Carolina Mantleslug recovery strategy
Read the recovery strategy for the Carolina Mantleslug, a slug at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Pivar, R.J., Nicolai, A. and Linton, J. 2023. Recovery Strategy for the Carolina Mantleslug (Philomycus carolinianus) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. v + 34 pp.
Cover illustration: Photo by A. Nicolai
© King’s Printer for Ontario, 2023
ISBN 978-1-4868-7052-3 HTML
ISBN 978-1-4868-7051-6 PDF
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
- Robert J. Pivar — Natural Resource Solutions Inc.
- Annegret Nicolai — Living Lab CLEF/Université Rennes 1
- Jessica Linton — Natural Resource Solutions Inc.
Declaration
The recovery strategy for the Carolina Mantleslug (Philomycus carolinianus) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada — Canadian Wildlife Service, Ontario
Executive summary
Carolina Mantleslug is a terrestrial land slug with an adult size of 6 to 10 centimetres and an ash-coloured mantle covering the entire body. The mantle is marbled dark grey to brown with two central lines of black dots. The slug is usually inactive when seen, so the head is not visible. An upper pair of light grey tentacles may extend from beneath the mantle, but the lower pair of tentacles is not usually visible.
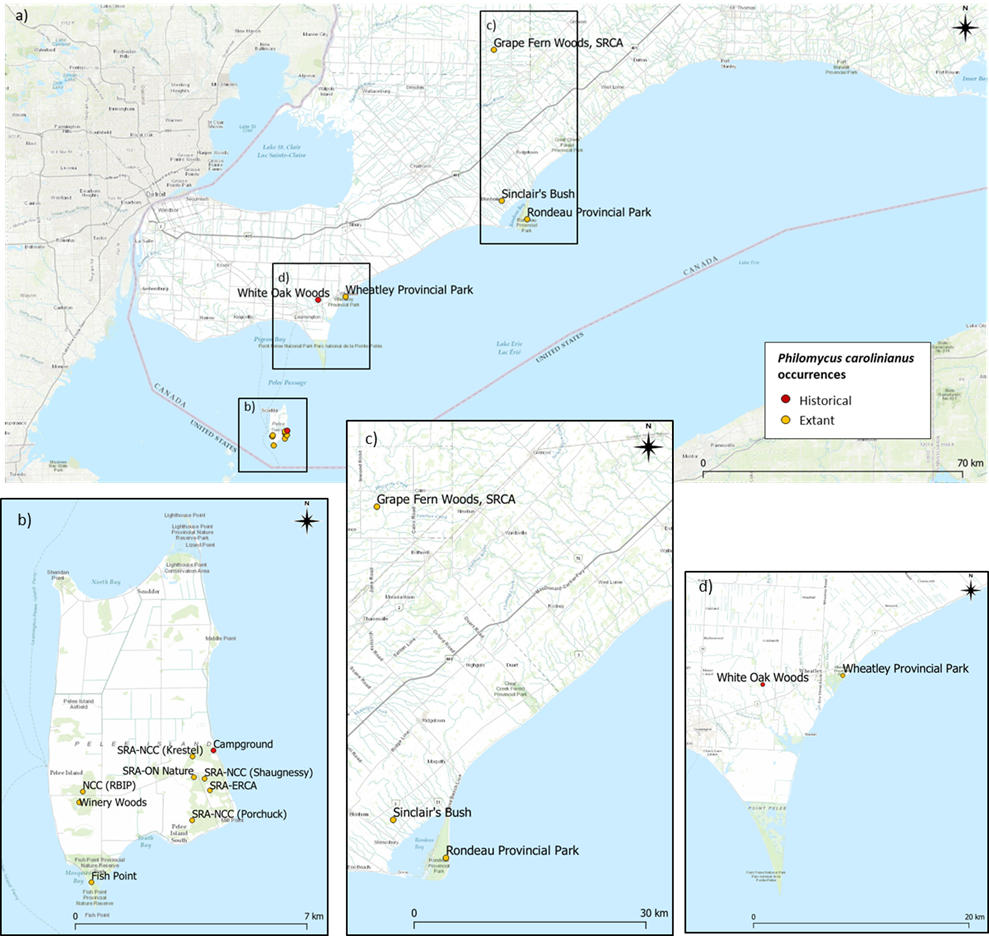
Currently, the Ontario range of Carolina Mantleslug includes at least seven known subpopulations: Pelee Island (Stone Road Alvar area, including the campground; Fish Point Provincial Nature Reserve; and Richard & Beryl Ivey Property and Winery Woods)), Wheatley Provincial Park, Grape Fern Woods, Rondeau Provincial Park and Sinclair’s Bush. It is uncertain if the Leamington subpopulation is extant, although habitat appears intact at White Oak Woods near Leamington.
Carolina Mantleslug is currently listed as threatened on the Species at Risk in Ontario (SARO) List. Key threats for this species include climate change (droughts, changes in frost regimes), prescribed burns, and habitat modifications due to invasive species such as plants, earthworms and other gastropods. Additionally, pollution and any direct and indirect human impacts (e.g., habitat alteration) specific to remaining sites are threats.
The recommended recovery goal for Carolina Mantleslug is to ensure the long-term persistence of extant subpopulations. To achieve this goal, recommended recovery objectives are identified below.
- Engage the scientific community, government land managers, land trusts, conservation organizations and private landowners in surveying suitable habitats to determine the current range extent in southwestern Ontario.
- Assess and mitigate threats at all known extant and historical occurrence sites in Ontario.
- Conduct and/or support research that fills knowledge gaps related to biology, population size, and habitat requirements that inform recovery efforts.
- Enhance and/or create habitat, where feasible and necessary, to increase habitat availability for extant subpopulations.
Information on the spatial limits of habitat used and dispersal by Carolina Mantleslug is lacking. When information on home range size, dispersal ability and key habitat features critical for supporting the species lifecycle becomes available, the area prescribed as habitat could be described more precisely and should be revisited. Based on the best information available, it is recommended that the entire Ecological Land Classification (ELC) ecosites occupied by extant subpopulations be prescribed as habitat in a habitat regulation, because given the rarity of the species, it may be present throughout the habitat but not be detected everywhere. It is recommended that the regulated area should be defined using a contiguous ecological area encompassing all occupied ecosites and any suitable unoccupied ecosites immediately adjacent to occupied ecosites. This recommendation increases the probability that all habitat elements necessary for foraging, mating, nesting, aestivating and hibernating for several generations are included.
It is further recommended for the species that a buffer of 90 metres be added to the defined ELC ecosite polygons and adjacent suitable unoccupied connection ecosites to allow for population augmentation, to maintain important microhabitat and its properties (e.g., leaf litter, decaying logs), to reduce edge effects and to account for temporary use of neighbouring habitat. Habitat known to be unsuitable (e.g., roads, farmland, urban areas, gardens, parks on the mainland, and lakes) should be excluded from this buffer.
1.0 Background information
1.1 Species assessment and classification
The following list provides assessment and classification information for the Carolina Mantleslug (Philomycus carolinianus). Note: The Glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Threatened
- SARO List History: Threatened (2022)
- COSEWIC Assessment History: Threatened (2019)
- SARA Schedule 1: No schedule, no status
- Conservation Status Rankings: G-rank: G5; N-rank: N1N2; S-rank: S1S2.
1.2 Species description and biology
Species description
Carolina Mantleslug (Philomycus carolinianus (Bosc, 1802)) is a terrestrial land slug in the family Philomycidae. It is a large slug with an adult size of 6 to 10 cm (measured as body length in active individuals) and an ash-coloured mantle covering the entire body (Pilsbry 1948). The mantle is marbled dark grey to brown with two central lines of black dots (Figure 1) (COSEWIC 2019). The slug is usually inactive when seen, so the head is not visible. An upper pair of light grey tentacles may extend from beneath the mantle, but the lower pair of tentacles is not usually visible (COSEWIC 2019).

Figure 1. Carolina Mantleslug (Philomycus carolinianus). Photos by A. Nicoliai
Care must be taken when identifying Carolina Mantleslug because its mantle colouration is highly variable within the species and is therefore often confused with other Philomycus species in many collections (e.g., Oughton 1948) (COSEWIC 2019). Based on external morphology alone, it can be confused with slugs in the genera Pallifera and Megapallifera. These genera are distinguished from Philomycus internally by their absence of a dart (Pilsbry 1948). It appears that Megapallifera mutabilis and P. carolinianus coexist in the same habitats. Genetic analysis is generally required to definitively identify Carolina Mantleslug. Specimens of Carolina Mantleslug from Pelee Island have been sequenced by the Biodiversity Institute of Ontario and their genetic fingerprints are available from the BOLD website (BOLDsystems 2022).
Species biology
Carolina Mantleslug is an air-breathing slug that is a simultaneous hermaphrodite (possesses both male and female reproductive organs) and lays eggs (Pilsbry 1948). Generally, both members of a mating pair exchange sperm and produce eggs; self-fertilization is possible but may result in lower reproductive success (COSEWIC 2019, White-McLean 2012). Reproduction usually occurs in spring in temperate regions (COSEWIC 2019) and clutch size ranges between 65 and 75 eggs with a hatching success varying between 40 and 75% (White-McLean 2012). Embryonic development has a duration of 22 to 45 days (White-McLean 2012). Under laboratory conditions, reproductive size (about 4.5 cm) was reached between 120 and 220 days after hatching (White-McLean 2012). May 2018 fieldwork in southwestern Ontario resulted in many records of large juveniles, suggesting that they hatched in the summer the year before (COSEWIC 2019). It is thought that the slugs may reach sexual maturity after one year under natural conditions since they hatch during the summer and growth is seasonal (COSEWIC 2019). Carolina Mantleslug, like other slugs of the same size, such as Tawny Garden Slug (Limax flavus), may live three to four years (Welter-Schulter 2012). The generation time is estimated to be about two years (COSEWIC 2019). Some reproductive processes in Carolina Mantleslug are sensitive to diet and temperature (White-McLean 2012), the latter being relevant to the threat of climate change (COSEWIC 2019). Laboratory studies found that at temperatures ranging from 10 to 21 degrees Celsius, hatching success is highest, and it decreases by more than half at 25 to 29 degrees (White-McLean 2012). Embryonic development is fastest at 25 degrees Celsius (about 17 days) and reduced at lower temperatures (White-McLean 2012). This indicates that eggs laid in fall may overwinter and hatch in spring, as observed in other slug species with freeze-tolerant eggs, such as Arion species (Ansart and Nicolai unpubl. data). The diet of Carolina Mantleslug is uncertain, although observations of its location, the colour of its feces and the colour of nearby fungi and lichen, suggest that it eats fungi and lichen (Nicolai, pers. obs.). It may also feed on decaying wood or other decaying plant material.
Carolina Mantleslug is crepuscular (active at dawn or dusk) or nocturnal, but will emerge from under logs or from holes in logs during the daytime provided the log is moist (Pilsbry 1948). In Ontario, it is found in leaf litter in moist conditions, but is only found under or in logs during dry summers (COSEWIC 2019). Carolina Mantleslug may have increased drought resistance because it has a high desiccation (loss of moisture to the point of drying out) tolerance (Thompson et al. 2006) and forms huddles of several individuals, which has been shown to reduce water loss by 34% in Limax species (Cook 1981). Carolina Mantleslug is more active at warmer temperatures (25 ˚C) and inactive at cooler temperatures (15 ˚C) (Rising and Armitage 1969). Carolina Mantleslug hibernates, as indicated by their low metabolic rate at five degrees Celsius (Rising and Armitage 1969), as opposed to European slugs, such as Arion species (Slotsbo et al. 2012) and Deroceras species (Storey et al. 2007). European species from these genera are found in Canada in Carolina Mantleslug habitat and may stay active under the insulating snow cover (COSEWIC 2019). The European species are freeze-tolerant. Their body fluids freeze between minus one and minus five degrees Celsius, allowing them to survive freezing for up to two days (Storey et al. 2007; Slotsbo et al. 2012). Carolina Mantleslug may exhibit similar freeze tolerance, but this has not been confirmed.
Physiological processes of Carolina Mantleslug may be impacted by heavy metals and pesticides in the soil as they accumulate in tissues (Barker 2001). Please refer to section 1.6 of this document for more details.
Carolina Mantleslug is a mostly inactive slug, resulting in very low dispersal capability compared to introduced exotic slug species, such as Arion or Deroceras species (COSEWIC 2019). While the exact dispersal capability of Carolina Mantleslug is unknown, Arion species have been recorded moving several metres per day with a mean speed of 11 cm/minute (Honek and Martinkova 2011). Eggs and immature stages are not known to be dispersed by wind and the likelihood of aerial or aquatic transport of adults is unknown, but likely small (COSEWIC 2019). However, some slug species can survive periods in water, and may be transported by water, such as exotic Arion species (Nicolai pers. obs.) and Sheathed Slug (Zacoleus idahoensis) (COSEWIC 2016). Slugs may also disperse through passive transportation by rafting on floating objects such as logs (Vagvolgyi 1975).
Due to the poor dispersal capability of Carolina Mantleslug, it is unlikely that dispersal from populations in the United States into Ontario is occurring (COSEWIC 2019). Historical and current habitat loss and degradation are likely factors preventing expansion outside the current occupied sites (COSEWIC 2019). These are both important factors to consider for species at the edge of their range, such as Carolina Mantleslug in Ontario, that may need to expand their range northward due to climate change (Gibson et al. 2009). Furthermore, since Carolina Mantleslug is not linked to human activities it is unlikely to be accidentally introduced as an accidental stowaway on vehicles or clothing. Similarly, because the species does not forage for fresh plant material, it is unlikely to be accidentally introduced to new areas via transporting horticultural or agricultural goods (Robinson 1999; Robinson and Slapcinsky 2005).
Carolina Mantleslug is a known host for the nematode Meningeal Worm (Parelaphostrongylus tenuis), a parasite of deer in North America (Rowley et al. 1987), but generally the parasites of Philomycidae are understudied. Nonetheless, information on parasites of other slug and land snail families is suggestive of avenues of investigation that may lead to greater knowledge. Trematodes and free swimming or attached flagellates have been observed in Polygyridae snails (Barger and Hnida 2008; Barger 2011; Current 2007). Parasitic mites and nematodes are also commonly observed in snails in general and can cause high mortality, reproductive disturbance, and reduced cold hardiness (Baur and Baur 2005; Morand et al. 2004; Örstan 2006). Slugs can also disperse other organisms essential for litter decomposition, including nematodes by transitorily ingesting them (Peterson et al. 2015), and oribatid mites through ingestion and egestion (Turke et al. 2018).
Gastropods are an important food source to a large variety of taxa, including salamanders, frogs, toads, turtles, snakes, lizards, birds, shrews, voles, moles, rats, mice, chipmunks, squirrels, sciomyzid fly larvae, firefly larvae, parasitic wasp larvae, beetles, ants, spiders and harvestman (Jordan and Black 2012). Predators specific to Carolina Mantleslug are unknown, but are likely to include many of the above-mentioned taxa.
1.3 Distribution, abundance and population trends
Carolina Mantleslug has a range across eastern North America. The northern limit is southern Ontario, Michigan and Vermont, while the east-west distribution is from Maine to Minnesota in the north and Florida to Texas in the south (COSEWIC 2019). In Canada, the current range of Carolina Mantleslug includes at least seven known subpopulations across Pelee Island, Wheatley Provincial Park, Grape Fern Woods, Rondeau Provincial Park and Sinclair’s Bush (COSEWIC 2019) (Figure 2). The Pelee Island population is divided into three subpopulations (Stone Road Alvar area [SRA], including the campground; Fish Point Provincial Nature Reserve; and Richard & Beryl Ivey Property [RBIP] of the Nature Conservancy of Canada [NCC] and Winery Woods) following NatureServe’s Element Occurrence standards (2022), which account for dispersal barriers (roads, unsuitable habitat) and distance (up to 1 km between subpopulations when habitat is suitable for connectivity). Carolina Mantleslug is also historically known from Leamington, Ontario, but has not been observed at this location since 1994 (COSEWIC 2019). Due to a lack of access during recent gastropod surveys conducted by Nicolai (2013-2019), it is uncertain if the Leamington subpopulation is extant, although habitat appears intact at White Oak Woods near Leamington (COSEWIC 2019). The Rondeau population appears to be quite strong, with specimens easily found when conditions are favourable, as recently as summer 2022, while Wheatley populations have not been monitored in recent years (Kaija pers. comm. 2022).
There are several potential recent Carolina Mantleslug observations on iNaturalist (iNaturalist 2022), however, due to its similar morphology to other species of Philomycus and Megapallifera, these records are difficult to verify without dissections or the use of genetic data (Nicolai pers. obs.). Some of these iNaturalist records might indicate that Carolina Mantleslug is extant in new sites (i.e., Clear Creek, Harrow) within its current range, but species verification is needed before these new sites can be confirmed. The size of the Canadian population of Carolina Mantleslug is unknown and data collected so far are insufficient to determine trends and fluctuations (COSEWIC 2019).
Migration between extant subpopulations is not likely because of distance and unsuitable land (agriculture, roads, urban areas, water bodies, etc.) between them.
1.4 Habitat needs
In Canada, Carolina Mantleslug mostly lives in low wet forests and riparian areas along the Lake Erie shore (Grimm 1996). In the US, Hubricht (1985) described Carolina Mantleslug habitat as floodplains, but also mountains up to 2,000 feet (610 m) in elevation. Specific microhabitat conditions for Carolina Mantleslug are not known, though general assumptions can be made that, like most slug species, it can be found under decaying logs, in leaf litter and require moisture. During surveys carried out by Nicolai from 2013 to 2019 the species was found in riparian wet forest and on the floor of older-growth deciduous forest growing on sandy or rocky soil, with abundant, well-decayed wood (COSEWIC 2019). On Pelee Island, Carolina Mantleslug is found in forests consisting of oak (Quercus species), maple (Acer species), mulberry (Morus species), ash (Fraxinus species) and hickory (Carya species) (COSEWIC 2019). The forest composition in its Wheatley Provincial Park habitat is composed of chestnut (Castanea dentata), Sassafras (Sassafras albidum), Black Gum (Nyssa sylvatica) and Pin Oak (Quercus palustris) (COSEWIC 2019). In Rondeau Provincial Park, the deciduous forest is mainly American Beech (Fagus grandifolia) and Sugar Maple (Acer saccharum) and smaller populations of basswood (Tilia species), tulip tree (Liriodendron tulipifera), White Ash (Fraxinus americana) andGreen Ash (Fraxinus pennsylvanica) (Dobbyn and Pasma 2012). The Rondeau Provincial Park forest habitat grows on sandy ridges that form sloughs which may be flooded for most of the year (COSEWIC 2019). Sinclair’s Bush is a deciduous forest and includes species of conservation concern, including Pawpaw Tree (Asimina triloba) and tulip tree (COSEWIC 2019).
Some species use different habitat patches in different seasons. For example, the Roman snail (Helix pomatia), uses nettle patches for reproduction, shrub patches for feeding, and forest soils for overwintering (Nietzke 1970). This behaviour is unknown in Carolina Mantleslug and requires further investigation. Since Carolina Mantleslug seems to be primarily a fungivore (organism that consumes fungi), the presence of a diverse mushroom and lichen community is an important habitat requirement and are present in all known occupied sites (COSEWIC 2019). It has been recorded feeding on Honey Fungus (Armillaria mellea), Gilled Bolete (Phylloporus boletinoides), Lurid Bolete (Boletus luridiceps) and Olivespore Bolete (Boletus oliveisporus) (White-McLean 2012). This is not an exhaustive list of fungi consumed by Carolina Mantleslug as information regarding its diet is lacking.
1.5 Limiting factors
In Ontario, Carolina Mantleslug is near the northern limits of its distribution and further expansion north is likely limited by harsh winters, human-caused habitat fragmentation and loss (Gibson et al. 2009), and physical barriers, such as large bodies of water (COSEWIC 2019). Low dispersal ability restricts gene flow among subpopulations (COSEWIC 2019), and may result in limited genetic and phenotypic differentiation, potentially reducing the fitness of a subpopulation (Fitzpatrick and Reid 2019). Population growth at the microhabitat scale is likely limited by the availability of moist refuges that buffer environmental fluctuations (Burch and Pearce 1990).
1.6 Threats to survival and recovery
A threat assessment for Carolina Mantleslug was compiled in its 2019 COSEWIC report and was based on knowledge of the extant subpopulations on Pelee Island, Rondeau Provincial Park, Grape Fern Woods and Wheatley Provincial Park. The threats below are organized from their highest to lowest impact, according to the assessment in the COSEWIC report (2019). A threat assessment for the Sinclair’s Bush subpopulation has not yet been conducted.
Climate change and severe weather
Foden et al. (2013) presented a systematic trait-based framework for assessing species’ vulnerability to climate change, and within this framework, Carolina Mantleslug can be considered highly vulnerable because it is exposed to climate change (spring frosts, absence of snow cover, droughts), is sensitive to its specific microhabitat conditions and it has a low adaptive capacity (low dispersal capabilities and it lives in small, isolated patches of natural habitat) (COSEWIC 2019). However, since it is more drought-tolerant than other gastropod species it may be able to persist at some level of climate change (COSEWIC 2019). Climate change models suggest that southwestern Ontario will experience an increase in extreme weather events, including droughts, floods and temperature extremes (Varrin et al. 2007). In the Lake Erie basin, summer precipitation is likely to decline while winter precipitation is likely to increase, according to a study by McDermid et al. (2015). Snails may be vulnerable to increasing average temperatures accompanied by increased incidences of drought (Pearce and Paustian 2013) and spring frost (Augspurger 2013), though there is no similar information available on slugs. Since Carolina Mantleslug is found mainly in floodplains and higher mountain areas, this suggests that it relies on moisture and lower temperatures in summer (COSEWIC 2019).
Despite Carolina Mantleslug being a specialist of wet forest, unusually high floods in the winter and spring can increase mortality when slugs are inactive (COSEWIC 2019). Pelee Island and Grape Fern Woods are both seasonally flooded wet forest (MNR 2005; NCC 2008), and with increased precipitation due to climate change, flooding can be expected over a larger area, especially in areas that are just barely above the lake level (COSEWIC 2019). Pelee Island ranges from 175 to 183 metres above sea level, with the lake level being 173 metres (Natural Resources Canada 2019).
The threat of habitat shifting and alteration also exists in known habitats of Carolina Mantleslug. Fish Point Provincial Nature Reserve has a population of Carolina Mantleslug that lives in the wet forest near the east shore, which could gradually erode in the future (COSEWIC 2019). A substantial part of the forest on the southern tip of the island was lost during the winter of 2018/2019; although this erosion is usually a slow process, the high lake level combined with heavier storms in the future could accelerate this habitat loss (COSEWIC 2019). Rondeau Provincial Park experiences similar erosion to its marshland, which may affect water levels in the forest habitat (COSEWIC 2019). It is unknown how these changes may impact gastropod communities in the future, but this type of habitat loss should be monitored as a possible threat or barrier to recovery (COSEWIC 2019).
Prescribed fire
Prescribed burns are an important management tool for prairie and forest conservation (Williams 2000), and are used to limit the invasion of exotic species (Brooks and Lusk 2008) and to promote growth and reproduction of native prairie species (Towne and Owensby 1984). Burning directly and indirectly affects survival of ground dwelling animals, including snails and other gastropods (Nekola 2002), by reducing and modifying organic substrates used as shelters, increasing soil evaporation and destroying the upper part of the soil and leaf litter habitat, which are important for the survival of litter-soil organisms (Bellido 1987; Knapp et al. 2009). Following prescribed burns in Oregon, Duncan (2005) found that slugs were not found at over a quarter of the sites that supported them during pre-fire surveys, and suggested that at sites where slugs persisted, they survived fires in deep fissures in coarse rock substrate or other underground refuges. Duncan (2005) also suggested that the distribution of microhabitats that allow for vertical movements during fires is important for the long-term viability of slug populations within the landscape. It is unknown if similar refuges exist within Carolina Mantleslug habitat in Ontario (COSEWIC 2019). Decaying logs are an important microhabitat of Carolina Mantleslug (COSEWIC 2019) and a summary report by the Department of Sustainability and Environment (2003) found that during and after fires, small, unburnt patches (as small as 1 m2) act as significant habitat for invertebrates and other animals, with fallen logs being the most important association with unburnt patches. This suggests that low-intensity burns may leave fallen logs intact, which may provide a refuge for Carolina Mantleslug.
Sections of the Stone Road Alvar on Pelee Island were burned by Ontario Nature and Essex Regional Conservation Authority in 1993, 1997, 1999 and 2005 (NCC 2008), as well as in 2019 accompanied by an impact study implemented by Ontario Nature, including snail monitoring done by A. Nicolai. Although Carolina Mantleslug has only been found in the wooded part of Stone Road Alvar, the threat from fire should be considered given the risk of fire reaching forested habitat (COSEWIC 2019). During these studies it was found that fire resulted in some gastropod mortality and that because of the patchiness of the fire, and the fact that the highest density of snails was observed in a small area that was burned (surrounded by unburned habitat), recolonization was fast and pre-burn densities were reached three years post-burn (Nicolai, unpublished data). These findings do not directly apply to Carolina Mantleslug, as it is found in wooded habitat. Direct impacts from fire on slug populations are reduced when available habitat is widespread and recolonization from nearby areas is possible. However, when habitat areas are small, large fires are considered detrimental to subpopulations. Small, patchy fires that are restricted to some parts of the area may be less harmful (Driscoll et al. 2021).
Invasive species
Several highly invasive plant species in southern Ontario, including Garlic Mustard (Alliaria petiolata), are found on Pelee Island in Carolina Mantleslug habitat. Garlic Mustard is known to displace native vegetation and alter soil nutrient cycles, which slows restoration of native plant species such as spring ephemeral wildflowers (Catling et al. 2015). Stoll et al. (2012) found that invasive knotweed (Fallopia species) in Switzerland caused a significant reduction in large and long-lived snail species, but not in slugs or small, short-lived snails, while Ruckli et al. (2013) found that gastropod abundance and richness increased in forests invaded by the invasive plant Himalayan Balsam (Impatiens glandulifera). European Common Reed (Phragmities australis) is a highly invasive plant with wide-reaching ecological impacts, which may be a threat in parts of Carolina Mantleslug’s range, such as in Rondeau Provincial Park (MNRF 2019). Further research is needed to determine how invasive plants and the ecosystem modifications they generate impact Carolina Mantleslug and gastropods in general.
Introduced non-native earthworms have become established in Canada and have altered forest floor habitats by reducing or eliminating the natural leaf litter layer, and by digging up and mixing the mineral soil with the organic surface layer (CABI 2016). Through these habitat alterations, invasive earthworms may indirectly alter terrestrial snail communities (Forsyth et al. 2016). Invasive earthworms are present on the north shore of Lake Erie (Evers et al. 2012), Pelee Island (Reynolds 2011) and elsewhere in Ontario (Reynolds 2014). The Asian genus Amynthas has been introduced to Essex County (Reynolds 2014) and is known to quickly reduce surface leaf litter where gastropods live (Qiu and Turner 2017). Other indirect effects could result from earthworms feeding on forest plant seeds (Cassin and Kotanen 2016) or by altering plant-fungi mutualisms (Paudel et al. 2016), thereby affecting understory vegetation composition (Drouin et al. 2016) and potentially reducing available fungi.
Exotic terrestrial gastropods are also a potential threat (Whitson 2005; Grimm et al. 2010) to Carolina Mantleslug. Several species of exotic gastropods are widespread in southern Ontario, and more specifically on Lake Erie islands and the mainland of southwestern Ontario (the carnivorous Draparnaud’s Glass Snail (Oxychilus draparnaudi) and Cellar Glass Snail (Oxychilus cellarius)). These non-native gastropods may directly affect native species (COSEWIC 2019; Mahlfeld 2000) through aggression (Kimura and Chiba 2010), density effects, food competition (Baur and Baur 1990) and competition for shelter (COSEWIC 2019).
Competition for food with other sympatric slugs in Ontario, such as Changeable Mantleslug (Megapallifera mutabilis), or exotic species, is a possibility for Carolina Mantleslug in southwestern Ontario (COSEWIC 2019). Aggressive behaviour of Leopard Slug (Limax maximus) has been shown to considerably reduce reproductive success of two Arion species in British Columbia (Rollo 1983). Leopard slug is introduced in Ontario and has been observed in areas close to Carolina Mantleslug, including near Rondeau and Wheatley Provincial Park, and on Pelee Island (iNaturalist 2022). Introduced exotic gastropods in Ontario, such as Grove Snail (Cepaea nemoralis) and various species of slugs, mainly Grey Fieldslug (Deroceras reticulatum) or Dusky Arion (Arion fuscus/subfuscus), likely share a similar diet to Carolina Mantleslug and therefore might be in direct competition for food sources, especially in habitats where these species distributions overlap (COSEWIC 2019).
Birds introduced to some parts of Ontario for recreational hunting, such as Wild Turkeys (Meleagris gallopavo) (native to mainland Ontario but introduced to Pelee Island) and Ring-necked Pheasants (Phasianus colchicus) may pose a threat to Carolina Mantleslug because both species are omnivorous and feed on gastropods (Sandilands 2005). These bird species have recently been identified as ongoing threats to other threatened or endangered gastropods (COSEWIC 2017; 2018). Their impacts on Carolina Mantleslug are unknown, but may be reduced relative to other species, as Carolina Mantleslug typically stays under logs, making it less accessible to birds (COSEWIC 2019).
Human disturbance
Gastropod populations may be fragmented by paved roads or tracks as narrow as three metres (Wirth et al. 1999) because snails tend not to cross roads (Baur and Baur 1990). These barriers likely also affect Carolina Mantleslug because of its low dispersal capabilities and reliance on moist conditions. Reck and van der Reer (2015) cite a study by Martin and Roweck (1988) who documented local extinctions in a population of Rotund Disc (Discus rotundatus) in Germany after its original habitat became unsuitable. Roads acted as a barrier to movement and mating possibilities, thus reducing gene flow. This conclusion could also be applicable to slugs with low dispersal abilities (COSEWIC 2019; Kaija pers. comm. 2022). Road mortality has been recognized as a threat for wildlife in protected areas, such as Point Pelee National Park (Parks Canada 2007), but since Carolina Mantleslug rarely moves away from under logs, it is not likely to be affected by road mortality (COSEWIC 2019).
The historical decline of this species is likely a result of habitat loss and degradation. According to the Essex Region Conservation Authority (ERCA) (2002), most of the forest cover in the historical range of this species was cleared for agriculture use during the 1800s. Roughly 5% of the original forest cover remains in southwestern Ontario, much of which remains in parks and conservation authority lands, with smaller privately owned fragments often less than 10 ha in size (ERCA 2002).
Mushroom picking may be a potential threat to Carolina Mantleslug (COSEWIC 2019) since it is known to consume mushrooms, which are an important habitat requirement for the species. While there is no data indicating how mushroom picking may affect Carolina Mantleslug, four edible mushrooms in Ontario used by humans (Northern Bushcraft 2018) may also be consumed by Carolina Mantleslug, especially Golden Chanterelle (Cantharellus cibarius) (White-McLean 2012). However, mushroom picking is not a common activity in any of the areas containing Carolina Mantleslug, and is therefore not considered to be a major threat.
Trampling is a negligible threat to this species because they live under logs and rocks, but displacement of these habitats and leaf litter may alter the microhabitat conditions (COSEWIC 2019). While there is a short loop trail, large parts of Stone Road Alvar are not accessible due to high vegetation density and absence of trails (COSEWIC 2019).
Pollution
Heavy metals and road salt are a threat to gastropods (Viard et al. 2004) because they decrease food consumption, growth and fecundity (Laskowski and Hopkin 1996) as a result of accumulation in the soil and food plants (Notten et al. 2005). These are particularly a threat where the species habitat is within close proximity to roads. Road density is low on Pelee Island, but Rondeau and Wheatley Provincial Parks have more roadways, some of which undergo winter maintenance, including salt application (Kaija pers. comm. 2022).
The effects of pesticides, other than molluscicides, on terrestrial gastropods are poorly known. Laboratory studies have shown that some herbicides increase mortality of aquatic snails that are infected with parasites (Koprivnikar and Walker 2011) and could affect reproduction in terrestrial snails (Druart et al. 2011), while other studies have found that terrestrial gastropods were not impacted by herbicides in agricultural (Roy et al. 2003) or forested (Hawkins et al. 1997) landscapes. The increasingly used neonicotinoid insecticides were found not to be harmful to Grey Fieldslug (Douglas and Tooker 2015). The effects of pesticides on Carolina Mantleslug are unknown, however agricultural land is adjacent to wooded areas on Pelee Island and in Grape Fern Woods, which may expose slugs to pesticide drift (COSEWIC 2019).
1.7 Knowledge gaps
Distribution and population sizes
Most of the known extant and historical occurrence sites in Ontario of Carolina Mantleslug were surveyed from 2013 to 2019, but some known occurrence sites on private property were not accessed leaving the potential for the species to have small subpopulations remaining (COSEWIC 2019). It is unknown if populations still persist in other habitat types where historical surveys were less common. Because current distribution data are incomplete, population trends and dynamics in Ontario are unknown, and threats to any extant populations are either site-specific (e.g., prescribed burns) or global (e.g., climate change). Minimum viable population size is also unknown for this species, and is important when determining potential for recovery of subpopulations.
Species ecology
The likelihood of ongoing decline is difficult to predict because of the limited biological knowledge available for the species. Basic biological knowledge, such as diet, predators/parasites, habitat requirements, dispersal strategies and the impact of pollutants and invasive species would provide better insight into the factors that are most important for the survival or decline of this species, as well as provide important insights into recovery viability. Continuing to monitor the effects of climate change and how it impacts the biology of Carolina Mantleslug will also help understand this threat and determine recovery viability.
1.8 Recovery actions completed or underway
To date, no species-specific recovery actions have been implemented for Carolina Mantleslug.
A study of prescribed burn impacts on species at risk on Stone Road Alvar implemented by Ontario Nature will include targeted surveys in 2022 and 2023. The same study included gastropod surveys from 2017-2020 implemented by A. Nicolai, but Carolina Mantleslug was not found in the burn area. On Nature Conservancy of Canada land on Pelee Island where the slug occurs, gastropod-focused habitat enhancement, public outreach for awareness, and surveys are conducted by trained staff (Croswaithe pers. comm. 2019).
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal for Carolina Mantleslug is to ensure the long-term persistence of extant subpopulations.
2.2 Recommended protection and recovery objectives
The recovery goal for this species is focused on mitigating threats and enhancing habitat to allow for long-term population persistence and expansion in Ontario. To achieve this goal, recommended recovery objectives are identified below.
- Engage the scientific community, government land managers, land trusts, conservation organizations and private landowners in surveying suitable habitats to determine the current range extent in southwestern Ontario.
- Assess and mitigate threats at all known extant and historical occurrence sites in Ontario.
- Conduct and/or support research that fills knowledge gaps related to biology, population size, and habitat requirements that inform recovery efforts.
- Enhance and/or create habitat, where feasible and necessary, to increase habitat availability for extant subpopulations.
2.3 Recommended approaches to recovery
Table 1. Recommended approaches to recovery of the Carolina Mantleslug in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Research |
| Knowledge gaps:
|
| Critical | Short-term | Communication; Education and Outreach |
| Knowledge gaps:
|
| Critical | Short-term | Inventory; Monitoring and Assessment |
| Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Management |
| Threats:
|
| Critical | Short-term | Management |
| Threats:
Knowledge gaps:
|
| Critical | Long-term | Monitoring and Assessment |
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Research |
| Knowledge gaps:
|
| Beneficial | Long-term | Management; Protection |
| Knowledge gaps:
|
| Critical | Short-term | Research |
| Threats:
|
| Critical | Long-term | Research |
| Threats:
Knowledge gaps:
|
| Critical | Short-term | Research |
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Management; Protection; Stewardship |
| Threats:
|
| Beneficial | Long-term | Management; Protection |
| Threats:
|
| Beneficial | Long-term | Monitoring and Assessment |
| Threats:
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
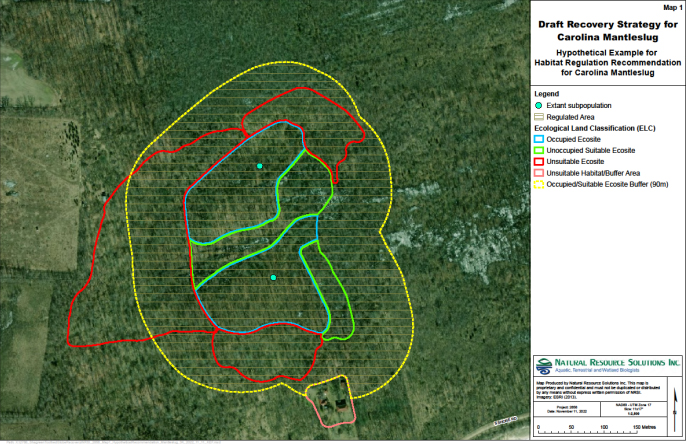
Information on the spatial limits of habitat used and dispersal by Carolina Mantleslug is lacking. When information on home range size, dispersal ability and key habitat features critical for supporting the species lifecycle becomes available, the area prescribed as habitat could be described more precisely and should be revisited. Based on the best information available, it is recommended that the regulated area should be defined using a contiguous ecological area encompassing all occupied ecosites and any suitable unoccupied ecosites immediately adjacent to occupied ecosites. Ecosites represent the recurring plant species patterns in a given habitat that are maintained by a variety of environmental factors, and defined by geology, soils and vegetation (Lee et al. 1998).
It is important to protect entire ecosites occupied by extant subpopulations because given the rarity of the species, it may be present throughout the habitat but not detected everywhere. Protecting adjacent suitable but unoccupied ecosites is also recommended. Like other slug and snail species, Carolina Mantleslug may use habitat patches within different ecosites in different seasons for various biological functions such as feeding and aestivation/hibernation (Burch and Pearce 1990). Including unoccupied suitable ecosites adjacent to occupied ecosites increases the probability that all habitat elements necessary for foraging, mating, nesting, aestivating and hibernating for several generations are included. Including adjacent suitable ecosites also supports natural expansion and recolonization of areas that were historically occupied, that may only be currently unoccupied due a combination of historical disturbance, ongoing restoration processes and slow recolonization speed. Suitable ecosites are those that provide forested and/or wooded habitat and have substantial leaf litter and decaying logs/plant material, all of which provide moist microhabitat sites for hibernation, aestivation and egg-laying. As more research and monitoring is completed to address knowledge gaps, these ecosite types and features may be further refined.
It is further recommended that a buffer of 90 metres be added to the defined ELC ecosite polygons (inclusive of both occupied ecosites and adjacent suitable unoccupied ecosites). Harper et al. (2005) reviewed 44 published studies on direct and indirect forest edge effects to determine the mean distance of influence on forest structure, processes, and biodiversity. While depth of influence varied greatly across measures and regions, based on this analysis, it is believed that a buffer distance of 90 metres is necessary to maintain important microhabitat properties and to reduce edge effects. The buffer may include habitat unsuitable for long-term occupancy, but should exclude habitat known to be unsuitable for maintaining microhabitat, such as human-modified landscapes, existing infrastructure and waterbodies (e.g., roads, farmland, urban areas, gardens, parks on the mainland and lakes). While the primary intent of the 90-metre buffer is to maintain suitable microhabitat, this buffer also accounts for temporary use of unsuitable neighbouring habitat based on the longest short-term dispersal distance measured in Polygyridae (32 m) (Edworthy et al. 2012).
A visual depiction of the area for consideration in developing a habitat regulation is shown in Figure 3. This recommendation, including the buffer, takes into account the current and historical range of Carolina Mantleslug to directly protect the species, to allow natural expansion and recolonization, and to connect ecosites that are spread over the former range of the species within Ontario.
Glossary
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
- 1 = critically imperiled
- 2 = imperiled
- 3 = vulnerable
- 4 = apparently secure
- 5 = secure
- NR = not yet ranked
- Dart
- A calcium carbonate spike located in a sac to aid in reproduction.
- Ecosite
- A mappable, landscape unit integrating a consistent set of environmental factors and vegetation characteristics.
- ELC
- Ecological Land Classification.
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Mantle
- The dorsal (back/upper side) body wall which covers the visceral mass (softer tissue, containing most of the internal organs).
- Molluscicides
- Pesticides for use against molluscs.
- Phenotypic differentiation
- variation in observable characteristics of an individual resulting from the interaction of its genes with the environment.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008 (Ontario Regulation 230/08).
- Sympatric
- Two or more species that exist in the same geographic area.
List of abbreviations
- BOLDsystems
- Barcode of Life Data System
- CLEF
- Conference and Labs of the Evaluation Forum
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- DNA
- Deoxyribonucleic acid
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
References
- Augspurger, C.K. 2013. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology 94:41–50.
- Barger, M.A. 2011. Tests of ecological equivalence of two species of terrestrial gastropods as second intermediate hosts of Panopistus pricei (Trematoda: Brachylaimidae). Journal of Parasitology 97:8–13.
- Barger, M.A. and J.A. Hnida. 2008. Survey of trematodes from terrestrial gastropods and small mammals in southeastern Nebraska, USA. Comparative Parasitology 75:308–314.
- Barker, G.M. 2001. The Biology of Terrestrial Molluscs. CABI Publishing, New York, New York. 558 pp.
- Baur, A., and B. Baur. 1990. Are roads barriers to dispersal in the land snail Arianta arbustorum? Canadian Journal of Zoology 68:613–617.
- Baur, A. and B. Baur. 2005. Interpopulation variation in the prevalence and intensity of parasitic mite infection in the land snail Arianta arbustorum. Invertebrate Biology 124:194–201.
- Bellido, A. 1987. Field Experiment about direct effect of a heathland prescribed fire on microarthropod community. Revue d’Ecologie et de Biologie du Sol 24:603–633.
- BOLDsystems (Barcode of Life Data System). 2022. Retrieved [June 2022], from the online database.
- Brooks, M. and M. Lusk. 2008. Fire Management and Invasive Plants: a Handbook. United States Fish and Wildlife Service, Arlington, Virginia, 27 pp.
- Burch, J.B. and T.A. Pearce. 1990. Terrestrial gastropods. Pp. 201–309. In: D.L. Dindal (ed.). Soil Biology Guide. John Wiley and Sons, New York, New York.
- CABI (CAB International). 2016. Invasive Species Compendium. Datasheet Lumbricus rubellus.[accessed June 2022].
- Cassin, C.M., and P.M. Kotanen. 2016. Invasive earthworms as seed predators of temperate forest plants. Biological Invasions 18:1567–1580.
- Catling, P.M., G. Mitrow, and A. Ward. 2015. Major invasive alien plants of natural habitats in Canada. 12. Garlic Mustard, Alliaire officinale: Alliaria petiolata (M. Bieberstein) Cavara & Grande. CBA/ABC Bulletin 48(2):51–60.
- Cook, A. 1981. Huddling and the control of water loss by the slug Limax pseudoflavus Evans. Animal Behavior 29:289–298.
- COSEWIC. 2016. COSEWIC assessment and status report on the Sheathed Slug Zacoleus idahoensis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 51 pp.
- COSEWIC. 2017. COSEWIC assessment and status report on the Eastern Banded Tigersnail Anguispira kochi kochi and the Western Banded Tigersnail Anguispira kochi occidentalis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 82 pp.
- COSEWIC. 2018. COSEWIC assessment and status report on Striped Whitelip Webbhelix multilineata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 62 pp.
- COSEWIC. 2019. COSEWIC assessment and status report on the Carolina Mantleslug Philomycus carolinianus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 54 pp.
- Current, W.L. 2007. Cryptobia sp. in the snail Triodopsis multilineata (Say): fine structure of attached flagellates and their mode of attachment to the spermatheca. Journal of Eukaryotic Microbiology 27:278–287.
- Department of Sustainability and Environment. 2003. Ecological impacts of fuel reduction burning in a mixed eucalypt foothill forest — summary report (1984-1999). Fire Research Report No. 57, Deparment of Sustainability and Environment, Victoria, Australia. xiv + 86 pp.
- Dobbyn, S., and L. Pasma. 2012. A Life Science Inventory and Evaluation of Rondeau Provincial Park. Ontario Parks, Southwest Zone, London, Ontario. viii + 206 pp. + 2 maps.
- Douglas, M.R., and J.F. Tooker. 2015. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environmental Science and Technology 49:5088–5097.
- Driscoll, D.A, D. Armenteras, A.F. Bennett, L. Brotons, M.F. Clarke, T.S. Doherty, A. Haslem, L.T. Kelly, C.F. Sato, H. Sitters, N. Aquilué, K. Bell, M. Chadid, A. Duane, M.C. Meza-Elizalde, K.M. Gilijohann, T.M. González, R. Jambhekar, J. Lazzari, A. Morán-Ordóñez, T. Wevill. 2021. How fire interacts with habitat loss and fragmentation. Biological Reviews 96:976–998.
- Drouin, M., R. Bradley, and L. Lapointe. 2016. Linkage between exotic earthworms, understory vegetation and soil properties in sugar maple forests. Forest Ecology and Management 364:113–121.
- Druart, C.M., M. Millet, R. Scheifler, O. Delhomme, and A. de Vaufleury. 2011. Glyphosate and glufosinate-based herbicides: fate in soil, transfer to, and effects on land snails. Journal of Soil Sediments 11:1373–1384.
- Duncan, N. 2005. Monitoring of sensitive mollusk populations following low-intensity wildfire in old growth coniferous forest. Unpublished report prepared for USDI Bureau of Land Management, Roseburg District Office, Oregon 97470, USA.
- Edworthy, A.B., K.M.M. Steensma, H.M. Zandberg, and P.L. Lilley. 2012. Dispersal, home-range size, and habitat use of an endangered land snail, the Oregon forestsnail (Allogona townsendiana). Canadian Journal of Zoology 90(7):875–884.
- ERCA (Essex Region Conservation Authority). 2002. Essex Region Biodiversity Conservation Strategy — Habitat Restoration and Enhancement Guidelines (Comprehensive Version). Dan Lebedyk, Project Coordinator, Essex, Ontario. 181 pp.
- Evers, A.K., A.M. Gordon, P.A. Gray, and W.I. Dunlop. 2012. Implications of a potential range expansion of invasive earthworms in Ontario’s forested ecosystems: a preliminary vulnerability analysis. Climate Change Research Report CCRR-23. Science and Information Resources Division. Ontario Ministry of Natural Resources, Ottawa, Ontario. 46 pp.
- Fitzpatrick, S.W. and B.N. Reid. 2019. Does gene flow aggravate or alleviate maladaptation to environmental stress in small populations? Evolutionary Applications 12(7):1402–1416.
- Foden, W.B., S.H.M. Butchart, S.N. Stuary, J.-C. Vié, H.R. Akçakaya, A. Angulo, L.M. DeVantier, A. Gutsche, E. Turak, L. Cao, S.D. Donner, V. Katariya, R. Bernard, R.A. Holland, A.F. Hughes, S.E. O’Hanlon, S.T. Garnett, C.H. Şekercioğlu, and G.M. Mace. 2013. Identifying the world’s most climate change vulnerable species: a systematic trail-based assessment of all birds, amphibians and corals. PLoS ONE 8(6):e65427.
- Forsyth, R.G., P. Catling, B. Kostiuk, S. McKay-Kuja, and A. Kuja. 2016. Pre-settlement snail fauna on the Sandbanks Baymouth Bar, Lake Ontario, compared with nearby contemporary faunas. Canadian Field-Naturalist 130:152–157.
- Gibson, S.Y., R.C. Van der Marel, and B.M. Starzomski. 2009. Climate change and conservation of leading-edge peripheral populations. Conservation Biology 23:1369–1373.
- Grimm, F.W. 1996. Terrestrial molluscs. In: I.M. Smith (ed.). Assessment of species diversity in the Mixedwood Plains ecosystem. Ecological Monitoring and Assessment Network, Canada.
- Grimm, F.W., R.G. Forsyth, F.W. Schueler, and A. Karstad. 2010. Identifying Land Snails and Slugs in Canada: Introduced Species and Native Genera. Canadian Food Inspection Agency, Ottawa, Ontario. 168 pp.
- Harper, K.A., S.E. MacDonald, P.J. Burton, J. Chen, K.D. Brosofske, S.C. Daunders, E.S. Euskirchen, D. Roberts, M.S. Jaiteh, and P. Esseen. 2005. Edge influence on forest structure and composition in fragmented landscapes. Conservation Biology 19(3):768–782.
- Hawkins, J.W., M.W. Lankester, R.A. Lautenschlager, and F.W. Bell. 1997. Effects of alternative conifer release treatments on terrestrial gastropods in northwestern Ontario. The Forestry Chronicle 73(1):91–98.
- Honek, A., and Z. Martinkova. 2011. Body size and colonization of cereal crops by the invasive slug Arion lusitanicus. Annals of Applied Biology 158:79–86.
- Hubricht, L. 1985. The distributions of the native land mollusks of the Eastern United States. Fieldiana Zoology 24:47–171.
- iNaturalist. 2022. iNaturalist Research-grade Observation. iNaturalist.org. Occurrence dataset [Retrieved July 2022].
- Jordan, S.F. and S.H. Black. 2012. Effects of forest land management on terrestrial mollusks: a literature review. USDA Forest Service, Region 6 USDI Oregon/Washington, Bureau of Land Management. 87 pp.
- Kimura, K. and S. Chiba. 2010. Interspecific interference competition alters habitat use patterns in two species of land snails. Evolutionary Ecology 24:815–825.
- Knapp, E.E., B.L. Estes, and C.N. Skinner. 2009. Ecological effects of prescribed fire season: a literature review and synthesis for managers. USA General Technical Report. Albany, California. 80 pp.
- Koprivnikar, J. and P.A. Walker. 2011. Effects of the herbicide Atrazine’s metabolites on host snail mortality and production of trematode cercariae. Journal of Parasitology 97:822–827.
- Laskowski, R. and S.P. Hopkin. 1996. Effect of Zn, Cu, Pb, and Cd on fitness in snails (Helix aspersa). Ecotoxicology and Environmental Safety 34:59–69.
- Lebourcq, G. 2020. Biologie de la conservation : Comment évaluer et intégrer les exigences écologiques des gastéropodes terrestres cryptiques ? — Le cas de l’espèce protégée Elona quimperiana. Master 1 thesis, University Rennes 1, France, 24 pp.
- Lee, H., W. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhlig, and S. McMurray. 1998. Ecological Land Classification for Southern Ontario. SCSS Field Guide FG-02. North Bay: Ontario Ministry of Natural Resources. 225 pp.
- Mahlfeld, K. 2000. Impact of introduced gastropods on molluscan communities, northern North Island. Conservation advisory notes no 277, Department of Conservation, Wellington, New Zealand. 18 pp.
- McDermid, J., S. Fera, and A. Hogg. 2015. Climate change projections for Ontario: an updated synthesis for policymakers and planners. Ontario Ministry of Natural Resources and Forestry, Science and Research Branch, Peterborough, Ontario. Climate Change Research Report CCRR-44.
- MNR (Ministry of Natural Resources). 2005. Fish Point and Lighthouse Point Park Management Plan. Queen’s Printer for Ontario. 25 pp.
- MNRF (Ministry of Natural Resources and Forestry). 2019. Invasive Phragmites Control at Long Point Region and Rondeau Provincial Park Implementation Plan — 2019. 24 pp.
- Morand, S., M.J. Wilson, and D.M. Glen. 2004. Nematodes (Nematoda) parasitic in terrestrial gastropods. Pp. 525–558. In: G. Barker (ed.). Natural Enemies of Terrestrial Mollusks. CABI Publishing, Cambridge, Massachusetts.
- NatureServe. 2022. NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. [Accessed: October 24, 2022].
- Natural Resources Canada. 2019. The Atlas of Canada — Toporama. Search for Pelee Island, Essex, Ontario (Island) [accessed 17 June 2022].
- NCC (Nature Conservancy of Canada). 2008. Management Guidelines: Pelee Island Alvars. NCC — Southwestern Ontario Region, London, Ontario. 43 pp.
- Nekola, J.C. 2002. Effects of fire management on the richness and abundance of central North American grassland land snail faunas. Animal Biodiversity and Conservation 25(2):53–66.
- Nietzke, G. 1970. Die Weinbergschnecke. 2. Auflage, Verlag Eugen Ulmer, Stuttgart. 155 pp.
- Northern Bushcraft. 2018. Wild Edible Mushrooms of Ontario. [accessed 17 October 2022].
- Notten, M.J.M., A.J.P. Oosthoek, J. Rozema, and J. Aerts. 2005. Heavy metal concentrations in a soil-plant-snail food chain along a terrestrial soil pollution gradient. Environmental Pollution 138:178–190.Örstan, A. 2006. Rearing terrestrial gastropoda. Pp. 287–293. In: C.F. Sturm, T.A. Pearce, and A. Valdés (eds.). The Mollusks: A Guide to Their Study, Collection, and Preservation. American Malacological Society, Pittsburgh, Pennsylvania. 445 pp.
- Örstan, A. 2006. Rearing terrestrial gastropoda. Pp. 287–293. In: C.F. Sturm, T.A. Pearce, and A. Valdés (eds.). The Mollusks: A Guide to Their Study, Collection, and Preservation. American Malacological Society, Pittsburgh, Pennsylvania. 445 pp.
- Oughton, J. 1948. A zoogeographical Study of Land Snails in Ontario. University of Toronto Press, Toronto, Ontario. 128 pp.
- Parks Canada. 2007. Point Pelee National Park of Canada State of the Park Report 2006. Her Majesty the Queen in Right of Canada. Leamington, Ontario. 44 pp.
- Paudel, S., T. Longcore, B. MacDonald, M.K. McCormick, K. Szlavecz, G.W.T. Wilson, and S.R. Loss. 2016. Belowground interactions with aboveground consequences: invasive earthworms and arbuscular mycorrhizal fungi. Ecology 97:605–614.
- Pearce, T.A. and M.E. Paustian. 2013. Are temperate land snails susceptible to climate change through reduced altitudinal ranges? A Pennsylvania example. American Malacological Bulletin 31:213–224.
- Peterson C., R.J. Hermann, M.-C. Barg, R. Schalkowski, P. Dirksen, C. Barbosa, and H. Schulenburg. 2015. Travelling at a slug’s pace: possible invertebrate vectors of Caenorhabditis nematodes. BMC Ecology:15–19.
- Pilsbry, H.A. 1948. Land Mollusca of North America (North of Mexico). Volume 2, Part 2. Academy of Natural Sciences of Philadelphia, Monograph 3:i–xvii + 521–1113.
- Qiu, J. and M.G. Turner. 2017. Effects of non-native Asian earthworm invasion on temperate forest and prairie soils in the Midwestern US. Biological Invasions 19:73–88.
- Reck, H., and R. van der Reer. 2015. Insects, snail and spiders: the role of invertebrates in road ecology. Pp. 247–257. In: R. van der Reer, D.J. Smith, and C. Grilo (eds.). Handbook of Road Ecology. John Wiley & Sons, Oxford, United Kingdom.
- Reynolds, J.W. 2011. The earthworms (Oligochaeta: Lumbricidae) of Pelee Island, Ontario, Canada. Megadrilogica 15(3):23–33.
- Reynolds, J.W. 2014. A checklist by counties of earthworms (Oligochaeta: Lumbricidae, Megascolecidae and Sparganophilidae) in Ontario, Canada. Megadrilogica 16:111–135.
- Rising, T.L., and K.B. Armitage. 1969. Acclimation to temperature by the terrestrial gastropods, Limax maximus and Philomycus carolinianus: oxygen consumption and temperature preference. Comparative Biochemistry and Physiology 30:1091–1114.
- Robinson, D.G. 1999. Alien invasions: the effects of the global economy on non-marine gastropod introduction into the United States. Malacologia 41:413–438.
- Robinson, D., and J. Slapcinsky. 2005. Recent introductions of alien gastropods into North America. American Malacological Bulletin 20:89–93.
- Rollo, C.D. 1983. Consequences of competition on the reproduction and mortality of three species of terrestrial slugs. Researches on Population Ecology 25:20–43.
- Rowley, M.A., E.S. Loker, J.F. Pagels, and R.J. Montali. 1987. Terrestrial gastropod hosts of Parelaphostrongylus tenuis at the National Zoological Park’s Conservation and Research Center, Virginia. Journal of Parasitology 73:1084–1089.
- Roy, D.B., D.A. Bohan, A.J. Haughton, M.O. Hill, J.L. Osborne, S.J. Clark, J.N. Perry, P. Rothery, R.J. Scott, D.R. Brooks, G.T. Champion, C. Hawes, M.S. Heard, and L.G. Firbank. 2003. Invertebrates and vegetation of field margins adjacent to crops subject to contrasting herbicide regimes in the Farm Scale Evaluations of genetically modified herbicide-tolerant crops. Philosophical Transactions of the Royal Society London B 358:1879–1898.
- Ruckli, R., H-P. Rusterholz and B. Baur. 2012. Invasion of Impatiens glandulifera affects terrestrial gastropods by altering microclimate. Acta Oecologica 47:16–23.
- Sandilands, A. 2005. Birds of Ontario: Habitat Requirements, Limiting Factors, and Status. Volume 1. Nonpasserines: Waterfowl through Cranes. University of British Columbia Press. Vancouver, British Columbia. 365 pp.
- Slotsbo, S., L.M. Hansen, K. Jordaens, T. Backeljau, A. Malmendal, N.C. Nielsen, and M. Holmstrup. 2012. Cold tolerance and freeze-induces glucose accumulation in three terrestrial slugs. Comparative Biochemistry and Physiology Part A 161:443–449.
- Stoll, P., K. Gatzsch, H.-P. Rusterholz, B. Baur. 2012. Response of plant and gastropod species to knotweed invastion. Basic and Applied Ecology 13(3):232–240.
- Storey K.B., J.M. Storey, and T.A. Churchill. 2007. Freezing and anoxia tolerance of slugs: a metabolic perspective. Journal of Comparative Physiology Part B 177:833–840.
- Thompson, J.M., A.G. Appel, J.L. Sibley, G.J. Keever, and W.G. Foshee III. 2006. Comparative water relations of three sympatric terrestrial slugs: (Stylomatophora: Agriolimacidae, Limacidae, and Philomycidae). Journal of Alabama Academy of Sciences 77:181–192.
- Towne, G., and C. Owensby. 1984. Long-term effects of annual burning at different dates in ungrazed Kansas tallgrass prairie. Journal of Range Management 37:392–397.
- Turke, M., M. Lange, and N. Eisenhauer. 2018. Gut shuttle service: endozoochory of dispersal-limited snail fauna. Behavioral Ecology 186:655–664.
- Vagvolgyi, J. 1975. Body size, aerial dispersal, and origin of Pacific land snail fauna. Systematic Zoology 24:465–488.
- Varrin, R., J. Bowman, and P.A. Gray. 2007. The known and potential effects of climate change on biodiversity in Ontario’s terrestrial ecosystems: case studies and recommendations for adaptation. Climate Change Research Report CCRR-09. Ontario Ministry of Natural Resources and Forestry. Queen’s Printer for Ontario, Toronto, Ontario. 47. 1379 pp.
- Viard, B., F. Pihan, S. Promeyrat, and S.J.-C. Pihan. 2004. Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: bioaccumulation in soil, Graminaceae and land snails. Chemosphere 55:1349–1359.
- Welter-Schulter, F. 2012. European Non-Marine Molluscs, a Guide for Species Identification. Planet Poster Editions, Göttingen, Germany. 679 pp.
- White-Mclean, J.A. 2012. Identification of agriculturally important mollusks to the U.S. and observation on select Florida species. Ph.D. thesis, University of Florida Gainesville, Florida. 467 pp.
- Whitson, M. 2005. Cepaea nemoralis (Gastropoda, Helicidae): the invited invader. Journal of the Kentucky Academy of Science 66:82–88.
- Williams, G.W. 2000. Reintroducing Indian type fire: implications for land managers. Fire Management Today 60(3):40–48.
- Wirth, T., P. Oggier, and B. Baur. 1999. Effect of road width on dispersal and population genetic structure in the land snail Helicella itala. Journal of Nature Conservation 8:23–29.
Personal communications
- Crosthwaite, J. 2019. Verbal and e-mail communication. March 8, 2022. Conservation Biology Coordinator — Southwestern Ontario, Nature Conservancy of Canada.
- Kaija, J. 2022. Verbal and e-mail communication. September 8, 2022. Assistant Ecologist — Southwest Zone, Ontario Parks.