The Spatial Distribution and Morphological Characteristics of Chum Salmon (Oncorhynchus keta) in South Korea
Abstract
:1. Introduction
2. Materials and Methods
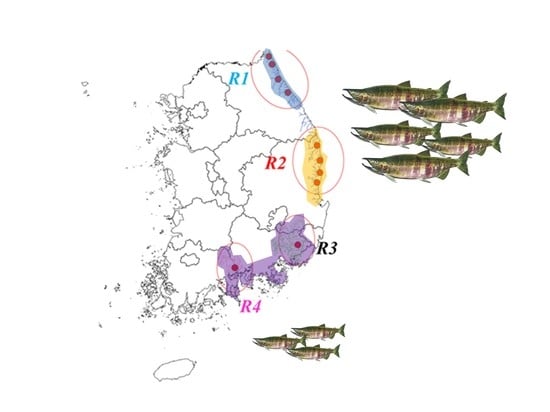
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Morphological Characteristic Differences
3.2. Regional Sex Ratio Differences
3.3. Correlation of Distribution with Water Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Region | St. Number | Year | Catch | The Number of Female | Female Ratio |
|---|---|---|---|---|---|
| R1 | St. 1 | 2014 | 2214 | 919 | 0.4151 |
| R1 | St. 1 | 2015 | 532 | 245 | 0.4605 |
| R1 | St. 1 | 2016 | 1564 | 831 | 0.5313 |
| R1 | St. 1 | 2018 | 1627 | 772 | 0.4745 |
| R1 | St. 1 | 2019 | 166 | 56 | 0.3373 |
| R1 | St. 2 | 2014 | 1638 | 718 | 0.4383 |
| R1 | St. 2 | 2016 | 1370 | 634 | 0.4628 |
| R1 | St. 2 | 2018 | 3430 | 1382 | 0.4029 |
| R1 | St. 2 | 2019 | 1057 | 364 | 0.3444 |
| R1 | St. 3 | 2014 | 31,833 | 15,261 | 0.4794 |
| R1 | St. 3 | 2015 | 18,151 | 9108 | 0.5018 |
| R1 | St. 3 | 2016 | 11,262 | 6342 | 0.5631 |
| R1 | St. 3 | 2018 | 6635 | 3010 | 0.4537 |
| R1 | St. 3 | 2019 | 2790 | 1208 | 0.433 |
| R1 | St. 4 | 2014 | 4011 | 2139 | 0.5333 |
| R1 | St. 4 | 2015 | 4249 | 2062 | 0.4853 |
| R1 | St. 4 | 2016 | 3462 | 1674 | 0.4835 |
| R1 | St. 4 | 2018 | 1971 | 876 | 0.4444 |
| R1 | St. 4 | 2019 | 457 | 110 | 0.2407 |
| Region | St. Number | Year | Catch | The Number of Female | Female Ratio |
|---|---|---|---|---|---|
| R2 | St. 6 | 2004 | 454 | 191 | 0.4229 |
| R2 | St. 6 | 2005 | 205 | 77 | 0.3756 |
| R2 | St. 6 | 2006 | 1221 | 534 | 0.4373 |
| R2 | St. 6 | 2007 | 1615 | 691 | 0.4279 |
| R2 | St. 6 | 2008 | 375 | 135 | 0.36 |
| R2 | St. 6 | 2009 | 706 | 266 | 0.3768 |
| R2 | St. 6 | 2010 | 1145 | 504 | 0.4402 |
| R2 | St. 6 | 2011 | 727 | 365 | 0.5021 |
| R2 | St. 6 | 2012 | 1286 | 541 | 0.4207 |
| R2 | St. 6 | 2013 | 1286 | 561 | 0.4362 |
| R2 | St. 6 | 2014 | 2091 | 881 | 0.4213 |
| R2 | St. 6 | 2015 | 1339 | 522 | 0.3898 |
| R2 | St. 6 | 2016 | 1077 | 488 | 0.4531 |
| R2 | St. 6 | 2017 | 1136 | 451 | 0.397 |
| R2 | St. 6 | 2018 | 442 | 145 | 0.3281 |
| R2 | St. 6 | 2019 | 881 | 391 | 0.4438 |
| R2 | St. 6 | 2020 | 1651 | 822 | 0.4979 |
| Region | St. Number | Year | Catch | The Number of Sex-Identified Individuals | Female Ratio |
|---|---|---|---|---|---|
| R3 | St. 9 | 2020 | 197 | 61/95 | 0.391 |
| Region | St. Number | Year | Catch | The Number of Female | Female Ratio |
|---|---|---|---|---|---|
| R4 | St. 10 | 2004 | 163 | 75 | 0.4601 |
| R4 | St. 10 | 2005 | 243 | 102 | 0.4198 |
| R4 | St. 10 | 2006 | 387 | 165 | 0.4264 |
| R4 | St. 10 | 2007 | 419 | 179 | 0.4272 |
| R4 | St. 10 | 2008 | 68 | 29 | 0.4265 |
| R4 | St. 10 | 2009 | 96 | 37 | 0.3854 |
| R4 | St. 10 | 2010 | 54 | 27 | 0.5 |
| R4 | St. 10 | 2011 | 59 | 30 | 0.5085 |
| R4 | St. 10 | 2012 | 79 | 36 | 0.4557 |
| R4 | St. 10 | 2013 | 162 | 57 | 0.3519 |
| R4 | St. 10 | 2014 | 188 | 61 | 0.3245 |
| R4 | St. 10 | 2015 | 208 | 48 | 0.2308 |
| R4 | St. 10 | 2016 | 124 | 36 | 0.2903 |
| R4 | St. 10 | 2017 | 265 | 88 | 0.3321 |
| R4 | St. 10 | 2018 | 704 | 239 | 0.3395 |
| R4 | St. 10 | 2019 | 692 | 316 | 0.4566 |
| R4 | St. 10 | 2020 | 569 | 244 | 0.4288 |
| Parameters | Regions | Average | Standard Deviation | Standard Error | n |
|---|---|---|---|---|---|
| pH | R1 | 8.95 | 0.46 | 0.15 | 9 |
| R2 | 7.83 | 0.36 | 0.07 | 24 | |
| R4 | 7.57 | 0.19 | 0.05 | 17 | |
| DO (mg/L) | R1 | 10.79 | 1.16 | 0.39 | 9 |
| R2 | 9.62 | 1.12 | 0.23 | 24 | |
| R4 | 9.28 | 1.19 | 0.29 | 17 | |
| BOD (mg/L) | R1 | 0.93 | 0.29 | 0.1 | 9 |
| R2 | 0.68 | 0.22 | 0.04 | 24 | |
| R4 | 0.75 | 0.19 | 0.05 | 17 | |
| TN (mg/L) | R1 | 1.25 | 0.36 | 0.12 | 9 |
| R2 | 1.3 | 0.96 | 0.196 | 24 | |
| R4 | 1.56 | 0.29 | 0.071 | 17 | |
| TP (mg/L) | R1 | 0.014 | 0.003 | 0.001 | 9 |
| R2 | 0.017 | 0.009 | 0.002 | 24 | |
| R4 | 0.043 | 0.012 | 0.003 | 17 | |
| Temperature (°C) | R1 | 15.64 | 1.78 | 0.595 | 9 |
| R2 | 17.77 | 1.87 | 0.38 | 24 | |
| R4 | 18.76 | 1.14 | 0.28 | 17 | |
| Conductivity (µS/cm) | R1 | 227.63 | 119.67 | 39.89 | 9 |
| R2 | 154.61 | 54.05 | 11.03 | 24 | |
| R4 | 4534.29 | 5326.83 | 1291.95 | 17 |
| Parameters | Source of Variance | Sum of Squares | df | Mean Square | F | Significance |
|---|---|---|---|---|---|---|
| pH | Between groups | 11.72 | 2 | 5.86 | 53.31 | <0.01 |
| Within group | 5.17 | 47 | 0.11 | |||
| Total | 16.89 | 49 | ||||
| DO (mg/L) | Between groups | 13.72 | 2 | 6.86 | 5.18 | <0.01 |
| Within group | 62.22 | 47 | 1.32 | |||
| Total | 75.95 | 49 | ||||
| BOD (mg/L) | Between groups | 0.40 | 2 | 0.20 | 3.98 | <0.05 |
| Within group | 2.37 | 47 | 0.05 | |||
| Total | 2.77 | 49 | ||||
| TN (mg/L) | Between groups | 0.85 | 2 | 0.42 | 0.85 | 0.44 |
| Within group | 23.55 | 47 | 0.50 | |||
| Total | 24.39 | 49 | ||||
| TP (mg/L) | Between groups | 0.008 | 2 | 0.004 | 47.13 | <0.01 |
| Within group | 0.004 | 47 | <0.001 | |||
| Total | 0.012 | 49 | ||||
| Temperature (°C) | Between groups | 56.14 | 2 | 28.07 | 10.44 | <0.01 |
| Within group | 126.40 | 47 | 2.69 | |||
| Total | 182.54 | 49 | ||||
| Conductivity (µS/cm) | Between groups | 2.13 × 109 | 2 | 1.07 × 109 | 11.04 | <0.01 |
| Within group | 4.54 × 109 | 47 | 9.66 × 107 | |||
| Total | 6.67 × 108 | 49 |
References
- Jobling, M.; Arnesen, A.M.; Benfey, T.; Carter, C.; Hardy, R.; Le Francois, N.R.; O’Keefe, R.; Koskela, J.; Lamarre, S.G. The salmonids (family: Salmonidae). In Finfish Aquaculture Diversification, 1st ed.; Le Francois, N., Jobling, M., Eds.; MPG Books Group: London, UK, 2010; p. 234. [Google Scholar]
- Norden, C.R. Comparative osteology of representative salmonid fishes, with particular reference to the grayling (Thymallus arcticus) and its phylogeny. J. Fish. Res. Board Can. 1961, 18, 679–791. [Google Scholar] [CrossRef]
- Groot, C.; Margolis, L. Pacific Salmon Life Histories, 1st ed.; UBCPress: Vancouver, BC, Canada, 1991; pp. 234–237. [Google Scholar]
- Bilby, R.; Beach, E.W.; Fransen, B.R.; Walter, J.K.; Bisson, P.A. Transfer of Nutrients from Spawning Salmon to Riparian Vegetation in Western Washington. Trans. Am. Fish. Soc. 2003, 132, 733–745. [Google Scholar] [CrossRef]
- Kumala, S.; Haapasaari, P.; Karjalaien, T.P.; Kuikka, S.; Pakarinen, T.; Romakkaniemi, A.; Vuorinen, P.J. Ecosystem services provided by Baltic salmon- a regional perspective to the socio-economic benefits associated with a keystone migratory species. In Socio-Economic Importance of Ecosystem Services in the Nordic Countries-Scoping Assessment in the Context of The Economics of Ecosystems and Biodiversity (TEEB); Kettunen, M., Koljonen, M.-L., Kallio-Nyberg, I., Förster, J., Eds.; Nordic Council of Ministers: Copenhagen, Denmark, 2012; pp. 266–276. [Google Scholar]
- Barton, L.H. Tanana River, Alaska, Fall Chum Salmon Radio Telemetry Study. In Fishery Research Bulletin; Gunstrom, G.K., Fried, S.M., Buklis, L.S., Schmidt, D.C., Eds.; Alaska Department of Fish and Game Devision of Commercial Fisheries: Juneau, Alaska, 1992; Volume 92-01, pp. 1–22. [Google Scholar]
- Seeb, L.W.; Crane, P.A.; Kondzela, C.M.; Wilmot, R.L.; Urawa, S.; Varnavskaya, N.V.; Seeb, J.E. Migration of pacific rim chum salmon on the high seas: Insight from genetic data. Environ. Biol. Fishes 2004, 69, 21–36. [Google Scholar] [CrossRef]
- Esteve, M. Observations of spawning behavior in Salmoninae: Salmo, Oncorhynchus and Salvelinus. Rev. Fish Biol. 2005, 15, 1–21. [Google Scholar] [CrossRef]
- Urawa, S.; Sato, S.; Crane, P.A.; Agler, B.; Josephson, R.; Azumaya, T. Stock-specific Ocean Distribution and Migration of Chum Salmon in the Bering Sea and North Pacific Ocean. NPAFC 2009, 5, 131–146. [Google Scholar]
- Lee, H.S.; Seong, K.B.; Lee, C.H. History and status of the chum salmon enhancement program in Korea. The Sea 2007, 12, 73–80. [Google Scholar]
- Miyakoshi, Y.; Nagato, M.; Kitada, S.; Kaeriyama, M. Historical and Current Hatchery Programs and Management of Chum Salmon in Hokkaido, Northern Japan. Rev. Fish. Sci. 2013, 21, 469–479. [Google Scholar] [CrossRef]
- Beacham, T.D.; Murray, C.B. Comparative developmental biology of chum salmon (Oncorhynchus keta) from the Fraser River, British Columbia. Can. J. Fish. Aquat. Sci 1986, 43, 252–262. [Google Scholar] [CrossRef]
- Kijima, A.; Fujio, Y. Relationship between average heterozygosity and river population size in chum salmon (Oncorhynchus keta). Bull. Japan. Soc. Sci. Fish 1984, 50, 603–608. [Google Scholar] [CrossRef]
- Okazaki, T. Distribution, migration and possible origins of genetically different populations of chum salmon Oncorhynchus keta along the eastern coast of northern Japan. Fish. Sci. 1986, 52, 983–994. [Google Scholar] [CrossRef] [Green Version]
- Pease, A.A.; González, A.A.; Rodiles-Hernández, R.; Winemiller, K.O. Functional diversity and trait-environment relationships of stream fish assemblages in a large tropical catchment. Freshw. Biol. 2012, 57, 1060–1075. [Google Scholar] [CrossRef]
- Fryxell, D.C.; Arnett, H.A.; Apgar, T.M.; Kinnison, M.T.; Palkovacs, E.P. Sex ratio variation shapes the ecological effects of a globally introduced freshwater fish. Proc. Royal Soc. B 2015, 282, 20151970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.E.; Kim, G.G.; Lee, Y.H. Genetic similarity-dissimilarity among Korea Chum salmons of each stream and their relationship with Japan salmons. The Sea 2007, 12, 94–101. [Google Scholar]
- Kwon, O.N.; Kim, J.K.; Yoon, M.G.; Kim, D.H.; Hong, K.E. Marine prey selectivity of released juvenile chum salmon (Oncorhynchus keta) during early marine migration in Korean waters. J. Kor. Soc. Fish. Mar. Edu. 2014, 26, 421–429. [Google Scholar]
- Seo, H.; Kim, S.; Seong, K.; Kang, S. Variability in scale growth rates of chum salmon (Oncorhynchus keta) in relation to climate changes in the late 1980s. Prog. Oceanogr. 2006, 68, 205–216. [Google Scholar] [CrossRef]
- Gleick, P.H. Making every drop count. Sci. Am. 2001, 284, 40–45. [Google Scholar] [CrossRef]
- Fukuwaka, M.; Suzuki, T. Early sea mortality of chum salmon juveniles in the open coastal waters of the Japan Sea. NPAFC 2001, 2, 7–8. [Google Scholar]
- Battin, J.; Wiley, M.W.; Ruckelshaus, M.H.; Palmer, R.N.; Korb, E.; Bartz, K.K.; Maki, H. Projected impacts of climate change on salmon habitat restoration. PNAS 2007, 104, 6720–6725. [Google Scholar] [CrossRef] [Green Version]
- Bams, R.A.; Lam, C.N.H. Influence of deteriorating water quality on growth and development of chum salmon (Oncorhynchus keta) larvae in a Japanese-style keeper channel. Can. J. Fish. Aquat. 1983, 40, 2098–2104. [Google Scholar] [CrossRef]
- Ko, E.J.; Do, Y.; Kim, H.; Song, H.S.; Wood, T.S.; Chon, T.S. Effective detection methods for Pectinatella magnifica Leidy 1851 colony distribution using statoblasts. Bio. Invasions 2021, 23, 981–987. [Google Scholar] [CrossRef]
- Jeong, K.S.; Kim, D.K.; Joo, G.J. Delayed influence of dam storage and discharge on the determination of seasonal proliferations of Microcystis aeruginosa and Stephanodiscus hantzschii in a regulated river system of the lower Nakdong River (South Korea). Water Res. 2007, 41, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H. The Geography of Korea. Soc. Stud. 1988, 79, 141–145. [Google Scholar] [CrossRef]
- Normile, D. Restoration or devastation? Science 2010, 327, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Mukhopadhyay, M.; Mitra, P.; Bagchi, M.; Karamkar, H. Impact of Farakka barrage on the hydrology and fishery of Hoogly estuary. Estuaries 1996, 19, 710–722. [Google Scholar] [CrossRef]
- Tekile, A.; Kim, I.; Kim, J. Mini-review on river eutrophication and bottom improvement techniques, with special emphasis on the Nakdong River. J. Environ. Sci. 2015, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.D.; Jang, M.H.; Jo, H.B.; Jeong, K.S.; Kim, G.Y.; Joo, G.J. Changes of fish assemblages after construction of an estuary barrage in the lower Nakdong River, South Korea. Limnology 2016, 17, 183–197. [Google Scholar] [CrossRef]
- Hooper, T.; Austen, M. Tidal barrages in the UK: Ecological and social impacts, potential mitigation, and tools to support barrage planning. Renew. Sust. Energ. Rev. 2013, 23, 289–298. [Google Scholar] [CrossRef]
- Ellis, D.V. Swimming speeds of sockeye and coho salmon on spawning migration. J. Fish. Res. Board Can. 1966, 23, 181–187. [Google Scholar] [CrossRef]
- Akita, M.T.; Makiguchi, H.; Nii, H.; Nakao, K.; Sandahl, J.F.; Ueda, H. Upstream migration of chum salmon through a restored segment of the Shibetsu River. Ecol. Freshw. 2006, 15, 125–130. [Google Scholar] [CrossRef]
- Water Environment Information System. Available online: http://water.nier.go.kr/ (accessed on 29 May 2021).
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Ruggerone, G.T.; Irvine, J.R. Number and biomass of natural and hatchery-origin pink salmon, chum salmon, and sockeye salmon in the north Pacific Ocean, 1925–2015. Mar. Coast. Fish. 2018, 10, 152–168. [Google Scholar] [CrossRef]
- Brykov, V.A.; Kukhleysky, A.D.; Shevlyakov, E.A. Sex ratio control in pink salmon (Oncorhynchus gorbuscha) and chum salmon (O. keta) populations: The possible causes and mechanism of changes in the sex ratio. Russ. J. Genet. 2008, 44, 786–792. [Google Scholar] [CrossRef]
- Craig, J.K.; Foote, C.J.; Wood, C.C. Evidence of Temperature-Dependent Sex Determination in Sockeye Salmon (Oncorhynchus nerka). Can. J. Fish. Aquat. Sci. 1996, 53, 141–147. [Google Scholar] [CrossRef]
- Alderdice, D.F.; Wickett, W.P.; Brett, J.R. Some effects of temporary exposure to low dissolved oxygen levels on Pacific salmon eggs. J. Fish. Res. Board Can. 1958, 15, 229–250. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Nagorski, S.; Hudson, J.; Pyare, S. Interactive physical and biotic factors control dissolved oxygen in salmon spawning streams in coastal Alaska. Aquat. Sci. 2019, 81, 1–11. [Google Scholar] [CrossRef]
- Beacham, T.D.; Murray, C.B. Effect of female size, egg size, and water temperature on development biology of chum salmon (Oncorhynchus keta) from the Nitinat River, British Columbia. Can. J. Fish. Aquat. Sci. 1985, 42, 1755–1765. [Google Scholar] [CrossRef]
- Zinichev, V.V.; Zotin, A.I. Selected temperature and optimums for development in prolarvae and larvae of chum salmon, Oncorhynchus keta. J. Ichthyol. 1987, 27, 141–144. [Google Scholar]
- Jensen, K.; Snekvik, C. Low pH levels wipe out salmon and trout populations in southernmost Norway. Ambio 1972, 1, 223–225. [Google Scholar]
- Rosseland, B.O.; Sevaldrub, I.; Svalastog, D.; Muniz, I.P. Studies on freshwater fish populations—effects of acidification on reproduction, population structure, growth and food selection. In Proceedings of the Ecological impact of acid precipitation, Sandefjord, Norway, 11 March 1980; Drabløs, D., Tollan, A., Eds.; SNSF-prosjektet, As-NLH. pp. 336–337. [Google Scholar]
- Rombough, P.J. Effects of low pH on eyed embryos and alevins of Pacific salmon. Can. J. Fish. Aquat. 1983, 40, 1575–1582. [Google Scholar] [CrossRef]
- Park, E.O.; Suh, H.L.; Soh, H.Y. Spatio-temporal distribution of Acartia (Copepoda: Calanoida) species along a salinity gradient in the Seomjin River estuary, South Korea. J. Nat. Hist. 2015, 79, 2799–2812. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Kaeriyama, M.; Seo, H.; Qin, Y. Effect of global warming on the life history and population dynamics of Japanese chum salmon. Fish. Sci. 2014, 80, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Aziz, O.I.; Nathan, J.M.; Katherine, W.M. Potential climate change impacts on thermal habitats of Pacific salmon (Oncorhynchus keta spp.) in the North Pacific ocean and adjacent seas. Can. J. Fish. Aquat. Sci. 2011, 68, 1660–1680. [Google Scholar] [CrossRef]
- Myeong, J.G.; Kim, Y.U. Morphological Study of Oncorhynchus spp. In Korea-V. Comparison of Skeletal Characters of Chum Salmon, O. keta, Masu Salmon O. masou and Rainbow Trout O. mykiss. Korean J. Fish. Aquat. Sci. 1996, 29, 208–229. [Google Scholar]
- Kohan, M.L.; Mueter, F.J.; Orsi, J.A.; McPhee, M.V. Variation in size, condition, and abundance of juvenile chum salmon (Oncorhynchus keta) in relation to marine factors in Southeast Alaska. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 165, 340–347. [Google Scholar] [CrossRef]
- Marr, J.C. Age, length and weight studies of three species of Columbia River salmon (Oncorhynchus keta, O. gorbuscha and O. kisutch); Department of Research Fish Commission of the State of Oregon: Salem, OR, USA, 1944; Volume 2, pp. 157–197. [Google Scholar]
- Liu, W.; Zhan, P.R.; Tang, F.J. Study on morphological development of fall chum salmon (Oncorhynchus keta) otoliths in Heilongjiang River. Acta Hydrobiol. Sin. 2010, 34, 1069–1076. [Google Scholar] [CrossRef]
- Toshihiko, S.; Kentaro, H.; Kei, S.; Kyuji, W.; Kengo, S.; Yukihiro, H.; Shigeto, K.; Tomoki, S.; Fumihisa, T.; Shupei, S. Stock composition of adult chum salmon Oncorhynchus keta caught in a setnet fishery estimated using genetic identification, scale patterns, and otolith thermal marking. Fish Sci. 2020, 86, 271–286. [Google Scholar]
- Froese, R.; Thorson, J.T.; Reyes, R.B., Jr. A Bayesian approach for estimating length weight relationships in fishes. J. Appl. Ichthyol. 2014, 30, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Allendorf, F.W.; Ryman, N.; Utter, F. Genetics and Fishery Management: Past, Present, and Future, 1st ed.; University of Washington Press: Seattle, DC, USA, 1987; pp. 1–19. [Google Scholar]
- Wimberger, P.H. Plasticity of fish body shape, the effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biol. J. Linn. Soc. Lond. 1992, 45, 197–218. [Google Scholar] [CrossRef]
- FishBase. Available online: https://www.fishbase.se (accessed on 1 June 2021).
- Marthers, R.G.; De carlos, M.; Crowley, K.; Teangana, D.Ó. A review of the potential effect of Irish hydroelectric installations on Atlantic salmon (Salmo Sar L.) populations, with particular reference to the river Erne. Proc. R. Ir. Acad. 2002, 102B, 69–79. [Google Scholar] [CrossRef]
- Mee, D.M.; Kirkpatrick, A.J.; Stonehewer, R.O. Post impoundment fishery investigations on the Tawe Barrage, South Wales. In Barrages: Engineering Design & Environmental Impacts; Burt, N., Watts, J., Eds.; John Wiley & Sons Ltd: West Sussex, UK, 1996; pp. 395–408. [Google Scholar]
- Russell, I.C.; Moore, A.; Ives, S.; Kell, L.T.; Ives, M.J.; Stonehewer, R.O. The migratory behaviour of juvenile and adult salmonids in relation to an estuarine barrage. Hydrobiologia 1998, 371, 321–334. [Google Scholar] [CrossRef]
- Plumb, J.M.; Perry, R.W.; Adams, N.S.; Rondorf, D.W. The effect of river impoundment and hatchery rearing on the migration behavior of juvenile steelhead in the lower Snake River, Washington. N. Am. J. Fish. Manag. 2006, 26, 438–452. [Google Scholar] [CrossRef]
- Hasler, A.D.; Allan, T.S.; Ross, M.H. Olfactory imprinting and homing in Salmon: Recent experiments in which salmon have been artificially imprinted to a synthetic chemical verify the olfactory hypothesis for salmon homing. Am. Sci. 1978, 66, 347–355. [Google Scholar] [PubMed]
- Salo, E.O. Life history of chum salmon (Oncorhynchus keta). In Pacific Salmon Life Histories, 2nd ed; Groot, C., Margolis, L., Eds.; UBCPress: Vancouver, BC, Canada, 1991; Volume 1, pp. 231–310. [Google Scholar]
- Zavolokin, A.V.; Zavolokin, E.A.; Khokhlov, Y.N. Changes in size and growth of anadromous chum salmon (Oncorhynchus keta) from 1962-2007. NPAFC 2009, 5, 157–163. [Google Scholar]
- Roff, D.A. The Evolution of Life Histories: Theory and Analysis, 1st ed.; Chapman & Hall, Ed.; Springer: New York, NY, USA, 1993. [Google Scholar]
- Fukuwaka, M. Density-dependence of chum salmon in coastal waters of the Japan Sea. NPAFC 2000, 2, 75–81. [Google Scholar]
- Azumaya, T.; Ishida, Y. An evaluation of the potential influence of SST and currents on the oceanic migration of juvenile and immature chum salmon (Oncorhynchus keta) by a simulation model. Fish. Oceanogr. 2004, 13, 10–23. [Google Scholar] [CrossRef]




| Sex | Total Number of Data (n) | Mean Length (cm) ±Standard Deviation | Mean Weight (Kg) ±Standard Deviation |
|---|---|---|---|
| Total | 4400 | 68.49 ± 5.4 | 2.66 ± 0.71 |
| Male | 2070 | 68.55 ± 5.9 | * 2.65 ± 0.75 |
| Female | 2330 | 68.42 ± 4.7 | * 2.69 ± 0.67 |
| Catch | Female Ratio | pH | DO | BOD | TN | TP | Temp | Conductivity | Distance | |
|---|---|---|---|---|---|---|---|---|---|---|
| Catch | 1.000 | 0.352 * | 0.429 ** | 0.369 * | 0.223 | 0.075 | −0.284 | −0.300 * | −0.164 | −0.397 ** |
| 0.019 | 0.004 | 0.014 | 0.145 | 0.626 | 0.062 | 0.048 | 0.288 | 0.008 | ||
| Female ratio | 0.352 * | 1.000 | 0.427 ** | 0.096 | 0.011 | 0.112 | −0.185 | −0.202 | −0.100 | −0.293 |
| 0.019 | 0.004 | 0.535 | 0.943 | 0.469 | 0.230 | 0.189 | 0.520 | 0.054 | ||
| pH | 0.429 ** | 0.427 ** | 1.000 | 0.344 * | 0.307 * | −0.109 | −0.479 ** | −0.573 ** | −0.349 * | −0.672 ** |
| 0.004 | 0.004 | 0.022 | 0.043 | 0.479 | 0.001 | 0.000 | 0.020 | 0.000 | ||
| DO | 0.369 * | 0.096 | 0.344 * | 1.000 | 0.056 | −0.136 | −0.333 * | −0.562 ** | −0.302 * | −0.397 ** |
| 0.014 | 0.535 | 0.022 | 0.717 | 0.378 | 0.027 | 0.000 | 0.047 | 0.008 | ||
| BOD | 0.223 | 0.011 | 0.307 * | 0.056 | 1.000 | 0.256 | 0.095 | −0.146 | 0.116 | −0.020 |
| 0.145 | 0.943 | 0.043 | 0.717 | 0.093 | 0.541 | 0.343 | 0.453 | 0.898 | ||
| TN | 0.075 | 0.112 | -0.109 | −0.136 | 0.256 | 1.000 | 0.555 ** | 0.113 | 0.133 | 0.566 ** |
| 0.626 | 0.469 | 0.479 | 0.378 | 0.093 | 0.000 | 0.465 | 0.389 | 0.000 | ||
| TP | −0.284 | −0.185 | −0.479 ** | −0.333 * | 0.095 | 0.555 ** | 1.000 | 0.475 ** | 0.562 ** | 0.852 ** |
| 0.062 | 0.230 | 0.001 | 0.027 | 0.541 | 0.000 | 0.001 | 0.000 | 0.000 | ||
| Temp. | −0.300 * | −0.202 | −0.573 ** | −0.562 ** | −0.146 | 0.113 | 0.475 ** | 1.000 | 0.319 * | 0.574 ** |
| 0.048 | 0.189 | 0.000 | 0.000 | 0.343 | 0.465 | 0.001 | 0.035 | 0.000 | ||
| Conductivity | −0.164 | −0.100 | −0.349 * | −0.302 * | 0.116 | 0.133 | 0.562 ** | 0.319 * | 1.000 | 0.520 ** |
| 0.288 | 0.520 | 0.020 | 0.047 | 0.453 | 0.389 | 0.000 | 0.035 | 0.000 | ||
| Distance | −0.397 ** | −0.293 | −0.672 ** | −0.397 ** | −0.020 | 0.566 ** | 0.852 ** | 0.574 ** | 0.520 ** | 1.000 |
| 0.008 | 0.054 | 0.000 | 0.008 | 0.898 | 0.000 | 0.000 | 0.000 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, D.; Joo, G.-J.; Jung, E.; Gim, J.-S.; Seong, K.B.; Kim, D.-H.; Lineman, M.J.M.; Kim, H.-W.; Jo, H. The Spatial Distribution and Morphological Characteristics of Chum Salmon (Oncorhynchus keta) in South Korea. Fishes 2022, 7, 27. https://doi.org/10.3390/fishes7010027
Hong D, Joo G-J, Jung E, Gim J-S, Seong KB, Kim D-H, Lineman MJM, Kim H-W, Jo H. The Spatial Distribution and Morphological Characteristics of Chum Salmon (Oncorhynchus keta) in South Korea. Fishes. 2022; 7(1):27. https://doi.org/10.3390/fishes7010027
Chicago/Turabian StyleHong, Donghyun, Gea-Jae Joo, Eunsong Jung, Jeong-Soo Gim, Ki Baik Seong, Doo-Ho Kim, Maurice J. M. Lineman, Hyun-Woo Kim, and Hyunbin Jo. 2022. "The Spatial Distribution and Morphological Characteristics of Chum Salmon (Oncorhynchus keta) in South Korea" Fishes 7, no. 1: 27. https://doi.org/10.3390/fishes7010027