Recurrent Respiratory Infections in Children with Down Syndrome: A Review
Abstract
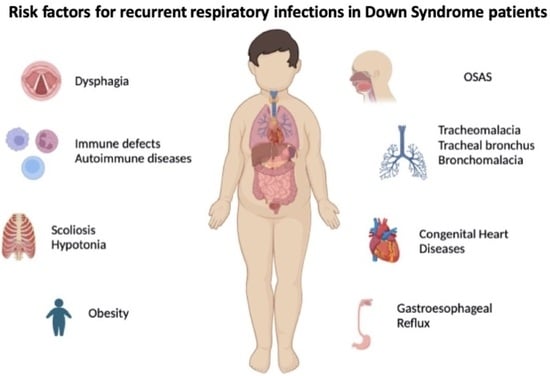
:1. Introduction
2. Materials and Methods
3. Epidemiology
4. Aetiology
4.1. Airway Malformations
4.2. Comorbidities Associated
4.2.1. Cardiovascular Involvement
4.2.2. Ear Nose Throat (ENT) Involvement
4.2.3. Gastrointestinal Involvement
4.2.4. Musculoskeletal Involvement and Obesity
4.2.5. Other Organs and Systems Involvement
4.3. Immunologic Impairments
4.3.1. Innate Immunity
4.3.2. Adaptive Immunity
5. Prevention
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National Population-Based Estimates for Major Birth Defects, 2010–2014. Birth Defects Res. 2020, 111, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Dott, B.; Alembik, Y.; Roth, M.P. Associated Congenital Anomalies among Cases with Down Syndrome. Eur. J. Med. Genet. 2015, 58, 674–680. [Google Scholar] [CrossRef]
- Santoro, S.L.; Chicoine, B.; Jasien, J.M.; Kim, J.L.; Stephens, M.; Bulova, P.; Capone, G. Pneumonia and Respiratory Infections in Down Syndrome: A Scoping Review of the Literature. Am. J. Med. Genet. Part A 2021, 185, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.J.; van Gameren-Oosterom, H.B.M.; Fekkes, M.; Dusseldorp, E.; De Vries, E.; van Wouwe, J.P. Significant Impact of Recurrent Respiratory Tract Infections in Children with Down Syndrome. Child Care Health Dev. 2013, 39, 801–809. [Google Scholar] [CrossRef]
- Bertrand, P.; Navarro, H.; Caussade, S.; Holmgren, N.; Sánchez, I. Airway Anomalies in Children with Down Syndrome: Endoscopic Findings. Pediatr. Pulmonol. 2003, 36, 137–141. [Google Scholar] [CrossRef]
- Ram, G.; Chinen, J. Infections and Immunodeficiency in Down Syndrome. Clin. Exp. Immunol. 2011, 164, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Bhayana, S.; Singh, A.; Kishore, J. Co-Morbidities Leading to Mortality or Hospitalization in Children with Down Syndrome and Its Effect on the Quality of Life of Their Parents. Indian J. Pediatr. 2014, 81, 1302–1306. [Google Scholar] [CrossRef]
- Skotko, B.G.; Davidson, E.J.; Weintraub, G.S. Contributions of a Specialty Clinic for Children and Adolescents with Down Syndrome. Am. J. Med. Genet. Part A 2013, 161, 430–437. [Google Scholar] [CrossRef]
- Nisihara, R.M.; Utiyama, S.R.R.; Oliveira, N.P.; Messias-Reason, I.J. Mannan-Binding Lectin Deficiency Increases the Risk of Recurrent Infections in Children with Down’s Syndrome. Hum. Immunol. 2010, 71, 63–66. [Google Scholar] [CrossRef]
- Jensen, K.M.; Sevick, C.J.; Seewald, L.A.S.; Halbower, A.C.; Davis, M.M.; McCabe, E.R.B.; Kempe, A.; Abman, S.H. Greater Risk of Hospitalization in Children with down Syndrome and OSA at Higher Elevation. Chest 2015, 147, 1344–1351. [Google Scholar] [CrossRef]
- Hilton, J.M.; Fitzgerald, D.A.; Cooper, D.M. Respiratory Morbidity of Hospitalized Children with Trisomy 21. J. Paediatr. Child Health 1999, 35, 383–386. [Google Scholar] [CrossRef]
- Medrano, C.; Garcia-Guereta, L.; Grueso, J.; Insa, B.; Ballesteros, F.; Casaldaliga, J.; Cuenca, V.; Escudero, F.; Garcia de la Calzada, L.; Luis, M.; et al. Respiratory Infection in Congenital Cardiac Disease. Hospitalizations in Young Children in Spain during 2004 and 2005: The CIVIC Epidemiologic Study. Cardiol. Young 2007, 17, 360–371. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Z.; Bai, Z.; MacDonald, N.E. A 4 Year Prospective Study to Determine Risk Factors for Severe Community Acquired Pneumonia in Children in Southern China. Pediatr. Pulmonol. 2013, 48, 390–397. [Google Scholar] [CrossRef]
- De Lausnay, M.; Verhulst, S.; Boel, L.; Wojciechowski, M.; Boudewyns, A.; Van Hoorenbeeck, K. The Prevalence of Lower Airway Anomalies in Children with Down Syndrome Compared to Controls. Pediatr. Pulmonol. 2020, 55, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Park, J.J.; Shi, T.; Torres, F.M.; Bont, L.; Nair, H.; Grobbee, D.E.; Greenough, A.; Manzoni, P.; Papadopoulos, N.; et al. The Burden of Respiratory Syncytial Virus (RSV) Associated Acute Lower Respiratory Infections in Children with Down Syndrome: A Systematic Review and Meta-Analysis. J. Glob. Health 2017, 7, 020413. [Google Scholar] [CrossRef]
- Löwensteyn, Y.N.; Phijffer, E.W.E.M.; Simons, J.V.L.; Scheltema, N.M.; Mazur, N.I.; Nair, H.; Bont, L.J. Respiratory Syncytial Virus-Related Death in Children with Down Syndrome: The RSV GOLD Study. Pediatr. Infect. Dis. J. 2020, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, A.A.; Castro-Rodriguez, J.A. Down Syndrome and the Risk of Severe RSV Infection: A Meta-Analysis. Pediatrics 2018, 142, e20180225. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Rivelli, A.; Chaudhari, S.; Chicoine, L.; Jia, G.; Rzhetsky, A.; Chicoine, B. Prevalence of Infectious Diseases among 6078 Individuals With Down Syndrome in the United States. J. Patient Cent. Res. Rev. 2022, 9, 64–69. [Google Scholar] [CrossRef]
- Landes, S.D.; Stevens, J.D.; Turk, M.A. Cause of death in adults with Down syndrome in the United States. Disabil. Health J. 2020, 13, 100947. [Google Scholar] [CrossRef] [PubMed]
- Uppal, H.; Chandran, S.; Potluri, R. Risk Factors for Mortality in Down Syndrome. J. Intellect. Disabil. Res. 2015, 59, 873–881. [Google Scholar] [CrossRef]
- Hamilton, J.; Yaneza, M.M.C.; Clement, W.A.; Kubba, H. The Prevalence of Airway Problems in Children with Down’s Syndrome. Int. J. Pediatr. Otorhinolaryngol. 2016, 81, 1–4. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Call, E.; Kelly, J. Diagnosis and Therapy for Airway Obstruction in Children With Down Syndrome. Arch. Otolaryngol. Neck Surg. 2003, 129, 642–645. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.L.; Sulek, M.; Nihill, M.; Duncan, N.O.; Friedman, E.M. Tenuous Airway in Children with Trisomy 21. Laryngoscope 1997, 107, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, A.; Song, W.J.; Almaghlouth, F.; Kemp, S.; Polkey, M.; Hull, J.H. Detection and diagnosis of large airway collapse: A systematic review. ERJ Open Res. 2021, 7, 00055–02021. [Google Scholar] [CrossRef] [PubMed]
- Fockens, M.M.; Hölscher, M.; Limpens, J.; Dikkers, F.G. Tracheal Anomalies Associated with Down Syndrome: A Systematic Review. Pediatr. Pulmonol. 2021, 56, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.; Alexopoulou, E.; Antón-Pacheco, J.L.; Bhatt, J.M.; Bush, A.; Chang, A.B.; Charatsi, A.M.; Coleman, C.; Depiazzi, J.; Douros, K.; et al. ERS Statement on Tracheomalacia and Bronchomalacia in Children. Eur. Respir. J. 2019, 54, 1900382. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Castillo-Corullón, S.; Pérez-Ruiz, E.; Luna, M.C.; Antón-Pacheco, J.L.; Mondejar-Lopez, P.; De-la-Serna, O.; Villa, J.R.; Osona, B.; Torres-Borrego, J.; et al. Spanish Multicentre Study on Morbidity and Pathogenicity of Tracheal Bronchus in Children. Pediatr. Pulmonol. 2019, 54, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.C.; Ciet, P.; Sodhi, K.S.; Lee, E.Y. Updates on MRI Evaluation of Pediatric Large Airways. AJR Am. J. Roentgenol. 2017, 208, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Lagan, N.; Huggard, D.; Mc Grane, F.; Leahy, T.R.; Franklin, O.; Roche, E.; Webb, D.; O’ Marcaigh, A.; Cox, D.; El-Khuffash, A.; et al. Multiorgan involvement and management in children with Down syndrome. Acta Paediatr. 2020, 109, 1096–1111. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Constantine, A.; Clift, P.; Condliffe, R.; Moledina, S.; Jansen, K.; Inuzuka, R.; Veldtman, G.R.; Cua, C.L.; Tay, E.L.W.; et al. Cardiovascular Complications of Down Syndrome: Scoping Review and Expert Consensus. Circulation 2023, 147, 425. [Google Scholar] [CrossRef]
- Bush, D.; Galambos, C.; Ivy, D.D.; Abman, S.H.; Wolter-Warmerdam, K.; Hickey, F. Clinical Characteristics and Risk Factors for Developing Pulmonary Hypertension in Children with Down Syndrome. J. Pediatr. 2018, 202, 212–219.e2. [Google Scholar] [CrossRef]
- Anil, M.A.; Shabnam, S.; Narayanan, S. Feeding and swallowing difficulties in children with Down syndrome. J. Intellect. Disabil. Res. 2019, 63, 992–1014. [Google Scholar] [CrossRef]
- Leder, S.B.; Karas, D.E. Fiberoptic Endoscopic Evaluation of Swallowing in the Pediatric Population. Laryngoscope 2000, 110, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A.; Shepherd, N.; Duvall, N.; Jenkinson, S.B.; Jalou, H.E.; Givan, D.C.; Steele, G.H.; Davis, C.; Bull, M.J.; Watkins, D.U.; et al. Clinical Identification of Feeding and Swallowing Disorders in 0–6 Month Old Infants with Down Syndrome. Am. J. Med. Genet. A 2019, 179, 177. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Maybee, J.; Moran, M.K.; Wolter-Warmerdam, K.; Hickey, F. Clinical Characteristics of Dysphagia in Children with Down Syndrome. Dysphagia 2016, 31, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Alsubie, H.S.; Rosen, D. The evaluation and management of respiratory disease in children with Down syndrome (DS). Paediatr. Respir. Rev. 2018, 26, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Weijerman, M.E.; Brand, P.L.; van Furth, M.A.; Broers, C.J.; Gemke, R.J. Recurrent wheeze in children with Down syndrome: Is it asthma? Acta Paediatr. 2011, 100, e194–e197. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.; Killeen, O.G. Musculoskeletal anomalies in children with Down syndrome: An observational study. Arch. Dis. Child. 2019, 104, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Cunin, V. Early-onset scoliosis: Current treatment. Orthop. Traumatol. Surg. Res. 2015, 101 (Suppl. 1), S109–S118. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.D.; Nayak, A.; Karnad, S.D.; Doctor, K.N. Gross motor dysfunction and balance impairments in children and adolescents with Down syndrome: A systematic review. Clin. Exp. Pediatr. 2022, 65, 142–149. [Google Scholar] [CrossRef]
- Ostrzyżek-Przeździecka, K.; Panczyk, M.; Bronikowski, M.; Gąsior, J.S.; Feleszko, W. Association of low physical activity with higher respiratory tract infections frequency among pre-school children. Pediatr. Res. 2023, 94, 594–602. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.Y.; Mullen, J.; Celedón, J.C. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J. Allergy Clin. Immunol. Pract. 2018, 6, 570–581.e10. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Sabiu, S. Adipose tissue inflammation linked to obesity: A review of current understanding, therapies and relevance of phyto-therapeutics. Heliyon 2023, 10, e23114. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Sugiyama, S.; Shinozuka, O.; Yamazaki, T.; Ohyama, T.; Ishikawa, I. Defective Neutrophil Chemotaxis in Down’s Syndrome Patients and Its Relationship to Periodontal Destruction. J. Periodontol. 1989, 60, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Licastro, F.; Melotti, C.; Parente, R.; Davis, L.J.; Chiricolo, M.; Zannotti, M.; Barboni, F. Derangement of Non-Specific Immunity in Down Syndrome Subjects: Low Leukocyte Chemiluminescence Activity after Phagocytic Activation. Am. J. Med. Genet. Suppl. 1990, 7, 242–246. [Google Scholar] [CrossRef]
- Huggard, D.; McGrane, F.; Lagan, N.; Roche, E.; Balfe, J.; Leahy, T.R.; Franklin, O.; Moreno, A.; Melo, A.M.; Doherty, D.G.; et al. Altered Endotoxin Responsiveness in Healthy Children with Down Syndrome. BMC Immunol. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. The CD14+ CD16+ Blood Monocytes: Their Role in Infection and Inflammation. J. Leukoc. Biol. 2007, 81, 584–592. [Google Scholar] [CrossRef]
- Huggard, D.; Doherty, D.G.; Molloy, E.J. Immune Dysregulation in Children with Down Syndrome. Front. Pediatr. 2020, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Huggard, D.; Koay, W.J.; Kelly, L.; McGrane, F.; Ryan, E.; Lagan, N.; Roche, E.; Balfe, J.; Leahy, T.R.; Franklin, O.; et al. Altered Toll-Like Receptor Signalling in Children with Down Syndrome. Mediat. Inflamm. 2019, 2019, 4068734. [Google Scholar] [CrossRef]
- Cossarizza, A.; Ortolani, C.; Forti, E.; Montagnani, G.; Paganelli, R.; Zannotti, M.; Marini, M.; Monti, D.; Franceschi, C. Age-Related Expansion of Functionally Inefficient Cells with Markers of Natural Killer Activity in Down’s Syndrome. Blood 1991, 77, 1263–1270. [Google Scholar] [CrossRef]
- Li, Y.Y.; Alexandrov, P.N.; Pogue, A.I.; Zhao, Y.; Bhattacharjee, S.; Lukiw, W.J. miRNA-155 upregulation and complement factor H deficits in Down’s syndrome. Neuroreport 2012, 23, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, T.; Huttunen, K.; Lassila, O.; Henttonen, M.; Säkkinen, A.; Linna, S.L.; Tiilikainen, A. Natural Killer Cell Function in Trisomy-21 (Down’s Syndrome). Clin. Exp. Immunol. 1982, 47, 735. [Google Scholar] [PubMed]
- Kusters, M.A.A.; Verstegen, R.H.J.; Gemen, E.F.A.; De Vries, E. Intrinsic Defect of the Immune System in Children with Down Syndrome: A Review. Clin. Exp. Immunol. 2009, 156, 189. [Google Scholar] [CrossRef] [PubMed]
- Rigas, D.A.; Elsasser, P.; Hecht, F. Impaired in Vitro Response of Circulating Lymphocytes to Phytohemagglutinin in Down’s Syndrome: Dose- and Time-Response Curves and Relation to Cellular Immunity. Int. Arch. Allergy Immunol. 1970, 39, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Cetiner, S.; Demirhan, O.; Inal, T.C.; Tastemir, D.; Sertdemir, Y. Analysis of Peripheral Blood T-Cell Subsets, Natural Killer Cells and Serum Levels of Cytokines in Children with Down Syndrome. Int. J. Immunogenet. 2010, 37, 233–237. [Google Scholar] [CrossRef]
- Kusters, M.A.; Jol-Van Der Zijde, E.C.M.; Gijsbers, R.H.J.M.; De Vries, E. Decreased Response after Conjugated Meningococcal Serogroup C Vaccination in Children with Down Syndrome. Pediatr. Infect. Dis. J. 2011, 30, 818–819. [Google Scholar] [CrossRef]
- Kusters, M.A.A.; Manders, N.C.C.; De Jong, B.A.W.; Van Hout, R.W.N.M.; Rijkers, G.T.; De Vries, E. Functionality of the Pneumococcal Antibody Response in down Syndrome Subjects. Vaccine 2013, 31, 6261–6265. [Google Scholar] [CrossRef]
- Kusters, M.A.; Jol-Van Der Zijde, C.M.; Van Tol, M.J.; Bolz, W.E.; Bok, L.A.; Visser, M.; De Vries, E. Impaired Avidity Maturation after Tetanus Toxoid Booster in Children with Down Syndrome. Pediatr. Infect. Dis. J. 2011, 30, 357–359. [Google Scholar] [CrossRef]
- Valentini, D.; Marcellini, V.; Bianchi, S.; Villani, A.; Facchini, M.; Donatelli, I.; Castrucci, M.R.; Marasco, E.; Farroni, C.; Carsetti, R. Generation of switched memory B cells in response to vaccination in Down syndrome children and their siblings. Vaccine 2015, 33, 6689–6696. [Google Scholar] [CrossRef]
- Okamoto, K.; Morio, T.; Nakamura, Y.; Hataya, H.; Mizuta, K.; Mori, M. Hospitalisations due to respiratory syncytial virus infection in children with Down syndrome before and after palivizumab recommendation in Japan. Acta Paediatr. 2021, 110, 1299–1306. [Google Scholar] [CrossRef]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Simões, E.A.F.; Madhi, S.A.; Muller, W.J.; Atanasova, V.; Bosheva, M.; Cabañas, F.; Baca Cots, M.; Domachowske, J.B.; Garcia-Garcia, M.L.; Grantina, I.; et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: A pooled analysis of randomised controlled trials. Lancet Child Adolesc. Health 2023, 7, 180–189. [Google Scholar] [CrossRef]
- Jones, J.M.; Fleming-Dutra, K.E.; Prill, M.M.; Roper, L.E.; Brooks, O.; Sánchez, P.J.; Kotton, C.N.; Mahon, B.E.; Meyer, S.; Long, S.S.; et al. Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Valentini, D.; Di Camillo, C.; Mirante, N.; Marcellini, V.; Carsetti, R.; Villani, A. Effects of Pidotimod on recurrent respiratory infections in children with Down syndrome: A retrospective Italian study. Ital. J. Pediatr. 2020, 46, 31. [Google Scholar] [CrossRef] [PubMed]



| System Involved | Malformation |
|---|---|
| Airways malformations | Upper airways malformations |
| Laryngomalacia | |
| Subglottic stenosis | |
| Tracheomalacia | |
| Bronchomalacia | |
| Tracheal bronchus | |
| Obstructive Sleep Apnoea | |
| Cardiovascular involvement | Congenital Heart Diseases |
| PPHN | |
| Pulmonary Hypertension | |
| ENT involvement | Dysphagia |
| Gastrointestinal involvement | Gastroesophageal Reflux |
| Other systems involved | Musculoskeletal |
| Overweight—Obesity | |
| Other causes for hospitalisation | |
| Immunologic impairment | Innate immunity |
| Adaptive immunity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghezzi, M.; Garancini, N.; De Santis, R.; Gianolio, L.; Zirpoli, S.; Mandelli, A.; Farolfi, A.; D’Auria, E.; Zuccotti, G.V. Recurrent Respiratory Infections in Children with Down Syndrome: A Review. Children 2024, 11, 246. https://doi.org/10.3390/children11020246
Ghezzi M, Garancini N, De Santis R, Gianolio L, Zirpoli S, Mandelli A, Farolfi A, D’Auria E, Zuccotti GV. Recurrent Respiratory Infections in Children with Down Syndrome: A Review. Children. 2024; 11(2):246. https://doi.org/10.3390/children11020246
Chicago/Turabian StyleGhezzi, Michele, Nicolò Garancini, Raffaella De Santis, Laura Gianolio, Salvatore Zirpoli, Anna Mandelli, Andrea Farolfi, Enza D’Auria, and Gian Vincenzo Zuccotti. 2024. "Recurrent Respiratory Infections in Children with Down Syndrome: A Review" Children 11, no. 2: 246. https://doi.org/10.3390/children11020246