Physicochemical, Pasting, and Thermal Properties of Native Corn Starch–Mung Bean Protein Isolate Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling Power, Water Absorption Capacity, and Solubility
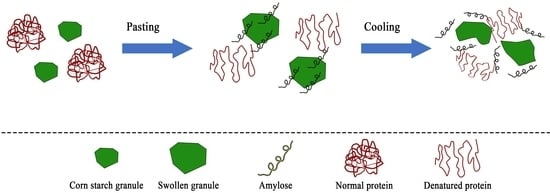
2.2. Pasting Properties
2.3. Thermal Properties
2.4. FT-IR Spectroscopy Analysis
2.5. Freeze-Thaw Stability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Isolation of Mung Bean Protein
4.3. Preparation of NCS/MBPI Blends
4.4. Swelling Power (SP), Water Absorption Capacity (WAC), and Solubility
4.5. Rapid Visco Analyzer (RVA) Measurements
4.6. Differential Scanning Calorimeter (DSC)
4.7. Fourier Transform Infrared (FT-IR) Spectroscopy
4.8. Evaluation of Freeze-Thaw Stability
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.-N.; Zhang, B.; Chen, B.; Chen, H.-Q. Effects of oligosaccharides on pasting, thermal and rheological properties of sweet potato starch. Food Chem. 2017, 230, 516–523. [Google Scholar] [CrossRef]
- Shams-Abadi, S.T.; Razavi, S.M.A. Cress seed gum improves rheological, textural and physicochemical properties of native wheat starch-sucrose mixture. Int. J. Biol. Macromol. 2021, 181, 945–955. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Han, Q.; Cao, C.; Liu, Q.; Kong, B. Short-term retrogradation behaviour of corn starch is inhibited by the addition of porcine plasma protein hydrolysates. Int. J. Biol. Macromol. 2018, 115, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jiang, L.; Wang, W.; Xiao, Y.; Liu, S.; Luo, Y.; Shen, M.; Xie, J. Effects of Mesona chinensis Benth polysaccharide on physicochemical and rheological properties of sweet potato starch and its interactions. Food Hydrocoll. 2020, 99, 105371. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Singh, A.; Geveke, D.J.; Yadav, M.P. Improvement of rheological, thermal and functional properties of tapioca starch by using gum arabic. LWT 2017, 80, 155–162. [Google Scholar] [CrossRef]
- Narciso, J.O.; Brennan, C. Whey and Pea Protein Fortification of Rice Starches: Effects on Protein and Starch Digestibility and Starch Pasting Properties. Starch-Stärke 2018, 70, 1700315. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, F.; Goff, H.D.; Li, Y. Study on starch-protein interactions and their effects on physicochemical and digestible properties of the blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Yadav, B.S.; Yadav, R.B.; Dangi, N. Assessing the influence of lentil protein concentrate on pasting and rheological properties of barley starch. J. Food Meas. Charact. 2020, 14, 1623–1633. [Google Scholar] [CrossRef]
- Zheng, M.; Xiao, Y.; Yang, S.; Liu, M.; Feng, L.; Ren, Y.; Yang, X.; Lin, N.; Liu, J. Effect of adding zein, soy protein isolate and whey protein isolate on the physicochemical and in vitro digestion of proso millet starch. Int. J. Food Sci. Technol. 2020, 55, 776–784. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Zhao, T.; Gong, T.; Zou, L.; Guo, Y. Young apple polyphenols postpone starch digestion in vitro and in vivo. J. Funct. Foods 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Kong, X.-R.; Zhu, Z.-Y.; Zhang, X.-J.; Zhu, Y.-M. Effects of Cordyceps polysaccharides on pasting properties and in vitro starch digestibility of wheat starch. Food Hydrocoll. 2020, 102, 105604. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Fang, Y.; Zhang, W.; Zhang, B. DSC study on the thermal properties of soybean protein isolates/corn starch mixture. J. Therm. Anal. 2014, 115, 1633–1638. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Zhang, B.; Fu, X.; Li, L.; Huang, Q. Structure, physicochemical and in vitro digestion properties of ternary blends containing swollen maize starch, maize oil and zein protein. Food Hydrocoll. 2018, 76, 88–95. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.; Zheng, H.; Brennan, C. The effects of dairy ingredients on the pasting, textural, rheological, freeze-thaw properties and swelling behaviour of oat starch. Food Chem. 2018, 245, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Aldred, P.; Panozzo, J.; Kasapis, S.; Adhikari, B. Rheological and microstructural characteristics of lentil starch–lentil protein composite pastes and gels. Food Hydrocoll. 2014, 35, 226–237. [Google Scholar] [CrossRef]
- Ribotta, P.D.; Colombo, A.; León, A.E.; Añón, M.C. Effects of Soy Protein on Physical and Rheological Properties of Wheat Starch. Starch-Stärke 2007, 59, 614–623. [Google Scholar] [CrossRef]
- Jekle, M.; Mühlberger, K.; Becker, T. Starch–gluten interactions during gelatinization and its functionality in dough like model systems. Food Hydrocoll. 2016, 54, 196–201. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, H.; Ai, L.; Xiong, W. Insight into protein-starch ratio on the gelatinization and retrogradation characteristics of reconstituted rice flour. Int. J. Biol. Macromol. 2020, 146, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-H.; Donner, E.; Yada, R.Y.; Liu, Q. Physicochemical properties and in vitro starch digestibility of potato starch/protein blends. Carbohydr. Polym. 2016, 154, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-chemical and functional properties of legume protein, starch, and dietary fiber—A review. Legume Sci. 2022, 4, e117. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ariahu, C.C.; Abu, J.O. Chemical composition, functional and pasting properties of cassava starch and soy protein concentrate blends. J. Food Sci. Technol. 2013, 50, 1179–1185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Qiao, D.; Zhao, S.; Lin, Q.; Wang, J.; Xie, F. Starch-based food matrices containing protein: Recent understanding of morphology, structure, and properties. Trends Food Sci. Technol. 2021, 114, 212–231. [Google Scholar] [CrossRef]
- Sehrawat, N.; Yadav, M.; Kumar, S.; Upadhyay, S.K.; Singh, M.; Sharma, A.K. Review on health promoting biological activities of mungbean: A potent functional food of medicinal importance. Plant Arch. 2020, 20, 2969–2975. [Google Scholar]
- Yi-Shen, Z.; Shuai, S.; FitzGerald, R. Mung bean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res. 2018, 62, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branch, S.; Maria, S. Evaluation of the functional properties of mung bean protein isolate for development of textured vegetable protein. Int. Food Res. J. 2017, 24, 1595–1605. [Google Scholar]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients 2019, 11, 1238. [Google Scholar] [CrossRef] [Green Version]
- El-Adawy, T.A. Functional properties and nutritional quality of acetylated and succinylated mung bean protein isolate. Food Chem. 2000, 70, 83–91. [Google Scholar] [CrossRef]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Tarahi, M.; Hedayati, S.; Shahidi, F. Effects of Mung Bean (Vigna radiata) Protein Isolate on Rheological, Textural, and Structural Properties of Native Corn Starch. Polymers 2022, 14, 3012. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.; Niakousari, M. Microstructure, pasting and textural properties of wheat starch-corn starch citrate composites. Food Hydrocoll. 2018, 81, 1–5. [Google Scholar] [CrossRef]
- Sun, Q.; Xiong, C.S.L. Functional and pasting properties of pea starch and peanut protein isolate blends. Carbohydr. Polym. 2014, 101, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cheng, J.; Lin, Q.; Wang, Q.; Wang, J.; Yu, G. Effects of endogenous proteins and lipids on structural, thermal, rheological, and pasting properties and digestibility of adlay seed (Coix lacryma-jobi L.) starch. Food Hydrocoll. 2021, 111, 106254. [Google Scholar] [CrossRef]
- Ribotta, P.D.; Colombo, A.; Rosell, C.M. Enzymatic modifications of pea protein and its application in protein–cassava and corn starch gels. Food Hydrocoll. 2012, 27, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lai, R.; Wang, X.; Wang, H.; Liu, Y. Preparation and Characterization of Composites of Hydroxypropyl Tapioca Starch and Zein. Starch-Stärke 2020, 72, 1900204. [Google Scholar] [CrossRef]
- Bejosano, F.P.; Corke, H. Effect of Amaranthus and buckwheat proteins on the rheological properties of maize starch. Food Chem. 1999, 65, 493–501. [Google Scholar] [CrossRef]
- Colombo, A.; Ribotta, P.D.; León, A.E. Thermal and Rheological Behavior of Peanut Protein Concentrate and Starch Composites. J. Am. Oil Chem. Soc. 2014, 91, 1911–1920. [Google Scholar] [CrossRef]
- Colombo, A.; León, A.E.; Ribotta, P.D. Rheological and calorimetric properties of corn-, wheat-, and cassava-starches and soybean protein concentrate composites. Starch-Stärke 2011, 63, 83–95. [Google Scholar] [CrossRef]
- Hedayati, S.; Shahidi, F.; Majzoobi, M.; Koocheki, A.; Farahnaky, A. Structural, rheological, pasting and textural properties of granular cold water swelling maize starch: Effect of NaCl and CaCl2. Carbohydr. Polym. 2020, 242, 116406. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; King, J. Pasting and Crystalline Property Differences of Commercial and Isolated Rice Starch with Added Amino Acids. J. Food Sci. 2003, 68, 832–838. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Moghadam, M.; Amirsalehi, A.; Emam-Djomeh, Z. Mung bean protein as a promising biopolymeric vehicle for loading of curcumin: Structural characterization, antioxidant properties, and in vitro release kinetics. J. Drug Deliv. Sci. Technol. 2021, 61, 102148. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Anbarani, N.M.; Razavi, S.M.A.; Taghizadeh, M. Impact of sage seed gum and whey protein concentrate on the functional properties and retrogradation behavior of native wheat starch gel. Food Hydrocoll. 2021, 111, 106261. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, B.; Li, M.-N.; Xie, Y.; Chen, H.-Q. Effects of glutenin and gliadin modified by protein-glutaminase on pasting, rheological properties and microstructure of potato starch. Food Chem. 2018, 253, 148–155. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Tarahi, M.; Shahidi, F.; Hedayati, S. A novel starch from bitter vetch (Vicia ervilia) seeds: A comparison of its physicochemical, structural, thermal, rheological, and pasting properties with conventional starches. Int. J. Food Sci. Technol. 2022, 57, 6833–6842. [Google Scholar] [CrossRef]
- Hoover, R.; Senanayake, S. Composition and physicochemical properties of oat starches. Food Res. Int. 1996, 29, 15–26. [Google Scholar] [CrossRef]



| MBPI (%) | Pasting Temperature (°C) | Viscosity (cP) | |||
|---|---|---|---|---|---|
| Peak | Breakdown | Setback | Final | ||
| 0 | 77.98 ± 0.02 a | 4691 ± 17 e | 1992 ± 20 e | 3063 ± 18 a | 5762 ± 15 a |
| 2 | 77.06 ± 0.03 b | 5002 ± 14 d | 2397 ± 25 d | 2738 ± 37 b | 5343 ± 25 b |
| 4 | 77.04 ± 0.01 b | 5282 ± 22 c | 2685 ± 22 c | 2612 ± 18 c | 5209 ± 13 c |
| 6 | 76.86 ± 0.06 c | 5578 ± 10 b | 3049 ± 23 b | 2475 ± 44 d | 5004 ± 12 d |
| 8 | 76.53 ± 0.06 d | 5648 ± 13 a | 3173 ± 19 a | 2400 ± 37 e | 4875 ± 28 e |
| MBPI (%) | Thermal Parameters | |||
|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | |
| 0 | 69.69 ± 0.42 e | 73.45 ± 0.40 e | 77.75 ± 0.35 d | 10.85 ± 0.23 a |
| 2 | 70.43 ± 0.12 d | 74.71 ± 0.19 d | 80.78 ± 0.20 c | 9.77 ± 0.07 b |
| 4 | 71.24 ± 0.12 c | 75.36 ± 0.24 c | 81.17 ± 0.17 bc | 9.54 ± 0.11 b |
| 6 | 71.69 ± 0.14 b | 76.10 ± 0.16 b | 81.43 ± 0.19 b | 9.11 ± 0.09 c |
| 8 | 72.21 ± 0.20 a | 76.72 ± 0.15 a | 82.26 ± 0.24 a | 8.79 ± 0.15 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarahi, M.; Shahidi, F.; Hedayati, S. Physicochemical, Pasting, and Thermal Properties of Native Corn Starch–Mung Bean Protein Isolate Composites. Gels 2022, 8, 693. https://doi.org/10.3390/gels8110693
Tarahi M, Shahidi F, Hedayati S. Physicochemical, Pasting, and Thermal Properties of Native Corn Starch–Mung Bean Protein Isolate Composites. Gels. 2022; 8(11):693. https://doi.org/10.3390/gels8110693
Chicago/Turabian StyleTarahi, Mohammad, Fakhri Shahidi, and Sara Hedayati. 2022. "Physicochemical, Pasting, and Thermal Properties of Native Corn Starch–Mung Bean Protein Isolate Composites" Gels 8, no. 11: 693. https://doi.org/10.3390/gels8110693