Non-Deep Physiological Dormancy in Seed and Germination Requirements of Lysimachia coreana Nakai
Abstract
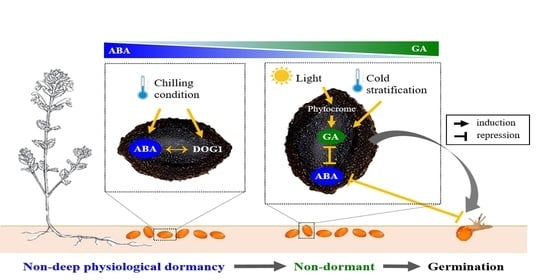
:1. Introduction
2. Materials and Methods
2.1. Seed Material and Collection
2.2. Morphological Characteristics of the Seeds
2.3. Effects of Temperature Treatment on Seed Germination
2.4. Effects of GA3 Treatment and Temperature Treatment on Germination
2.5. Effects of Warm and/or Cold Stratification Periods on Germination
2.6. Germination Characteristics Analysis
2.7. Statistical Analyses
3. Results
3.1. Morphological Characteristics of Seeds
3.2. Effects of Temperature Treatment on Seed Germination
3.3. Effects of GA3 Treatment and Temperature Treatment on Germination
3.4. Effects of Warm and/or Cold Stratification Periods on Germination
3.5. Comparison of Seed Germination According to Ga3 and Stratification under Optimal Germination Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Kang, H.; Yang, W. Climate change-induced water stress suppresses the regeneration of the critically endangered forest tree Nyssa yunnanensis. PLoS ONE 2017, 12, e0182012. [Google Scholar] [CrossRef] [PubMed]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil. 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Cochrane, A.M.; Daws, M.I.; Hay, F.R. Seed-based approach for identifying flora at risk from climate warming. Austral Ecol. 2011, 36, 923–935. [Google Scholar] [CrossRef]
- Carón, M.M.; De Frenne, P.; Brunet, J.; Chabrerie, O.; Cousins, S.A.O. Interacting effects of warming and drought on regeneration and early growth of Acer pseudoplatanus and A. platanoides. Plant Biol. 2015, 17, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, V.; Barbera, A.C.; Maucieri, C.; Gimma, G.; Scalisi, C.; Patanè, C. Evaluation of variability to drought and saline stress through the germination of different ecotypes of carob (Ceratonia siliqua L.) using a hydrotime model. Ecol. Eng. 2016, 95, 557–566. [Google Scholar] [CrossRef]
- Kim, Y.R.; Tae, K.H.; Sim, J.K.; Ko, S.C. A palynotaxonomic study on the genus Lysimachia in Korea. Korean J. Plant Taxon. 1993, 23, 43–56. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The control of seed dormancy and germination by temperature, light and nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Pawłowski, T.A. Proteomic approach to analyze dormancy breaking of tree seeds. Plant Mol. Biol. 2010, 73, 15–25. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Hu, X.W.; Huang, X.W.; Wang, Y.R. Hormonal and temperature regulation of seed dormancy and germination in Leymus chinensis. Plant Growth Regul. 2012, 67, 199–207. [Google Scholar] [CrossRef]
- Baskin, C.C.; Meyer, S.E.; Baskin, J.M. Two types of morphophysiological dormancy in seeds of two genera (Osmorhiza and Erythronium) with an arcto-tertiary distribution pattern. Am. J. Bot. 1995, 82, 293–298. [Google Scholar] [CrossRef]
- Chen, D.L.; Luo, X.P.; Yuan, Z.; Bai, M.J.; Hu, X.W. Seed dormancy release of Halenia elliptica in response to stratification temperature, duration and soil moisture content. BMC Plant Biol. 2020, 20. [Google Scholar] [CrossRef]
- Stokes, P. Temperature and seed dormancy. In Encyclopedia of Plant Physiology, 3rd ed.; Lang, A., Ed.; Differenzierung und Entwicklung/Differentiation and Development. Handbuch der Pflanzenphysiologie/Encyclopedia of Plant Physiology; Springer: Berlin, Germany, 1965; Volume 15, pp. 2393–2450. [Google Scholar]
- Cuena-Lombraña, A.; Porceddu, M.; Dettori, C.A.; Bacchetta, G. Discovering the type of seed dormancy and temperature requirements for seed germination of Gentiana lutea L. subsp. lutea (Gentianaceae). J. Plant Ecol. 2018, 11, 308–316. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.D.; Kim, S.D.; Kim, H.H.; Kim, J.H.; Lee, J.W.; Yun, T.; Lee, C.H. Effects of storage condition, growth regulator, and inorganic salt on the germination of Lysimachia davurica. Kor. J. Hort. Sci. Technol. 2003, 21, 34–38. [Google Scholar]
- Dillon, K.; Reichard, S.H. Effect of Temperature on the Seed Germination of Garden Loosestrife (Lysimachia vulgaris L.). Nat. Areas J. 2014, 34, 212–215. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; pp. 10–11. [Google Scholar]
- Royal Botanic Gardens, Kew. Millennium Seed Bank Partnership. Available online: https://www.kew.org/msbp (accessed on 22 April 2019).
- Vandelook, F.; Bolle, N.; Van Assche, J.A. Seed dormancy and germination of the European Chaerophyllum temulum (Apiaceae), a member of a trans-atlantic genus. Ann. Bot. 2007, 100, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Seed Analysts (AOSA). Rules for testing seeds. J. Seed Technol. 1990, 12, 1–112. [Google Scholar]
- Soltani, E.; Ghaderi-Farlias, F.; Baskin, C.C.; Baskin, J.M. Problems with using mean germination time to calculate rate of seed germination. Aust. J. Bot. 2015, 63, 631–635. [Google Scholar] [CrossRef]
- Scott, S.J.; Jones, R.A.; Williams, W.A. Review of data analysis methods for seed germination. Crop Sci. 1984, 24, 1160–1162. [Google Scholar] [CrossRef]
- Basserdorf, C.H. International Rules for Seed Testing, 3rd ed.; International Seed Testing Association, ISTA: Wallisellen, Switzerland, 2010. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2021-2. 2021. Available online: https://www.iucnredlist.org (accessed on 13 July 2021).
- Phartyal, S.S.; Kondo, T.; Hoshino, Y.; Baskin, C.C.; Baskin, J.M. Morphological dormancy in seeds of the autumn-germinating shrub Lonicera caerulea var. emphyllocalyx (Caprifoliaceae). Plant Species Biol. 2009, 24, 20–26. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, ecology, and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Oh, H.J.; Shin, U.S.; Lee, S.Y.; Kim, S.Y.; Jeong, M.J. Non-deep physiological dormancy in seeds of Euphorbia jolkinii Boiss. Native to korea. J. Ecol. Environ. 2021. [CrossRef]
- Nikolaeva, M.G. Factors controlling the seed dormancy pattern. In The Physiology and Biochemistry of Seed Dormancy and Germination, 2nd ed.; Khan, A.A., Ed.; North-Holland Publishing: Amsterdam, The Netherlands, 1977; pp. 51–74. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. The natural history of soil seed banks of arable land. Weed Sci. 2006, 54, 549–557. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of non-deep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef]
- Baskin, J.M.; Nan, X.; Baskin, C.C. A comparative study of seed dormancy and germination in an annual and perennial species of Senna (Fabaceae). Seed Sci. Res. 1998, 8, 501–512. [Google Scholar] [CrossRef]
- George, D.W. High-temperature seed dormancy in wheat (Triticum aestivum L.). Crop Sci. 1967, 7, 249–253. [Google Scholar] [CrossRef]
- Olsson, G.; Mattson, B. Seed dormancy in wheat under different weather conditions. Cereal Res. Commun. 1976, 4, 181–185. [Google Scholar]
- Strand, E. A seed dormancy index for selection of cereal cultivars resistant to preharvest sprouting. Cereal Res. Commun. 1980, 8, 219–223. [Google Scholar]
- Vegis, A. Dormancy in higher plants. Ann. Rev. Plant Physiol. 1964, 15, 185–224. [Google Scholar] [CrossRef]
- Weisner, L.E.; Grabe, D.F. Effect of temperature preconditioning and cultivar on ryegrass (Lolium sp.) seed dormancy. Crop Sci. 1972, 12, 760–764. [Google Scholar] [CrossRef]
- Nyachiro, J.M.; Clarke, F.R.; DePauw, R.M.; Knox, R.E.; Armstrong, K.C. Temperature effects on seed germination and expression of seed dormancy in wheat. Euphytica 2002, 126, 123–127. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Germination ecophysiology of herbaceous plant species in a temperate region. Am. J. Bot. 1988, 75, 286–305. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds, 3rd ed.; Cambridge University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Poschlod, P.; Abedi, M.; Bartelheimer, M.; Drobnik, J.; Rosbakh, S.; Saatkamp, A. Seed ecology and assembly rules in plant communities. In Vegetation Ecology, 2nd ed.; Maarel, E.V., Franklin, J., Eds.; John Wiley & Sons: Chichester, UK, 2013; pp. 164–202. [Google Scholar]
- Rosbakh, S.; Poschlod, P. Initial temperature of seed germination as related to species occurrence along a temperature gradient. Funct. Ecol. 2015, 29, 5–14. [Google Scholar] [CrossRef]
- Kondo, T.; Mikubo, M.; Yamada, K.; Walck, J.L.; Hidayati, S.N. Seed dormancy in Trillium camschatcense (Melanthiaceae) and the possible roles of light and temperature requirements for seed germination in forests. Am. J. Bot. 2011, 98, 215–226. [Google Scholar] [CrossRef]
- Belderrok, B. Seed dormancy problems in cereals. Field Crop Abstr. 1968, 21, 203–211. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 3rd ed.; Academic/Elsevier: San Diego, CA, USA, 2014. [Google Scholar]
- Corbineau, F.; Bianco, J.; Garello, G.; Come, D. Breakage of Pseudotsuga menziesii seed dormancy by cold treatment as related to changes in seed ABA sensitivity and ABA levels. Physiol. Plant 2002, 114, 313–319. [Google Scholar] [CrossRef]
- Bianco, J.; Garello, G.; Le Page-Degivry, M.T. Release of dormancy in sunflower embryos by dry storage: Involvement of gibberellins and abscisic acid. Seed Sci Res. 1994, 4, 57–62. [Google Scholar] [CrossRef]
- Wang, M.; Heimovaara-Dijkstra, S.; Van Duijn, B. Modulation of germination of embryos isolated from dormant and non-dormant barley grains by manipulation of endogenous abscisic acid. Planta 1995, 195, 586–592. [Google Scholar] [CrossRef]
- Le Page-Degivry, M.T.; Bianco, J.; Barthe, P.; Garello, G. Changes in hormone sensitivity in relation to onset and breaking of sunflower embryo dormancy. In Plant Dormancy: Physiology, Biochemistry and Molecular Biology; CAB International: Oxon, UK, 1996; pp. 221–231. [Google Scholar]
- Wang, M. The role of abscisic acid in the regulation of barley grain germination. Seed Sci. Technol. 1996, 25, 67–74. [Google Scholar]
- Yoshioka, T.; Endo, T.; Satoh, S. Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol. 1998, 39, 307–312. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates Inc: Sunderland, UK, 2012. [Google Scholar]
- Kim, H.M.; Kim, J.H.; Lee, D.H.; Jung, Y.H.; Park, C.Y.; Lee, M.H.; Kim, K.M.; Lee, J.H.; Na, C.S. Non-deep simple morphophysiological dormancy and germination characteristics of Gentiana triflora var. japonica (Kusn.) H. Hara (Gentianaceae), a rare perennial herb in Korea. Plants 2021, 10, 1979. [Google Scholar] [CrossRef]
- Yang, L.E.; Peng, D.L.; Li, Z.M.; Huang, L.; Yang, J.; Sun, H. cold stratification, temperature, light, GA3, and KNO3 effects on seed germination of Primula beesiana from Yunnan, China. Plant Divers. 2020, 42, 168–173. [Google Scholar] [CrossRef]
- González-López, Ó.; Casquero, P.A. Effects of GA3 pregerminative treatment on Gentiana lutea L. var. aurantiaca germination and seedlings morphology. Sci. World J. 2014, 2014, 751279. [Google Scholar]
- Naseri, B.; Tabari, M.; Phartyal, S.S.; Abedi, M. Deep physiological dormancy in seeds of Balkan maple (Acer hyrcanum): A rare tree in the Hyrcanian Mountain forests of Iran. Seed Sci. Technol. 2018, 46, 473–482. [Google Scholar] [CrossRef]
- Kim, H.J.; Na, H. Seed dormancy and germination in Oenanthe stolonifera as affected by temperature and gibberellic acid. Hortic. Environ. Biotechnol. 2021, 62, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Sun, D.L.; He, M. Optimum germination conditions of Lysimachia davurica seeds. J. Northeast For. Univ. 2014, 42, 27–29. [Google Scholar]
- Lee, S.Y.; Rhie, Y.H.; Kim, K.S. Dormancy breaking and germination requirements of seeds of Thalictrum uchiyamae (Ranunculaceae) with underdeveloped embryos. Sci. Hortic. 2018, 231, 82–88. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Lu, J.J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Seed germination ecology of the cold desert annual Isatis violascens (brassicaceae): Two levels of physiological dormancy and role of the pericarp. PLoS ONE 2015, 10, e0140983. [Google Scholar] [CrossRef]
- Moncaleano-Escandon, J.; Silva, B.C.F.; Silva, S.R.S.; Granja, J.A.A.; Alves, M.C.J.L.; Pompelli, M.F. Germination responses of Jatropha curcas L. seeds to storage and aging. Ind. Crops Prod. 2013, 44, 684–690. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ogawa, M.; Kuwahara, A.; Hanada, A.; Kamiya, Y.; Yamaguchi, S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 2004, 16, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H. Practical methods for rapid seed germination from seed coat-imposed dormancy of Prunus yedoensis. Sci. Hortic. 2019, 243, 451–456. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S.Y.; Rhie, Y.H.; Lee, J.H.; Kim, S.Y.; Lee, K.C. Seed dormancy type and germination characteristics in Tiarella polyphylla D. Don native to Korea. Korean J. Plant Res. 2018, 31, 363–371. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Sul, J.H.; Cho, K.H. Effect of preservation period, light, temperature and priming on the seed germination of Lysimachia mauritiana. Kor. J. Env. Eco. 1998, 12, 9–13. [Google Scholar]
- Norbert, L.; Stefanie, K.; Andrea, J. ISTA Working Sheets on Tetrazolium Testing, 3rd ed.; International Seed Testing Association-ISTA: Zurich, Switzerland, 2003; p. 171. [Google Scholar]



| T (°C) | GA3 (mg·L−1) | GR (%) | T50 (Days) | GI |
|---|---|---|---|---|
| 25 | Control | 4.0 ± 2.5 h | 10.3 ± 3.0 ns | 0.4 ± 0.3 d |
| 100 | 38.0 ± 6.6 def | 19.5 ± 6.4 ns | 6.9 ± 1.9 abcd | |
| 250 | 66.0 ± 4.0 abc | 10.8 ± 1.6 ns | 8.1 ± 1.1 abcd | |
| 500 | 30.0 ± 12.3 defg | 12.6 ± 1.1 ns | 3.7 ± 1.4 bcd | |
| 20 | Control | 2.0 ± 2.0 h | - | 0.2 ± 0.2 d |
| 100 | 24.0 ± 5.1 efgh | 16.7 ± 3.3 ns | 5.0 ± 1.5 bcd | |
| 250 | 52.0 ± 2.0 bcd | 20.2 ± 4.8 ns | 10.8 ± 1.6 abcd | |
| 500 | 48.0 ± 5.8 cde | 20.0 ± 0.9 ns | 9.9 ± 1.6 abcd | |
| 15 | Control | 0.0 ± 0.0 h | - | - |
| 100 | 16.0 ± 8.1 fgh | 27.5 ± 0.9 ns | 4.4 ± 2.3 abcd | |
| 250 | 36.0 ± 9.8 defg | 24.0 ± 1.5 ns | 8.8 ± 2.5 abcd | |
| 500 | 48.0 ± 3.7 cde | 20.5 ± 1.8 ns | 11.5 ± 0.8 abc | |
| 25/15 | Control | 14.0 ± 4.0 fgh | 16.1 ± 2.3 ns | 2.6 ± 0.8 bcd |
| 100 | 32.0 ± 2.0 defg | 28.4 ± 2.5 ns | 8.5 ± 0.8 abcd | |
| 250 | 82.0 ± 3.7 a | 13.3 ± 0.9 ns | 13.3 ± 1.1 ab | |
| 500 | 12.0 ± 3.7 gh | 18.8 ± 3.2 ns | 2.2 ± 0.7 cd | |
| 20/10 | Control | 2.0 ± 2.0 h | - | 0.7 ± 0.7 d |
| 100 | 32.0 ± 2.0 defg | 28.4 ± 2.5 ns | 8.5 ± 0.8 abcd | |
| 250 | 76.0 ± 5.1 ab | 20.9 ± 0.9 ns | 16.3 ± 0.8 a | |
| 500 | 16.0 ± 5.1 fgh | 21.5 ± 4.9 ns | 4.2 ± 1.6 bcd | |
| 5 | Control | 0.0 ± 0.0 h | - | - |
| 100 | 0.0 ± 0.0 h | - | - | |
| 250 | 0.0 ± 0.0 h | - | - | |
| 500 | 2.0 ± 2.0 h | 36.0 ± 0.0 ns | 0.7 ± 0.7 d | |
| p-value | T 1 | ∗∗ | ∗∗∗ | ∗ |
| GA3 | ∗∗∗ | ∗∗∗ | ∗∗∗ | |
| T × GA3 | ∗∗∗ | ns | ∗∗∗ |
| T (°C) | S (week) | GR (%) | T50 (days) | GI |
|---|---|---|---|---|
| 25 | CS-6 | 16.0 ± 6.8 cd | 2.4 ± 0.4 a | 0.4 ± 0.2 b |
| CS-9 | 42.0 ± 8.6 abcd | 2.9 ± 0.2 ab | 1.4 ± 0.3 ab | |
| 20 | CS-6 | 32.0 ± 3.7 abcd | 4.4 ± 0.7 ab | 2.4 ± 0.7 ab |
| CS-9 | 52.0 ± 5.6 ab | 3.7 ± 0.5 ab | 2.5 ± 0.6 ab | |
| 15 | CS-6 | 12.0 ± 3.7 d | 3.5 ± 0.4 ab | 0.5 ± 0.2 b |
| CS-9 | 22.0 ± 3.7 bcd | 3.8 ± 0.3 ab | 0.9 ± 0.2 b | |
| 25/15 | CS-6 | 46.0 ± 9.3 abc | 4.4 ± 0.5 ab | 2.5 ± 0.7 ab |
| CS-9 | 56.0 ± 4.0 a | 3.9 ± 0.4 ab | 3.0 ± 0.6 ab | |
| 20/10 | CS-6 | 36.0 ± 2.5 abcd | 8.1 ± 0.4 bc | 3.6 ± 0.6 ab |
| CS-9 | 48.0 ± 8.6 ab | 3.8 ± 0.9 ab | 2.4 ± 0.7 ab | |
| 5 | CS-6 | 24.0 ± 5.1 bcd | 24.0 ± 1.0 d | 5.8 ± 1.3 a |
| CS-9 | 16.0 ± 6.0 cd | 9.9 ± 2.3 c | 1.6 ± 0.7 ab | |
| p-value | T 1 | ∗∗∗ | ∗∗∗ | ∗∗∗ |
| S 2 | ∗∗∗ | ∗∗∗ | Ns | |
| T × S | ns | ∗∗∗ | ∗∗ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.G.; Im, J.H.; Kwak, M.J.; Park, C.H.; Lee, M.H.; Na, C.S.; Woo, S.Y. Non-Deep Physiological Dormancy in Seed and Germination Requirements of Lysimachia coreana Nakai. Horticulturae 2021, 7, 490. https://doi.org/10.3390/horticulturae7110490
Baek SG, Im JH, Kwak MJ, Park CH, Lee MH, Na CS, Woo SY. Non-Deep Physiological Dormancy in Seed and Germination Requirements of Lysimachia coreana Nakai. Horticulturae. 2021; 7(11):490. https://doi.org/10.3390/horticulturae7110490
Chicago/Turabian StyleBaek, Saeng Geul, Jin Hyun Im, Myeong Ja Kwak, Cho Hee Park, Mi Hyun Lee, Chae Sun Na, and Su Young Woo. 2021. "Non-Deep Physiological Dormancy in Seed and Germination Requirements of Lysimachia coreana Nakai" Horticulturae 7, no. 11: 490. https://doi.org/10.3390/horticulturae7110490