Constitutional Isomers
Constitutional isomers are molecules that have the same molecular formula but they have a different connectivity of atoms in the molecules. It’s best if I illustrate the idea of constitutional isomers with an example. Suppose, we have a molecule with a molecular formula C2H6O. Building the Lewis structures for this molecule reveals the two possibilities for the atom connections:
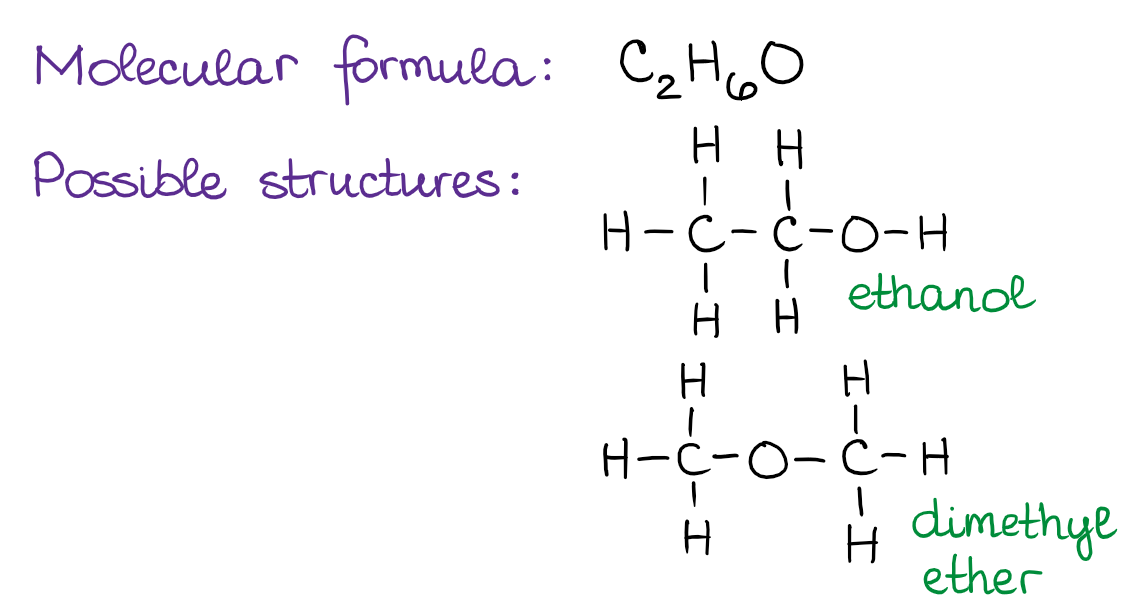
The two molecules above, ethanol (ethyl alcohol) and dimethyl ether, are example of constitutional isomers. They both have exactly the same atoms in the same ratios in the molecule. However, the connections between those atoms, or in other words, the constitution of the molecule, is different. The important thing about the constitutional isomers, is to realize that those are different molecules and, therefore, have different properties. You’ll see examples of constitutional isomers many times in your course, so it’s very important to be able to spot those.
Hydrogen Deficiency Index (HDI)
or
Degree of Unsaturation (DU)
Both of those terms refer to the same thing. The exact term your instructor will use will depend on their personal preferences or the book choice. While HDI is usually not introduced till we look into spectroscopy, I believe, it’s a very potent tool to know early in the semester. And frankly, I’m a little confused why it’s virtually never taught here. Anyways, back to HDI and why it’s so cool.
HDI is a useful tool to estimate the structural motifs in the molecule. Basically, each degree of unsaturation or HDI unit corresponds to either a π-bond or a cyclic motif in the molecule. Recall that double and triple bonds have π-bonds. The more “correct” definition of HDI is the number of H2 equivalents required to saturate molecule to have only open-chain single bonds structure. Which, if you ask me, sounds ugly, so I’m never going to repeat it again. 🤣
Let’s look at a few examples and see how molecular features relate to the units of HDI:
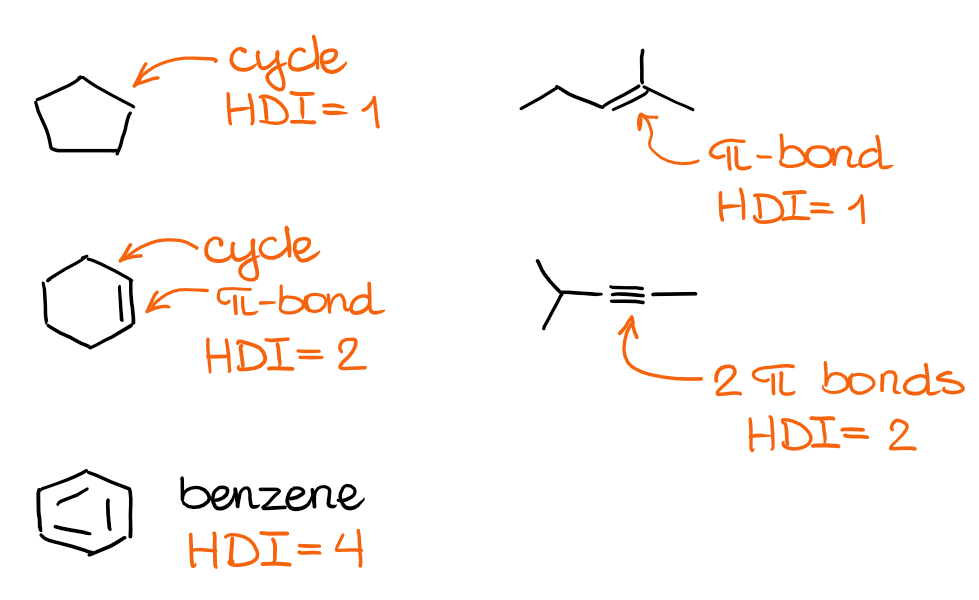
The combination of the cycles and double or triple bonds in your molecule will give you the HDI value. But how is this useful for constitutional isomers? Well, since the constitutional isomers have the same molecular formula, they will have the same HDI indexes too! This way, if you know the HDI for a molecule, you can automatically draw various constitutional isomers with the correct structural motifs. So, is there a way to calculate the HDI from the molecular formula alone? There sure is!
Calculating HDI from the Molecular Formula
To calculate HDI we use a very simple formula. You might want to memorize it as it’s a very useful one, especially, if you’re trying to draw constitutional isomers for a bunch of different molecules.
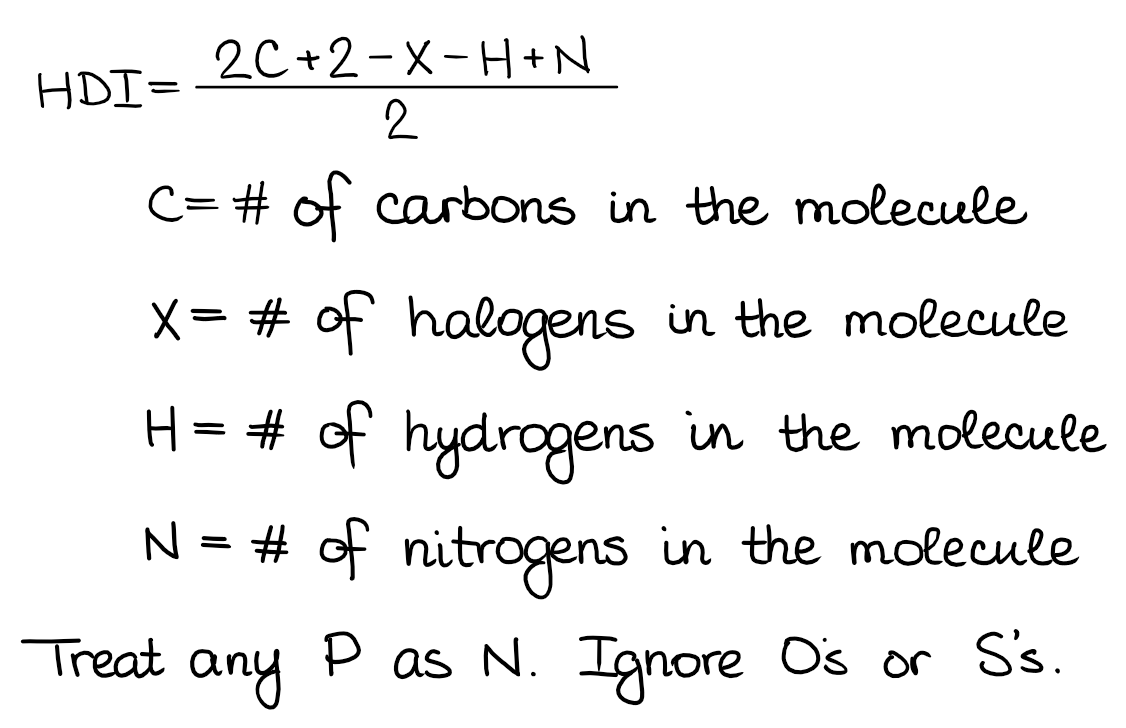
The formula I’m showing here has a few different forms. So, if your instructor or a textbook uses a different one, don’t worry, they all give the same result. I personally like this one, so I’m gonna stick with it. Let’s look at a few example using this formula:
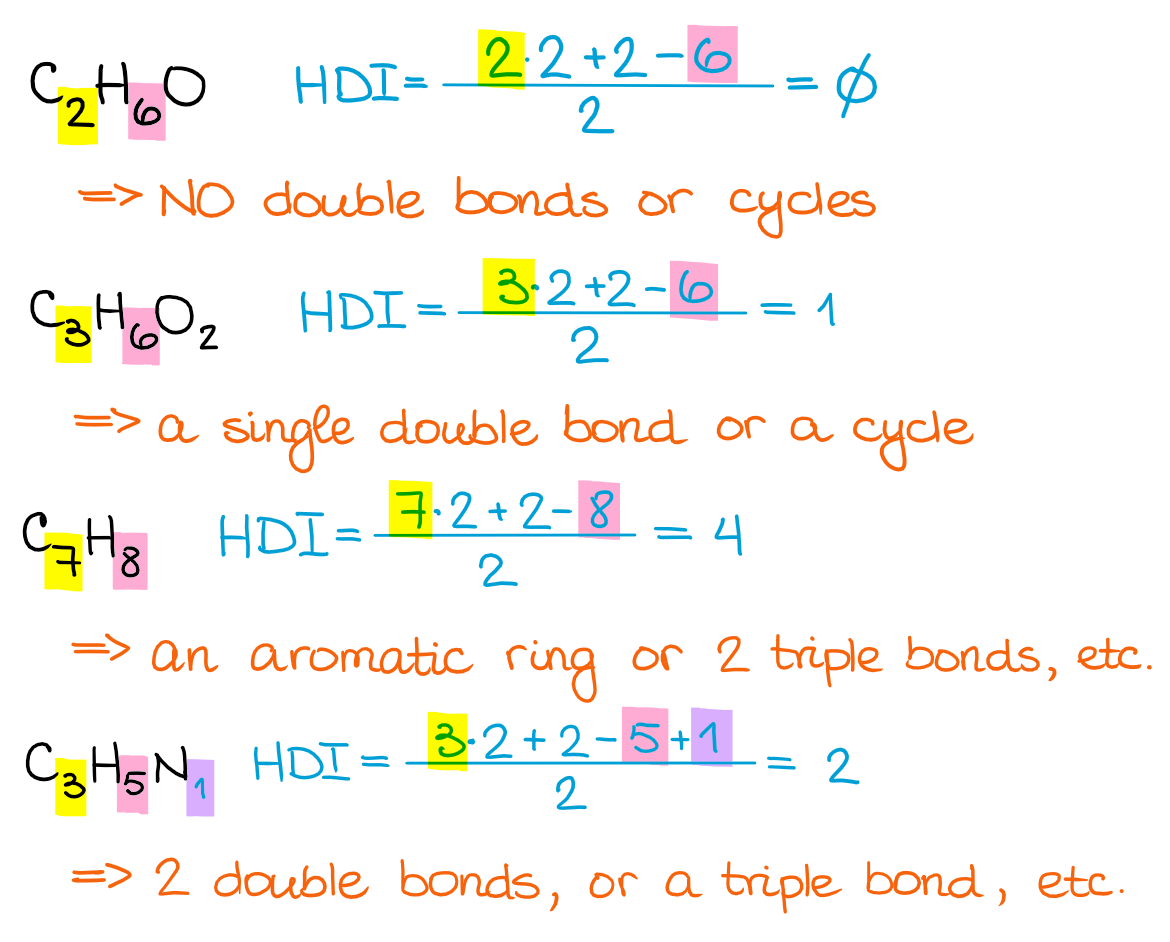
As you can see, the higher the HDI, the more possibilities you can get for the structural motifs in your molecule. So, while it’s a powerful tool, it’s not the “instant answer” formula, especially when it comes to more complex molecules.
How to Approach Problems Using HDI Formula
So, when you need to come up with the possible constitutional isomers for a molecule, the first thing you wanna look at is going to be the molecular formula. From there we’ll get the HDI and have some idea about what sort of structural features to look for in our molecule.
Let’s come up with all possible constitutional isomers for a molecule with the molecular formula C3H6O.
Step 1: Calculate the HDI from the Molecular Formula
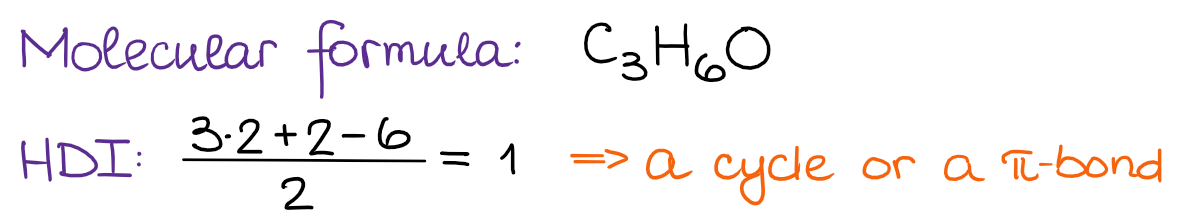
Based on our HDI, we know that this molecule must contain a double bond or a cycle. But not those two together since the HDI = 1.
Step 2: Draw Possible COnstitutional Isomers Starting from Linear (Simplest) Molecules
First, I always go with the possible linear structures for my constitutional isomers.
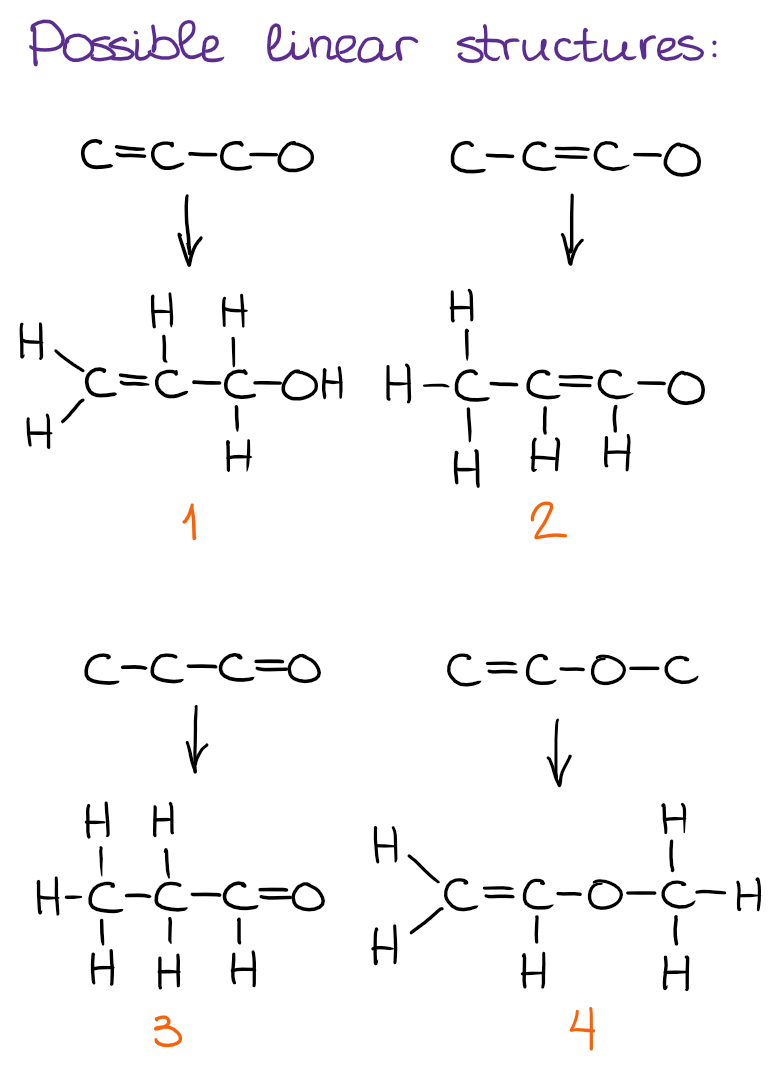
To make it easier, I suggest you come up with the core of the structure and then add the necessary number of hydrogens. Be careful not to add to many and be consistent with the bonding patterns that we normally observe in organic molecules!
After we’re done with the linear molecules, time to see if we can make any branches to the side from the core atoms we have in hand:
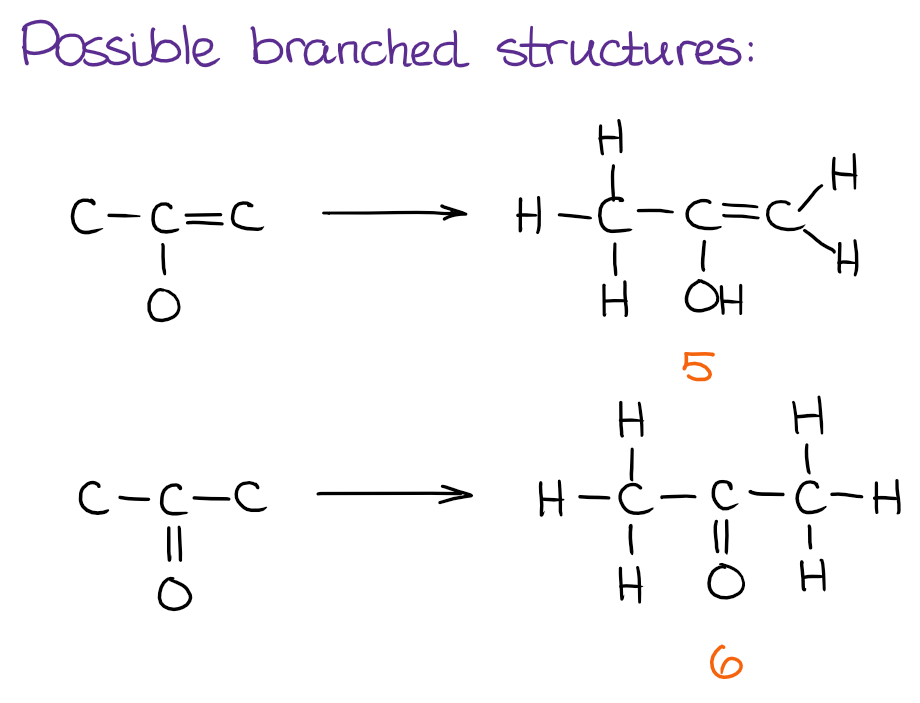
And finally, we can see if there are any cyclic structures we can put together:
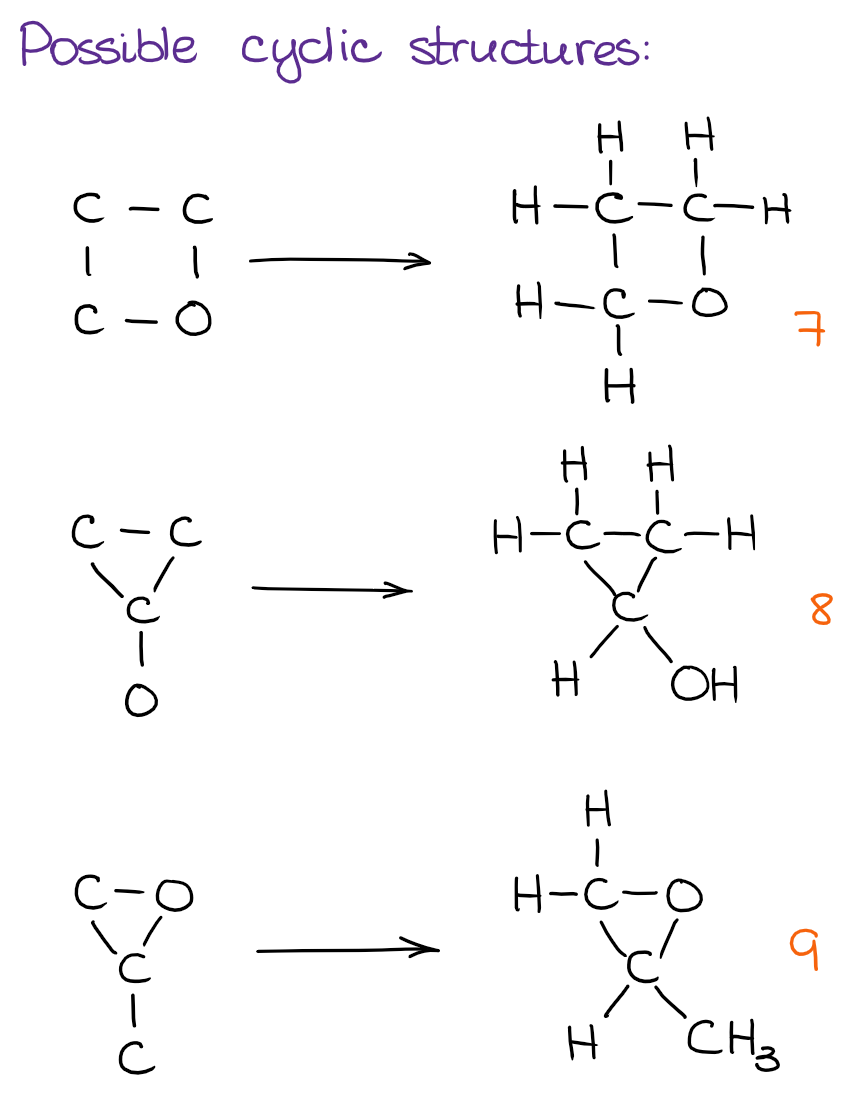
Using this simple approach, we can quickly and efficiently go through the possibilities with a very low chance of missing any structures. While the number of possible constitutional isomers grows exponentially with the number of atoms in the molecule, I don’t anticipate your instructor giving you anything that would have more than a dozen of structures on the test. However, a molecule with 5-7 constitutional isomers is a fair game! As you can see, the molecule above has 9. And I have seen this exact molecule on a few tests in the previous years. Also, be very careful if your instructor is asking for just constitutional isomers or for all possible isomers. If we’re not limited to the simple cases, you’ll also have to check for stereochemistry, which is a new layer of complexity. I’m going to talk about those some other time though.
