Abstract
Postnatal growth failure (PGF) in preterm infants remains an important clinical issue. In this study, we analysed the incidence of PGF among very low birth weight (VLBW) infants and evaluated the risk factors for PGF based on the data of 2799 VLBW infants obtained from the Korean Neonatal Network database from 2013 to 2014. PGF was defined as a decrease in weight Z score between birth and discharge of more than −1.28 using the Fenton growth charts. Risk factors were evaluated in relation to birth weight for gestational age, namely small (SGA) or appropriate (AGA) for gestational age, using propensity score matching used for between-group differences. The overall incidence of PGF was 45.5%, with a rate of 68.9% in the SGA group and 36.2% in the AGA group. PGF was negatively correlated with gestation and birth weight; additionally, PGF was associated with a higher incidence of co-morbidities. Predictors of PGF in the SGA group were respiratory distress syndrome and days to attain 100 mL/kg of enteral feeding. The only predictor of PGF in the AGA group was days to attain 100 mL/kg of enteral feeding. Early initiation and aggressive progression of enteral nutrition may decrease the incidence of PGF.
Similar content being viewed by others
Introduction
The goal of nutrition for premature infants is to duplicate the in-utero growth rate and body composition of a foetus of the same gestational age1. However, achieving this weight gain goal is difficult and, consequently, preterm infants are frequently significantly underweight at the time of hospital discharge2.
Recently, the Vermont Oxford Network, which evaluates the postnatal growth of preterm infants, defined postnatal growth failure (PGF) as a discharge weight that is lower than the 10th percentile for postmenstrual age. Among infants registered in this Network, the prevalence rate of PGF is 50.3%3. Kelleher reported that birth weight was significantly lower in low-birth-weight children with failure to thrive than in those without failure to thrive4. As well, small for gestational age (SGA) infants had poorer weight gain than appropriate for gestational age (AGA) infants5, and SGA infants remained at a lower body weight until the age of 3–6 years6.
Factors such as weight, gestational age, length of hospital stay, presence of respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), and sepsis have been associated with post-discharge growth and development in preterm infants7. According to a National Institute of Child Health and Human Development study on VLBW infants, nutritional intake deficits persist to some degree during the hospital stay, and infants with BPD, necrotizing enterocolitis (NEC), or late-onset sepsis demonstrate slower growth. Other studies have shown that infants with PGF were more likely to be SGA and to require significantly more days of mechanical ventilation, oxygen and time to regain birth weight that AGA infants8,9. Poor nutritional intake is common in VLBW in the early postnatal months2.
Growth impairment during early infancy can have permanent detrimental effects2. Impaired foetal and postnatal growth has been associated with neurodevelopmental delay, ischemic heart disease, impaired glucose tolerance, type-II diabetes mellitus, hypertension, and metabolic syndrome10,11. Measures of PGF for preterm infants have varied among studies, with no universally agreed upon criteria to define PGF. Generally, growth failure is considered when a baby’s weight is below the 10th percentile (≤−1.28 Z-score), but with different reference charts and variable postnatal periods having been used12. In this study, we defined postnatal weight growth as the change in weight Z score between birth and discharge, using the Fenton growth chart as a reference13.
Using this criterion, we evaluated the incidence of PGF among VLBW infants enrolled in the Korean Neonatal Network and investigated predictive factors of PGF through a comparison of PGF and non-PGF infants. In order to minimize errors due to the influence of SGA status, we also analysed the independent risk factors affecting postnatal growth in SGA and AGA groups separately, after propensity-score matching.
Results
Patients’ characteristics
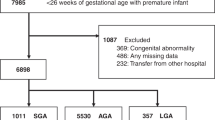
Complete clinical data were available for 2799 patients, and the overall incidence of PGF was 45.9% (1286 of 2799; Fig. 1). The mean birth weight was 1132.91 g, with a mean gestational age of 28.98 weeks, respectively. The mean Z score of body weight at birth and at discharge was −0.45 and −1.78, respectively, with 29.7% of infants (830 of 2799) classified as SGA (Table 1).
Clinical characteristics of the PGF and non-PGF groups
Significant differences between the PGF and non-PGF groups were identified for gestational age, birth weight and Apgar score. The incidence of PGF in SGA group was greater than in the AGA group (Table 1). As well, the incidence of co-morbidities (RDS; BPD; patent ductus arteriosus, PDA; NEC; intraventricular haemorrhage, IVH; periventricular leukomalacia, PVL; sepsis; and retinopathy of prematurity, ROP) was greater in the PGF than non-PGF group (Table 2).
Risk factors for postnatal growth failure among AGA infants
In the AGA group, after matching for gestational age, the incidence of oligohydramnios, RDS, drug therapy for PDA, and sepsis were higher in the PGF than non-PGF group. The duration of ventilation, hospitalization and parenteral nutrition, as well as the time needed to achieve 100 ml/kg of enteral feeding, were longer for the PGF than non-PGF group (Table 3). On Cox regression analysis, RDS (odds ratio [OR], 1.012, 95% confidence interval [CI] 1.008–1.050), and days to achieve 100 ml/kg of enteral feeding (OR 1.030, 95% CI 1.015–1.045) were retained as significant risk factors for PGF among AGA infants (Table 4).
Risk factors for postnatal growth failure among SGA infants
Among SGA infants, after matching for gestational age, the incidence of chorioamnionitis, BPD (moderate or severe), PVL, and congenital anomalies was higher in the PGF than non-PFG group. The Apgar score at 5 min was significantly lower in the PGF than non-PGF group. The duration of ventilation, hospitalization, parenteral nutrition, and achievement of 100 ml/kg of enteral feeding were also significantly longer in the PGF than the non-PGF group (Table 5). On Cox regression analysis, days to achieve 100 ml/kg of enteral feeding (OR 1.028, 95% CI 1.006–1.050) was retained as the only significant risk factor for PGF among SGA infants (Table 4).
Discussion
As advances in neonatal care have significantly improved the survival rate of VLBW preterm infants, continuous growth monitoring from the time of birth is a good predictor of clinical status, outcomes of treatment and nutritional status. In particular, since growth has a direct effect on neurological development, monitoring of growth patterns is very important for NICU clinicians8,14. Therefore, our analysis of postnatal growth patterns, using the data obtained from 55 NICU centres in Korea, is very important to ascertain an accurate epidemiology of PGF in Korea.
Despite differences in the definition of PGF, reference standards used and the population studied, PGF is a universal problem in preterm infants. The reported incidence of PGF, however, has varied depending on the reference for growth used. Using the Fenton growth curves, as per our study, one study reported an incidence of PGF of 32.6% among surviving infants of <31 week gestation, where PGF was defined by a discharge weight below the 10th percentile15. According to the 2013 data from the Vermont Oxford Network, 50.3% of VLBW infants were discharged with a body weight below the 10th percentile. In accordance with previous studies, our study identified an incidence of PGF of 45.9% among surviving VLBW infants in Korea, based on a definition of PGF as a Z-score change from birth to discharge of >−1.28.
Various factors affect postnatal growth. In a prospective study of dietary intake and growth, PGF was significantly correlated with prematurity2. In our study, PGF was associated with a lower gestational age and a significantly lower birth weight, with these infants having a lower Apgar scores at birth than non-PGF infants. The PGF group also developed RDS, air leak, and pulmonary hypertension more frequently, requiring a longer duration of ventilation support than the non-PGF group. Compared to the non-PGF group, the PGF group had a higher incidence of sepsis and NEC during the admission period, longer duration of total parenteral nutrition (TPN), and time to full oral feeding. In addition, as the period of oxygen-use became longer, outcomes of BPD, IVH and PVL were significantly more frequent than among non-PGF infants. Our findings were consistent with those of previous studies, in which PGF in VLBW infants has been associated with late-onset sepsis, surgical NEC, ROP, IVH, BPD, and a longer post-natal hospital stay16,17.
Recent studies have demonstrated a direct relationship between growth achieved before 40 weeks gestation and neurodevelopmental outcome14,15. In addition, long-term follow-up studies have shown that, preterm gestational age, length of hospital stay, and the presence of RDS, BPD, and sepsis all affect growth and development7. Considering the burden of neurodevelopmental impairments to families and societies, reducing the risk for PGF during hospitalization is important.
SGA infants had higher risk of adverse neonatal growth than AGA infants. To accurately identify risk factors of PGF, we used propensity score matching for birth weight and gestation, with an equal number of PGF and non-PGF infants in both AGA and SGA groups. The frequency of RDS, PDA treatment and sepsis were significantly higher among PGF than non-PGF infants in AGA group. In contrast, in the SGA group, chorioamnionitis as a maternal factor, and BPD and PVL as infant factors were significantly more common among PGF than non-PGF infants. The difference in risk factors for PGF between the AGA and SGA groups may be explained by intrauterine infection caused by chorioamnionitis in SGA, which appears to increase the risk for BPD and PVL. After adjusting for potential confounding variables, a longer time to reach full enteral feeding was a significant risk factor for PGF in both AGA and SGA infants. This finding supports previous studies which have suggested that aggressive nutrition should be used to prevent PGF18,19,20. Wilson et al. reported that aggressive nutritional regimen (enteral feeding + parenteral), initiated from post-natal day 1, reduced the incidence of PGF, 59% compared to 82% among preterm infants without nutritional intervention18,21. As well, the aggressive nutrition regimen lowered the incidence of adverse clinical or metabolic sequelae. The ESPGHAN Committee recommended enteral nutrient supplementation for preterm VLBW infants, including the use of fortified human milk and formula designed for premature infants, providing a reasonable range of energy intake of 110–135 kcal/kg/day22,23. Our data also identified achieving early full enteral nutrition and reducing the number of days of TPN as being necessary to prevent PGF.
The growth velocity of preterm infants has been evaluated using various methods, with the most frequently reported method after 2005 being the Z scores measured from birth to discharge12, with Fenton’s growth chart being the most commonly used reference for the calculation of Z-scores and, therefore, for the identification of PGF9,12. Accordingly, we used the Z-score change in body weight from birth to discharge to define PGF.
The limitations of our study need to be acknowledged. Complete and informative data on nutritional practices, specifically the composition and volume of feeding, as well as the timing and rate of feeding over the study period, were not available. However, the changes in growth cannot be explained about the patient characteristics or major morbidities independently. As well, growth was monitored only at birth and at discharge. Variations about the definition of PGF and the reference standards may have contributed confounding factors for the comparisons among studies. Differences in clinical factors and site variations could not be controlled for. Therefore, nutritional practice may have influenced the measurement improvement in weight gain.
In summary, we have described the occurrence of PGF in a large national neonatal cohort in Korea, providing the first report of national growth-outcome data for VLBW infants in Korea. We have also identified the duration of TPN and days to achieve enteral feeding of 100 ml/kg as independent risk factors of PGF among VLBW infants, which could play a role in reducing PGF. As a suggestion for future research, including long-term growth and neurodevelopmental outcomes of PGF infants may allow for the discovery and assessment of additional clinical predictors.
In conclusion, VLBW infants are at high risk for PGF and, therefore, greater attention to optimising nutritional intake, either enterally or parenterally, may decrease the incidence of PGF.
Materials and Methods
Study population
We performed a cohort study using prospectively collected data from 55 Korean Neonatal Network centres. Records of a total of 3522 VLBW infants, born between January 2013 and December 2014, and registered in the Korean Neonatal Network were reviewed. We excluded 723 VLBW infants who died or were transferred or discharged later than postmenstrual age (PMA) 51 weeks. A small number (0.01%) of the data records were incorrectly recorded, resulting in an unexplained error. The data of the remaining 2799 infants were included in our analysis. For analysis, we classified infants into the PGF and non-PGF groups, and compared maternal demographics and neonatal characteristics between groups. After propensity-score matching for gestational age and birth weight, PGF (n = 375) and non-PGF (n = 375) groups were analysed in infants born AGA and infants born SGA, PGF (n = 176) and non-PGF (n = 176) groups.
Data collection and analysis
Trained research assistants prospectively gathered maternal, delivery and neonatal data up to the time of discharge, according to the operational procedures24. Gestational age was calculated according to maternal last menstrual period, early pregnancy ultrasound examination findings or the new Ballard score. Weight, head circumference and height were measured at birth and at discharge. To control for variation in gestational age and postnatal age, body weight was converted into a Z-score using the Fenton growth chart25,26. To ascertain the degree of postnatal growth, the Z-score at birth was subtracted from the Z-score at discharge. Postnatal growth was defined as the change in the Z-score for weight between birth and discharge. PGF was defined as a decrease in weight Z score between birth and discharge of more than −1.28 using the Fenton growth charts13,27. SGA was defined as birth weight below the 10th percentile according to the intrauterine growth charts of Lubencho28.
Antenatal corticosteroid exposure was defined as the mother received one or more doses of any corticosteroid. Pulmonary haemorrhage was defined as massive and significant pulmonary haemorrhage that destabilized vital signs. PDA was defined as the use of medication or surgical treatment for therapeutic and/or prophylactic purposes. IVH was graded according to the method of Papile et al.29. NEC was classified according to the system of Bell et al.30. BPD was defined by the NIH classification31.
Statistical analysis
Unadjusted comparisons of maternal demographics and neonatal characteristics between the non-PGF and PGF groups were performed using a chi-squared or Fisher’s exact test for categorical data and Student’s t-test for continuous data. To adjust for confounding effects of gestational age and birth weight, propensity-score matching was performed. Propensity-score matching is the method for controlling covariate imbalance that produces the selection bias. After matching, univariate analyses for continuous variables were performed using paired t-tests. Categorical variables were examined using a Cox regression analysis. Multivariate survival analyses, adjusted for the factors found to be significant on univariate analysis, were also performed to identify independent risk factors of PGF. The relationship between Z-score of the weight, height and head circumference at discharge and at birth were evaluated using Pearson’s correlation coefficients. The correlation between changes in Z score of the weight, height and head circumference and Z score at birth were analyzed. All statistical analyses were performed using SPSS software version 21.0 (IBM Corp., Chicago, IL, USA) and SAS version 9.2 (SAS Institute, Cary, NC, USA). P-values < 0.05 were considered to be statistically significant.
Ethics statement
The data registration was approved by the institutional review board of every hospital participating in the Korean Neonatal Network (KNN). All protocols and methods in this study were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained prospectively from parents of infants registered in the KNN.
The dataset generated and analysed during the current study are not publicly available due to the research of Korea Centres for Disease Control and Prevention, but are available from the corresponding author on reasonable request.
References
American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics 75, 976–986 (1985).
Embleton, N. E., Pang, N. & Cooke, R. J. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 107, 270–273 (2001).
Horbar, J. D. et al. Weight Growth Velocity and Postnatal Growth Failure in Infants 501 to 1500 Grams: 2000–2013. Pediatrics 136, e84–92 (2015).
Kelleher, K. J. et al. Risk factors and outcomes for failure to thrive in low birth weight preterm infants. Pediatrics 91, 941–948 (1993).
Pilling, E. L., Elder, C. J. & Gibson, A. T. Growth patterns in the growth-retarded premature infant. Best Pract Res Clin Endocrinol Metab 22, 447–462 (2008).
Monset-Couchard, M., de Bethmann, O. & Relier, J. P. Long term outcome of small versus appropriate size for gestational age co-twins/triplets. Arch Dis Child Fetal Neonatal Ed 89, F310–314 (2004).
Lima, P. A., Carvalho, M., Costa, A. C. & Moreira, M. E. Variables associated with extra uterine growth restriction in very low birth weight infants. J Pediatr (Rio J) 90, 22–27 (2014).
Senterre, T. & Rigo, J. Optimizing early nutritional support based on recent recommendatio ns in VLBW infants and postnatal growth restriction. J Pediatr Gastroenterol Nutr 53, 536–542 (2011).
Fenton, T. R. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr 3, 13 (2003).
Lapillonne, A. & Griffin, I. J. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr 162, S7–16 (2013).
Barker, D. J. The fetal and infant origins of adult disease. BMJ 301, 1111 (1990).
Fenton, T. R. et al. Preterm Infant Growth Velocity Calculations: A Systematic Review. Pediatrics 139 (2016).
Lin, Z. et al. Quantification of EUGR as a Measure of the Quality of Nutritional Care of Premature Infants. PLoS One 10 (2015).
Ehrenkranz, R. A. et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117, 1253–1261 (2006).
Belfort, M. B. et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 128, e899–906 (2011).
Griffin, I. J., Tancredi, D. J., Bertino, E., Lee, H. C. & Profit, J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch Dis Child Fetal Neonatal Ed 101, F50–55 (2014).
Clark, R. H., Thomas, P. & Peabody, J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 111, 986–990 (2003).
Ehrenkranz, R. A. Extrauterine growth restriction: is it preventable? J Pediatr (Rio J) 90, 1–3 (2013).
Rover, M. M., Viera, C. S., Silveira, R. C., Guimaraes, A. T. & Grassiolli, S. Risk factors associated with growth failure in the follow-up of very low birth weight newborns. J Pediatr (Rio J) 92, 307–313 (2016).
Loui, A. et al. Growth in high risk infants <1500 g birthweight during the first 5 weeks. Early Hum Dev 84, 645–650 (2008).
Wilson, D. C. et al. Randomised controlled trial of an aggressive nutritional regimen in sick very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 77, F4–11 (1997).
Agostoni, C. et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 50, 85–91 (2015).
Tsang R, U. R., Koletzko, B. & Zlotkin, S. Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines. 415–416 (2005).
Chang, Y. S., A, S. & Park, W. S. Establishment of the Korean NeonatalNetwork (KNN). Neonatal Med 20, 169–178 (2013).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13, 59 (2013).
Fenton, T. R. & Sauve, R. S. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr 61, 1380–1385 (2007).
Cooke, R. J., Ainsworth, S. B. & Fenton, A. C. Postnatal growth retardation: a universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed 89, F428–430 (2004).
Lubchenco, L. O., Hansman, C. & Boyd, E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics 37, 403–408 (1966).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92, 529–534 (1978).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 187, 1–7 (1978).
Ehrenkranz, R. A. et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 116, 1353–1360 (2005).
Acknowledgements
This research was supported by a fund (code 2016-ER6307-00) by Research of Korea Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Contributions
Lee S.M. and Kim N.H. performed research and analysed data. Park M.S., Park K.I., Namgung R. designed the study and analysed data. Lee S.M. and Jeon J.H. wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.M., Kim, N., Namgung, R. et al. Prediction of Postnatal Growth Failure among Very Low Birth Weight Infants. Sci Rep 8, 3729 (2018). https://doi.org/10.1038/s41598-018-21647-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21647-9
This article is cited by
-
An exploratory study of clinical factors associated with IGF-1 and IGFBP-3 in preterm infants
Pediatric Research (2024)
-
Racial and ethnic disparities in postnatal growth among very low birth weight infants in California
Journal of Perinatology (2023)
-
Impact of time to full enteral feeding on long-term neurodevelopment without mediating by postnatal growth failure in very-low-birth-weight-infants
Scientific Reports (2023)
-
Development and validation of machine learning-based clinical decision support tool for identifying malnutrition in NICU patients
Scientific Reports (2023)
-
The association between BMI trajectories and bronchopulmonary dysplasia among very preterm infants
Pediatric Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.