Basic Intersexuality (Abnormal Hermaphroditism) in the Blackmouth Catshark, Galeus melastomus, (Rafinesque, 1810), from the Southern Tyrrhenian Sea (Central Mediterranean Sea)
Abstract
:1. Introduction
2. Materials and Methods
Histological Assessment
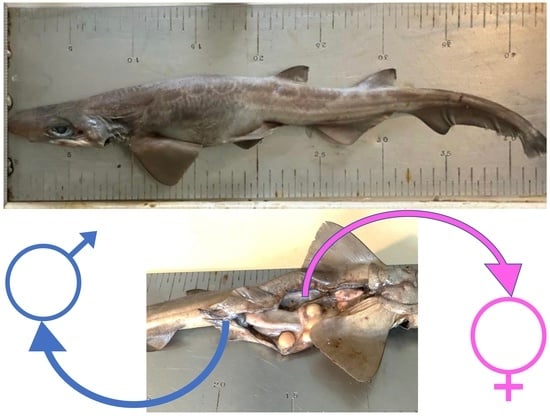
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bornatowski, H.; Abilhoa, V. Record of an anomalous embryo of Rhinobatos percellens (Elasmobranchii: Rhinobatidae) in the southern coast of Brazil. Mar. Biodivers. Rec. 2009, 2, e36. [Google Scholar] [CrossRef]
- Capapé, C.; Ali, M. Record of dicephalous embryo in Longnose Spurdog Squalus blainvillei (Chondrichthyes: Squalidae) from the Syrian coast (Eastern Mediterranean). In ANNALES Series Historia Naturalis; Scientific and Research Center of the Republic of Slovenia: Pirano, Slovenia, 2017; Volume 27, pp. 59–62. [Google Scholar]
- Castro-Aguirre, J.L.; Torres-Villegas, J.R. Sobre un Caso de Bicefalia Funcional en Rhinoptera Steindachneri Evermann y Jenkins (Chondricthys, Elasmobranchii, Batoidei), Capturado en la Costa Occidental de Baja California, Mexico. Cienc. Mar. 1979, 6, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Delpiani, S.M.; Deli Antoni, M.Y.; Barbini, S.A.; Figueroa, D.E. First record of a dicephalic specimen of tope Galeorhinus galeus (Elasmobranchii: Triakidae). J. Fish Biol. 2011, 78, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Ehemann, N.; Marín-Sanz, J.; Barany-González, M. Nota: Two cases of two-head shark embryos, smalleye smooth-hound Mustelus higmani and the blue shark Prionace glauca. Bol. Investig. Mar. Y Costeras 2016, 45, 149–153. [Google Scholar] [CrossRef]
- Escobar-Sánchez, O.; Galván-Magaña, F.; Downton-Hoffmann, C.A.; Carrera-Fernández, M.; Alatorre-Ramírez, V.G. First record of a morphological abnormality in the longtail stingray Dasyatis longa (Myliobatiformes: Dasyatidae) in the Gulf of California, Mexico. Mar. Biodivers. Rec. 2009, 2, e26. [Google Scholar] [CrossRef]
- Galván-Magaña, F.; Escobar-Sánchez, O.; Carrera-Fernández, M. Embryonic bicephaly in the blue shark, Prionace glauca, from the Mexican Pacific Ocean. Mar. Biodivers. Rec. 2011, 4, e1. [Google Scholar] [CrossRef]
- Atz, J.W. Intersexuality in fishes. In Intersexuality in Vertebrates Including Man; Marshall, A.J., Armstrong, C.N., Eds.; Academic Press: New York, NY, USA, 1964; pp. 145–232. [Google Scholar]
- Capapé, C.; El Kamel-Moutalibi, O.; Mnasri, N.; Boumaïza, M.; Reynaud, C. A case of hermaphroditism in tortonese’s stingray, Dasyatis tortonesei (Elasmobranchii: Rajiformes: Dasyatidae) from the lagoon of bizerte, Tunisia. Acta Ichthyol. Piscat. 2012, 42, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.T.; Feldheim, K.A.; Poulakis, G.R.; Chapman, D.D. Facultative parthenogenesis in a critically endangered wild vertebrate. Curr. Biol. 2015, 25, R446–R447. [Google Scholar] [CrossRef] [Green Version]
- Gianeti, M.D.; Vooren, C.M. A hermaphrodite guitarfish, Rhinobatos horkelii (Müller & Henle, 1841) (Rajiformes: Rhinobatidae), from southern Brazil. Cah. Biol. Mar. 2007, 48, 407–409. [Google Scholar]
- González-gonzález, L.V. Primer Reporte de Mono Clasper en Zapteryx Exasperata Para El Golfo de California, México Genéticas O. Compendio de Resúmenes Orales Y Carteles del XV Congreso Nacional de Ictiología, V Simposio Latinoamericano de Ictiología; I Simposio Internacional de Genómica de Peces: Aguascalientes, Mexico, 2016; Volume 9, pp. 213–214. [Google Scholar]
- Haas, D.L.; Ebert, D.A. First Record of Hermaphroditism in the Bering Skate, Bathyraja interrupta. Northwest Nat. 2008, 89, 181–185. [Google Scholar] [CrossRef]
- Costa, M.E.; Borges, T.C.; Capapé, C. Cases of abnormal hermaphroditism in velvet belly Etmopterus spinax (Chondrichthyes: Etmopteridae) from the southern coast of Portugal. Cah. Biol. Mar. 2013, 54, 309–317. [Google Scholar] [CrossRef]
- Ball, R.E.; Jones, C.S.; Lynghammar, A.; Noble, L.R.; Griffiths, A.M. The first confirmed cases of full albinism in rajid species. J. Fish Biol. 2013, 82, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Becerril-García, E.E.; Tamburin, E.; González-Armas, R.; Galván-Magaña, F. First record of albinism in the swell shark, Cephaloscyllium ventriosum (Elasmobranchii: Carcharhiniformes: Scyliorhinidae). Acta Ichthyol. Piscat. 2017, 47, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Souissi, J.B.; Golani, D.; Méjri, H.; Salem, M.B.; Capapé, C. First confirmed record of the Halave’s Guitarfish, Rhinobatos halavi (Forsskål, 1775) (Chondrichthyes: Rhinobatidae) in the Mediterranean Sea with a description of a case of albinism in elasmobranchs. Cah. Biol. Mar. 2007, 48, 67–75. [Google Scholar]
- Mulas, A.; Bellodi, A.; Porcu, C.; Cau, A.; Coluccia, E.; Demurtas, R.; Marongiu, M.F.; Pesci, P.; Follesa, M.C. Living naked: First case of lack of skin-related structures in an elasmobranch, the blackmouth catshark (Galeus melastomus). J. Fish Biol. 2020, 97, 1252–1256. [Google Scholar] [CrossRef]
- Bottaro, M.; Ferrando, S.; Gallus, L.; Girosi, L.; Vacchi, M. First record of albinism in the deep-water shark Dalatias licha. Mar. Biodivers. Rec. 2008, 1, e10. [Google Scholar] [CrossRef]
- Moore, A.B.M. Morphological abnormalities in elasmobranchs. J. Fish Biol. 2015, 87, 465–471. [Google Scholar] [CrossRef]
- Smith, C.L.; Armstrong, C.N.; Marshall, A.J. Intersexuality in Vertebrates Including Man. Copeia 1967, 1967, 692. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Greco, S.; Sophronidis, K.; Lembo, G.; Giordano, D.; Argyri, A. Geographical distribution, abundance and some population characteristics of the species of the genus Pagellus (Osteichthyes: Perciformes) in different areas of the Mediterranean. Sci. Mar. 2002, 66, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Busalacchi, B.; Bottari, T.; Giordano, D.; Profeta, A.; Rinelli, P. Distribution and biological features of the common pandora, Pagellus erythrinus (Linnaeus, 1758), in the southern Tyrrhenian Sea (Central Mediterr). Helgol. Mar. Res. 2014, 68, 491–501. [Google Scholar] [CrossRef] [Green Version]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef] [PubMed]
- Iglésias, S.P.; Sellos, D.Y.; Nakaya, K. Discovery of a normal hermaphroditic chondrichthyan species: Apristurus longicephalus. J. Fish Biol. 2005, 66, 417–428. [Google Scholar] [CrossRef]
- Daniel, J.F. The Elasmobranch Fishes. [With Plates]; University of California Press: Berkeley, CA, USA, 1928. [Google Scholar]
- Irvine, S.B.; Laurenson, L.J.B.; Stevens, J.D.; Martin, R.A.; MacKinlay, D. Hermaphroditism in the Southern Lanternshark, Etmopterus granulosus. Biology of Deep Sea Elasmobranchs. In Biology of Deep Sea Elasmobranchs, Proceedings of the International Congress on the Biology of Fish, Vancouver, BC, Canada, 21-25 July 2002; University of British Columbia: Vancouver, BC, Canada, 2002; Volume 1, pp. 49–54. [Google Scholar]
- Jones, A.A.; Potter, I.C. Description of the reproductive tract and gonad histology of a second form of hermaphroditism in the Port Jackson shark Heterodontus portusjacksoni. J. Mar. Biol. Assoc. UK 2009, 89, 1403–1407. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.A.; White, W.T.; Potter, I.C. A hermaphroditic Port Jackson shark, Heterodontus portusjacksoni, with complete and separate female and male reproductive tracts. J. Mar. Biol. Assoc. UK 2005, 85, 1171–1172. [Google Scholar] [CrossRef] [Green Version]
- Pratt, H.L. Reproduction in the Blue Shark, Prionace Glauca. Fish. Bull. 1979, 77, 445–470. [Google Scholar]
- Capapé, C.; Zahnd, J. Cas d’hermaphrodisme chez Scyliorhinus canicula (Linné, 1758). Bull. Inst. Océanographique 1974, 3, 131–138. [Google Scholar]
- Capapè, C.; Chadli, A.; Baouendi, A. A case of hermaphroditism in Scyliorhinus stellaris (Linné, 1758) (Pisces, Scyliorhinidae): Morphological and histological study. Arch. Inst. Pasteur Tunis 1979, 56, 343–351. [Google Scholar]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Hendon, J.M.; Koester, D.M.; Hoffmayer, E.R.; Driggers, W.B.; Cicia, A.M. Occurrence of an Intersexual Blacktip Shark in the Northern Gulf of Mexico, with Notes on the Standardization of Classifications for This Condition in Elasmobranchs. Mar. Coast. Fish. 2013, 5, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Dodd, J.M. Reproduction in cartilaginous fishes (chondrichthyes). In Fish Physiology; Elsevier: Amsterdam, Netherlands, 1983; Volume 9, pp. 31–95. ISBN 1546-5098. [Google Scholar]
- Luer, C.A. Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes; JHU Press: Baltimore, MD, USA, 2000; Volume 2000, ISBN 0801860482. [Google Scholar]
- King, A.D. Hermaphroditism in the Common dogfish (Scyliorhinus caniculus). J. Zool. 1966, 148, 312–314. [Google Scholar] [CrossRef]
- Irvine, S.B. Age, Growth and Reproduction of Deepwater Dogfishes from Southeastern Australia; Deakin University: Victoria, Australia, 2004; p. 397. [Google Scholar]
- Zhao, Y.; Chen, M.; Jiang, C.; Yang, S.; Xiao, J. Occurrence of an Intersexual Pacific Spadenose Shark Scoliodon macrorhynchos from the Southern Taiwan Strait. Mar. Coast. Fish. 2017, 9, 573–576. [Google Scholar] [CrossRef] [Green Version]
- D’Iglio, C.; Savoca, S.; Rinelli, P.; Spanò, N. Diet of the Deep-Sea Shark Galeus melastomus Rafinesque, 1810, in the Mediterranean Sea: What We Know and What We Should Know. Sustainability 2021, 13, 3962. [Google Scholar] [CrossRef]
- Rinelli, P.; Bottari, T.; Florio, G.; Romeo, T.; Giordano, D.; Greco, S. Observations on distribution and biology of Galeus melastomus (Chondrichthyes, Scyliorhinidae) in the southern Tyrrhenian Sea (central Mediterranean). Cybium 2005, 29, 41–46. [Google Scholar]
- Van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; Eschmeyer, W.N.; Froese, R. Pisces. Collect. Patrim. Nat. 2001, 50, 357–374. [Google Scholar]
- Compagno, L.J. V Sharks of the world. An annotated and illustrated catalouge of shark species to date. Part II (Carcharhiniformes). FAO Fish. Synopsis 1984, 4, 250–655. [Google Scholar]
- D’Iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and Ecological Aspects of the Blackmouth Catshark (Galeus melastomus Rafinesque, 1810) in the Southern Tyrrhenian Sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Navarro, J.; Coll, M.; Aguzzi, J.; Cardona, L.; Sáez-Liante, R. Feeding ecology and trophic position of three sympatric demersal chondrichthyans in the northwestern Mediterranean. Mar. Ecol. Prog. Ser. 2015, 524, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Yemisken, E.; Navarro, J.; Forero, M.; Megalofonou, P.; Eryilmaz, L. Trophic partitioning between abundant demersal sharks coexisting in the North Aegean Sea. J. Mar. Biol. Assoc. UK 2019, 99, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Serena, F.; Mancusi, C.; Ungaro, N.; Hareide, N.R.; Guallart, J.; Coelho, R.; Crozier, P. Galeus melastomus. IUCN Red List Threat. Species 2009, e.T161398A124477972. [Google Scholar] [CrossRef]
- Psomadakis, P.N.; Giustino, S.; Vacchi, M. Mediterranean fish biodiversity: An updated inventory with focus on the Ligurian and Tyrrhenian seas. Zootaxa 2012, 3263, 1–46. [Google Scholar] [CrossRef]
- Sion, L.; Bozzano, A.; D’Onghia, G.; Capezzuto, F.; Panza, M. Chondrichthyes species in deep waters of the Mediterranean Sea. Sci. Mar. 2004, 68, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Ragonese, S.; Di Stefano, L.; Bianchini, M.L. Catture e selettività di pesci cartilaginei nella pesca dei gamberi rossi nello Stretto di Sicilia. Biol. Mar. Medit. 2000, 7, 400–401. [Google Scholar]
- Rey, J.; de Sola, L.G.; Massutí, E. Distribution and biology of the blackmouth catshark Galeus melastomus in the Alboran Sea (Southwestern Mediterranean). J. Northwest Atl. Fish. Sci. 2005, 35, 215–223. [Google Scholar] [CrossRef]
- Ramírez-Amaro, S.; Ordines, F.; Terrasa, B.; Esteban, A.; García, C.; Guijarro, B.; Massutí, E. Demersal chondrichthyans in the western Mediterranean: Assemblages and biological parameters of their main species. Mar. Freshw. Res. 2016, 67, 636–652. [Google Scholar] [CrossRef] [Green Version]
- Gouraguine, A.; Hidalgo, M.; Moranta, J.; Bailey, D.M.; Ordines, F.; Guijarro, B.; Valls, M.; Barberá, C.; De Mesa, A. Elasmobranch spatial segregation in the western Mediterranean. Sci. Mar. 2011, 75, 653–664. [Google Scholar] [CrossRef]
- Bertrand, J.; Teresa Spedicato, M. International Bottom Trawl Survey in the Mediterranean Instruction Manual. 2017, p. 106. Available online: https://archimer.ifremer.fr/doc/00002/11321/ (accessed on 4 May 2022).
- Bertrand, J.A.; Relini, L.O.; Papaconstantinou, C.; Jukic-Peladic, S.; Souplet, A.; de Sola, L.G.; Piccinetti, C.; Kavadas, S.; Rossi, M. Mediterranean marine demersal resources: The Medits international trawl survey (1994–1999). Sci. Mar. 2002, 66 (Suppl. 2), 103–124. [Google Scholar]
- Perdichizzi, A.; D’Iglio, C.; Giordano, D.; Profeta, A.; Ragonese, S.; Rinelli, P. Comparing life-history traits in two contiguous stocks of the deep-water rose shrimp Parapenaeus longirostris (H. Lucas, 1846) (Crustacea: Decapoda) in the Southern Tyrrhenian Sea (Central Mediterranean Sea). Fish. Res. 2022, 248, 106206. [Google Scholar] [CrossRef]
- Follesa, M.C.; Carbonara, P. Atlas of the Maturity Stages of Mediterranean Fishery Resources. In Studies and Reviews n. 99; FAO: Rome, Italy, 2019; ISBN 9789251319758. [Google Scholar]
- Lauriano, E.R.; Pergolizzi, S.; Aragona, M.; Montalbano, G.; Guerrera, M.C.; Crupi, R.; Faggio, C.; Capillo, G. Intestinal immunity of dogfish Scyliorhinus canicula spiral valve: A histochemical, immunohistochemical and confocal study. Fish Shellfish Immunol. 2019, 87, 490–498. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Checklist of living Chondrichthyes. In Reproductive Biology and Phylogeny of Chondrichthyes; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 513–517. [Google Scholar]
- Yano, K.; Tanaka, S. Hermaphroditism in the lantern shark Etmopterus unicolor (Squalidae, chondrichthyes). Jpn. J. Ichthyol. 1989, 36, 338–345. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.C.; Sosa-Nishizaki, O. Reproductive biology of the brown smoothhound shark Mustelus henlei, in the northern Gulf of California, México. J. Fish Biol. 2008, 73, 782–792. [Google Scholar] [CrossRef]
- Kousteni, V.; Megalofonou, P. Reproductive Biology of Squalus Blainvillei (Risso, 1826) in the Eastern Mediterranean Sea. Rapp. Comm. Int. Mer Médit. 2010, 39, 562. [Google Scholar]
- Springer, S.; Lowe, R.H. A New Smooth Dogshark, Mustelus higmani, from the Equatorial Atlantic Coast of South America. Copeia 1963, 1963, 245. [Google Scholar] [CrossRef]
- Veríssimo, A.; Gordo, L.; Figueiredo, I. Reproductive biology and embryonic development of Centroscymnus coelolepis in Portuguese mainland waters. ICES J. Mar. Sci. 2003, 60, 1335–1341. [Google Scholar] [CrossRef]
- Templeman, R. Characteristics Between Populations of Thorny Skate (Raja radiata) in the Northwest Atlantic. J. Northwest Atl. Fish. Sci. 1987, 7, 155–167. [Google Scholar] [CrossRef]
- Castro, J.I. Biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bull. Mar. Sci. 1996, 59, 508–522. [Google Scholar]
- Yano, K. Studies on Morphology, Phylogeny, Taxonomy and Biology of Japanese Squaloid Sharks, Order Squaliformes. Doctoral Dissertation, Tokai University, Shimizu, Shizouka, Japan, 1985. [Google Scholar]
- Dalù, M.; Consalvo, G.C.; Romanelli, M. A hermaphrodite specimen of Torpedo torpedo (Chondrichthyes, Torpedinidae). Biol. Mar. Mediterr. 2003, 10, 792–794. [Google Scholar]
- Semper, C. Das Urogenitalsystem der Plagiostomen Und Seine Bedeutung Für das Uebrigen Wirbelthiere; Druck und Verlag der Stahel’schen Buch-und Kunsthandlung: Würzburg, Germany, 1875. [Google Scholar]
- Daniel, J.F. The Elasmobranch Fishes; University of California: Oakland, CA, USA, 1934. [Google Scholar]
- Braccini, J.M. An abnormal hermaphrodite piked spurdog, Squalus megalops, schooling with mature males. Mar. Biodivers. Rec. 2009, 2, e132. [Google Scholar] [CrossRef]
- Soto-López, K.; Ochoa-Báez, R.I.; Tovar-Ávila, J.; Galván-Magaña, F. Reproductive biology of the brown smooth-hound shark, Mustelus henlei (Chondrichthyes: Triakidae), off northwestern Mexico based on macroscopic and histological analyses. Ciencias Mar. 2018, 44, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Serrano-López, J.N.; Soto-López, K.; Ochoa-Báez, R.I.; O’Sullivan, J.; Galván-Magaña, F. Morphometry and histology to assess the maturity stage of three endangered devil ray species (Elasmobranchii: Mobulidae) from the Gulf of California. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1624–1635. [Google Scholar] [CrossRef]
- Capapé, C.; Kassar, A.; Reynaud, C.; Hemida, F. Atypical characteristics in blackmouth catshark, Galeus melastomus (Chondrichthyes: Scyliorhinidae) from the Algerian coast (southern Mediterranean Sea). Thalass. Salentina 2019, 41, 23–32. [Google Scholar] [CrossRef]
- Quignard, J.P.; Negla, N. Anomalies au niveau du système génital chez les sélaciens rajiformes. Trav. Du Lab. Biol. Halieut. L’université Rennes 1971, 5, 121–124. [Google Scholar]
- Arthur, D.R. Abnormalities in the Sexual Apparatus of the Common Dogfish (Scyliorhinus caniculus). In Proceedings of the Linnean Society of London; Oxford University Press: New York, NY, USA, 1950; Volume 162, pp. 52–56. [Google Scholar]
- Baker, J.R.; Oxon, B.A. An Hermaphrodite Dogfish (Scyliorhinus canicula). J. Anat. 1919, 58, 335. [Google Scholar]
- Fuller, A.S.; Zacharov, J.M. Abnormalities in the Urinogenital System of the Common Dogfish. Nature 1960, 185, 50. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Iglio, C.; Albano, M.; Famulari, S.; Spanò, N.; Rinelli, P.; Savoca, S.; Capillo, G. Basic Intersexuality (Abnormal Hermaphroditism) in the Blackmouth Catshark, Galeus melastomus, (Rafinesque, 1810), from the Southern Tyrrhenian Sea (Central Mediterranean Sea). Fishes 2022, 7, 120. https://doi.org/10.3390/fishes7030120
D’Iglio C, Albano M, Famulari S, Spanò N, Rinelli P, Savoca S, Capillo G. Basic Intersexuality (Abnormal Hermaphroditism) in the Blackmouth Catshark, Galeus melastomus, (Rafinesque, 1810), from the Southern Tyrrhenian Sea (Central Mediterranean Sea). Fishes. 2022; 7(3):120. https://doi.org/10.3390/fishes7030120
Chicago/Turabian StyleD’Iglio, Claudio, Marco Albano, Sergio Famulari, Nunziacarla Spanò, Paola Rinelli, Serena Savoca, and Gioele Capillo. 2022. "Basic Intersexuality (Abnormal Hermaphroditism) in the Blackmouth Catshark, Galeus melastomus, (Rafinesque, 1810), from the Southern Tyrrhenian Sea (Central Mediterranean Sea)" Fishes 7, no. 3: 120. https://doi.org/10.3390/fishes7030120