The Greek warrior helmet: A look at wolf-hirschhorn syndrome
Received: 05-Dec-2022, Manuscript No. PULJCGG-22-5838 ; Editor assigned: 08-Dec-2022, Pre QC No. PULJCGG-22-5838 (PQ); Reviewed: 22-Dec-2022 QC No. PULJCGG-22-5838 ; Revised: 23-Jan-2023, Manuscript No. PULJCGG-22-5838 (R); Published: 30-Jan-2023
Citation: Deines C. The Greek warrior helmet: A look at wolf-hirschhorn syndrome. J Clin Genet Genom 2023;6(1):1-4.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Wolf-Hirschhorn Syndrome (WHS) was the first human chromosomal deletion identified as a variable 4p chromosomal deletion syndrome. The phenotype can vary based on the size and placement of the deletion. Its core characteristics include mental retardation, craniofacial dysmorphology, epilepsy, and growth delay; however, there can be many other defects based on the specific gene deletions. Management of this syndrome is multifaceted, and it often involves multiple specialties and lifelong care. Thus, implications for advanced practice nurses includes accurate diagnosis and treatment of defects and comorbidities involving different body systems, collaboration with different medical teams, and early intervention.
Keywords
Chromosomal deletions; Wolf-Hirschhorn syndrome; 4p deletions; WHS; Management of WHS; Craniofacial dysmorphology
Introduction
Wolf-Hirschhorn syndrome is the first example of a classic human chromosomal deletion syndrome. It was clinically described in 1961 by Hirschhorn and then in 1965 by Wolf. Although originally seen as a single gene syndrome, it is now accepted as multigenic involving variable deletion of the distal short arm of chromosome. The core phenotypical feature of WHS is the “Greek warrior helmet” facies marked by dysmorphic facial features such as a prominent glabella, hypertelorism, a broad beaked nose, and frontal bossing. Other core features of WHS are intellectual disability, developmental delay, congenital hypotonia, and seizures. Because this syndrome can vary in deletion size, phenotypes and system wide defects can also vary. Heart, skeletal, genitourinary, neurologic, and gastrointestinal defects are often seen in infants with WHS, and management of this syndrome is life long and complex [1].
Definition of the disease
Wolf-Hirsch horn syndrome is a heterozygous 4p deletion syndrome found on the 4p16.3 region. The core characteristics of WHS are mental retardation, growth delay, epilepsy, and cranio facial abnormalities often referred to as the “Greek warrior helmet” facies. This syndrome can vary in the size of deletions and result in an array of differing phenotypes. It is a rare syndrome with an incidence of 1 in 50,000 to 1 in 20,000 in live births and a 2:1 female to male ratio. This deletion is de novo (present for the first time in one family member because of a variant,) in 50%-60% of cases. It also can be the result of an unbalanced translocation and is associated with partial trisomy of another region. There are several comorbidities associated with WHS and each child is affected differently [2].
Disease etiology and pathogenesis
Wolf-Hirschhorn syndrome is a sub-telomeric 4p deletion syndrome and is caused by a monosomy of part of the p arm (the short arm) of chromosome 4 and encompasses the 4p16.3 region. The development of the forebrain and craniofacial regions is complicated and involves regulation of many genetic cell types and interactions. Therefore, these regions are susceptible to disruptions caused by contiguous gene deletions. Many mental retardation cases involve sub-telomeric deletions because these regions are filled with genes and therefore subject to greater numbers of gene rearrangements. The 4p16.3 is a critical region for WHS and there are two region for WHS and there are two regions within that contain multiple genes. Several of these genes have been identified and play a role in the differing phenotypes. These genes include the core contributor, WHSCR, located at the 4p terminal. Other identified genes include WHSCR-1, WHSCR-2, and LETM1 [3].
Phenotype and presentation
The presentation of WHS is complex and variable. While WHS was originally hypothesized to be a single gene phenotype, it is now widely accepted that the pathogenesis of WHS is multigenic. This contiguous gene syndrome has two critical regions at 4p that contribute to the core phenotype. Within the first critical region are the WHSC1 and WHSC2. WHSC1 is a developmental gene and considered the candidate gene for growth delay and for the facial dysmorphisms. Other genes have been identified to play different roles in the clinical presentation. The LETM1 gene encodes mitochondrial protein and is a candidate gene for seizures. In addition, a new candidate gene for seizures is identified as CPLX1 and encodes for a presynaptic regulatory protein [4].
As previously stated, the various phenotypes are related to the size of the deletion and can be divided into three categories. The first includes individuals with deletion less than 3.5 Mb at 4p16-4pter. This size deletion always expresses a mild phenotype. The second category includes individuals with a deletion between 5 and 18 Mb and usually presents with the classic WHS phenotype. The third category is those with deletion larger than 22 Mb and is always associated with major malformations. The core phenotype of WHS includes pre and postnatal growth delay, intellectual disability, distinctive craniofacial features (“Greek warrior helmet” facies), and seizures [5].
Many of the organ systems and most parts of the body can potentially be affected by the different genes. Fetal phenotypes include major anomalies such as intrauterine growth restriction, microcephaly, cleft palate, corpus callosum agenesis, ventricular septal defect, diaphragmatic hernia, and renal hypoplasia. Minor anomalies of the fetus may include scalp defects, hypertelorism, pulmonary isomerism, common mesentery, hypospadias, and sacral dimple. The neonate with WHS will likely also have profound growth retardation and skeletal anomalies including long slender fingers with additional flexion creases, a long narrow chest with widely spaced nipples, duplication of thumbs and great toes, hypoplasia of pubic bones, vertebral and rib anomalies, defective calvarium ossification, scoliosis, kyphosis, osteoporosis, delayed bone maturation, and a sacral dimple. Central nervous system involvement includes profound mental retardation, microcephaly, seizures, congenital hypotonia with muscle hypertrophy and cranial malformations. The skull may have frontal bossing, high frontal hairline, hemangioma over forehead or glabella, and scalp defects. Defects with the eyes can include hypertelorism, down slanting palpebral fissures, epicanthal folds, strabismus, coloboma, hyperplasia of orbital ridges, ectopic pupils, cataracts, and esotropia.
The mouth and ears are also affected. A child with WHS may have a short upper lip, a short philtrum, a cleft lip or palate, a bifid uvula, a carp like mouth, micrognathia or retrognathia, and oligodontia. The ears are low set, misshapen and may have preauricular dimples. Sensorineural hearing loss is common as well as chronic otitis media. Possible cardiovascular defects include atrial septal defect, ventricular septal defect, persistent left superior vena cava, and valve abnormalities. Pulmonary defects may include bilateral bilobed or trilobed lungs and diaphragmatic hernia.
The GI anomalies include diastasis recti, umbilical or inguinal hernias, accessory spleens, absent gallbladder, or intestinal malrotation. Genitourinary anomalies are seen in 25% of cases and include hypo plastic kidneys, cystic dysplastic kidneys, unilateral renal agenesis, hydronephrosis, exstrophy of the bladder, hypoplastic external genitalia, cryptorchidism, and hypospadias in males, and hypoplastic Mullerian derivatives (agenesis of vagina, cervix, or uterus; hypoplastic uterus). Other phenotypical characteristics include a thin corpus collosum, ventriculomegaly, craniofacial asymmetry, hypertelorism, wide nasal bridge, cystic hygroma, cleft lip/palate, mispositioned ears, midline scalp defects, cardiac defects, diaphragmatic hernia, intestinal defects, and renal anomalies [6-8].
Case Presentation
Rayah Dye was born with a 27.5 Mb deletion on the 4p chromosome and presents with classic WHS phenotypical features. Rayah’s parents knew at 27 weeks gestation she had a 4p deletion and she was not expected to survive much past birth. On the third day of life she was sent home on palliative care. At home, she suffered a hypoxic event and her parents rushed her to the hospital knowing that their child could be saved, and this event was not just part of the dying process. Rayah spent several weeks in the hospital where she needed help breathing and frequent suctioning because she was unable to clear her airway.
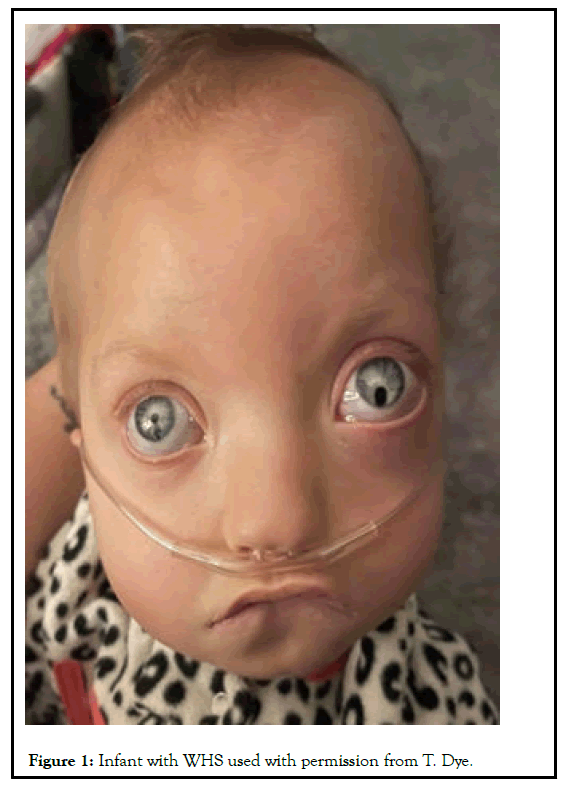
She was at high risk for infection due to her weakened immune system. After several surgeries, Rayah was discharged to home with a low flow nasal cannula and a gastrostomy tube. At the time of this publication Rayah was two years old (Figure 1) [9].
As seen in Figure 1, Rayah has the “Greek warrior helmet” facies and craniofacial asymmetry with a broad beaked nose, a prominent glabella, hypertelorism, coloboma in the left eye, bulging eyes, a cleft palate, and a downturned mouth. In Figure 2 you can see she has long slender fingers as well as a long slender chest. As with many children with WHS, Rayah has multi system involvement and has had several surgeries. She was born with a ventricular septal defect in her heart and tetralogy of fallout which required open heart surgery to correct. She also had gastrointestinal issues requiring a gastrostomy tube to be placed at five months old and at fifteen months old had a gut malrotation surgery. She suffers with bilateral hearing loss and musculoskeletal issues. When asked about the experience of raising a child with Wolf-Hirschhorn syndrome, Tiffany Dye (Rayah’s mother) commented, “raising a child with WHS scared me at first not knowing all the medical stuff it entailed. But now that I have my reigns on everything, I feel so honored to be her mom because she is a blessing all in herself and i get the privilege to be part of that blessing”.
Results and Discussion
Management
Management for the neonate born with WHS can be difficult, involves a multidisciplinary approach, and varies depending on the specific defects of the child. Early intervention programs are imperative to improve motor development, speech and language skills, social skills, and overall cognition. Feeding difficulties and failure to thrive are common and may need to be managed by gavage feeding, gastrostomy and partial fundoplication. Seizure activity is present in 75% of WHS cases and are managed with anticonvulsants including bromide therapy for seizure control. Common skeletal abnormalities are clubfoot, scoliosis, and kyphosis requiring orthopedic care. Heart defects may be present and will need to be addressed by cardiology. In 25% of cases there will be genitourinary complications and nephrology/urology will need to be consulted. Approximately 50% of individuals will have some type of dental abnormality and will be followed by orthodontia [10].
Recurrence risk and genetic counseling
Because different mechanisms can lead to the 4p deletions, it is essential to determine these mechanisms for counseling and the recurrence risk estimation. About 55% of WHS cases are de novo terminal or interstitial 4p deletions and therefore have no significant increased risk for future siblings. However, 40%-45% of WHS cases result from an unbalanced translocation involving 4p and can be inherited from a parent, so there is a risk of an unbalanced product in offspring. These families should be notified that other family members can be carriers for the same translocation and are at increased risk for having children with 4p deletion. The remaining 5% of cases are due to complex chromosome rearrangements including ring chromosome 4 or inverted duplications. The patient with WHS is unlikely to reproduce due to mental retardation. In families where one parent is known to be a carrier of a chromosome rearrangement, prenatal diagnosis is available by amniocentesis, CVS, or PUBS. Some distinct phenotypes can be detected by ultrasound. The most common findings are intrauterine growth restriction and microcephaly. Other defects that may be noted on ultrasound are increased nuchal fold thickness, hypertelorism with prominent glabella, cleft lip, diaphragmatic hernia, and anomalous urinary stream from the ventral surface of the penis. Chromosomal analysis including karyotyping, FISH, and whole chromosome painting can also be offered to families. As seen in the case study, families used to be told to take their baby home and not get too attached because the baby would not survive more than a few days. Now, research has shown that these babies can survive but the prognosis for children with WHS depends on the size of the deletion and the comorbidities. Mortality rate in the first two years is 21% and cases with large de novo deletions more likely to die than those with smaller deletions. The cause of death is attributed to birth anoxia, withdrawal of treatment after premature delivery, lower respiratory tract infections, sudden unexplained death, and congenital anomaly complications.
Implications for advanced practice nursing
Due to the variable presentation and the multigenic nature of WHS, the advanced practitioner needs to be acutely aware of how a child with WHS will present as well as the possible sequelae that comes with a WHS diagnosis. During the acute neonatal phase while the infant is in the NICU, the advanced nurse will need to coordinate with different teams including but not limited to cardiology, ENT, ophthalmology, nephrology, physical and speech therapy, neurology, urology, surgery, and immunology. Diagnostic investigation will include conventional cytogenetic studies which are the most common method to detect chromosome arm 4p deletions. Florescence In Situ Hybridization (FISH) studies can help diagnose patients with very small deletions or cryptic translocation. A chromosome microarray is the most useful test because it can detect all currently known deletions of WHS critical region. Other diagnostic testing includes an immune workup to detect Immunoglobulin A (IgA) deficiency, impaired polysaccharide responsiveness, and T-cell immunity, radiography to assess bon maturation, fusion of vertebrae, fused ribs, dislocated hips, and club feet. In addition, EEG monitoring for seizures may be necessary, echocardiography to detect heart defects, renal ultrasound to detect renal anomalies, MRI and CT scans to determine brain pathology, swallow study for feeding difficulty, audio logic and otologic evaluation, ophthalmic examination, and developmental testing. Moreover, specific growth charts for children with WHS are recommended as standard growth charts are not applicable [11,12].
The emotional toll this diagnosis has on the family is significant and it is critical that the practitioner helps provide opportunities for support such as WHS support groups, psychological support, grief support, and child life specialist if there are other children in the family. It is important to support the family in their decision making regarding procedures, surgeries, and wishes for the child. Of equal importance, the practitioner should not give false hope, rather, should gently and compassionately inform the family of what the future may look like for them and their child.
Conclusion
In summary, Wolf-Hirsch horn syndrome is a complex and variable chromosomal deletion syndrome located on the 4p16.3 region. Because of the variability, the phenotype of children with WHS can differ greatly. Those with a large deletion of 22Mb or larger will likely present with the core WHS features including mental and growth retardation, craniofacial abnormalities, and seizures. Management for WHS is complex and requires an interdisciplinary approach. The implications for the advanced practice nurse are many and requires collaboration with multiple specialties to manage the variable defects and comorbidities that present with WHS.
References
- Battaglia A, Carey JC. The delineation of the Wolf‐Hirschhorn syndrome over six decades: Illustration of the ongoing advances in phenotype analysis and cytogenomic technology. Am J Med Genet. 2021;185(9):2748-55.
[Crossref] [Google Scholar] [PubMed]
- Barrie ES, Alfaro MP, Pfau RB, et al. De novo loss of function variants in NSD2 (WHSC1) associate with a subset of Wolf–Hirschhorn syndrome. Mol Case Stud. 2019;5(4):004044.
[Crossref] [Google Scholar] [PubMed]
- Bergemann AD, Cole F, Hirschhorn K. The etiology of Wolf–Hirschhorn syndrome. Trends Genet. 2005;21(3):188-95.
[Crossref] [Google Scholar] [PubMed]
- Berrocoso S, Amayra I, Lazaro E, et al. Coping with Wolf-Hirschhorn syndrome: Quality of life and psychosocial features of family careers. Orphanet J Rare Dis. 2020;15(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Correa T, Mergener R, Leite J, et al. Cytogenomic integrative network analysis of the critical region associated with Wolf-Hirschhorn syndrome. Biomed Res Int. 2018.
[Crossref] [Google Scholar] [PubMed]
- Derar N, Al-Hassnan ZN, Al-Owain M, et al. De novo truncating variants in WHSC1 recapitulate the Wolf–Hirschhorn (4p16. 3 microdeletion) syndrome phenotype. Genet Med. 2019;21(1):185-8.
[Crossref] [Google Scholar] [PubMed]
- Hirschhorn K, Cooper HL, Firschein IL. Deletion of short arms of chromosome 4–5 in a child with defects of midline fusion. Humangenetik. 1965;1(5):479-82.
[Crossref] [Google Scholar] [PubMed]
- Limeres J, Serrano C, de Nova JM, et al. Oral manifestations of Wolf-Hirschhorn syndrome: Genotype-phenotype correlation analysis. J Clin Med. 2020;9(11):3556.
[Crossref] [Google Scholar] [PubMed]
- Rjiba K, Ayech H, Kraiem O, et al. Disorders of sex development in Wolf–Hirschhorn syndrome: A genotype–phenotype correlation and MSX1 as candidate gene. Mol Cytogenet. 2021;14:1-9.
[Crossref] [Google Scholar] [PubMed]
- Wolf U, Reinwein H, Porsch R, et al. Deficiency on the short arm of a chromosome no.4. Humangenetik. 1965;1:397-413.
- Xing Y, Holder JL, Liu Y, et al. Prenatal diagnosis of Wolf-Hirschhorn syndrome: From ultrasound findings, diagnostic technology to genetic counseling. Arch Gynecol Obstet. 2018;298:289-295.
[Crossref] [Google Scholar] [PubMed]
- Zollino M, Doronzio PN. Dissecting the Wolf-Hirschhorn syndrome phenotype: WHSC1 is a neurodevelopmental gene contributing to growth delay, intellectual disability, and to the facial dysmorphism. J Hum Genet. 2018;63:859-861.
[Crossref] [Google Scholar] [PubMed]