A Real-Time PCR Method for the Authentication of Common Cuttlefish (Sepia officinalis) in Food Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. FINS Identification of Samples
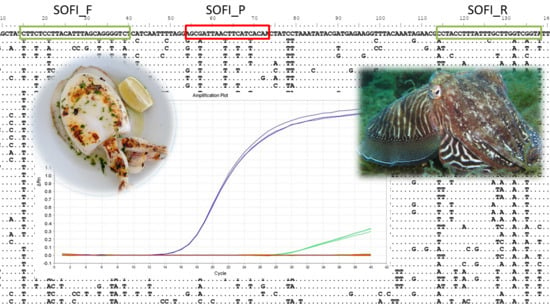
2.3. RT-PCR Design

2.4. Real-Time PCR Conditions and Data Treatment
3. Results
3.1. Efficiency and Detection Limit
3.2. Inclusivity and Specificity
3.3. Application to Commercial Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Fishery and Aquaculture Statistics; 2017/FAO annuaire; FAO: Rome, Italy, 2019. [Google Scholar]
- Fisheries and Aquaculture Software. FishStatJ—Software for Fishery and Aquaculture Statistical Time Series. In FAO Fisheries and Aquaculture Department; FAO: Rome, Italy, 2016; Updated 21 July 2016; Available online: http://www.fao.org/fishery/Rome (accessed on 6 November 2019).
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:EN:PDF (accessed on 25 October 2011).
- Regulation (EU) No 1379/2013 of the European Parliament and of the Council of 11 December 2013 on the common organisation of the markets in fishery and aquaculture products, amending Council Regulations (EC) No 1184/2006 and (EC) No 1224/2009 and repealing Council Regulation (EC) No 104/2000. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:354:0001:0021:EN:PDF (accessed on 11 December 2013).
- FDA, Food and Drug Administration; U.S. Department of Health and Human Services. Fish and Fishery Products: Hazards and Controls Guidance, 4th ed.; Chapter 3; IFAS-University of Florida: Gainesville, FL, USA, 2019.
- Warner, K.; Mustain, P.; Lowell, B.; Geren, S.; Talmage, S. Deceptive Dishes: Seafood Swaps Found Worldwide; Oceana Reports; Oceana: New York, NY, USA, 2016; Available online: www.oceana.org (accessed on 12 December 2019).
- Espiñeira, M.; Vieites, J.M. Rapid method for controlling the correct labeling of products containing common octopus (Octopus vulgaris) and main substitute species (Eledone cirrhosa and Dosidicus gigas) by fast real-time PCR. Food Chem. 2012, 135, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Santaclara, F.J.; Espiñeira, M.; Vieites, J.M. Genetic identification of squids (families Ommastrephidae and Loliginidae) by PCR–RFLP and FINS methodologies. J. Agric. Food Chem. 2007, 55, 9913–9920. [Google Scholar] [CrossRef] [PubMed]
- Guardone, L.; Tinacci, L.; Costanzo, F.; Azzarelli, D.; D’Amico, P.; Tasselli, G.; Magni, A.; Guidi, A.; Nucera, D.; Armani, A. DNA barcoding as a tool for detecting mislabeling of fishery products imported from third countries: An official survey conducted at the Border Inspection Post of Livorno-Pisa (Italy). Food Control 2017, 80, 204–216. [Google Scholar] [CrossRef]
- Espiñeira, M.; Vieites, J.M.; Santaclara, F.J. Species authentication of octopus, cuttlefish, bobtail and bottle squids (families Octopodidae, Sepiidae and Sepiolidae) by FINS methodology in seafoods. Food Chem. 2010, 121, 527–532. [Google Scholar] [CrossRef]
- Chapela, M.J.; Sotelo, C.G.; Calo-Mata, P.; Pérez-Martín, R.I.; Rehbein, H.; Hold, G.L.; Quinteiro, J.; Rey-Méndez, M.; Rosa, C.; Santos, A.T. Identification of cephalopod species (Ommastrephidae and Loliginidae) in seafood products by Forensically Informative Nucleotide Sequencing (FINS). J. Food Sci. 2002, 67, 1672–1676. [Google Scholar] [CrossRef]
- Chapela, M.J.; Sotelo, C.G.; Pérez-Martín, R.I. Molecular identification of cephalopod species by FINS and PCR-RFLP of a cytochrome b gene fragment. Eur. Food Res. Technol. 2003, 217, 524–529. [Google Scholar] [CrossRef]
- Sin, Y.W.; Yau, C.; Chu, K.H. Morphological and genetic differentiation of two loliginid squids, Uroteuthis (Photololigo) chinensis and Uroteuthis (Photololigo) edulis (Cephalopoda: Loliginidae), in Asia. J. Exp. Mar. Biol. Ecol. 2009, 369, 22–30. [Google Scholar] [CrossRef]
- Undheim, E.A.; Norman, J.A.; Thoen, H.H.; Fry, B.G. Genetic identification of Southern Ocean octopod samples using mtCOI. C. R. Biol. 2010, 333, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Cerioli, M.; Colombo, M.M.; Marchisio, E.; Malandra, R.; Renon, P. A simple polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method for the differentiation of cephalopod mollusc families Loliginidae from Ommastrephidae, to avoid substitutions in fishery field. Food Control 2002, 13, 185–190. [Google Scholar] [CrossRef]
- Herrero, B.; Lago, F.C.; Vieites, J.M.; Espiñeira, M. Rapid method for controlling the correct labeling of products containing European squid (Loligo vulgaris) by fast real-time PCR. Eur. Food Res. Technol. 2012, 234, 77–85. [Google Scholar] [CrossRef]
- Ye, J.; Feng, J.; Liu, S.; Zhang, Y.; Jiang, X.; Dai, Z. Identification of four squid species by quantitative real-time polymerase chain reaction. Mol. Cell. Probes 2016, 30, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Feng, J.; Dai, Z.; Meng, L.; Zhang, Y.; Jiang, X. Application of Loop-Mediated Isothermal Amplification (LAMP) for Rapid Detection of Jumbo Flying Squid Dosidicus gigas (D’Orbigny, 1835). Food Anal. Methods 2017, 10, 1452–1459. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuceicl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Dudley, J.; Nei, M.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Millar, W.; Lipman, D.J. Gapped BLAST and PSIBLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorak, M.T. Real-Time PCR; Taylor & Francis Group: New York, NY, USA, 2006. [Google Scholar]
- Resolución de 26 de enero de 2018, de la Secretaría General del Mar, por la que se establece y se publica el listado de denominaciones comerciales de especies pesqueras y de acuicultura admitidas en España; BOE-A-2018-2884; Boletín oficial del Estado núm. 53, de 1 de marzo de 2018; Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2018; pp. 25487–25513.
- ORDEN de 17 de enero de 1986 sobre norma reguladora del comercio exterior de las conservas de cefalópodos; Boletín oficial del Estado Núm. 30; Ministerio de Economía y Hacienda: Madrid, Spain, 1986; pp. 4682–4684.
- Kutyavin, I.V.; Afonina, I.A.; Mills, A.; Gorn, V.V.; Lukhtanov, E.A.; Belousov, E.S.; Singer, M.J.; Walburger, D.K.; Lokhov, S.G.; Gall, A.A.; et al. 3’-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res. 2000, 28, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Luekasemsuk, T.; Panvisavas, N.; Chaturongakul, S. TaqMan qPCR for detection and quantification of mitochondrial DNA from toxic pufferfish species. Toxicon 2015, 102, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.J.R.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Exploiting 16S rRNA gene for the detection and quantification of fish as a potential allergenic food: A comparison of two real-time PCR approaches. Food Chem. 2018, 245, 1034–1041. [Google Scholar] [CrossRef]


| Sample Code | Species | Common Name (FAO) | Geographic Origin | Ct Mean ± SD |
|---|---|---|---|---|
| SOFF2 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Vigo | 12.98 ± 0.32 |
| SOFF3 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Cambados | 14.05 ± 0.26 |
| SOFF4 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Cambados | 14.80 ± 0.04 |
| SOFF5 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Cambados | 16.16 ± 0.35 |
| SOFF6 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Vigo | 13.57 ± 0.70 |
| SOFF7 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Vigo | 15.37 ± 0.10 |
| SOFF8 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Vigo | 13.51 ± 0.07 |
| SOFF9 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27) | 14.11 ± 0.20 |
| SOFF10 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27) | 15.65 ± 0.21 |
| SOFF11 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27) | 13.86 ± 0.48 |
| SOFF12 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27) | 13.25 ± 0.39 |
| SOFF15 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Algarve | 12.72 ± 0.06 |
| SOFF16 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Algarve | 12.59 ± 0.19 |
| SOFF17 | Sepia officinalis | Common cuttlefish | Atlantic, Northeast (FAO 27.9) Algarve | 13.98 ± 0.12 |
| SBER 2 | Sepia betheloti | African cuttlefish | Atlantic, Eastern Central (FAO 34) | ≥40 |
| SBER 3 | Sepia betheloti | African cuttlefish | Atlantic, Eastern Central (FAO 34) | ≥40 |
| SORB 4 | Sepia orbygniana | Pink cuttlefish | Atlantic, Northeast (FAO 27) | ≥40 |
| SORB 5 | Sepia orbygniana | Pink cuttlefish | Atlantic, Northeast (FAO 27) | ≥40 |
| SPHA 1 | Sepia pharaonis | Pharaon cuttlefish | Indian Ocean, Western (FAO 51) | ≥40 |
| LVUL 2 | Loligo vulgaris | European squid | Western Central Atlantic (FAO 31) | 27.00 ± 0.16 |
| LVUL 1 | Loligo vulgaris | European squid | Atlantic, Northeast (FAO 27) | 26.03 ± 0.28 |
| LVUL 5 | Loligo vulgaris | European squid | Atlantic, Northeast (FAO 27) | 29.15 ± 0.14 |
| LVUL 3 | Loligo vulgaris | European squid | Western Central Atlantic (FAO 31) | ≥40 |
| LVUL 4 | Loligo vulgaris | European squid | Western Central Atlantic (FAO 31) | ≥40 |
| LVUL 6 | Loligo vulgaris | European squid | Western Central Atlantic (FAO 31) | ≥40 |
| LVUL 7 | Loligo vulgaris | European squid | Western Central Atlantic (FAO 31) | ≥40 |
| LVUL 8 | Loligo vulgaris | European squid | Western Central Atlantic (FAO 31) | 29.80 ± 0.40 |
| LREY 1 | Loligo reynaudi | Cape Hope squid | Atlantic, Southeast (FAO 47) | ≥40 |
| IILL 2 | Illex illecebrosus | Northern Shortfin squid | Atlantic, Northwest (FAO 21) | ≥40 |
| TEBL 1 | Todaropsis eblanae | Lesser flying squid | Atlantic, Northeast (FAO 27) | ≥40 |
| TPAC 3 | Todarodes pacificus | Japanese flying squid | Pacific, Northwest (FAO 61) | ≥40 |
| ICOI 10 | Illex coindetii | Southern shortfin squid | Atlantic, Northeast (FAO 27) | ≥40 |
| LGAH 9 | Loligo gahi | Patagonian squid | Pacific, Southeast (FAO 87) | ≥40 |
| MHYA 8 | Martialia hyadesi | Sevenstar flying squid | Atlantic, Antarctic (FAO 48) | ≥40 |
| NSLO6 | Nototodarus sloanii | Wellington flying squid | Pacific, Southwest (FAO 81) | ≥40 |
| TSAG 1 | Todarodes sagittatus | European flying squid | Atlantic, Northeast (FAO 27) | ≥40 |
| OVUL 142 | Octopus vulgaris | Common octopus | Atlantic, Northeast (FAO 27) | ≥40 |
| OCYA 3 | Octopus cyanea | Big blue octopus | Pacific, Western Central (FAO 71) | ≥40 |
| OCYA 4 | Octopus cyanea | Big blue octopus | Pacific, Western Central (FAO 71) | ≥40 |
| OMIM 1 | Octopus mimus | Changos octopus | Pacific, Southeast (FAO 87) | ≥40 |
| ECIR 143 | Eledone cirrhosa | Horned octopus | Atlantic, Northeast (FAO 27) | ≥40 |
| DGIG 1 | Dosidicus gigas | Jumbo squid | Pacific, Southeast (FAO 87) | ≥40 |
| AMEM 1 | Amphioctopus membranaceus | Webfoot octopus | Indian Ocean, Western (FAO 51) | ≥40 |
| Sample Code | Type of Processing | Type of Establishment | Species Declared | Species Identified by FINS | Ct Mean ± SD |
|---|---|---|---|---|---|
| S1 | Frozen | Supermarket | Sepia spp. | Sepia pharaonis | 29.77 ± 0.62 |
| S2 | Frozen | Supermarket | Sepia spp. | Sepia pharaonis | 27.68 ± 0.06 |
| S3 | Frozen | Supermarket | Sepia spp. | Sepia sp (not S. officinalis) | ≥40 |
| S4 | Frozen | Supermarket | “Sepia” | Sepia sp (not S. officinalis) | 31.85 ± 0.26 |
| S5 | Canned | Supermarket | “Sepia” | Sepia officinalis | 16.91 ± 0.47 |
| S6 | Frozen | Supermarket | Sepia spp. | Sepia sp (not S. officinalis) | ≥40 |
| S7 | Cooked | Supermarket | Sepia officinalis | Sepia officinalis | 17.88 ± 0.94 |
| S8 | Canned | Supermarket | “Sepia” | Sepia officinalis | 15.41 ± 0.03 |
| S10 | Grilled | Restaurant | “Choco” | Sepia officinalis | 13.70 ± 0.06 |
| S11 | Frozen | Supermarket | Sepia aculeata | Sepia sp (not S. officinalis) | ≥40 |
| S12 | Frozen | Supermarket | Sepiella spp. | Sepiella inermis | ≥40 |
| S13 | Frozen | Supermarket | Sepia pharaonis | Sepia aculeata | ≥40 |
| S14 | Thawed | Supermarket | Sepia officinalis | Sepia officinalis | 14.03 ± 0.28 |
| S15 | Grilled | Restaurant | “Sepia” | Sepia bertheloti | 26.08 ± 0.11 |
| S16 | Thawed | Supermarket | Sepia officinalis | Sepia officinalis | 13.43 ± 0.06 |
| S17 | Canned | Supermarket | “Sepia” | Sepia pharaonis | 23.62 ± 0.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, A.; Ramilo-Fernández, G.; Sotelo, C.G. A Real-Time PCR Method for the Authentication of Common Cuttlefish (Sepia officinalis) in Food Products. Foods 2020, 9, 286. https://doi.org/10.3390/foods9030286
Velasco A, Ramilo-Fernández G, Sotelo CG. A Real-Time PCR Method for the Authentication of Common Cuttlefish (Sepia officinalis) in Food Products. Foods. 2020; 9(3):286. https://doi.org/10.3390/foods9030286
Chicago/Turabian StyleVelasco, Amaya, Graciela Ramilo-Fernández, and Carmen G. Sotelo. 2020. "A Real-Time PCR Method for the Authentication of Common Cuttlefish (Sepia officinalis) in Food Products" Foods 9, no. 3: 286. https://doi.org/10.3390/foods9030286