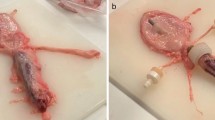
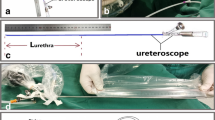
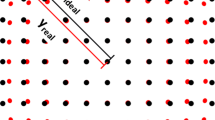
Abstract
The ureteric wall is a complex multi-layered structure. The ureter shows variation in passive mechanical properties, histological morphology and insertion forces along the anatomical length. Ureter mechanical properties also vary depending on the direction of tensile testing and the anatomical region tested. Compliance is greatest in the proximal ureter and lower in the distal ureter, which contributes to the role of the ureter as a high-resistance sphincter. Similar to other human tissues, the ureteric wall remodels with age, resulting in changes to the mechanical properties. The passive mechanical properties of the ureter vary between species, and variation in tissue storage and testing methods limits comparison across some studies. Knowledge of the morphological and mechanical properties of the ureteric wall can aid in understanding urine transport and safety thresholds in surgical techniques. Indeed, various factors alter the forces required to insert access sheaths or scopes into the ureter, including sheath diameter, safety wires and medications. Future studies on human ureteric tissue both in vivo and ex vivo are required to understand the mechanical properties of the ureter and how forces influence these properties. Testing of instrument insertion forces in humans with a focus on defining safe upper limits and techniques to reduce trauma are also needed. Last, evaluation of dilatation limits in the mid and proximal ureter and clarification of tensile strength anisotropy in human specimens are necessary.
Key points
-
The ureter shows variation in passive mechanical properties along the anatomical length. The proximal and distal ureter have the highest and lowest compliance, respectively.
-
The uniaxial tensile strength of the ureter is anisotropic between axial and circumferential directions; however, results vary between studies, and further experiments are needed in human tissues.
-
The passive mechanical properties of the ureter change with age, probably owing to the remodelling of the ureter wall.
-
Various factors affect the forces required to insert ureteroscopes and ureteral access sheaths into the ureter, including sheath diameter, medications and the use of safety wires.
-
Excessive insertion and withdrawal forces are associated with substantial ureteral trauma, and further testing of insertion forces in humans with a focus on defining a safe upper limit are required.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gregersen, H. et al. Regional differences exist in elastic wall properties in the ureter. Scand. J. Urol. Nephrol. 30, 343–348 (1996).
Knudsen, L. et al. Elastic wall properties and collagen content in the ureter: an experimental study in pigs. Neurourol. Urodyn. 13, 597–606 (1994). discussion 606-8.
Sokolis, D. P. Identification and characterisation of regional variations in the material properties of ureter according to microstructure. Comput. Methods Biomech. Biomed. Eng. 17, 1653–1670 (2014).
Griffiths, D. J. Flow of urine through the ureter: a collapsible, muscular tube undergoing peristalsis. J. Biomech. Eng. 111, 206–211 (1989).
Simeone, C. et al. [Structure, physiology and physiopathology of the ureter]. Arch. Ital. Urol. Nefrol. Androl. 65, 21–25 (1993).
Fung, Y.-C., Biomechanics: mechanical properties of living tissues. (Springer Science & Business Media, 2013).
Hansen, I. & Gregersen, H. Morphometry and residual strain in porcine ureter. Scand. J. Urol. Nephrol. 33, 10–16 (1999).
Petsepe, D. C. et al. Regional and age-dependent residual strains, curvature, and dimensions of the human ureter. Proc. Inst. Mech. Eng. H. 232, 149–162 (2018).
Ordon, M., Schuler, T. D. & Honey, R. J. Ureteral avulsion during contemporary ureteroscopic stone management: “the scabbard avulsion”. J. Endourol. 25, 1259–1262 (2011).
Türk, C. et al. EAU guidelines on interventional treatment for urolithiasis. Eur. Urol. 69, 475–482 (2016).
Wein, A.J. et al. Campbell-Walsh urology: expert consult premium edition: enhanced online features and print, 4-volume set. (Elsevier Health Sciences, 2011).
Novaes, H. F. et al. Analysis of ureteral length in adult cadavers. Int. Braz. J. Urol. 39, 248–256 (2013). discussion 256.
Osman, F. et al. Ureteral motility. Acta Physiol. Hung. 96, 407–426 (2009).
Hammad, F. T. Electrical propagation in the renal pelvis, ureter and bladder. Acta Physiol. 213, 371–383 (2015).
Venkatesh, R. et al. Impact of a double-pigtail stent on ureteral peristalsis in the porcine model: initial studies using a novel implantable magnetic sensor. J. Endourol. 19, 170–176 (2005).
Griffiths, D.J. & C. Notschaele, The mechanics of urine transport in the upper urinary tract: 1. The dynamics of the isolated bolus. Neurourol. Urodyn. 2, https://doi.org/10.1002/nau.1930020209 (2005).
Kinn, A. C. & Lykkeskov-Andersen, H. Impact on ureteral peristalsis in a stented ureter. An experimental study in the pig. Urol. Res. 30, 213–218 (2002).
Boone, A. W. & Smith, A. G. The elastic properties of normal ureter. J. Urol. 73, 481–486 (1955).
Yin, F. C. & Fung, Y. C. Mechanical properties of isolated mammalian ureteral segments. Am. J. Physiol. 221, 1484–1493 (1971).
Weiss, R. M., Bassett, A. L. & Hoffman, B. F. Dynamic length-tension curves of cat ureter. Am. J. Physiol. 222, 388–393 (1972).
Apter, J. T. & Mason Dynamic mechanical properties of mammalian ureteral muscle. Am. J. Physiol. 221, 266–272 (1971).
Sokolis, D. P. Multiaxial mechanical behaviour of the passive ureteral wall: experimental study and mathematical characterisation. Comput. Methods Biomech. Biomed. Eng. 15, 1145–1156 (2012).
Sokolis, D. P. et al. Age- and region-related changes in the biomechanical properties and composition of the human ureter. J. Biomech. 51, 57–64 (2017).
Rassoli, A. et al. Biaxial mechanical properties of human ureter under tension. Urol. J. 11, 1678–1686 (2011).
Stemper, B. D. et al. Mechanics of fresh, refrigerated, and frozen arterial tissue. J. Surg. Res. 139, 236–242 (2007).
Shilo, Y. et al. Evaluation of the tensile strength of the human ureter–preliminary results. J. Endourol. 28, 1470–1473 (2014).
Sokolis, D. P. In vitro study of age-related changes in human ureteral failure properties according to region, direction, and layer. Proc. Inst. Mech. Eng. H. 233, 570–583 (2019).
Hanczar, M., Moazen, M. & Day, R. The significance of biomechanics and scaffold structure for bladder tissue engineering. Int. J. Mol. Sci. 22, 12657 (2021).
Natali, A. N. et al. Bladder tissue biomechanical behavior: experimental tests and constitutive formulation. J. Biomech. 48, 3088–3096 (2015).
Lichtenstein, O. et al. Static and dynamic mechanical properties of the carotid artery from normotensive and hypertensive rats. Hypertension 32, 346–350 (1998).
Monson, K. L. et al. Axial mechanical properties of fresh human cerebral blood vessels. J. Biomech. Eng. 125, 288–294 (2003).
Cunnane, E. M. et al. Mechanical, compositional and morphological characterisation of the human male urethra for the development of a biomimetic tissue engineered urethral scaffold. Biomaterials 269, 120651 (2021).
Zhao, J. B. et al. Morphological properties and residual strain along the small intestine in rats. World J. Gastroenterol. 8, 312–317 (2002).
Fung, Y. C. What are the residual stresses doing in our blood vessels? Ann. Biomed. Eng. 19, 237–249 (1991).
Moulton, D. E. & Goriely, A. Circumferential buckling instability of a growing cylindrical tube. J. Mech. Phys. Solids 59, 525–537 (2011).
Pedro, R. N. et al. In vitro evaluation of ureteral perforation forces. Urology 70, 592–594 (2007). discussion 594–595.
Vahidi, B. & Fatouraee, N. A biomechanical simulation of ureteral flow during peristalsis using intraluminal morphometric data. J. Theor. Biol. 298, 42–50 (2012).
Vahidi, B. et al. A mathematical simulation of the ureter: effects of the model parameters on ureteral pressure/flow relations. J. Biomech. Eng. 133, 031004 (2011).
Hosseini, G. et al. Simulation of the upper urinary system. Crit. Rev. Biomed. Eng. 41, 259–268 (2013).
Imamura, T. et al. Morphological profile of atypical femoral fractures: age-related changes to the cross-sectional geometry of the diaphysis. J. Anat. 235, 892–902 (2019).
Saini, A., Berry, C. & Greenwald, S. Effect of age and sex on residual stress in the aorta. J. Vasc. Res. 32, 398–405 (1995).
Coentro, J. Q. et al. Collagen quantification in tissue specimens. Methods Mol. Biol. 1627, 341–350 (2017).
Lareu, R. R. et al. Essential modification of the Sircol Collagen Assay for the accurate quantification of collagen content in complex protein solutions. Acta Biomater. 6, 3146–3151 (2010).
De Coninck, V. et al. Systematic review of ureteral access sheaths: facts and myths. BJU Int. 122, 959–969 (2018).
Tefik, T. et al. Impact of ureteral access sheath force of insertion on ureteral trauma: in vivo preliminary study with 7 patients. Ulus. Travma Acids. Cerrahi Derg. 24, 514–520 (2018).
Traxer, O. & Thomas, A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J. Urol. 189, 580–584 (2013).
Fukui, S. et al. Examining the impact of different properties of ureteral access sheaths in reducing insertion force during retrograde intrarenal surgery: an in vitro study. J. Endourol. 35, 1757–1763 (2021).
Patel, N. & Monga, M. Ureteral access sheaths: a comprehensive comparison of physical and mechanical properties. Int. Braz. J. Urol. 44, 524–535 (2018).
Eandi, J. A., Hu, B. & Low, R. K. Evaluation of the impact and need for use of a safety guidewire during ureteroscopy. J. Endourol. 22, 1653–1658 (2008).
Ulvik, Ø. & Wentzel-Larsen, T. A novel method to measure the mechanical pushing and pulling forces during ureteroscopy in a normal clinical setting. J. Endourol. 27, 625–630 (2013).
Ulvik, Ø., Wentzel-Larsen, T. & Ulvik, N. M. A safety guidewire influences the pushing and pulling forces needed to move the ureteroscope in the ureter: a clinical randomized, crossover study. J. Endourol. 27, 850–855 (2013).
Graversen, J. A. et al. The effect of extralumenal safety wires on ureteral injury and insertion force of ureteral access sheaths: evaluation using an ex vivo porcine model. Urology 79, 1011–1014 (2012).
Harper, J. D. et al. Comparison of a novel radially dilating balloon ureteral access sheath to a conventional sheath in the porcine model. J. Urol. 179, 2042–2045 (2008).
Kaler, K. S. et al. Ureteral access sheath deployment: how much force is too much? Initial studies with a novel ureteral access sheath force sensor in the porcine ureter. J. Endourol. 33, 712–718 (2019).
Koo, K. C. et al. Efficacy and safety of ultrasonic longitudinal-axis vibration for the reduction of ureteral access sheath insertion force: a randomized controlled trial in a porcine model. J. Endourol. 33, 140–145 (2019).
Koo, K. C. et al. The impact of preoperative α-adrenergic antagonists on ureteral access sheath insertion force and the upper limit of force required to avoid ureteral mucosal injury: a randomized controlled study. J. Urol. 199, 1622–1630 (2018).
Lildal, S. K. et al. Pharmacological relaxation of the ureter when using ureteral access sheaths during ureterorenoscopy: a randomized feasibility study in a porcine model. Adv. Urol. 2016, 8064648 (2016).
Patel, R. M. et al. Analysis of ureteral diameter and peristalsis in response to irrigant fluid temperature changes in an in vivo porcine model. J. Endourol. 35, 1236–1243 (2021).
Tapiero, S. et al. Determining the safety threshold for the passage of a ureteral access sheath in clinical practice using a purpose-built force sensor. J. Urol. 206, 364–372 (2021).
A. Skolarikos, et al. EAU Guidelines on Urolithiasis (2022).
Dickstein, R. J. et al. Is a safety wire necessary during routine flexible ureteroscopy? J. Endourol. 24, 1589–1592 (2010).
Ulvik, Ø. et al. Ureteroscopy with and without safety guide wire: should the safety wire still be mandatory? J. Endourol. 27, 1197–1202 (2013).
Stern, K. L. et al. The safety wire with a ureteral access sheath — does it hurt more than it helps? Can. J. Urol. 26, 9733–9735 (2019).
Park, A. & Venkatesh, R. Understanding the ureter: challenges and opportunities. J. Endourol. 30, S34–S36 (2016).
Jiang, P. et al. The impact of one week of pre-stenting on porcine ureteral luminal circumference. J. Endourol. 36, 885–890 (2022).
Kaler, K. S. et al. Medical impulsive therapy (MIT): the impact of 1 week of preoperative tamsulosin on deployment of 16-French ureteral access sheaths without preoperative ureteral stent placement. World J. Urol. 36, 2065–2071 (2018).
Lu, C. et al. Endoscopic balloon dilatation in the treatment of benign ureteral strictures: a meta-analysis and systematic review. J. Endourol. 33, 255–262 (2019).
Huffman, J. L. & Bagley, D. H. Balloon dilation of the ureter for ureteroscopy. J. Urol. 140, 954–956 (1988).
Selmy, G. et al. Effect of balloon dilation of ureter on upper tract dynamics and ureteral wall morphology. J. Endourol. 7, 211–219 (1993).
Collyer, W. C. et al. Assessment of optimal balloon size for rupture of the ureteropelvic junction and mid-ureter in a porcine model. J. Endourol. 15, 937–942 (2001).
Galal, H. et al. Management of ureteral strictures by different modalities and effect of stents on upper tract drainage. J. Endourol. 7, 411–417 (1993).
Kuntz, N. J. et al. Balloon dilation of the ureter: a contemporary review of outcomes and complications. J. Urol. 194, 413–417 (2015).
Lim, G. W. et al. Retrograde balloon dilation as a therapeutic option for post-gynecologic surgery ureteral stricture followed by ureteroureterostomy: a comparative study regarding stricture length. Yeungnam Univ. J. Med. 35, 179–186 (2018).
Sugita, Y., Clarnette, T. D. & Hutson, J. M. Retrograde balloon dilatation for primary pelvi-ureteric junction stenosis in children. Br. J. Urol. 77, 587–589 (1996).
de la Rosette, J. J. M. C. H., Skrekas, T. & Segura, J. W. Handling and prevention of complications in stone basketing. Eur. Urol. 50, 991–999 (2006).
Sarkissian, C. et al. Tissue damage from ultrasonic, pneumatic, and combination lithotripsy. J. Endourol. 29, 162–170 (2015).
Lange, D. et al. Ureteral stent-associated complications — where we are and where we are going. Nat. Rev. Urol. 12, 17–25 (2015).
Corneli, A. et al. The patient voice: stent experiences after ureteroscopy — insights from in-depth interviews with participants in the USDRN STENTS nested qualitative cohort study. J. Endourol. 37, 642–653 (2023).
Ramsay, J. W. et al. The effects of double J stenting on unobstructed ureters. An experimental and clinical study. Br. J. Urol. 57, 630–634 (1985).
Scotland, K. B. et al. Indwelling stents cause obstruction and induce ureteral injury and fibrosis in a porcine model. BJU Int. 131, 367–375 (2023).
Reicherz, A. et al. Indwelling stents cause severe inflammation and fibrosis of the ureter via urothelial–mesenchymal transition. Sci. Rep. 13, 5492 (2023).
van Mastrigt, R., Glerum, J. J. & Tauecchio, E. A. Variation of passive mechanical properties of the ureter along its length. Urol. Int. 36, 145–151 (1981).
Acknowledgements
Funding for this review was provided by the MD StAR programme of the Royal College of Surgeons of Ireland.
Author information
Authors and Affiliations
Contributions
S.O.M. and S.M.C. researched data for the article. S.O.M., E.M.C., M.T.W. and N.F.D. contributed substantially to discussion of the content. S.O.M., E.M.C. and N.F.D. wrote the article. S.O.M., E.M.C., C.V.C., F.J.O.B. and N.F.D. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Dimitrios Sokolis, Dirk Lange and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Anisotropy
-
Variation in mechanical properties depending on the direction of testing.
- Balloon dilatation
-
Strictures or orifices might be dilated using a balloon. This procedure is usually carried out under radiological guidance using specific devices and can be performed in the urinary tract and other organ systems such as the gastrointestinal tract.
- Benchtop testing
-
Performing experiments in the laboratory setting, typically used to evaluate the correctness of a technique or model.
- Circumferential strain
-
Change in length along the circumferential axis of the structure (ureter).
- Compliance
-
The ease with which a structure elastically deforms in response to force.
- Contrast extravasation
-
The leakage of contrast on imaging. In the ureter, contrast extravasation might suggest perforation or rupture.
- Distensibility
-
Capacity of the ureter to dilate in response to intraluminal pressure.
- Elastic modulus
-
A unit of measurement that describes the ability of a tissue to withstand elastic deformation under stress sand maintain its shape.
- Hydronephrosis
-
Dilatation of the ureter and renal pelvis.
- Laplace’s law
-
A physiological concept that describes the relationship between wall tension, intraluminal pressure and radius. Wall tension Is equal to intraluminal pressure multiplied by radius, and is correlates inversely with wall thickness.
- Newton
-
Amount of force required to make a mass of 1 kilogram accelerate at a rate of 1 metre per second square; in practical terms, this force can be compared with that exerted by an average sized apple on the hand of a person who is holding it.
- Reference state
-
Position from which testing should ideally be performed to ensure that all external and internal forces have been removed.
- Safety wire
-
The use of a wire placed in the renal pelvis during ureterorenoscopy to enable the placement of a guidewire or insertion of instruments in a safe manner.
- Serial dilatation
-
Dilatation of an orifice or structure by starting with a small size of dilator and steadily increasing the size of dilator in increments.
- Sirius red staining
-
A standard staining technique to assess the organization of collagen fibres in tissues.
- Traxer
-
A visual five-point scale used to measure ureteral wall injury. One and five refer to the least and most severe injury, respectively.
- Ureteral access sheath insertion force
-
The force required by a surgeon or a device user to insert an access sheath into the ureter.
- Ureteric access sheath
-
Commonly used devices that are inserted into the ureter through the urethra over a guidewire to enable easy access to the proximal ureter and kidney and to ensure continuous irrigation throughout flexible ureterorenoscopy. These devices are removed at the end of the procedure. The French (Fr) system is used for the sizing, with the first number referring to the size of the inner dilator, and the second number referring to the size of the outer sheath (for example, 12/14 Fr).
- Ureterotomy
-
Cutting into or opening of a ureter.
- Wall tension
-
The reaction force through which the ureter wall resists the forces attempting to expand it.
- Zero-stress state
-
The state with which all external and internal forces are removed. This state provides the reference state from which the stress–strain response can be examined.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Meara, S., Cunnane, E.M., Croghan, S.M. et al. Mechanical characteristics of the ureter and clinical implications. Nat Rev Urol 21, 197–213 (2024). https://doi.org/10.1038/s41585-023-00831-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-023-00831-1