Abstract
Heterocyclic amines (HCAs) are formed by cooking protein-rich foods, for instance, meat and fish, and are listed as possible human carcinogens. In the present study, the presence of five potential HCAs (IQ, MeIQ, MeIQx, 4,8-DiMeIQx, and PhIP) in cooked camel meat burgers was analyzed for the first time. The analysis was performed in home-cooked and fast-food burger samples containing food additives. The applied cooking technique for the home-cooked samples was pan frying for a controlled cooking time and temperature. In the control cooked meat samples (samples that contained no food additives), the concentrations of MeIQx, 4,8-DiMeIQx, and PhIP ranged from 2.47 ng/g to 4.89 ng/g, whereas IQ and MeIQ were found to be below the limit of quantification. The concentrations contents of MeIQx, 4,8-DiMeIQx, and PhIP in the home-cooked and fast-food samples ranged from 1.52 ng/g to 2.13 ng/g and 1.85 ng/g to 3.46 ng/g, respectively. IQ and MeIQ were not detected in either type of sample. In comparison to the control samples, the home-cooked and fast-food samples produced lower levels of HCAs. Such observations could result from the existence of antioxidants in incorporated food additives, which induce pro-oxidative effects with the successive formation and/or scavenging of free radicals.
Similar content being viewed by others
Introduction
Humans are incessantly exposed to unpredictable levels of hazardous chemicals in food, water, and air1. Human exposure to such chemicals can contribute to the development of various cancers2. Heterocyclic amines (HCAs) are known to be foodborne mutagens/carcinogens, detected at low concentrations (parts per billion) in food containing protein-rich meat or fish processed under household cooking conditions1, 3,4,5. The levels of HCAs produced in meat products are mainly dependent on the kinds of meat product, cooking time, and temperature6, 7. To date, more than twenty-five HCAs have been identified in cooked meat products, and they are usually formed from a nonenzymatic chemical reaction (Maillard reaction) between reducing sugars and amino acids that occurs under normal cooking conditions8,9,10. The intake of HCAs through our diet (especially the consumption of cooked meat) has also been considered a cause of various cancers11,12,13,14,15. A number of epidemiological studies have shown a relationship between high cooked-meat intake and the risk of developing cancer11,12,13,14,15. Previously published studies have reported positive relationships between a high cooked-meat intake and the development of several cancers, such as bladder16, colorectal17, 18, breast19, colon, rectum, kidney20, and prostate cancer21.
Based on such findings, the International Agency for Research on Cancer (IARC) has categorized 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), and 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ) as possible human carcinogens, whereas 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) has been categorized as a probable human carcinogen 22. Recently, the National Toxicology Program (NTP) also listed PhIP, MeIQx, MeIQ, and IQ as reasonably anticipated to be a human carcinogen 23. To illustrate the characteristics of real HCA exposure in humans, numerous investigations have examined meat products either thermally processed at home or from fast-food outlets. HCA concentrations in home-cooked meat products have been quantified in numerous studies in Saudi Arabia5, Brazil24, USA25, Singapore26, Spain3, 27, Denmark28, Switzerland29, and Korea30. HCA concentrations in fast-food meat products have also been quantified in a number of investigations in Sweden31, USA32,33,34, Thailand34, Canada35, and the United Kingdom36. These studies have produced valuable data relating to the HCA levels in meat products and can act as important markers from a community health perspective. In addition, these data can also offer information on aspects that affect HCA occurrence and specify means of diminishing or removing such carcinogens.
Camel meat is one of the most commonly eaten meats in the Saudi Arabian diet and, thus, could be a significant source of HCA exposure. Over recent years, fast food has become one of the main channels in the food consumer service industry, especially in Saudi Arabia, where fast-food sales in 2015 totaled 22.6 billion Saudi riyal37. Among the types of fast food, burger meat consumption has grown by 11% due to due to its remarkable appeal to the public37. Therefore, the study of the relationship between HCAs and their role in the etiology of human cancer requires the precise determination of HCAs in such meat products. The objectives of the present investigation were to determine, for the first time, the formation of HCAs in camel meat burgers, to reveal new potential sources of HCAs, and to determine how the concentrations and kinds of HCA could be affected by the addition of food additives typically used in cooking methods in Saudi Arabia.
Results and Discussion
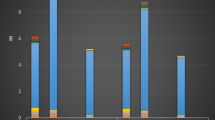
In this study, two types of camel meat burger (home- cooked and fast- food) were analyzed for five potential HCAs. Only the superficial layer of each meat sample was studied since HCAs primarily occur in this layer. The quantity of HCAs obtained in the superficial meat layer was converted into the quantity of HCAs in each entire sample by considering the ratio between the weight of the superficial meat layer and that of the whole meat sample. To check the performance of the method, quality control parameters, such as the linearity, limit of detection (LOD, signal-to-noise ratio 3:1), limit of quantification (LOQ, signal-to-noise ratio 10:1), and recovery values, were studied. The obtained LOD and recovery values are detailed in Table 1. The LOD values ranged between 0.01 ng/g and 0.03 ng/g, while the recovery values ranged from 54% to 65%. The LOD and recovery values were found to be in good agreement with those previously obtained from cooked beef and camel meat products5, 27, 38, 39. The LOD and recovery values in beef and camel meats were comparable, which may be due to the similarity of the meat composition5 and of the SPE techniques applied, with the exception of the extraction solvent being dichloromethane instead of ethyl acetate39, 40. Calibration curves for the studied HCAs were constructed and found to be linear, with correlation coefficients (r 2) greater than 0.998. Table 2 illustrates the mean values of the HCAs and their equivalent standard deviations achieved from three standard addition calibrations spiked at different levels. In the control samples, the signal-to-noise ratio values for IQ and MeIQ were found to be <10; thus, the outcomes were demonstrated to be below the limit of quantification. Furthermore, IQ and MeIQ were not detected in either the home-cooked or fast-food burger samples, and the results indicate the two as not detected. As an example, Fig. 1 displays the UPLC-MS/MS chromatograms of the HCAs in the home-cooked burger samples. It can be seen that the detected concentrations of HCAs varied substantially: whereas PhIP was detected at high concentrations, MeIQx and 4,8-DiMeIQx were detected at lower concentrations. Chromatograms relating to IQ and MeIQ are not presented because they were either not detected or found to be below the limit of quantification in the analyzed samples. It can also be seen that good UPLC-MS/MS sensitivity was attained during HCA determination, while six SRM transitions from the triple quadrupole instrument were acquired at identical times.
Typically, MeIQx, 4,8-DiMeIQx, and PhIP were detected most often in the studied samples, whereas IQ and MeIQ were only detected in the control samples. Among them, PhIP was produced at high concentrations, ranging from 2.13 ng/g to 4.89 ng/g. However, MeIQx and, 4,8-DiMeIQx were produced at lower concentrations, ranging from 1.33 ng/g to 2.47 ng/g. The values obtained for MeIQx, 4,8-DiMeIQx, and PhIP in the control meat samples were found to be in good agreement with those achieved in previous studies5, 38. Generally, IQ and MeIQ were either not detected or generated at very low concentrations in the meat samples, which might be because elevated thermal processing temperatures are necessary for their formation41. In contrast, MeIQx and 4,8-DiMeIQx are typically formed at higher concentrations under household cooking conditions41. PhIP is usually found at higher concentrations in such types of meat, and the concentrations found in the current study were in good agreement with those reported by previous studies34, 42. Nonetheless, in other high-protein foods, such as cooked chicken and fish (swordfish), PhIP was found in very high concentrations (>100 ng/g), especially in swordfish43, 44. The HCA levels in such foods are higher than those detected in the present study and these food products are typically defined as being extremely HCA contaminated43, 44. In addition, PhIP could also be the main HCA in the human diet.
Compared with the control samples, lower concentrations of HCAs were formed in the home-cooked and fast-food samples. MeIQx, 4,8-DiMeIQx, and PhIP were detected in both the sample types at concentrations ranging from 1.33 ng/g to 3.46 ng/g cooked meat, while IQ and MeIQ were not produced in any of the burger samples. These results illustrate that the addition of food additives in both the home-cooked and fast-food samples was sufficient to reduce HCA formation. It can also be observed from Table 2 that the home-cooked samples produced relatively lower concentrations of HCAs than the fast-food samples, which may be due to either the longer cooking time and higher temperature or the food additives incorporated during food preparation45, 46. In previous studies, authors reported lower concentrations of HCAs in meat burgers than in other thermally processed meat products, signifying that the use of food additives diminished the formation of HCAs27. Khan et al. described the effect of food additives on the formation of HCAs in cooked meat products and found a large reduction in HCAs38. For instance, the addition of garlic, ginger, pepper, tomato, and onion reduced the amounts of MeIQx (50–76%), 4,8-DiMeIQx (47–80%), and PhIP (45–78%)38. A number of researchers have determined that plants of the genus Allium have positive antioxidant effects45, 47, 48, likely because of their numerous antioxidant constituents, such as polyphenolics and flavonoids45. This study was the first to determine the HCA concentration in cooked camel meat burgers, which are frequently eaten in Saudi Arabia. Overall, the results revealed that the concentration of HCAs changed with the addition of food additives, as well as with the cooking time and temperature.
Conclusions
The concentrations of five potential HCAs were identified for the first time in camel meat burgers, either home- cooked or from fast-food outlets in Saudi Arabia. The results demonstrated that HCAs were detected in all of the analyzed burger samples. The concentrations of HCAs in the home-cooked and fast-food burgers were lower than in the control burger samples. In the latter, the concentrations of MeIQx, 4,8-DiMeIQx, and PhIP ranged from 2.47 ng/g to 4.89 ng/g, whereas the concentrations of IQ and MeIQ were found to be below the limit of quantification. By contrast, the concentrations of MeIQx, 4,8-DiMeIQx, and PhIP in the home-cooked and fast-food burger samples were relatively lower, ranging from 1.52 ng/g to 3.46 ng/g. IQ and MeIQ were not detected in either type of sample. Such variations could be due to the applied cooking time and temperature, as well as the existence of antioxidants in incorporated food additives, which play an important role in the formation of HCAs. The data obtained from the cooked camel meat burgers could be applied to measure HCA exposure, especially in the Saudi Arabian population, and could be used in epidemiology studies.
Materials and Methods
Chemicals and reagents
HPLC-grade ethyl acetate, methanol, and acetonitrile were obtained from Merck (Darmstadt, Germany). Formic acid (98%), ammonium formate, and ammonium acetate were purchased from Merck (Darmstad, Germany). Sodium hydroxide and ammonia solution (25%) were purchased from BDH Laboratory Supplies (Poole, UK) and Panreac Química (Barcelona, Spain), respectively. All chemicals were of analytical/reagent grade.
The HCAs 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx), 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), and 2-amino-3,4,7,8-tetramethyl-imidazo [4,5-f]quinoxaline (4,7,8-TriMeIQx) (Fig. 2) were obtained from Toronto Research Chemicals (Toronto, Canada). 4,7,8-TriMeIQx was used as an internal standard (I.S.), and the purity of the HCAs was >99%.
Water purification was performed using a Milli-Q water purification system, model Advantage A10 (Millipore Corporation, Bedford, USA). Stock standard solutions of each HCA at a level of 150 µg/g were prepared in organic solvent (methanol) and used for additional dilutions. Standard mixed solutions of the HCAs at levels ranging from 0.003 µg/g to 1.0 µg/g, containing the internal standard 4,7,8-TriMeIQx (0.5 µg/g), were prepared by weight to establish a linear range over which to construct the calibration curves. Before being injected into the ultra-performance liquid chromatography (UPLC) system, the HCA solutions and samples were filtered through a 0.45-µm PTFE filter (Macherey-Nagel GmbH, Düren, Germany). Octadecylsilane C18 (100 mg), Bond Elut propylsulfonyl silica PRS (500 mg) solid-phase extraction (SPE) cartridges, stopcocks, and coupling pieces were obtained from Varian (Harbor City, USA). An Extrelut NT20 extraction column was purchased from Merck (Darmstadt, Germany). Inert diatomaceous earth material was supplied from Agilent Technologies (Apple Valley, USA). Glass vials (40 mL) with screw caps containing a PTFE seal (Thermo Scientific, Rockwood, USA) were used to hold the meat samples.
To assess the cooking temperature of the camel meat, an insulated type K wire probe with the Normadics TC6 software (Cole-Parmer, Vernon Hills, USA) was used. Ultra-Turrax® T25 digital (IKA®, Staufen, Germany) and Microtron® MB800 (Kinematica AG, Littau, Switzerland) mixers were used to blend the heat-treated meat samples. For solid-phase extraction (SPE) and solvent evaporation, Visiprep™ and Visidry™ vacuum manifolds (Supelco, Gland, Switzerland) were applied.
Sample cooking conditions
Twenty camel meat burger samples were obtained from various local fast-food outlets in Riyadh, Saudi Arabia. The samples were ground with a food processor (Microtron MB800), sieved, bottled, and stored at −30 °C until analysis. For the preparation of the three control and three home-cooked burger samples, fresh camel meat and food additives were purchased from a local store in Riyadh, Saudi Arabia. The meat was ground and prepared in 50-g patties that were 5 cm in diameter and 0.5 cm in thickness. However, for the fast-food samples, the size varied. A detailed description of the camel meat burger preparation is provided in Table 3. In brief, the meat patties were pan fried on a non-stick frying pan (Tefal, Paris, France) using a Nippotec electric stove (Shanghai, China). To measure the cooking temperature of the meat patties, five insulated type K wire probes with the Normadics TC6 software were used. The insulated wire probes were applied to the center of the pan, to the lower and upper sides of the patty, between the pan and the patty, and in the center of the patty. The frying temperature of the meat samples ranged from 215 °C to 225 °C, the total cooking time of the patties was 4 min (2 min on each side). The temperature of the patties was permitted to decrease to ambient temperature after cooking. Then, the weight loss of the thermally processed patties was measured as the variance between the quantity of the meat sample before and after the cooking process (Table 3). The superficial layer of the cooked meat, approximately 2–3 mm, was separated, ground, sieved, bottled, and stored at −30 °C until HCA extraction.
HCA extraction
The extraction and purification of HCAs from the thermally processed camel meat samples were performed following formerly established SPE procedures40, 49. The refrigerated samples were removed and allowed to reach ambient temperature. Then, 3-g subsamples from the superficial layer meat samples were homogenized with a solution of sodium hydroxide (1 M) using an Ultra-Turrax® T25 digital blender followed by mixing with inert diatomaceous earth material (13 g). The sample was moved to an Extrelut column coupled to a Bond Elut PRS (500 mg) cartridge, which was preconditioned successively with HCl (15 mL, 0.1 M), water (10 mL), and methanol (5 mL). An organic solvent (ethyl acetate, 75 mL) was used to extract the HCAs from the diatomaceous earth using a coupled Bond Elut PRS (500 mg) cartridge. After complete elution of ethyl acetate from the cartridge, the PRS cartridge was desiccated under vacuum using Visidry™ vacuum manifolds and washed successively with methanol/water (6:4, v/v, 15 mL) and water (2 mL). Subsequently, a Bond Elut C18 (100 mg) cartridge was preconditioned successively with methanol (15 mL) and water (5 mL), and coupled with the PRS cartridge. The desorption of HCAs from the PRS cartridge to the C18 cartridge was performed using an ammonium acetate (0.5 M, 20 mL, pH 8.5) solution. Lastly, the C18 cartridge was washed with water (5 mL) followed by vacuum desiccation. The HCAs were eluted from the C18 cartridge to a 1.5-mL Eppendorf tube (Wesseling-Berzdorf, Germany) with a methanol/ammonia (9:1, v/v, 0.8 mL) mixed solution. Using a nitrogen stream, the obtained solvent mixture containing the HCAs was evaporated until complete dryness. Once dried completely, the extract was dissolved with a methanolic solution (0.1 mL) containing the internal standard 4,7,8-TriMeIQx (0.5 µg/g). Lastly, the methanolic solution was passed through a PTFE syringe filter (0.45 µm) and stored at 4 °C before being injected into the UPLC-MS/MS system.
To overcome sample matrix effects, HCA quantification and recovery were carried out in triplicate using the standard addition method. The standard addition method included three spiked meat samples at different concentrations (50%, 200%, and 400%) and two non-spiked meat samples. The recovery values were measured from the linear regression slope attained from a graph of the spiked HCA amount versus the measured HCA amount. To obtain statistical data, analysis of variance (ANOVA) was used.
HCA determination
Chromatographic separation of the HCAs was achieved on a rapid Acquity® UPLC technique equipped with a quaternary pump (Waters, Milford, USA). A reversed-phase Acquity® BEH C18 analytical column with dimensions of 50 mm × 2.1 mm i.d. and a 1.7 mm particle size (Waters, Milford, USA) was applied. A mixed solution of formic acid/ammonium formate (30 mM, pH 4.75) (A) and organic solvent acetonitrile (B) was used as a binary mobile phase at a flow rate of 1000 µL/min. The elution program of the mobile phase was as follows: 0–0.1 min, 5% B; 0.1–1.5 min, 5–30% B; 1.5–1.8 min, 30–60% B; 1.8–2.4 min, 60% B; 2.4–2.5 min, returned to its initial conditions; 2.5–3 min. Then, 10 µL of the sample was injected into the UPLC system39. To eliminate contamination during analysis, the column was rinsed with a mixture of methanol/water (50/50, v/v) for 10 min every 25 sample injections.
HCA detection was performed on a triple quadrupole mass spectrometer (MS/MS) (Micromass Quattro Premier, Milford, USA) fitted with an electrospray ionization (ESI) source. The mass spectrometer was operated in positive ionization mode. To acquire the UPLC-MS/MS data, selected reaction monitoring (SRM) was used. The optimized ESI source parameters were as follows: source temperature, 100 °C; desolvation temperature, 400 °C; cone voltage, 40 V; capillary voltage, 3.0 kV; cone gas, 49 L/h; desolvation gas, 804 L/h. A nitrogen generator model NM30LA (Peak Scientific, Inchinnan, UK) was used to supply the cone gas to the MS system. An argon gas cylinder was used to supply the collision gases to the MS system. Primary vacuum to the MS system was supplied using an Oerlikon rotary pump, model SOGEVACSV40BI (Cedex, France). The SRM parameters applied to the MS/MS system are given in Table 4. The MassLynx V4.1 software (Waters, Milford, USA) was used to obtain the UPLC-MS/MS data39.
References
Sugimura, T. Studies on environmental chemical carcinogenesis in Japan. Sci 233, 312–318, doi:10.1126/science.3088728 (1986).
Sugimura, T. Food and cancer. Toxicol 181–182, 17–21, doi:10.1016/S0300-483X(02)00250-0 (2002).
Khan, M. R. et al. Mutagenic heterocyclic amine content in thermally processed offal products. Food Chem. 112, 838–843, doi:10.1016/j.foodchem.2008.06.045 (2009).
Keşkekoğlu, H. & Üren, A. Inhibitory effects of pomegranate seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs after cooking by four different methods. Meat Sci. 96, 1446–1451, doi:10.1016/j.meatsci.2013.12.004 (2014).
Khan, M. R. et al. Solid phase extraction and ultra performance liquid chromatography-tandem mass spectrometric identification of carcinogenic/mutagenic heterocyclic amines in cooked camel meat. RSC Adv 5, 2479–2485, doi:10.1039/C4RA13967D (2015).
Gibis, M., Kruwinnus, M. & Weiss, J. Impact of different pan-frying conditions on the formation of heterocyclic aromatic amines and sensory quality in fried bacon. Food Chem. 168, 383–389, doi:10.1016/j.foodchem.2014.07.074 (2015).
Knize, M. G. et al. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem. Toxicol. 32, 595–603, doi:10.1016/0278-6915(94)90002-7 (1994).
Hodge, E. Dehydrated foods: chemistry of browning reactions in model systems. J. Agric. Food Chem. 1, 928–943, doi:10.1021/jf60015a004 (1953).
Dennis, C., Karim, F. & Smith, J. S. Evaluation of maillard reaction variables and their effect on heterocyclic amine formation in chemical model systems. J Food Sci 80, T472–T478, doi:10.1111/jfds.2015.80.issue-2 (2015).
Felton, J. S. & Knize, M. G. Heterocyclic-amine mutagens/carcinogens in foods. In Cooper, C. S. & Grover, P. L. (eds) Handbook of Experimental Pharmacology, Berlin-Heidelberg: Springer-Verlag, Germany, pp. 471–502 (1990).
Helmus, D. S. et al. Red meat-derived heterocyclic amines increase risk of colon cancer: a population-based case-control study. Nutr. Cancer 65, 1141–1150, doi:10.1080/01635581.2013.834945 (2013).
Sugimura, T. Multistep carcinogenesis: a 1992 perspective. Sci. 258, 603–607, doi:10.1126/science.1411570 (1992).
Sinha, R. et al. Dietary intake of heterocyclic amines, meat-derived mutagenic activity, and risk of colorectal adenomas. Cancer Epidemiol. Biomarkers Prev 10, 559–562 (2001).
Sugimura, T. Nutrition and dietary carcinogens. Carcinogenesis 21, 387–395, doi:10.1093/carcin/21.3.387 (2000).
Rohrmann, S., Hermann, S. & Linseisen, J. Heterocyclic aromatic amine intake increases colorectal adenoma risk: findings from a prospective European cohort study. Am J Clin Nutr 89, 1418–1424, doi:10.3945/ajcn.2008.26658 (2009).
Lin, J. et al. Red meat and heterocyclic amine intake, metabolic pathway genes, and bladder cancer risk. Cancer Res. 70, 2825–2825, doi:10.1158/1538-7445.AM10-2825 (2010).
Cross, A. J. et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 70, 2406–2414, doi:10.1158/0008-5472.CAN-09-3929 (2010).
Ollberding, N. J. et al. Meat consumption, heterocyclic amines and colorectal cancer risk: the Multiethnic Cohort Study. Int. J. Cancer 131, E1125–E1133, doi:10.1002/ijc.v131.7 (2012).
Sinha, R. et al. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a carcinogen in high-temperature-cooked meat, and breast cancer risk. J. Natl. Cancer Inst. 92, 1352–1354, doi:10.1093/jnci/92.16.1352 (2000).
Augustsson, K. et al. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet 353, 703–707, doi:10.1016/S0140-6736(98)06099-1 (1999).
Sander, A., Linseisen, J. & Rohrmann, S. Intake of heterocyclic aromatic amines and the risk of prostate cancer in the EPIC-Heidelberg cohort. Cancer Causes Cont 22, 109–114, doi:10.1007/s10552-010-9680-9 (2011).
The International Agency for Research on Cancer (IARC), Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines, and Mycotoxins. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, vol. 56, Lyon, France pp. 165–242 (1993).
The National Toxicology Program (NTP), Report on Carcinogens, Thirteenth Edition. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health USA (2014).
Iwasaki, M. et al. Heterocyclic amines content of meat and fish cooked by Brazilian methods. J. Food Compost. Anal 23, 61–69, doi:10.1016/j.jfca.2009.07.004 (2010).
Keating, G. A. & Bogen, K. T. Methods for estimating heterocyclic amine concentrations in cooked meats in the US diet. Food Chem. Toxicol. 39, 29–43, doi:10.1016/S0278-6915(00)00115-0 (2001).
Salmon, C. P. et al. Heterocyclic aromatic amines in domestically prepared chicken and fish from Singapore Chinese households. Food Chem. Toxicol. 44, 484–492, doi:10.1016/j.fct.2005.08.022 (2006).
Busquets, R. et al. Occurrence of heterocyclic amines in several home-cooked meat dishes of the Spanish diet. J. Chromatogr. B 802, 79–86, doi:10.1016/j.jchromb.2003.09.033 (2004).
Aaslyng, M. D. et al. Content of heterocyclic amines and polycyclic aromatic hydrocarbons in pork, beef and chicken barbecued at home by Danish consumers. Meat Sci. 93, 85–91, doi:10.1016/j.meatsci.2012.08.004 (2013).
Zimmerli, B. et al. Occurrence of heterocyclic aromatic amines in the Swiss diet: analytical method, exposure estimation and risk assessment. Food Addit. Contam. 18, 533–551, doi:10.1080/02652030119545 (2001).
Back, Y. M. et al. Analysis of heterocyclic amines and beta-carbolines by liquid chromatography-mass spectrometry in cooked meats commonly consumed in Korea. Food Addit. Contam. Part A 26, 298–305, doi:10.1080/02652030802526834 (2009).
Borgen, E. & Skog, K. Heterocyclic amines in some Swedish cooked foods industrially prepared or from fast food outlets and restaurants. Mol. Nutr. Food Res. 48, 292–298, doi:10.1002/mnfr.200400024 (2004).
Sullivan, K. M. et al. Detection of PhIP in grilled chicken entrées at popular chain restaurants throughout California. Nutr. Cancer. 60, 592–602, doi:10.1080/01635580801956519 (2008).
Knize, M. G. et al. Heterocyclic amine content in fast-food meat products. Food Chem. Toxicol. 33, 545–551, doi:10.1016/0278-6915(95)00025-W (1995).
Puangsombat, K. et al. Heterocyclic amine content in commercial ready to eat meat products. Meat Sci. 88, 227–233, doi:10.1016/j.meatsci.2010.12.025 (2011).
Klassen, R. D. et al. Heterocyclic aromatic amines in cooked hamburgers and chicken obtained from local fast food outlets in the Ottawa region. Food Res. Int. 35, 837–847, doi:10.1016/S0963-9969(02)00087-X (2002).
Lu, F., Kuhnle, G. K. & Cheng, Q. Heterocyclic amines and polycyclic aromatic hydrocarbons in commercial ready-to-eat meat products on UK market. Food Cont 73, 306–315, doi:10.1016/j.foodcont.2016.08.021 (2017).
Fast Food in Saudi Arabia, http://www.euromonitor.com/fast-food-in-saudi-arabia/report. Accesses on 29/11/2016.
Khan, M. R. et al. Effect of Natural Food Condiments on Carcinogenic/Mutagenic Heterocyclic Amines Formation in Thermally Processed Camel Meat. J. Food Process. Preserv. doi:10.1111/jfpp.12819 (2016).
Barcelo-Barrachina, E. et al. Ultra-performance liquid chromatography–tandem mass spectrometry for the analysis of heterocyclic amines in food. J. Chromatogr. A 1125, 195–203, doi:10.1016/j.chroma.2006.05.060 (2006).
Toribio, F. et al. Heterocyclic amines in griddled beef steak analysed using a single extract clean-up procedure. Food Chem. Toxicol. 45, 667–675, doi:10.1016/j.fct.2006.10.016 (2007).
Sinha, R. et al. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem. Toxicol. 36, 279–287, doi:10.1016/S0278-6915(97)00162-2 (1998).
Puangsombat, K. et al. Occurrence of heterocyclic amines in cooked meat products. Meat Sci. 90, 739–746, doi:10.1016/j.meatsci.2011.11.005 (2012).
Khan, M. R. et al. Identification of seafood as an important dietary source of heterocyclic amines by chemometry and chromatography–mass spectrometry. Chem. Res. Toxicol. 26, 1014–1022, doi:10.1021/tx4001682 (2013).
Khan, M. R. et al. Preparation and characterisation of fried chicken as a laboratory reference material for the analysis of heterocyclic amines. J. Chromatogr. B 877, 1997–2002, doi:10.1016/j.jchromb.2009.05.016 (2009).
Vitaglione, P. & Fogliano, V. Use of antioxidants to minimize the human health risk associated to mutagenic/carcinogenic heterocyclic amines in food. J. Chromatogr. B 802, 189–199, doi:10.1016/j.jchromb.2003.09.029 (2004).
Gibis, M. & Weiss, J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 134, 766–774, doi:10.1016/j.foodchem.2012.02.179 (2012).
Shon, M. Y. et al. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem. Toxicol. 42, 659–666, doi:10.1016/j.fct.2003.12.002 (2004).
Stoilova, I. et al. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 102, 764–770, doi:10.1016/j.foodchem.2006.06.023 (2007).
Gross, G. & Grüter, A. Quantitation of mutagegnic/carcinogenic heterocyclic aromatic amines in food products. J. Chromatogr. A 592, 271–278, doi:10.1016/0021-9673(92)85095-B (1992).
Acknowledgements
This study was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-AGR2594-02).
Author information
Authors and Affiliations
Contributions
M.R.K. performed the experimental work and statistical data analysis, and wrote the manuscript. M.N. and Z.A.A. reviewed/edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizwan Khan, M., Naushad, M. & Abdullah Alothman, Z. Presence of heterocyclic amine carcinogens in home-cooked and fast-food camel meat burgers commonly consumed in Saudi Arabia. Sci Rep 7, 1707 (2017). https://doi.org/10.1038/s41598-017-01968-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01968-x
This article is cited by
-
Dietary phytochemicals as the potential protectors against carcinogenesis and their role in cancer chemoprevention
Clinical and Experimental Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.