Abstract
Aortic regurgitation (AR) is a common valvular heart disease that unless appropriately managed is associated with morbidity and mortality. Left ventricular (LV) mechanics and aortic impedance are the main determinants of outcome in patients with AR and govern clinical management. Mild and moderate AR in individuals with normal LV dimensions are both generally benign. In the absence of symptoms and before LV dimensions increase, even severe AR is not generally associated with increased morbidity or mortality. Once LV enlargement occurs, however, symptoms and/or a decline in ejection fraction can develop, and both represent an indication for surgical intervention. Disease progression occurs at a variable rate, and is often insidious. Hence, symptoms do not correlate with objective evidence of ventricular dysfunction. Exercise testing can help highlight symptoms related to valve dysfunction. Asymptomatic patients with severe AR and preserved LV function can benefit from vasodilator drug therapy. Several agents from this class can reduce AR severity, but results are inconsistent. In this Review, we examine the epidemiology of AR in terms of the interplay between arterial and ventricular forces marking progression of disease over time, and analyze the practice guidelines regarding diagnosis and treatment.
Key Points
-
Aortic regurgitation (AR) is a common valvular pathology that when severe often requires valve replacement
-
Evaluation and risk stratification of patients with severe AR requires echocardiography
-
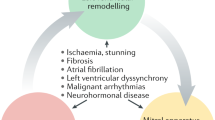
Complex interaction between ventricular and vascular forces governs progression from compensated AR to permanent left ventricular dysfunction
-
Ventricular dysfunction as a result of chronic AR is reversible when treatment is initiated early, but advanced adverse myocardial remodeling can be irreversible
-
Vasodilator drugs such as renin–angiotensin inhibitors can produce favorable myocardial remodeling and benefit patients with AR, but cannot replace definitive surgical treatment when regurgitation is severe
-
Early surgical intervention is generally indicated when AR is associated with expansion of the ascending aorta
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bonow RO et al. (1991) Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation 84: 1625–1635
Lebowitz NE et al. (2000) Prevalence and correlates of aortic regurgitation in American Indians: the Strong Heart Study. J Am Coll Cardiol 36: 461–467
Singh J et al. (1999) Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation. Am J Cardiol 83: 897–902
Aronow WS et al. (1997) Prevalence of echocardiographic findings in 554 men and in 1,243 women aged >60 years in a long-term health care facility. Am J Cardiol 79: 379–380
Kumpuris AG et al. (1982) Importance of preoperative hypertrophy, wall stress, and end-systolic dimensions echocardiographic predictors of normalization of left ventricular dilatation after valve replacement in chronic aortic insufficiency. Am J Cardiol 49: 1091–1100
Gaasch WH et al. (1983) Chronic aortic regurgitation: prognostic value of left ventricular end-systolic dimension and end-diastolic radius/thickness ratio. J Am Coll Cardiol 1: 775–782
Mirsky I et al. (1990) Clinical assessment of diastolic dysfunction. Prog Cardiovasc Dis 32: 291–318
Villari B et al. (1992) Effect of aortic valve stenosis (pressure overload) and regurgitation (volume overload) on left ventricular systolic and diastolic function. Am J Cardiol 69: 927–934
Wollmuth JR et al. (2006) Left ventricular wall stress in patients with severe aortic insufficiency with finite element analysis. Ann Thorac Surg 82: 840–846
Becker M et al. (2007) Impact of left ventricular loading conditions on myocardial deformation parameters: analysis of early and late changes of myocardial deformation parameters after aortic valve replacement. J Am Soc Echocardiogr 20: 681–689
Borer JS et al. (2002) Myocardial fibrosis in chronic aortic regurgitation: molecular and cellular responses to volume overload. Circulation 105: 1837–1842
Gupta A et al. (2006) Cellular response of human cardiac fibroblasts to mechanically simulated aortic regurgitation. Am J Ther 13: 8–11
Piper C et al. (2003) Remodeling of the cardiac extracellular matrix differs between volume- and pressure-overloaded ventricles and is specific for each heart valve lesion. J Heart Valve Dis 12: 592–600
Villari B (1993) Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol 22: 1477–1484
Milewicz DM et al. (2005) Treatment of aortic disease in patients with Marfan syndrome. Circulation 111: e150–e157
Grotenhuis HB et al. (2007) Reduced aortic elasticity and dilatation are associated with aortic regurgitation and left ventricular hypertrophy in nonstenotic bicuspid aortic valve patients. J Am Coll Cardiol 49: 1660–1665
Chaliki HP et al. (2002) Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 106: 2687–2693
Goldschlager N et al. (1973) The natural history of aortic regurgitation: a clinical and hemodynamic study. Am J Med 54: 577–588
Dujardin KS et al. (1999) Mortality and morbidity of aortic regurgitation in clinical practice: a long-term follow-up study. Circulation 99: 1851–1857
Bonow RO et al. (1983) The natural history of asymptomatic patients with aortic regurgitation and normal left ventricular function. Circulation 68: 509–517
Bonow RO et al. (1984) Reversal of left ventricular dysfunction after aortic valve replacement for chronic aortic regurgitation: influence of duration of preoperative left ventricular dysfunction. Circulation 70: 570–579
Bonow RO et al.; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists (2006) ACC/AHA 2006 Practice Guidelines for the Management of Patients With Valvular Heart Disease: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 48: e1–e148
Bonow RO et al. (1988) Long-term serial changes in left ventricular function and reversal of ventricular dilatation after valve replacement for chronic aortic regurgitation. Circulation 78: 1108–1120
Gaasch WH et al.(1978) Chronic aortic regurgitation: the effect of aortic valve replacement on left ventricular volume, mass and function. Circulation 58: 825–836
Klodas E et al. (1996) Surgery for aortic regurgitation in women: contrasting indications and outcomes compared with men. Circulation 94: 2472–2478
Babu AN et al.(2003) Eponyms and the diagnosis of aortic regurgitation: what says the evidence? Ann Intern Med 138: 736–742
Zoghbi WA et al. (2003) Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler schocardiography. J Am Soc Echocardiogr 16: 777–802
Tribouilloy CM et al. (1998) Application of the proximal flow convergence method to calculate the effective regurgitant orifice area in aortic regurgitation. J Am Coll Cardiol 32: 1032–1039
Schiller NB et al. (1989) Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2: 358–367
Bonow RO et al. (1998) ACC/AHA Guidelines for the Management of Patients With Valvular Heart Disease. Executive Summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease). J Heart Valve Dis 7: 672–707
Siemienczuk D et al. (1989) Chronic aortic insufficiency: factors associated with progression to aortic valve replacement. Ann Intern Med 110: 587–592
Lindsay J Jr et al. (1987) Prognostic implications of left ventricular function during exercise in asymptomatic patients with aortic regurgitation. Angiology 38: 386–392
Goldman ME et al. (1984) Relation between exercise-induced changes in ejection fraction and systolic loading conditions at rest in aortic regurgitation. J Am Coll Cardiol 3: 924–929
Wahi S et al. (2000) Exercise echocardiography predicts development of left ventricular dysfunction in medically and surgically treated patients with asymptomatic severe aortic regurgitation. Heart 84: 606–614
Borer JS et al. (1998) Prediction of indications for valve replacement among asymptomatic or minimally symptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation 97: 525–534
Vahanian A et al. (2007) Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 28: 230–268
Della Corte A et al. (2007) Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 31: 397–404
Gott VL et al. (1999) Replacement of the aortic root in patients with Marfan's syndrome. N Engl J Med 340: 1307–1313
Davies RR et al. (2002) Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 73: 17–27
Bentall H and De Bono A (1968) A technique for complete replacement of the ascending aorta. Thorax 23: 338–339
Birks EJ et al. (1999) Early and long-term results of a valve-sparing operation for Marfan syndrome. Circulation 100: II29–II35
de Oliveira NC et al. (2003) Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg 125: 789–796
Greenberg B et al. (1988) Long-term vasodilator therapy of chronic aortic insufficiency: a randomized double-blinded, placebo-controlled clinical trial. Circulation 78: 92–103
Lin M et al. (1994) Vasodilator therapy in chronic asymptomatic aortic regurgitation: enalapril versus hydralazine therapy. J Am Coll Cardiol 24: 1046–1053
Brower GL and Janicki JS (2001) Contribution of ventricular remodeling to pathogenesis of heart failure in rats. Am J Physiol Heart Circ Physiol 280: 674–683
Chandrashekhar Y (2007) Embracing diversity in remodeling: a step in therapeutic decision making in heart failure? J Am Coll Cardiol 49: 822–825
Schon HR et al. (1994) Effects of 12 months quinapril therapy in asymptomatic patients with chronic aortic regurgitation. J Heart Valve Dis 3: 500–509
Wisenbaugh T et al. (1994) Six month pilot study of captopril for mildly symptomatic, severe isolated mitral and isolated aortic regurgitation. J Heart Valve Dis 3: 197–204
Plante E et al. (2004) Effectiveness of beta-blockade in experimental chronic aortic regurgitation. Circulation 110: 1477–1483
Scognamiglio R et al. (1990) Long-term nifedipine unloading therapy in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol 16: 424–429
Banaszewski M et al. (1998) Captopril or nifedipine? Comparison of rest and exercise acute effects and long-term therapy in chronic isolated asymptomatic moderate to severe aortic regurgitation. J Heart Valve Dis 7: 488–499
Scognamiglio R et al. (1994) Nifedipine in asymptomatic patients with severe aortic regurgitation and normal left ventricular function. N Engl J Med 33: 689–694
Evangelista A et al. (2005) Long-term vasodilator therapy in patients with severe aortic regurgitation. N Engl J Med 353: 1342–1349
Vahanian A et al. (2007) Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 28: 230–268
Shores J et al. (1994) Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan's syndrome. N Engl J Med 330: 1335–1341
Dajani AS et al. (1997) Prevention of bacterial endocarditis—recommendations by the American Heart Association. Circulation 96: 358–366
Wilson W et al. (2007) Prevention of Infective Endocarditis. Guidelines From the American Heart Association. A Guideline From the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 115: 1–19
Horstkotte D et al. (2004) Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European society of cardiology. Eur Heart J 25: 267–276
Salem DN et al. (2004) Antithrombotic therapy in valvular heart disease—native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126 (Suppl): S457–S482
Habashi JP et al. (2006) Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121
Lacro RV et al. (2007) Rationale and design of a randomized clinical trial of β-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am Heart J 154: 624–631
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Goldbarg, S., Halperin, J. Aortic regurgitation: disease progression and management. Nat Rev Cardiol 5, 269–279 (2008). https://doi.org/10.1038/ncpcardio1179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1179
This article is cited by
-
Prognostic significance of myocardial fibrosis and CMR characteristics in bicuspid aortic valve with moderate and severe aortic insufficiency
European Radiology (2021)
-
Abnormal cisatracurium pharmacodynamics and pharmacokinetics among patients with severe aortic regurgitation during anesthetic induction
BMC Anesthesiology (2020)
-
Aortic Regurgitation
Current Cardiology Reports (2019)
-
Comparison of effects of losartan and metoprolol on left ventricular and aortic function at rest and during exercise in chronic aortic regurgitation
The International Journal of Cardiovascular Imaging (2018)
-
Aortic Regurgitation Generates a Kinematic Obstruction Which Hinders Left Ventricular Filling
Annals of Biomedical Engineering (2017)