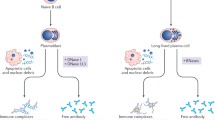
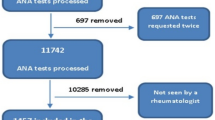
Abstract
Antinuclear antibodies (ANAs) are valuable laboratory markers to screen for and support the diagnosis of various rheumatic diseases (known as ANA-associated rheumatic diseases). The importance of ANA testing has been reinforced by the inclusion of ANA positivity as an entry criterion in the 2019 systemic lupus erythematosus classification criteria. In addition, specific ANAs (such as antibodies to Sm, double-stranded DNA (dsDNA), SSA/Ro60, U1RNP, topoisomerase I, centromere protein B (CENPB), RNA polymerase III and Jo1) are included in classification criteria for other rheumatic diseases. A number of techniques are available for detecting antibodies to a selection of clinically relevant antigens (such as indirect immunofluorescence and solid phase assays). In this Review, we discuss the advantages and limitations of these techniques, as well as the clinical relevance of the differences between the techniques, to provide guidance in understanding and interpreting ANA test results. Such understanding not only necessitates insight into the sensitivity and specificity of each assay, but also into the importance of the disease context and antibody level. We also highlight the value of titre-specific information (such as likelihood ratios).
Key points
-
Clinicians should be aware of the type of assay used for antinuclear antibody detection and the advantages and disadvantages of using immunofluorescence (IIF) assays and solid phase immunoassays (SPAs) for screening.
-
IIF assays can vary in performance between different laboratories, whereas the performance of fully automated assays is more consistent.
-
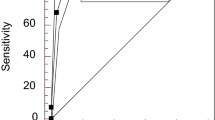
The performance characteristics of IIF assays and SPAs are disease-dependent; IIF assays are more sensitive than SPA for screening for systemic sclerosis (and systemic lupus erythematosus) but not Sjögren syndrome.
-
For both IIF assays and SPAs, no single cut-off has both good sensitivity and good specificity; combining IIF with SPA has the highest clinical value.
-
A dichotomous interpretation of test results overlooks important information at the antibody level; this limitation of antinuclear antibody testing can be overcome by reporting test result-specific likelihood ratios.
-
The differences among the assays are not only important considerations when screening and diagnosing patients, but also when using these assays for classifying patients (for example, for clinical trial enrolment).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mahler, M., Meroni, P. L., Bossuyt, X. & Fritzler, M. J. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J. Immunol. Res. 2014, 315179 (2014).
Meroni, P. L. & Schur, P. H. ANA screening: an old test with new recommendations. Ann. Rheum. Dis. 69, 1420–1422 (2010).
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 78, 1151–1159 (2019).
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 76, 9–16 (2017).
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 72, 1747–1755 (2013).
Alarcon-Segovia, D. & Villareal, M. Classification and diagnostic criteria for mixed connective tissue disease. in Mixed connective tissue diseases and antinuclear antibodies (eds Kasukawa, R. & Sharp, G. C.) 33–40 (Elsevier, 1987).
Lundberg, I. E. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 76, 1955–1964 (2017).
Damoiseaux, J. et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 78, 879–889 (2019).
Chan, E. K. et al. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014–2015. Front. Immunol. 6, 412 (2015).
Cavazzana, I. et al. Evaluation of a novel particle-based assay for detection of autoantibodies in idiopathic inflammatory myopathies. J. Immunol. Methods 474, 112661 (2019).
Agmon-Levin, N. et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 73, 17–23 (2014).
Tan, E. M. et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 40, 1601–1611 (1997).
Leuchten, N. et al. Detection of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res. 70, 428–438 (2018).
Op De Beeck, K. et al. Detection of antinuclear antibodies by indirect immunofluorescence and by solid phase assay. Autoimmun. Rev. 10, 801–808 (2011).
Willems, P. et al. Screening for connective tissue disease-associated antibodies by automated immunoassay. Clin. Chem. Lab. Med. 56, 909–918 (2018).
Willems, P. et al. Antinuclear antibody as entry criterion for classification of systemic lupus erythematosus: pitfalls and opportunities. Ann. Rheum. Dis. 78, e76 (2019).
Mariz, H. A. et al. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 63, 191–200 (2011).
Vulsteke, J. B. et al. Titre-specific positive predictive value of antinuclear antibody patterns. Ann. Rheum. Dis. pii: annrheumdis-2019–216245 (2019).
Albesa, R. et al. Increased prevalence of anti-DFS70 antibodies in young females: experience from a large international multi-center study on blood donors. Clin. Chem. Lab. Med. 57, 999–1005 (2019).
Conrad, K., Röber, N., Andrade, L. E. & Mahler, M. The clinical relevance of anti-DFS70 autoantibodies. Clin. Rev. Allergy Immunol. 52, 202–216 (2017).
Mahler, M., Andrade, L. E., Casiano, C. A., Malyavantham, K. & Fritzler, M. J. Anti-DFS70 antibodies: an update on our current understanding and their clinical usefulness. Expert. Rev. Clin. Immunol. 15, 241–250 (2019).
Bonroy, C. et al. The importance of detecting anti-DFS70 in routine clinical practice: comparison of different care settings. Clin. Chem. Lab. Med. 56, 1090–1099 (2018).
Bossuyt, X. et al. Detection of antinuclear antibodies by automated indirect immunofluorescence analysis. Clin. Chim. Acta 415, 101–106 (2013).
Claessens, J. et al. Solid phase assays versus automated indirect immunofluorescence for detection of antinuclear antibodies. Autoimmun. Rev. 17, 533–540 (2018).
Van Hoovels, L. et al. Analytical performance of the single well titer function of NOVA View®: good enough to omit ANA IIF titer analysis? Clin. Chem. Lab. Med. 56, 258–261 (2018).
Oyaert, M., Bossuyt, X., Ravelingien, I. & Van Hoovels, L. Added value of indirect immunofluorescence intensity of automated antinuclear antibody testing in a secondary hospital setting. Clin. Chem. Lab. Med. 54, e63–e66 (2016).
Schouwers, S. et al. Value-added reporting of antinuclear antibody testing by automated indirect immunofluorescence analysis. Clin. Chem. Lab. Med. 52, 547–551 (2014).
Bossuyt, X. et al. Harmonization of clinical interpretation of antinuclear antibody test results by solid phase assay and by indirect immunofluorescence through likelihood ratios. Autoimmun. Rev. 11, 102386 (2019).
Bossuyt, X. & Luyckx, A. Antibodies to extractable nuclear antigens in antinuclear antibody-negative samples. Clin. Chem. 51, 2426–2427 (2005).
Hoffman, I. E. A., Peene, I., Veys, E. & De Keyser, F. Detection of specific antinuclear reactivities in patients with negative anti-nuclear antibody immunofluorescence screening test. Clin. Chem. 48, 2171–2176 (2002).
Mahler, M. et al. International multicenter evaluation of autoantibodies to ribosomal P proteins. Clin. Vaccine Immunol. 13, 77–83 (2006).
Fritzler, M. J., Choi, M. Y. & Mahler, M. The anti-nuclear antibody (ANA) test in the diagnosis of anti-synthetase syndrome and other autoimmune myopathies (AIM). J. Rheumatol. 45, 444–445 (2018).
Ceribelli, A. et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res. Ther. 14, R97 (2012).
Stuhlmüller, B., Schneider, U., González-González, J. B. & Feist, E. Disease specific autoantibodies in idiopathic inflammatory myopathies. Front. Neurol. 10, 4382019 (2019).
Pisetsky, D. S., Thompson, D. K., Wajdula, J., Diehl, A. & Sridharan, S. Variability in antinuclear antibody testing to assess patient eligibility for clinical trials of novel treatments for systemic lupus erythematosus. Arthritis Rheumatol. 71, 1534–1538 (2019).
Pisetsky, D. S., Spencer, D. M., Lipsky, P. E. & Rovin, B. H. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann. Rheum. Dis. 77, 911–913 (2018).
Pregnolato, F., Borghi, M. O., Meroni, P. L. & Forum Interdisciplinare per la Ricerca sulle Malattie Autoimmuni (FIRMA) Study Group. Pitfalls of antinuclear antibody detection in systemic lupus erythematosus: the positive experience of a national multicentre study. Ann. Rheum. Dis. 78, e50 (2019).
Pham, B. N., Albarede, S., Guyard, A., Burg, E. & Maisonneuve, P. Impact of external quality assessment on antinuclear antibody detection performance. Lupus. 14, 113–119 (2005).
Van Blerk, M. et al. Current practices in antinuclear antibody testing: results from the Belgian External Quality Assessment Scheme. Clin. Chem. Lab. Med. 47, 102–108 (2009).
Van Hoovels, L., Schouwers, S., Van den Bremt, S. & Bossuyt, X. Variation in antinuclear antibody detection by automated indirect immunofluorescence analysis. Ann Rheum Dis. 78, e48 (2018).
van der Pol, P., Bakker-Jonges, L. E., Kuijpers, J. H. S. A. M. & Schreurs, M. W. J. Analytical and clinical comparison of two fully automated immunoassay systems for the detection of autoantibodies to extractable nuclear antigens. Clin. Chim. Acta. 476, 54–159 (2018).
Jeong, S. et al. Evaluation of an automated screening assay, compared to indirect immunofluorescence, an extractable nuclear antigen assay, and a line immunoassay in a large cohort of Asian patients with antinuclear antibody-associated rheumatoid diseases: a multicenter retrospective study. J. Immunol. Res. 2018, 9094217 (2018).
Robier, C., Amouzadeh-Ghadikolai, O., Stettin, M. & Reicht, G. Comparison of the clinical utility of the Elia CTD Screen to indirect immunofluorescence on HEp-2 cells. Clin. Chem. Lab. Med. 54, 365–370 (2016).
Otten, H. G. et al. Measurement of antinuclear antibodies and their fine specificities: time for a change in strategy? Clin. Exp. Rheumatol. 35, 462–470 (2017).
Bentow, C. et al. Clinical performance evaluation of a novel, automated chemiluminescent immunoassay, QUANTA Flash CTD Screen Plus. Immunol. Res. 61, 110–116 (2015).
Bizzaro, N. et al. The association of solid-phase assays to immunofluorescence increases the diagnostic accuracy for ANA screening in patients with autoimmune rheumatic diseases. Autoimmun. Rev. 17, 541–547 (2018).
Op De Beéck, K. et al. Antinuclear antibody detection by automated multiplex immunoassay in untreated patients at the time of diagnosis. Autoimmun. Rev. 12, 137–143 (2012).
de Almeida Brito, F. et al. Diagnostic Evaluation of ELISA and chemiluminescent assays as alternative screening tests to indirect immunofluorescence for the detection of antibodies to cellular antigens. Am. J. Clin. Pathol. 145, 323–331 (2016).
Orme, M. E., Andalucia, C., Sjölander, S. & Bossuyt, X. A hierarchical bivariate meta-analysis of diagnostic test accuracy to provide direct comparisons of immunoassays versus indirect immunofluorescence for initial screening of connective tissue diseases. Clin. Chem. Lab. Med. https://doi.org/10.1515/cclm-2020-0094 (2020).
Orme, M. E., Andalucia, C., Sjölander, S. & Bossuyt, X. A comparison of a fluorescence enzyme immunoassay versus indirect immunofluorescence for initial screening for connective tissue diseases: systematic literature review and meta-analysis of diagnostic test accuracy studies. Best. Pract. Res. Clin. Rheumatol. 32, 521–534 (2018).
Bossuyt, X. et al. Antinuclear antibodies by indirect immunofluorescence and solid phase assays. Ann. Rheum. Dis. 79, e65 (2019).
Bossuyt, X. & Fieuws, S. Detection of antinuclear antibodies: added value of solid phase assay? Ann. Rheum. Dis. 73, e10 (2014).
Meroni, P. L. et al. Unending story of the indirect immunofluorescence assay on HEp-2 cells: old problems and new solutions? Ann. Rheum. Dis. 78, e46 (2019).
Pisetsky, D. S., Spencer, D. M., Rovin, B. & Lipsky, P. E. Role of ANA testing in the classification of patients with systemic lupus erythematosus. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2019-216259 (2019).
Pisetsky, D. S., Spencer, D. M., Lipsky, P. E. & Rovin, B. H. Response to ‘Antinuclear antibodies by indirect immunofluorescence and solid phase assays’ by Bossuyt et al. Ann. Rheum. Dis. 79, e66 (2019).
Bizzaro, N. Can solid-phase assays replace immunofluorescence for ANA screening? Ann. Rheum. Dis. 79, e32 (2018).
Pisetsky, D. S., Bossuyt, X. & Meroni, P. L. ANA as an entry criterion for the classification of SLE. Autoimmun. Rev. 18, 102400 (2019).
Damoiseaux, J. et al. Autoantibodies in idiopathic inflammatory myopathies: clinical associations and laboratory evaluation by mono- and multispecific immunoassays. Autoimmun. Rev. 18, 293–305 (2019).
Cavazzana, I. et al. Testing for myositis specific autoantibodies: comparison between line blot and immunoprecipitation assays in 57 myositis sera. J. Immunol. Methods 433, 1–5 (2016).
Vulsteke, J. B. et al. Detection of myositis-specific antibodies. Ann. Rheum. Dis. 78, e7 (2019).
Platteel, A. C. M. et al. Frequencies and clinical associations of myositis-related antibodies in The Netherlands: a one-year survey of all Dutch patients. J. Transl Autoimmunity 2, 100013 (2019).
Infantino, M. et al. Combining immunofluorescence with immunoblot assay improves the specificity of autoantibody testing for myositis. Rheumatology 58, 1239–1244 (2019).
Picard, C. et al. Heterogeneous clinical spectrum of anti-SRP myositis and importance of the methods of detection of anti-SRP autoantibodies: a multicentric study. Immunol. Res. 64, 677–686 (2016).
Kaji, K. et al. Autoantibodies to RuvBL1 and RuvBL2: a novel systemic sclerosis-related antibody associated with diffuse cutaneous and skeletal muscle involvement. Arthritis Care Res. 66, 575–584 (2014).
Hennes, E. M. et al. International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 48, 169–176 (2008).
Alvarez, F. et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol. 31, 929–938 (1999).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 67, 145–172 (2017).
Martini, A. et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 46, 190–197 (2019).
Fierz, W. & Bossuyt, X. Likelihood ratios as value proposition for diagnostic laboratory tests. J. Appl. Lab. Med. 5, 1061–1069 (2020).
Choi, B. C. Slopes of a receiver operating characteristic curve and likelihood ratios for a diagnostic test. Am. J. Epidemiol. 148, 1127–1132 (1998).
Author information
Authors and Affiliations
Contributions
X.B. researched data for the article and wrote the article. P.L.M. and M.O.B. made substantial contributions to discussion of the content. E.D.L., M.O.B. and P.L.M. reviewed and edited the manuscript before submission
Corresponding author
Ethics declarations
Competing interests
X.B. has been a consultant for Inova Diagnostics and Thermo-Fisher and has received lecture fees from Inova Diagnostics, Menarini and Thermo Fisher. P.L.M. has been a consultant for Inova Diagnostics and Thermo-Fisher and has received lecture fees from Inova Diagnostics, and Thermo Fisher. E.D.L. and M.O.B. declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks L. Andrade, J. Damoiseaux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Specificity
-
The ability of a test to correctly exclude individuals who do not have the disease; the specificity is calculated as the fraction of individuals without the disease who test negative.
- Sensitivity
-
The ability of a test to correctly detect patients with a disease; the sensitivity of a test is calculated as the fraction of patients with the disease who test positive.
- Likelihood ratio
-
The ratio of the probability of a particular test result for a patient with a particular disease and the probability of the same test result for an individual without the disease.
Rights and permissions
About this article
Cite this article
Bossuyt, X., De Langhe, E., Borghi, M.O. et al. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat Rev Rheumatol 16, 715–726 (2020). https://doi.org/10.1038/s41584-020-00522-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-00522-w
This article is cited by
-
Phenotypic and Immunological Characterization of Patients with Activated PI3Kδ Syndrome 1 Presenting with Autoimmunity
Journal of Clinical Immunology (2024)
-
Diagnosis and management of autoimmune diseases in the ICU
Intensive Care Medicine (2024)
-
Contributions of Synthetic Chemicals to Autoimmune Disease Development and Occurrence
Current Environmental Health Reports (2024)
-
Negative ANA-IIF in SLE patients: what is beyond?
Clinical Rheumatology (2023)
-
Laboratory and clinical practices in antinuclear antibody detection and related antigens: recommendations from a Spanish multicentre survey
Immunologic Research (2023)