamedova, M.; Abubakirov, N. Triterpene glycosides of Salsola micranthera. I. Structures of salsolosides C and D. Chem. Nat. Compd. 1983, 19, 691–695. [Google Scholar] [CrossRef]
- Annaev, C.; Isamukhamedova, M.; Abubakirov, N. Triterpene glycosides of Salsola micranthera. II. The structure of salsoloside E. Chem. Nat. Compd. 1984, 20, 60–64. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Statti, G.A.; Menichini, F. In vitro cytotoxic activity of Salsola oppositifolia Desf.(Amaranthaceae) in a panel of tumour cell lines. Z. Naturforsch. C. 2008, 63, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Iannuzzi, A.M.; Moschini, R.; De Leo, M.; Pineschi, C.; Balestri, F.; Cappiello, M.; Braca, A.; Del-Corso, A. Chemical profile and nutraceutical features of Salsola soda (agretti): Anti-inflammatory and antidiabetic potential of its flavonoids. Food Biosci. 2020, 37, 100713. [Google Scholar] [CrossRef]
- Elsharabasy, F.S.; AL-Mushhin, A.A.; Araffa, S.; Farrag, A.R.H. Phytochemical screening and gastroprotective effect of the aerial parts of Salasola terrandra Forssk. Against aspirin induced gastric ulceration in rats. J. Pharm. Phytochem. 2015, 3, 221–232. [Google Scholar]
- Karawya, M.; Wassel, G.; Ruecker, G.; Baghdadi, H.; Ahmed, Z. Isolation of triacetonamine from Salsola tetrandra. Phytochemistry 1971, 10, 3303–3304. [Google Scholar] [CrossRef]
- Mohammadi, M.; Alaei, M.; Bajalan, I. Phytochemical screening, total phenolic and flavonoid contents and antioxidant activity of Anabasis setifera and Salsola tomentosa extracted with different extraction methods and solvents. Orient. Pharm. Exp. Med. 2016, 16, 31–35. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Bian, Z.-X.; Wu, T.-X. Systematic review of Chinese herbal medicine for functional constipation. World J. Gastroenterol. 2009, 15, 4886. [Google Scholar] [CrossRef]
- Gannoun, S.; Mahfoudhi, A.; Flamini, G.; Helal, A.; Mighri, Z. Chemical composition and antimicrobial activities of Tunisian Salsola vermiculata L. J. Chem. Pharm. Res. 2016, 8, 1087–1092. [Google Scholar]
- Al-Saleh, G.; Gamal El-Din, A.; Abbas, J.; Saeed, N. Phytochemical and Biological Studies of Medicinal Plants in Bahrain: The Family Chenopodiaceae—Part 2. Int. J. Pharmacogn. Phytochem. Res. 1997, 35, 38–42. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Al-Ghamdi, F.A.; Noubigh, A. Two new bioactive salsolanol and biphenylsalsinol from the aerial parts of Salsola villosa Delile. ex Schul.(Chenopodiaceae) growing in Saudi Arabia. Asian Pac. J. Trop. Biomed. 2015, 5, 624–628. [Google Scholar] [CrossRef] [Green Version]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010, 1, 80–89. [Google Scholar]
- Reynolds, J.; Noakes, T.; Schwellnus, M.; Windt, A.; Bowerbank, P. Non-steroidal antiinflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S. Afr. Med. J. 1995, 85, 517–522. [Google Scholar]
- Garcia, M.; Saenz, M.; Gomez, M.; Fernandez, M. Topical antiinflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 78–80. [Google Scholar]
- Shehata, I.A.; El-harshany, E.; Abdallah, H.M.; Esmat, A.; Abdel-Sattar, E.A. Anti-inflammatory activity of Kleinia odora. Eur. J. Integr. Med. 2018, 23, 64–69. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Elsharabasy, F.S.; Hosney, A.M. Chemical constituents from the aerial parts of Salsola inermis. Egypt. Pharm. J. 2013, 12, 90. [Google Scholar]
- Seo, J.H.; Jin, M.H.; Chang, Y.H. Anti-inflammatory effect of Salsola komarovii extract with dissociated glucocorticoid activity. BMC Complement. Med. Ther. 2020, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Yang, X.W.; Ma, C.M.; Cai, S.Q.; Li, J.N.; Shoyama, Y. A bioactive alkaloid from the flowers of Trollius chinensis. Heterocycles-Sendai Inst. Heterocycl. Chem. 2004, 63, 1443–1448. [Google Scholar] [CrossRef]
- Wang, S.; Yan, M.; Guo, Y.; Sun, R.; Jin, H.; Gong, Y. In vivo and in vitro effects of Salsola collina on gastrointestinal motility in rats. Iran. J. Basic Med. Sci. 2020, 23, 383. [Google Scholar] [PubMed]
- Mughal, T.; Naeem, I.; Aziz, M.T.; Ahsan, A. Antibacterial and synergistic studies of Salsola kali. J. App. Pharm. 2010, 1, 18–26. [Google Scholar] [CrossRef]
- Mahasneh, A.M.; Abbas, J.A.; El-Oqlah, A.A. Antimicrobial activity of extracts of herbal plants used in the traditional medicine of Bahrain. Phytother. Res. 1996, 10, 251–253. [Google Scholar] [CrossRef]
- Znini, M.; Bouklah, M.; Majidi, L.; Kharchouf, S.; Aouniti, A.; Bouyanzer, A.; Hammouti, B.; Costa, J.; Al-Deyab, S. Chemical composition and inhibitory effect of Mentha spicata essential oil on the corrosion of steel in molar hydrochloric acid. Int. J. Electrochem. Sci. 2011, 6, 691–704. [Google Scholar]
- Aggarwal, K.; Khanuja, S.; Ahmad, A.; Santha Kumar, T.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Boughalleb, N.; Trabelsi, L.; Harzallah-Skhiri, F. Antifungal activity from polar and non-polar extracts of some Chenopodiaceae wild species growing in Tunisia. Nat. Prod. Res. 2009, 23, 988–997. [Google Scholar] [CrossRef]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative stress in diabetes and Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, J.; Yang, L. Insights for oxidative stress and mTOR signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid. Med. Cell. Longev. 2017, 2017, 6437467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, P.P.; Xia, Q.; Sun, X.; Yu, H. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)—light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J. Environ. Sci. Health C 2012, 30, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.N.; Jin, S.; Park, H.-J.; Kwon, H.J.; Kim, B.W. Anti-oxidative and anti-cancer activities by cell cycle regulation of Salsola collina extract. Microbiol. Biotechnol. Lett. 2014, 42, 73–81. [Google Scholar] [CrossRef]
- Shehab, N.G.; Abu-Gharbieh, E.; Bayoumi, F.A. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complement. Altern. Med. 2015, 15, 401. [Google Scholar] [CrossRef] [Green Version]
- Aniss, H.A.; Said, A.E.M.; El Sayed, I.H.; Adly, C. Amelioration of adriamycin-induced cardiotoxicity by Salsola kali aqueous extract is mediated by lowering oxidative stress. Redox Rep. 2014, 19, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Tundis, R.; Menichini, F.; Conforti, F.; Loizzo, M.R.; Bonesi, M.; Statti, G.; Menichini, F. A potential role of alkaloid extracts from Salsola species (Chenopodiaceae) in the treatment of Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2009, 24, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Mahadeva, S.; Goh, K.-L. Epidemiology of functional dyspepsia: A global perspective. World J. Gastroenterol. 2006, 12, 2661. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Jabri, M.-A.; Mamadou, G.; Limas-Nzouzi, N.; Sebai, H.; El-Benna, J.; Marzouki, L.; Eto, B.; Amri, M. Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice. RSC Adv. 2016, 6, 44345–44353. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef]
- Aslam, N.; Janbaz, K.H. Antispasmodic and bronchorelaxant activities of Salsola imbricata are mediated through dual Ca+2 antagonistic and β-adrenergic agonistic effects. Pharm. Biol. 2017, 55, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.H.; Aziz, N.; Ghayur, M.N.; Gilani, A.-H. Pharmacological basis for the medicinal use of psyllium husk (Ispaghula) in constipation and diarrhea. Dig. Dis. Sci. 2011, 56, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, J. Antihypertensive effects of alcoholic extracts from Salsola. Mod. Food Sci. Technol. 2007, 23, 17–19. [Google Scholar]
- Assarehzadegan, M.A.; Sankian, M.; Jabbari, F.; Noorbakhsh, R.; Varasteh, A. Allergy to Salsola Kali in a Salsola incanescens-rich area: Role of extensive cross allergenicity. Allergol. Int. 2009, 58, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforov, S.; Semenov, A.; Syrchina, A. Effect of an aqueous extract of Salsola collina on the course of experimental cholelithiasis in rabbits. Pharm. Chem. J. 2002, 36, 496–499. [Google Scholar] [CrossRef]
- Ali, I.; Chaudhry, M.S.; Farooq, U. Camel rearing in Cholistan desert of Pakistan. Pak. Vet. J. 2009, 29, 85–92. [Google Scholar]
- Fowler, J.L.; Hageman, J.H.; Moore, K.J.; Suzukida, M.; Assadian, H.; Valenzuela, M. Salinity effects on forage quality of Russian thistle. Rangel. Ecol. Manag. 1992, 45, 559–563. [Google Scholar] [CrossRef]



















| Accepted Species in Genus Salsola | Synonyms |
|---|---|
| Salsola acanthoclada Botsch. | Nitrosalsola acanthoclada (Botsch.) Theodorova |
| Salsola africana (Brenan) Botsch. | Salsola dendroides var. africana Brenan |
| Salsola algeriensis Botsch. | Nitrosalsola algeriensis (Botsch.) Theodorova |
| Salsola angusta Botsch. | - |
| Salsola arbusculiformis Drobow | - |
| Salsola australis R.Br. | Kali australe (R.Br.) Akhani and Roalson Kali macrophyllum (R.Br.) Galasso and Bartolucci Salsola macrophylla R.Br. Salsola tragus var. australis (R.Br.) Bég. |
| Salsola austroiranica Akhani | - |
| Salsola austrotibetica Sukhor. | - |
| Salsola baranovii Iljin | - |
| Salsola brevifolia Desf. | Nitrosalsola brevifolia (Desf.) Theodorova |
| Salsola chellalensis Botsch. | Nitrosalsola chellalensis (Botsch.) Theodorova |
| Salsola chinghaiensis A.J.Li | - |
| Salsola collina Pall. | Kali collinum (Pall.) Akhani and Roalson Salsola chinensis Gand. Salsola erubescens Schrad. Salsola ircutiana Gand. Salsola kali subsp. collina (Pall.) O.Bolòs and Vigo |
| Salsola cruciata L.Chevall. ex Batt. and Trab. | Darniella cruciata (L.Chevall. ex Batt. and Trab.) Brullo |
| Salsola cyrenaica (Maire and Weiller) Brullo | Darniella cyrenaica Maire and Weiller Salsola sieberi subsp. cyrenaica (Maire and Weiller) Brullo and Furnari |
| Salsola daghestanica (Turcz. ex Bunge) Lipsky | Noaea daghestanica Turcz. ex Bunge |
| Salsola divaricata Masson ex Link | Salsola capensis Botsch. Salsola divaricata (Moq.) Ulbr. |
| Salsola drummondii Ulbr. | Salsola obpyrifolia Botsch. and Akhani |
| Salsola euryphylla Botsch. | - |
| Salsola foliosa (L.) Schrad. ex Schult. | Anabasis clavata S.G.Gmel. Anabasis foliata Pall. ex Bunge Anabasis foliosa L. Caspia foliosa (L.) Galushko Micropeplis foliosa (L.) G.L.Chu Neocaspia foliosa (L.) Tzvelev Salsola baccifera Pall. Salsola clavifolia Pall. |
| Salsola glomerata (Maire) Brullo | Darniella glomerata (Maire) Brullo |
| Salsola gobicola Iljin | Kali gobicola (Iljin) Brullo and Hrusa |
| Salsola grandis Freitag, Vural and Adigüzel | - |
| Salsola griffithii (Bunge) Freitag and Khani | Kali griffithii (Bunge) Akhani and Roalson Noaea griffithii Bunge |
| Salsola gymnomaschala Maire | Darniella gymnomaschala (Maire) Brullo Seidlitzia gymnomaschala (Maire) Iljin |
| Salsola gypsacea Botsch. | |
| Salsola halimocnemis Botsch. | Nitrosalsola gypsacea (Botsch.) Theodorova |
| Salsola hartmannii Sukhor. | - |
| Salsola ikonnikovii Iljin | Kali ikonnikovii (Iljin) Akhani and Roalson Salsola beticolor Iljin Salsola centralasiatica Iljin Salsola potaninii Iljin |
| Salsola jacquemontii Moq. | Kali jacquemontii (Moq.) Akhani and Roalson Kali nepalensis (Grubov) Brullo, Giusso and Hrusa Salsola nepalensis Grubov |
| Salsola junatovii Botsch. | - |
| Salsola kali L. | Corispermum pilosum Raf. Kali soda Moench Kali turgidum (Dumort.) Gutermann Salsola acicularis Salisb. Salsola aptera Iljin Salsola decumbens Lam. Salsola gmelinii Rouy Salsola kali var. apula Ten. Salsola kali subsp. austroafricana Aellen Salsola kali var. hirta Ten. Salsola kali var. mixta W.D.J.Koch Salsola kali var. rosacea Pall. Salsola kali var. rosacea Moq. Salsola kali var. rubella Moq. Salsola kali var. vulgaris W.D.J.Koch Salsola scariosa Stokes Salsola spinosa Lam. Salsola turgida Dumort. |
| Salsola kerneri (Wol.) Botsch. | - |
| Salsola komarovii Iljin | Kali komarovii (Iljin) Akhani and Roalson |
| Salsola laricifolia Litv. ex Drobow | - |
| Salsola longifolia Forssk. | Darniella longifolia (Forssk.) Brullo Darniella sinaica (Brullo) Brullo Salsola fruticosa Cav. Salsola longiflora J.F.Gmel. Salsola oppositifolia Sieber ex Moq. Salsola sieberi C.Presl Salsola sinaica Brullo Seidlitzia longifolia (Forssk.) Iljin |
| Salsola mairei Botsch. | Nitrosalsola mairei (Botsch.) Theodorova |
| Salsola makranica Freitag | - |
| Salsola masclansii G.Monts. and D.Gómez | - |
| Salsola melitensis Botsch. | Darniella melitensis (Botsch.) Brullo |
| Salsola monoptera Bunge | Kali monopterum (Bunge) Lomon. |
| Salsola omanensis Boulos | - |
| Salsola oppositifolia Desf. | Petrosimonia sibirica (Pall.) Bunge |
| Salsola pachyphylla Botsch. | - |
| Salsola papillosa (Coss.) Willk. | Salsola angularis Sennen |
| Salsola paulsenii Litv. | Kali paulsenii (Litv.) Akhani and Roalson Kali pellucidum (Litv.) Brullo, Giusso and Hrusa Salsola pellucida Litv. |
| Salsola pontica (Pall.) Iliin | Kali ponticum (Pall.) Sukhor. Kali tragus subsp. ponticum (Pall.) Mosyakin Salsola kali var. pontica Pall. Salsola kali subsp. pontica (Pall.) Mosyakin Salsola pontica var. glabra Tzvelev Salsola squarrosa subsp. pontica (Pall.) Mosyakin Salsola tragus subsp. pontica (Pall.) Rilke |
| Salsola praecox (Litv.) Litv. | Kali praecox (Litv.) Sukhor. Salsola elegantissima Iljin Salsola kali var. praecox Litv. Salsola paulsenii subsp. praecox (Litv.) Rilke |
| Salsola praemontana Botsch. | Nitrosalsola praemontana (Botsch.) Theodorova |
| Salsola ryanii Hrusa and Gaskin | Kali ryanii (Hrusa and Gaskin) Brullo and Hrusa |
| Salsola sabrinae Mosyakin | Salsola tragus subsp. grandiflora Rilke |
| Salsola schweinfurthii Solms | Darniella schweinfurthii (Solms) Brullo |
| Salsola sinkiangensis A.J.Li | Kali sinkiangense (A.J.Li) Brullo, Giusso and Hrusa |
| Salsola squarrosa Steven ex Moq. | Kali dodecanesicum C.Brullo, Brullo, Giusso and Ilardi Salsola controversa Tod. ex Lojac. Salsola squarrosa subsp. controversa (Tod. ex Lojac.) Mosyakin |
| Salsola strobilifera (Benth.) Mosyakin | Salsola australis var. strobilifera (Benth.) Domin Salsola kali var. strobilifera Benth. |
| Salsola subglabra Botsch. | Nitrosalsola subglabra (Botsch.) Theodorova |
| Salsola tamamschjanae Iljin | Kali tamamschjanae (Iljin) Akhani and Roalson |
| Salsola tamariscina Pall. | Caroxylon tamariscinum (Pall.) Moq. Kali tamariscinum (Pall.) Akhani and Roalson Salsola tamariscifolia Falk Salsola tenuifolia Falk |
| Salsola tragus L. | Kali tragus (L.) Scop. Salsola altaica (C.A.Mey.) Iljin Salsola brachypteris Moq. Salsola caroliniana Walter Salsola dichracantha Kitag. Salsola iberica (Sennen and Pau) Botsch. ex Czerep. Salsola kali var. brachypteris (Moq.) Benth. Salsola kali var. brevimarginata W.D.J.Koch Salsola kali var. caroliniana (Walter) Nutt. Salsola kali var. glabra Ten. Salsola kali subsp. iberica (Sennen and Pau) Rilke Salsola kali var. leptophylla Benth. Salsola kali subsp. ruthenica (Iljin) Soó Salsola kali var. tenuifolia Tausch Salsola kali var. tragus (L.) Moq. Salsola pestifer A.Nelson Salsola pseudotragus (Beck) Iljin Salsola ruthenica Iljin Salsola ruthenica var. filifolia A.J.Li Salsola ruthenica var. tragus (L.) Morariu Salsola tragus subsp. iberica Sennen and Pau Salsola tragus var. pseudocollina Tzvelev Salsola tragus var. tenuifolia (Tausch) Tzvelev |
| Salsola tunetana Brullo | Darniella tunetana (Brullo) Brullo |
| Salsola turcica Yild. | - |
| Salsola verticillata Schousb. | Darniella verticillata (Schousb.) Brullo Salsola deschaseauxiana Litard. and Maire Seidlitzia verticillata (Schousb.) Iljin |
| Salsola webbii Moq. | Anabasis tamariscifolia Webb Salsola ericoides Lag. ex Willk. and Lange |
| Salsola zaidamica Iljin | Kali zaidamicum (Iljin) Akhani and Roalson |
| Salsola zygophylla Batt. | Darniella zygophylla (Batt.) Brullo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murshid, S.S.A.; Atoum, D.; Abou-Hussein, D.R.; Abdallah, H.M.; Hareeri, R.H.; Almukadi, H.; Edrada-Ebel, R. Genus Salsola: Chemistry, Biological Activities and Future Prospective—A Review. Plants 2022, 11, 714. https://doi.org/10.3390/plants11060714
Murshid SSA, Atoum D, Abou-Hussein DR, Abdallah HM, Hareeri RH, Almukadi H, Edrada-Ebel R. Genus Salsola: Chemistry, Biological Activities and Future Prospective—A Review. Plants. 2022; 11(6):714. https://doi.org/10.3390/plants11060714
Chicago/Turabian StyleMurshid, Samar S. A., Dana Atoum, Dina R. Abou-Hussein, Hossam M. Abdallah, Rawan H. Hareeri, Haifa Almukadi, and RuAngelie Edrada-Ebel. 2022. "Genus Salsola: Chemistry, Biological Activities and Future Prospective—A Review" Plants 11, no. 6: 714. https://doi.org/10.3390/plants11060714