Structure and Biological Activity of a Turripeptide from Unedogemmula bisaya Venom.
Omaga, C.A., Carpio, L.D., Imperial, J.S., Daly, N.L., Gajewiak, J., Flores, M.S., Espino, S.S., Christensen, S., Filchakova, O.M., Lopez-Vera, E., Raghuraman, S., Olivera, B.M., Concepcion, G.P.(2017) Biochemistry 56: 6051-6060
- PubMed: 29090914
- DOI: https://doi.org/10.1021/acs.biochem.7b00485
- Primary Citation of Related Structures:
5VR1 - PubMed Abstract:
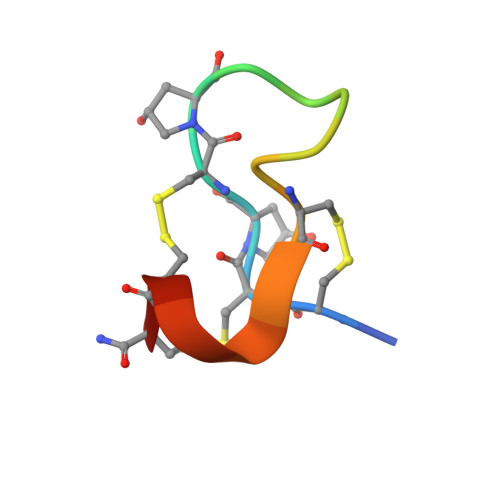
The turripeptide ubi3a was isolated from the venom of the marine gastropod Unedogemmula bisaya, family Turridae, by bioassay-guided purification; both native and synthetic ubi3a elicited prolonged tremors when injected intracranially into mice. The sequence of the peptide, DCCOCOAGAVRCRFACC-NH 2 (O = 4-hydroxyproline) follows the framework III pattern for cysteines (CC-C-C-CC) in the M-superfamily of conopeptides. The three-dimensional structure determined by NMR spectroscopy indicated a disulfide connectivity that is not found in conopeptides with the cysteine framework III: C 1 -C 4, C 2 -C 6 , C 3 -C 5 . The peptide inhibited the activity of the α9α10 nicotinic acetylcholine receptor with relatively low affinity (IC 50 , 10.2 μM). Initial Constellation Pharmacology data revealed an excitatory activity of ubi3a on a specific subset of mouse dorsal root ganglion neurons.
Organizational Affiliation:
Marine Science Institute, University of the Philippines , P. Velasquez Street, Diliman, Quezon City 1101, Philippines.