This article, the first in a two-part series, discusses the classification, pathophysiology and presentation of cardiomyopathies. It comes with a self-assessment enabling you to test your knowledge after reading it
Abstract
Cardiomyopathies are diseases characterised by structural and functional abnormalities of the myocardium. There are various types and subtypes, some of which have a strong genetic component. The understanding of cardiomyopathies has been enhanced by advances in the fields of medicine and genetics. This article – the first in a two-part series – describes the classification and pathophysiology of the main cardiomyopathies and their clinical presentation. The second article will cover diagnosis, management and the role of the multidisciplinary team in patient care.
Citation: Jarvis S (2019) Cardiomyopathies 1: classification, pathophysiology and symptoms. Nursing Times [online]; 115: 7, 38-42.
Author: Selina Jarvis is a research nurse and former Mary Seacole development scholar at Kingston and St George’s University of London and King’s Health Partners, Guy’s and St Thomas’ Foundation Trust.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Assess your knowledge and gain CPD evidence by taking the Nursing Times Self-assessment test
- Read part 2 of this series here
Introduction
Cardiomyopathies are a mixed group of diseases of the myocardium (cardiac muscle) defined by structural or functional abnormalities that negatively affect the pump function of the heart. In some types, there is obstruction to the outflow of blood during the cardiac cycle (Jarvis and Saman, 2018). Advances in clinical and genomic medicine have led to better detection and understanding of cardiomyopathies (some of which have a strong genetic component), while high-profile cases of sudden cardiac death have increased awareness of the conditions. This first article in a two-part series covers the classification, pathophysiology and presentation of cardiomyopathies.
Definition and classification
The American Heart Association describes cardiomyopathies as “a heterogeneous group of diseases of the myocardium associated with mechanical and/or electric dysfunction that usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilatation due to a variety of etiologies that are frequently genetic. Cardiomyopathies are either confined to the heart or part of generalised systemic disorders and often lead to cardiovascular death or progressive heart failure-related disability” (Maron et al, 2006).
Cardiomyopathies were previously divided into primary and secondary: a primary cardiomyopathy was confined to the cardiac muscle, while a secondary cardiomyopathy was part of a systemic disorder affecting multiple organs. Today, new and more-appropriate classifications are used.
The European Society of Cardiology orders cardiomyopathies according to morphological and functional phenotype (Elliott et al, 2008) (Fig 1). Cardiomyopathies can be further subdivided according to the underlying condition.

According to Elliott et al (2008), the main types of cardiomyopathies are:
- Dilated cardiomyopathy (DCM);
- Hypertrophic cardiomyopathy (HCM);
- Restrictive cardiomyopathy (RCM);
- Arrhythmogenic cardiomyopathy (ACM);
- Unclassified cardiomyopathies.
Since the publication of Elliott et al’s (2008) classification system, another – the MOGE(S) tool – has been proposed (Arbustini et al, 2013). This allows health professionals to stage the disease by addressing five simple attributes of cardiomyopathic disorders (Box 1), although it may not be appropriate for all patients.
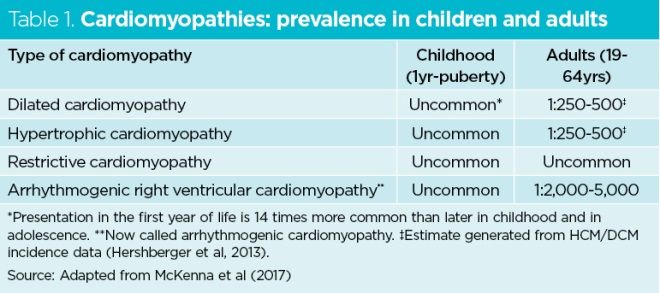
Table 1 shows the global prevalence of the main cardiomyopathies in children and adults. Patients with cardiomyopathies may be completely asymptomatic or experience a wide variety of signs and symptoms (Fig 2).
Box 1. MOGE(S) tool
M = morphological phenotype of the cardiomyopathy (for example, dilated or restrictive)
O = organ involvement
G = genetic or familial inheritance (for example, autosomal dominant)
E = etiological annotation, with details of genetic defect or underlying disease or cause
S = stage of disease
Source: Arbustini et al (2013)

Source: Peter Lamb
Dilated cardiomyopathy
The most common cause of heart failure (Weintraub et al, 2017), DCM is diagnosed on the basis that it cannot be explained by either abnormal loading conditions (increased blood pressure or volume) or coronary artery disease, where an ischaemic cardiomyopathy may occur (Elliott et al, 2008). DCM can develop at any age and is more common in males. In children, it accounts for around 60% of all cases of cardiomyopathy (Lipshultz et al, 2003).
The condition encompasses heterogeneous diseases characterised by inappropriate ventricular hypertrophy (thickening of the ventricle wall) or dilation of one or both ventricles (with thinning and enlargement). It occurs progressively (Weintraub et al, 2017) and can lead to decompensated heart failure. In the developed world, it is a common reason for needing a heart transplant (Maron et al, 2006).
DCM can be caused by an inherent problem in the myocardium. Approximately 20-48% of cases are genetic (Taylor et al, 2006), in which case the condition is called familial DCM. Gene defects affecting structural elements in the cardiac muscle cells (cardiomyocytes), ion channels, cytoskeleton and mitochondria have been identified (Braunwald, 2017; Sisakian, 2014).
DCM can also be secondary to systemic causes such as inflammation, malnutrition and infectious, autoimmune or endocrine diseases. In high-income countries, such as the UK, alcohol overuse contributes to 21-36% of cases (Weintraub et al, 2017). The risk of developing DCM as a result of alcohol misuse is influenced by various susceptibility factors, including those that are ethnic and genetic (Weintraub et al, 2017; Laonigro et al, 2009).
Table 2 outlines the causes of DCM. In many cases, no definitive cause is found (idiopathic DCM).

Approximately 80% of patients with DCM experience symptoms of heart failure such as dyspnoea, fatigue, chest pain, orthopnoea and paroxysmal nocturnal dyspnoea. They may also present with clinical features of an underlying systemic cause. To ascertain any underlying cause, it is important to take a detailed medical and family history, as well as enquiring about previous viral infections and drug and alcohol use.
On examination, the cardiac apex beat may be displaced due to ventricular dilation and there may be signs of congestive heart failure, such as swollen legs from peripheral and/or sacral oedema, crackles from pulmonary congestion and distended neck veins from raised internal jugular venous pressure. Auscultation may reveal a ‘gallop rhythm’ caused by an extra heart sound (S3) that occurs as part of congestive heart failure, or there may be features of mitral regurgitation occurring as a result of a dilated left ventricle.
Hypertrophic cardiomyopathy
First identified in 1957 at St George’s Hospital in London in eight patients who presented with asymmetrical cardiac septal thickening (hypertrophy) of the left ventricle, HCM has since been recognised globally, with a prevalence of 0.2% in the general population (Houston and Stevens, 2015). Patients may present with sudden cardiac death. Mortality is estimated at 1% a year (Houston and Stevens, 2015; Elliott et al, 2014).
HCM is a heterogeneous disorder characterised by left ventricular hypertrophy and, in some cases, left ventricular outflow tract obstruction (Houston and Stevens, 2015). In around 60% of adults and adolescents, the condition is caused by familial disease (Marian and Braunwald, 2017). A large number of genetic mutations in HCM have been described including those affecting proteins important for sarcomere function (a sarcomere is a basic unit of repeating contractile proteins that make up muscle cells). The normal mode of inheritance for such gene defects is autosomal dominant, although autosomal and sex-linked recessive patterns have also been described (Braunwald, 2017). Gene defects may include β-myosin heavy chain gene, myosin-binding protein C and troponin T account for 70-80% of all cases of inherited HCM (Marian and Braunwald, 2017; Sisakian, 2014). Some patients inherit more than one gene defect and may experience more severe disease.
Other causes of HCM include metabolic or neuromuscular diseases caused by genetic problems (5-10% of cases) (Houston and Stevens, 2015) and non-genetic causes such as amyloidosis, a rare condition in which the abnormal protein amyloid accumulates in the heart (Elliott et al, 2014).
Age is an important clue in HCM as the inherited metabolic or neuromuscular causes are more common in newborns and infants than in older children and adults (Elliott et al, 2014). Reconstructing a genetic history can help define the inheritance pattern of the disease. Key elements include:
- Family history of sudden cardiac death;
- Unexplained heart failure or arrhythmias;
- Signs and symptoms of an underlying systemic cause.
Some patients have few symptoms while others may experience chest pain, dyspnoea, palpitations and/or syncope. Patients with HCM can be at risk of sudden death in the absence of any previous symptoms (Houston and Stevens, 2015).
Examination does not necessarily reveal anything abnormal, as not all patients have left ventricular outflow obstruction caused by a hypertrophied ventricle. Possible signs include a prominent para-sternal lift, detected by placing the heel of the hand over the left parasternal region (side of the sternum); if the hand is lifted off the chest wall with each heartbeat, this indicates a prominent parasternal lift and may point towards ventricular hypertrophy.
Patients may also have distended neck veins from raised jugular venous pressure. Palpation of the apex beat of the heart may reveal an increased left ventricular apical impulse or, rarely, a systolic thrill. There may also be a pan-systolic murmur resulting from mitral valve regurgitation. Another type of murmur may be present, caused by turbulent flow through the outflow tract, which is mid-systolic with a crescendo-decrescendo sound.
Restrictive cardiomyopathy
Characterised by ventricular stiffness leading to reduced ventricular filling and diastolic volume during the cardiac cycle, RCM is suspected when patients have near-normal systolic function but diastolic dysfunction on echocardiography. Unlike the other cardiomyopathies, which are defined by morphological changes in the ventricles, RCM is defined based on the resulting haemodynamic problems (Sisakian, 2014). It is the least common cardiomyopathy and makes up 5% of paediatric cardiomyopathies (Muchtar et al, 2017).
There are several causes of RCM but in 50% no cause is identified (Muchtar et al, 2017). Certain types of RCM affect certain ethnic groups more than others; for instance, endomyocardial fibrosis is a cause of RCM that is most prevalent in tropical countries and sub-Saharan African countries, such as Cameroon (Muchtar et al, 2017; Chelo et al, 2015). In other regions, RMC more commonly occurs because of amyloidosis, sarcoidosis and haemochromatosis (Muchtar et al, 2017).
RCM can develop from a primary cause; for example, endomyocardial fibrosis. It can also be secondary to:
- Other systemic conditions that cause infiltration of the myocardium; for example, amyloidosis, sarcoidosis and radiation effects;
- Conditions that cause abnormal loading within the myocardial cells; for example, haemochromatosis (a state of iron overload), glycogen storage disorders or Fabry disease (caused by accumulation of globotriaosylceramide);
- Other types of cardiomyopathy causing a restrictive type of pathophysiology.
Table 3 summarises the causes of RCM.
RCM may be associated with symptoms and signs of congestive heart failure, such as peripheral oedema, raised jugular venous pressure and gallop rhythm, as well as features of an underlying systemic disease.

Arrhythmogenic cardiomyopathy
This genetic cardiomyopathy was first identified in 1700 in a family that had a specific dilation of the right ventricle (Braunwald, 2017). Since then, there have been several reports of patients with the condition, characterised by ventricle muscle that has been replaced with fibrofatty tissue.
Until recently, ACM was called arrhythmogenic right ventricular cardiomyopathy (Corrado et al, 2017), but it has become apparent that the left ventricle can be affected in up to 75% of patients (Sisakian, 2014; Falase and Ogah, 2012). The condition is a significant cause of sudden cardiac death due to electric instability and subsequent ventricular tachycardia or ventricular fibrillation (Sisakian, 2014).
Symptoms include:
- Palpitations or syncope;
- Signs of ventricular failure (for example, ascites, hepatic congestion, raised jugular venous pressure and marked oedema);
- Life-threatening arrhythmias.
Other cardiomyopathies
Peripartum cardiomyopathy
Peripartum cardiomyopathy is a rare life-threatening condition that occurs around the last month of pregnancy and up to six months postpartum. It has similar clinical characteristics to dilated cardiomyopathy (DCM) such as ventricle dilation and systolic dysfunction (Table 2).
Diagnosis is made after ruling out other causes of cardiomyopathy, as the condition shares many characteristics with other causes of systolic heart failure. Women may be diagnosed with peripartum cardiomyopathy if it develops in the absence of pre-existing heart failure or any other identifiable cause. The underlying pathology is poorly understood; one hypothesis is that prolactin may cause oxidative stress (Honigberg and Givertz, 2019).
Stress-induced cardiomyopathy
Stress-induced cardiomyopathy (or takotsubo cardiomyopathy) is often preceded by intense emotional or physical stress, with likely catecholamine release including adrenaline and noradrenaline. Oestrogen deficiency in postmenopausal women has also been implicated.
The condition shows characteristic features on echocardiography, including a hyperdynamic left ventricular segment and abnormal shape in systole resembling an octopus trap (called takotsubo in Japanese). Thus the apex of the heart may balloon but it is not strictly considered as a DCM as it has different clinical features of presentation. An electrocardiogram may show left ventricular contractile dysfunction and associated ST elevation. Many patients will need an angiogram to assess the coronary vasculature and rule out myocardial infarction.
In stress-induced cardiomyopathy, the functional and structural changes to the myocardium are reversible. They may resolve within days or weeks with treatment strategies that use diuretics and nitrates and are aimed at reducing life-threatening complications (Kato et al, 2017).
Left ventricular non-compaction
Left ventricular non-compaction (or spongy myocardium) is a rare congenital cardiomyopathy (Nunez-Gil and Feltes-Guzmán, 2012). It typically affects the apex of the heart and is defined by an altered myocardial wall with prominent trabeculae (irregular muscular columns projecting from the inner surface of the heart) and deep intratrabecular recesses, which results in a thickened myocardium with two layers (one non-compacted layer and one thin compacted layer).
There is continuity between the left ventricular cavity and deep intratrabecular recesses, which fill with blood from the ventricular cavity without evidence of communication with the epicardial coronary artery system (Attenhofer-Jost and Connolly, 2019). Complications include heart failure, arrhythmias and embolic events (Nunez-Gil and Feltes-Guzmán, 2012).
Histiocytoid cardiomyopathy
Histiocytoid cardiomyopathy (or Purkinje cell hamartoma) is a rare cardiomyopathy that presents between birth and the age of four years and is more common in females. It is associated with arrhythmias and sudden cardiac death as well as congenital heart defects. It may occur in conjunction with extra-cardiac features, including abnormalities of the nervous system and the eyes.
Histiocytoid cardiomyopathy is thought to be caused by a developmental defect of the Purkinje cells (part of the conducting system of the heart), but findings of excessive numbers of abnormally shaped mitochondria in cardiac tissue suggest that mitochondrial dysfunction may play a role (Shehata et al, 2015).
Conclusion
Patients with cardiomyopathies may experience a range of signs and symptoms, or none. It is important to take a careful clinical history of their presenting symptoms, along with relevant details of their medical history, medication use, family history and alcohol use.
Part 2 in this series will describe diagnosis and management, as well as the role of the multidisciplinary team in the care of patients with cardiomyopathies.
Key points
- Dilated cardiomyopathy is the most common cause of heart failure
- Up to 36% of cases of dilated cardiomyopathy can be due to alcohol misuse
- Several types of cardiomyopathy are associated with sudden cardiac death
- Stress-induced cardiomyopathy is often preceded by intense emotional or physical stress
- Many cardiomyopathies are rare diseases with a strong genetic component

- Test your knowledge with Nursing Times Self-assessment after reading this article. If you score 80% or more, you will receive a personalised certificate that you can download and store in your NT Portfolio as CPD or revalidation evidence.
- Take the Nursing Times Self-assessment for this article
Attenhofer-Jost CH, Connolly HM (2019) Isolated left ventricular noncompaction in adults: clinical manifestations and diagnosis.
Braunwald E (2017) Cardiomyopathies: an overview. Circulation Research; 121: 7, 711-721.
Chelo D et al (2015) Endomyocardial fibrosis in Sub Saharan Africa: the geographical origin, socioeconomic status, and dietary habits of cases reported in Yaounde, Cameroon. Annals of Pediatric Cardiology; 8: 3, 202-209.
Corrado D et al (2017) Arrhythmogenic cardio-myopathy. Circulation Research; 121: 7, 784-802.
Elliott PM et al (2014) 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). European Heart Journal; 35: 39, 2733-2779.
Elliott P et al (2008) Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. European Heart Journal; 29: 2, 270-276.
Falase AO, Ogah OS (2012) Cardiomyopathies and myocardial disorders in Africa: present status and the way forward. Cardiovascular Journal of Africa; 23: 10, 552-562.
Hershberger RE et al (2013) Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nature Reviews – Cardiology; 10: 9, 531-547.
Honigberg MC, Givertz MM (2019) Peripartum cardiomyopathy. British Medical Journal; 364: k5287.
Houston BA, Stevens GR (2015) Hypertrophic cardiomyopathy: a review. Clinical Medical Insights – Cardiology; 8: Suppl 1, 53-65.
Jarvis S, Saman S (2018) Cardiac system 1: anatomy and physiology. Nursing Times [online]; 114: 2, 34-37.
Kato K et al (2017) Takotsubo syndrome: aetiology, presentation and treatment. Heart; 103: 18, 1461-1469.
Laonigro I et al (2009) Alcohol abuse and heart failure. European Journal of Heart Failure; 11: 5, 453-462.
Lipshultz SE et al (2003) The incidence of pediatric cardiomyopathy in two regions of the United States. New England Journal of Medicine; 348: 17, 1647-1655.
Marian AJ, Braunwald E (2017) Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circulation Research; 121: 7, 749-770.
Maron BJ et al (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee: Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation; 113: 14, 1807-1816.
McKenna WJ et al (2017) Classification, epidemiology, and global burden of cardio-myopathies. Circulation Research; 121: 7, 722-730.
Muchtar E et al (2017) Restrictive cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circulation Research; 121: 7, 819-837.
Nunez-Gil IJ, Feltes-Guzmán G (2012) Left ventricular noncompaction. E-Journal of Cardiology Practice; 10: 31.
Shehata BM et al (2015) Exome sequencing of patients with histiocytoid cardiomyopathy reveals a de novo NDUFB11 mutation that plays a role in the pathogenesis of histiocytoid cardiomyopathy. American Journal of Medical Genetics. Part A; 167A: 9, 2114-2121.
Sisakian H (2014) Cardiomyopathies: evolution of pathogenesis concepts and potential for new therapies. World Journal of Cardiology; 6: 6, 478-494.
Taylor MR et al (2006) Cardiomyopathy, familial dilated. Orphanet Journal of Rare Diseases; 1, 27.
Weintraub RG et al (2017) Dilated cardiomyopathy. Lancet; 390: 10092, 400-414.
 Nursing Times Resources for the nursing profession
Nursing Times Resources for the nursing profession 

Have your say
or a new account to join the discussion.