What is postthrombotic venous obstruction and how can it be avoided?
obstruction and how can it be
avoided?
FACS, FACC
The Toledo Hospital, Toledo, OH, USA;
Adjunct Professor of Surgery,
University of Michigan
Abstract
Postthrombotic venous obstruction is part of the pathophysiology of a postthrombotic syndrome. When the obstruction occurs in the iliofemoral segment, postthrombotic morbidity is often severe. In a recent study, the intraluminal contents of chronically occluded postthrombotic common femoral veins were analyzed. Approximately 80% to 90% of the tissues analyzed were composed of type I collagen, with type III collagen comprising the remainder. Vascular endothelial growth factor receptor 2 (VEGFR2) was more abundant in young specimens (≤1 year after the acute DVT); angiopoieton-1 receptor (TIE-2) was observed more often and at higher concentrations in mature specimens (≥10 years after the acute DVT); and the CD31 ligand was found equally in both young and mature specimens. Postthrombotic endoluminal obstruction can be avoided if the initial obliterating thrombus is successfully removed during the course of treatment for acute DVT. In fact, randomized trials, registries, and large observational experiences have demonstrated a reduction in the incidence of postthrombotic syndrome after successful thrombus removal. Another randomized trial, the ATTRACT trial, has recruited 692 patients with acute DVT in order to evaluate whether there is a reduction in or elimination of the incidence of postthrombotic syndrome with anticoagulation plus catheter-directed thrombolysis vs anticoagulation alone– results will be available in 2017.
Introduction
Postthrombotic syndrome is the consequence of acute deep vein thrombosis (DVT) of the lower extremities. Ambulatory venous hypertension is the underlying pathophysiology resulting from venous valvular incompetence and postthrombotic luminal obstruction. Patients with iliofemoral DVT have the most frequent and severe postthrombotic morbidity and suffer the highest risk of recurrence.1-3 In a prospective observational study of patients treated for acute DVT with anticoagulation alone, Kahn et al2 observed that the most powerful predictor of severe postthrombotic syndrome was iliofemoral DVT.
Qvarfordt et al4 measured compartment pressures in patients with iliofemoral DVT before and after venous thrombectomy and showed that preoperative compartment pressures exceeded 35 mm Hg and dropped to 10 mm Hg or less following iliofemoral venous thrombectomy. In this setting, compartment pressures can be used as a surrogate for venous pressures. Labropoulos et al5 measured arm-foot pressure gradients in patients with chronic postthrombotic venous disease. Patients with iliofemoral venous disease had the highest resting and postocclusive hyperemic pressures compared with patients with infra-inguinal postthrombotic disease.
Treatment strategies of anticoagulation alone do not assure that the occlusive thrombus will resolve and they depend upon the body’s endogenous thrombolytic activity to recanalize the obstructive thrombus. Unfortunately, a thrombus in the iliofemoral venous system frequently persists, causing central venous obstruction. As mentioned earlier, patients with iliofemoral DVT treated with anticoagulation alone have the highest risk for severe postthrombotic syndrome. This is largely due to persistent obstruction of the major venous outflow tract of the lower extremities.
Luminal obstruction
Based on ultrasound findings and phlebography, the obstructive nature of the thrombus in the vein lumen has been variously described as chronic thrombus, intraluminal fibrosis, or scar tissue. Until recently, no definitive description of the human tissue that chronically obstructs postthrombotic veins has been provided. In an attempt to resolve the extreme morbidity of these patients, those presenting with incapacitating postthrombotic syndrome due to chronic iliofemoral and inferior vena cava occlusion are fully evaluated. If the common femoral vein is obstructed, it is recommended to perform a common femoral vein endophlebectomy followed by transluminal recanalization of the occluded iliac veins and inferior vena cava (if involved).6
In a recent study, Comerota et al7 analyzed the intraluminal contents of 18 chronically occluded postthrombotic common femoral vein specimens obtained from 16 patients undergoing endophlebectomy followed by intraluminal recanalization of their iliocaval venous segments. Specimens were studied using the hematoxylin/eosin and Masson’s trichrome stains for collagen, immunohistochemical collagen staining, and von Kossa stains. Young specimens (those ≤1 year from the acute DVT) and mature specimens (≥10 years from the acute DVT) were evaluated to study the evolution of the function of endothelial cells lining neovessels and recanalization channels. Antibodies to four biomarkers were used to examine the specific function of these endothelial cells. The biomarkers included vascular endothelial growth factor receptor 2 (VEGFR2), angiopoieton-1 receptor (TIE-2), platelet endothelial cell adhesion molecule 1 (PECAM1), which is also known as CD31, and von Willebrand factor (vWF).
VEGFR2 is an important signaling protein for vascular neogenosis and angiogenesis that stimulates monocyte and macrophage migration. VEGF receptors are typically found on young endothelial cells populating neovascular channels. There are numerous subtypes of VEGF receptors; however, VEGFR2 is the predominant mediator of the cellular responses to VEGF.8 TIE-2 is a tyrosine kinase receptor that is important for the development of blood vessels. TIE-2 promotes sprouting and branching from the primary capillary plexus and vascular remodeling, and it is necessary for normal embryonic vascular development and stabilization of blood vessels in adults.9 CD31 is a type 1 transmembrane glycoprotein that has a number of biologic functions, such as regulating vascular integrity and affecting cell survival.10 CD31 interacts with leukocytes to prevent transendothelial leukocyte migration and remove apoptotic leukocytes. Due to the sophisticated functions of CD31, it is thought that CD31 would most likely be expressed by mature endothelial cells. vWF is a glycoprotein produced by the endothelium, megokaryocytes, and subendothelial connective tissue11 that is important for maintaining hemostasis, and it is expected that mature endothelium would have a higher concentration of vWF.
Results
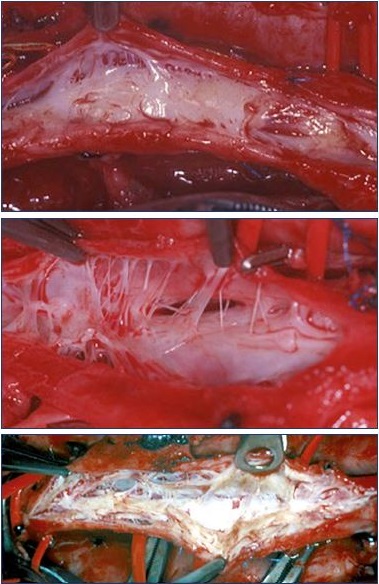
Figure 1 shows three typical endoluminal images observed after venotomy of the common femoral vein. In our experience, a thrombus was absent in all but one patient. The one patient in which a thrombus was present had a documented recurrent DVT 2.5 months prior to the venotomy The hematoxylin/eosin staining confirmed that abundant collagen, neovascularization, recanalization, and inflammation were present in the common femoral vein (Figure 2). The neovascular channels were observed in the loose collagen, whereas few neovascular channels (if any) occurred within the densely packed collagen. An interesting observation was the close proximity of recanalization channels to neovessels. This suggests that two processes–neovascularization and revascularization– are governed partly by a common stimulus.
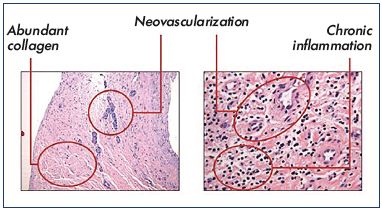
Figure 2. Hematoxylin/eosin staining showing abundant
collagen, neovascularization, recanalization, and inflammation
in the common femoral vein.
VEGFR2 was found in greater concentrations in younger specimens in both neovessels and recanalization channels. However, the neovessel endothelium was more densely stained than the recanalization endothelium. It is likely that VEGF plays a central role in both recanalization and neovascularization of the thrombus. CD31 was found in both young and mature specimens. CD31 has numerous physiological functions that include regulating vascular integrity, controlling cell survival, modulating angiogenesis and cell migration, and influencing vascular permeability. Which aspects of its many functions are operative in the earlier vs the later stages of thrombus resolution require further study. As anticipated, a greater number of channels were found in mature specimens expressing higher concentrations of vWF. Cells under the regulation of the endothelial–specific TIE-2 promoter were observed more often and at higher concentrations in mature specimens.
Can postthrombotic venous obstruction be avoided?
The answer to this question depends upon whether a strategy of thrombus removal is attempted and successful. The true question is “does a strategy of thrombus removal result in less postthrombotic morbidity?” Based upon current evidence, the answer to this question is yes!
Plate et al12-14 reported the short-term and long-term results of their randomized trial of venous thrombectomy plus anticoagulation vs anticoagulation alone for patients with iliofemoral DVT. They observed that iliofemoral venous patency was significantly better and venous pressures, leg edema, and postthrombotic morbidity were lower in patients randomized to venous thrombectomy. The evolution of catheter-based techniques has significantly reduced the need for venous thrombectomy. Integrating mechanical techniques with catheter-directed lysis has reduced the dose of the plasminogen activator, reduced the length of the hospital stay, and improved the efficiency of thrombus removal.15,16
Figure 3A is a photograph of a patient with severe acute phlegmasia cerulea dolens after 5 days of treatment with low-molecular-weight heparin. The patient was markedly uncomfortable and could not ambulate. The iliofemoral phlebogram (Figure 3B) shows extensive venous obstruction. Following pharmacomechanical thrombolysis, patency was restored to the femoral vein (Figure 3C), common femoral vein, and iliac venous system (Figure 3D). The patient had persistent obstruction of the common iliac vein, which was corrected with a 16-mm bare-metal stent (Figure 3E). At the 36-month follow-up, the physical examination was normal, the veins were patent with normal valve function, and the patient was fully active and asymptomatic (Figure 3F).
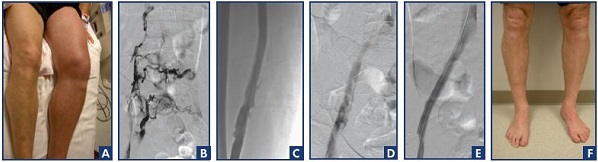
Figure 3. Posttreatment assessment of a patient with deep vein thrombosis treated by pharmacomechanical thrombolysis and
stenting after unsuccessful anticoagulation.
Photograph of a patient with severe acute phlegmasia cerulea dolens after 5 days of treatment with low-molecular-weight heparin
(Panel A). Iliofemoral phlebogram showing extensive venous obstruction (Panel B). Patency restoration to the femoral vein (Panel C)
and the iliac venous system (Panel D) after pharmacomechanical thrombolysis. The patient had persistent obstruction of the common
iliac vein, which was corrected with a 16-mm bare-metal stent (Panel E). At the 36-month follow-up, the physical examination was
normal and the veins were patent with normal valve function (Panel F).
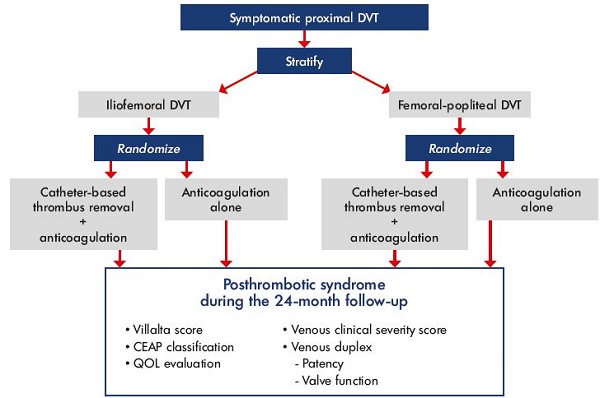
Figure 4. ATTRACT trial design.
Abbreviations: ATTRACT, Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis; CEAP, clinical,
etiological, anatomical, and pathophysiological classification; DVT, deep vein thrombosis; QOL, quality of life.
A cohort-controlled study of treatment for patients with iliofemoral DVT found that catheter-directed thrombolysis improved health-related quality of life compared with anticoagulation alone.17 Furthermore, postthrombotic morbidity was found to correlate with residual thrombus following catheter-directed thrombolysis.18 Therefore, when starting a strategy of thrombus removal, the goal should be to remove as much of the thrombus as possible and restore unobstructed venous drainage to the vena cava.
The CaVenT (Catheter-directed Venous thrombolysis in acute iliofemoral vein Thrombosis) study investigators randomized patients to anticoagulation plus catheter directed thrombolysis vs anticoagulation alone.19 They found significant benefit with catheter-directed thrombolysis, which was correlated with patency of the iliofemoral venous segment. Since the majority of patients entered into the trial had a patent iliac venous system, the number needed to treat to prevent one postthrombotic syndrome was seven. If all patients had had iliofemoral DVT, it is the author’s opinion that the number needed to treat to prevent postthrombotic syndrome would be much smaller, approaching unity.
The ATTRACT trial (Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis)20 is the largest trial to date randomizing patients with acute DVT to catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone. The target of 692 patients was reached in December 2014. The primary end point is postthrombotic syndrome at 2 years (Figure 4). Patients were stratified at entry according to the level of their acute DVT and whether the DVT involved the iliofemoral vein or the femoral popliteal vein. The final follow-up visits will occur in December 2016, at which time, the data will be analyzed, presented, and published. While the results of the ATTRACT trial are anxiously awaited, the current body of evidence strongly supports the adoption of a strategy of thrombus removal for patients with iliofemoral DVT. Of course, removing the acute thrombus will restore patency and eliminate the substrate for luminal obstruction, thereby significantly reducing the likelihood of severe postthrombotic morbidity.

REFERENCES
1. Akesson H, Brudin L, Dahlström JA, Eklöf B, Ohlin P, Plate G. Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg. 1990;4(1):43-48.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698-707.
3. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110(7):515-519.
4. Qvarfordt P, Eklöf B, Ohlin P. Intramuscular pressure in the lower leg in deep vein thrombosis and phlegmasia cerulae dolens. Ann Surg. 1983;197(4):450-453.
5. Labropoulos N, Volteas N, Leon M, et al. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch Surg. 1997;132(1):46-51.
6. Comerota AJ, Grewal NK, Thakur S, Assi Z. Endovenectomy of the common femoral vein and intraoperative iliac vein recanalization for chronic iliofemoral venous occlusion. J Vasc Surg. 2010;52(1):243-247.
7. Comerota AJ, Oostra C, Fayad Z, et al. A histological and functional description of the tissue causing chronic postthrombotic venous obstruction. Thromb Res. 2015;135(5):882-887.
8. Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19(10):2003-2012.
9. Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376(6535):70- 74.
10. Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23(6):953-964.
11. Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67(1):395-424.
12. Plate G, Einarsson E, Ohlin P, Jensen R, Qvarfordt P, Eklöf B. Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg. 1984;1(6):867-876.
13. Plate G, Akesson H, Einarsson E, Ohlin P, Eklöf B. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg. 1990;4(5):483-489.
14. Plate G, Eklöf B, Norgren L, Ohlin P, Dahlström JA. Venous thrombectomy for iliofemoral vein thrombosis—10-year results of a prospective randomised study. Eur J Vasc Endovasc Surg. 1997;14(5):367-374.
15. Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192(6):782-788.
16. Martinez Trabal JL, Comerota AJ, LaPorte FB, Kazanjian S, DiSalle R, Sepanski DM. The quantitative benefit of isolated, segmental, pharmacomechanical thrombolysis (ISPMT) for iliofemoral venous thrombosis. J Vasc Surg. 2008;48(6):1532-1537.
17. Comerota AJ, Throm RC, Mathias SD, Haughton S, Mewissen M. Catheterdirected thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000;32(1):130-137.
18. Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55(3):768-773.
19. Enden T, Haig Y, Klow NE, et al. Longterm outcome after additional catheterdirected thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38.
20. Comerota AJ. The ATTRACT trial: rationale for early intervention for iliofemoral DVT. Perspect Vasc Surg Endovasc Ther. 2009;21(4):221-224.
.