Abstract
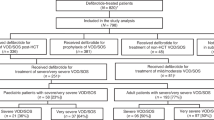
Severe hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially life-threatening complication of haematopoietic cell transplantation (HCT). This multinational, prospective, observational study (NCT03032016), performed by the EBMT, enrolled patients treated with defibrotide from April 2015 to July 2018. This analysis focused on defibrotide-treated patients with VOD/SOS post-HCT. The primary endpoint was incidence of serious adverse events (SAEs) of interest up to 12 months post-HCT in patients with severe VOD/SOS. Overall, 104 defibrotide-treated patients with VOD/SOS post-HCT were enrolled: 62 had severe VOD/SOS and comprised the primary study population, including 36 with multi-organ dysfunction/failure (MOD/MOF). SAEs of interest occurred in 20 of 62 (32%) severe VOD/SOS patients; the most common by category were infection (24%) and bleeding (13%). In patients with severe VOD/SOS, the Kaplan–Meier–estimated Day 100 survival rate was 73% (95% CI: 60%, 82%) with VOD/SOS resolution by Day 100 in 45 of 62 (73%) patients. MOD/MOF resolved in 19 of 36 (53%) patients with MOD/MOF at VOD/SOS diagnosis. Results from this multicentre registry study build on prior defibrotide studies supporting the utility of defibrotide for the treatment of VOD/SOS post-HCT. These results provide additional real-world evidence of the effectiveness and safety of defibrotide in patients with VOD/SOS post-HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All relevant data are provided within the paper and supporting files.
References
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transpl. 2010;16:157–68.
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transpl. 2018;53:138–45.
Dalle JH, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Transpl. 2016;22:400–9.
Richardson PG, Corbacioglu S, Ho VT, Kernan NA, Lehmann L, Maguire C, et al. Drug safety evaluation of defibrotide. Expert Opin Drug Saf. 2013;12:123–36.
Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–20.
Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4:332–46.
Carreras E, Diaz-Ricart M. Early complications of endothelial origin. In: Carreras E, Dufour C, Mohty M, Kroger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham (CH): Springer; 2019. p. 315–22.
Richardson PG, Triplett BM, Ho VT, Chao N, Dignan FL, Maglio M, et al. Defibrotide sodium for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Expert Rev Clin Pharm. 2018;11:113–24.
Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2015;168:481–91.
Defitelio® (defibrotide sodium) injection, for intravenous use [packet insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2016. https://pp.jazzpharma.com/pi/defitelio.en.USPI.pdf.
Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–65.
Kernan NA, Grupp S, Smith AR, Arai S, Triplett B, Antin JH, et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br J Haematol. 2018;181:816–27.
Corbacioglu S, Carreras E, Mohty M, Pagliuca A, Boelens JJ, Damaj G, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: final results from the International Compassionate-use Program. Biol Blood Marrow Transpl. 2016;22:1874–82.
Defitelio® (defibrotide sodium) [summary of product characteristics]. Villa Guardia, Italy: Gentium SpA; 2018. https://www.ema.europa.eu/en/documents/product-information/defitelio-epar-product-information_en.pdf.
Mohty M, Labopin M, Lebon D, Berceanu A, Jubert C, Yakoub-Agha I, et al. Efficacy and safety of defibrotide in the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome following haematopoietic stem cell transplantation: interim results from the DEFIFrance study. Poster presented at: 45th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT); 24–27 March 2019; Frankfurt, Germany.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transpl. 2016;51:906–12.
Mohty M, Malard F, Abecasis M, Aerts E, Alaskar AS, Aljurf M, et al. Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a position statement from an international expert group. Bone Marrow Transpl. 2019;55:485–95.
Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transpl. 2019;25:1271–80.
Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Remenyi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–e39.
Danylesko I, Shimoni A, Nagler A. Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transpl. 2012;47:5–14.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83.
Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729–36.
Cairo MS, Cooke KR, Lazarus HM, Chao N. Modified diagnostic criteria, grading classification and newly elucidated pathophysiology of hepatic SOS/VOD after haematopoietic cell transplantation. Br J Haematol. 2020;190:822–36.
Acknowledgements
This study was supported by Jazz Pharmaceuticals. Medical writing and editorial assistance were provided by Erica Chevalier-Larsen, PhD, CMPP™, of SciFluent Communications, Inc., and were financially supported by Jazz Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
MM, ML, and RJR designed the research study. MLB, DB, EC, SC, NM, KP, CR, RW, MZ, and SL performed the research. MM, NM, ML, RJ, VA, and RJR analysed the data. FC contributed essential reagents or tools. All authors wrote and critically revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
MM has received honoraria and research funding from Jazz Pharmaceuticals. DB and ML have received honoraria from Jazz Pharmaceuticals. RH, VA, and RJR are employees of and hold stock ownership and/or stock options in Jazz Pharmaceuticals. SL has received a consultancy honorarium from Jazz Pharmaceuticals. MLB, EC, SC, NM, KP, CR, RW, MZ and FC have no competing interests to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mohty, M., Battista, M.L., Blaise, D. et al. A multicentre, multinational, prospective, observational registry study of defibrotide in patients diagnosed with veno-occlusive disease/sinusoidal obstruction syndrome after haematopoietic cell transplantation: an EBMT study. Bone Marrow Transplant 56, 2454–2463 (2021). https://doi.org/10.1038/s41409-021-01265-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01265-2
This article is cited by
-
HokUS-10 scoring system predicts the treatment outcome for sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation
Scientific Reports (2023)
-
Hypofibrinolysis in pediatric patients with veno-occlusive disease in hematopoietic stem cell transplantation
Journal of Cancer Research and Clinical Oncology (2023)
-
Real-world use of defibrotide for veno-occlusive disease/sinusoidal obstruction syndrome: the DEFIFrance Registry Study
Bone Marrow Transplantation (2023)
-
Defibrotide-treated patients with anicteric or icteric veno-occlusive disease/sinusoidal obstruction syndrome after hematopoietic cell transplantation: an EBMT study
Bone Marrow Transplantation (2022)
-
Serum levels of albumin and creatinine predict the outcome of sinusoidal obstruction syndrome after allogeneic HSCT
Annals of Hematology (2022)