Abstracts
Astyanax multidens is redescribed based on syntypes and additional specimens from the rio Amazonas at Silves, Amazonas State and Óbidos, Pará State, and the rios Trombetas, Tapajós, Xingú, and Araguaia.Astyanax multidens is distinguished from congeners by the unique combination of five to seven maxillary teeth, 31 to 34 pored lateral-line scales, 20 to 23 branched anal-fin rays, and a dark triangular blotch restricted to the middle caudal-fin rays. Astyanax multidens was collected syntopically with Jupiaba paranatinga in the rio Tapajós basin and with Jupiaba cf. essequibensis in the rio Xingu basin, which have paired anteriorly oriented pelvic-fin spines, a antipredatory mechanism. Based on the external morphological similarity betweenAstyanax multidens and the two species ofJupiaba, their sympatric occurrence, and their distinct phylogenetic position, we interpret this a case of Batesian mimicry.
Jupiaba; Mimicry; Neotropical; Taxonomy
Astyanax multidens é redescrita com base no exame dos síntipos e espécimes adicionais do rio Amazonas em Silves, AM e Óbidos, PA e rios Trombetas, Tapajós, Xingú e Araguaia. Astyanax multidensdistingue-se dos congêneres pela exclusiva combinação de cinco a sete dentes no maxilar, 31 a 34 escamas perfuradas na linha lateral, 20 a 23 raios ramificados na nadadeira anal, e uma mácula triangular escura restrita aos raios medianos da nadadeira caudal. Astyanax multidens foi coletada sintopicamente com Jupiaba paranatinga na bacia do rio Tapajós, e com Jupiabacf. essequibensis na bacia do rio Xingu, que possuem espinho pélvico voltado para frente, um mecanismo antipredatório. Baseado na similaridade morfológica externa entre Astyanax multidense estas duas espécies de Jupiaba, sua ocorrência simpátrica, e sua distante posição filogenética, nós interpretamos como um caso de mimetismo Batesiano.
Introduction
The family Characidae is the fourth largest family of fishes in the World, with over 1.100 valid species, being smaller only than the freshwater families Cyprinidae, Cichlidae, and the mainly marine Gobiidae (Nelson, 2006; Eschmeyer & Fricke, 2012). Interestingly, contrary to these three families, the Characidae is much more geographically limited, being restricted to South America, from Texas (U.S.A.) to Argentina. The family is experiencing a high annual rate of species description, with 46 new species in 2010, despite the still prevalent poor knowledge about the taxonomy and interrelationships of species. Half of the species of the Characidae were listed as incertae sedis by Lima et al. (2003). Most of the larger genera, such as Astyanax Baird & Girard, Moenkhausia Eigenmann, HyphessobrysonDurbin, and Hemigrammus Gill, are now considered to be not monophyletic (Lima et al., 2003), with numerous species level problems.
Astyanax is the most species rich genus in Characiformes, with approximately 130 valid species (Eschmeyer & Fricke, 2012), of which almost 15% (18 species) were described in the last five years (e.g., Zanata & Camelier, 2008; Marinho & Lima, 2009; Bertaco et al., 2010; Garavello & Sampaio, 2010), and about one-third described in the last 10 years (42 species from 2002 to 2011) (e.g., Azpelicueta et al., 2002; Garutti, 2003; Bertaco & Lucena, 2006). Similarly increasing are the contributions through redescriptions of poorly known species (e.g., Garutti, 2003; Mirande et al., 2006; Garutti & Langeani, 2009; Pavanelli & Oliveira, 2009; Azpelicueta & Loureiro, 2009; Almirón et al., 2010; Bertaco et al., 2010; Bertaco & Lucena, 2010; Soneira et al., 2010).
Astyanax is primarily characterized by having two series of teeth on the premaxilla with the inner tooth row consisting of five teeth, complete lateral line, and caudal fin not covered by scales (Eigenmann, 1917; 1921), but has been considered to be not monophyletic for over 40 years (Rosen, 1972; Weitzman & Fink, 1983; Weitzman & Malabarba, 1998; Lima et al., 2003). Furthermore, recent phylogenetic analyses based on molecular data, including only very few species (two or three in each study) have also demonstrated the non-monophyletic condition of Astyanax (Calcagnotto et al., 2005; Javonillo et al., 2009; Oliveira et al., 2011). In a comprehensive phylogenetic analysis of the Characidae, Mirande (2010) recovered the species of Astyanax in three different monophyletic groups with A. latens Mirande, Aguilera & Azpelicueta closely related to two species of Hyphessobrycon (in the Hyphessobrycon luetkenii clade), A. parisAzpelicueta, Almirón & Casciotta the sister to a large characid clade, andAstyanax spp.(15 species) in what he termedAstyanax clade which also includes Hyphessobrycon anisitsi (Eigenmann), Markiana nigripinnis (Perugia), and Psellogrammus kennedyi (Eigenmann). Mirande's (2010)Astyanax clade is supported by a single non-exclusive synapomorphy: the presence of one or no maxillary teeth.
We recently collected specimens of a small-sized characid widely distributed in the rio Tapajós basin which proved to be Astyanax multidens Eigenmnann, 1908. This is a poorly known species described more than a century ago and only cited in fish catalogs, or as citations of samples from the rio Paraguay basin. The aim of the present contribution is to redescribe Astyanax multidensbased on syntypes and recently collected specimens.
Material and Methods
Counts and measurements were taken from seven syntypes and 30 non-type specimens, according to Fink & Weitzman (1974), with the addition of head depth measured at the vertical through the posteriormost tip of bony opercle, and longitudinal scale rows below lateral line counted to the pelvic-fin insertion. Counts are followed by their frequencies in parentheses. Measurements are given as percents of standard length (SL), except for subunits of the head given as percents of head length. Counts of vertebrae, supraneurals, gill-rakers of the first arch, tooth cusps, smaller dentary teeth, procurrent caudal-fin ray and position of the pterygiophores were taken from four cleared and stained (cs) specimens, prepared following Taylor & van Dyke (1985). Vertebral count includes the four vertebrae of the Weberian apparatus and the fused PU1 + U1 as a single element.Counts observed in the syntype series are marked with an asterisk. Meristic data for other species ofAstyanax were taken for direct examination of specimens (see Comparative material) or from original description or redescriptions (e.g., Eigenmann, 1927; Garutti, 2003; Melo & Buckup, 2006; Pavanelli & Oliveira, 2009).
Institutional abbreviations are: ANSP, Academy of Natural Sciences, Philadelphia; DZSJRP, Departamento de Zoologia e Botânica, Universidade Estadual Paulista "Júlio de Mesquita Filho", Câmpus de São Jose do Rio Preto, São José do Rio Preto; FMNH, Field Museum of Natural History, Chicago; INPA, Instituto Nacional de Pesquisas da Amazônia, Manaus; MCP, Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge; MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro; MZUSP, Museu de Zoologia, Universidade de São Paulo, São Paulo; NMW, Naturhistorisches Museum Wien, Vienna; USNM, National Museum of Natural History, Smithsonian Institution, Washington, D.C.
Results
Astyanax multidens Eigenmann, 1908 Figs. 1-4
Astyanax multidens: (a) syntype, MCZ 89559, 29.2 mm SL, rio Amazonas at Óbidos, (b) syntype, MCZ 89559, 30.0 mm SL, rio Amazonas at Óbidos, (c) MCZ 20826, 36.3 mm SL, rio Amazonas at Óbidos.
Astyanax multidens: (a) MZUSP 96606, 44.6 mm SL, rio Peixoto de Azevedo, tributary of rio Teles Pires, (b) MZUSP 99818, 29.5 mm SL, rio Teles Pires, (c) MZUSP 94171, 25.7 mm SL, rio Culuene, tributary of rio Xingu, (d) MZUSP 52228, 25.2 mm SL, rio água Fria, rio Araguaia basin.
Upper and lower jaws of Astyanax multidens (lateral view, right side), MZUSP 96726, 44.3 mm SL. Scale bar 1 mm.
Astyanax multidens, photographed live, MZUSP 91955, ca. 25 mm SL, rio Culuene, tributary of rio Xingu (photo by Leandro Sousa).
Astyanax multidens Eigenmann, 1908: 98 [Óbidos and Silva (=Silves), Lake Saraca].- Eigenmann, 1910: 434 [citation].- Eigenmann, 1921: plate 50, fig. 4 [illustration].- Eigenmann, 1927: 323 [literature compilation].- Eigenmann, 1927: 328 [literature compilation; considered to be allied to A. guianensis].- Fowler, 1943: 3 [similarity to A. chaparae].- Fowler, 1948: 55-56 [literature compilation; in part, not rio Paraguay].- Géry, 1977: 426 [identification key].- Vari & Howe, 1991: 7 [catalogue of type specimens].- Lima et al., 2003: 111 [literature compilation].- Limaet al., 2007: 46 [literature compilation].
Diagnosis. Astyanax multidens is distinguished from all congeners exceptA. angustifrons (Regan), A. aurocaudatusEigenmann, A. gisleni Dahl, A. goyanensis(Miranda Ribeiro), A. guaporensis Eigenmann,A. guianensis Eigenmann, A. henseli Melo & Buckup, A. leopoldi Géry, Planquette & Le Bail,A. nasutus Meek, A. nicaraguensisEigenmann, A. superbus Myers, and A. totaeHaluch & Abilhoa by having five to seven maxillary teeth (vs. less than five). Astyanax multidensis distinguished from the aforementioned species, except A. angustifrons, A. guaporensis, A. guianensis, A. leopoldi, A. nicaraguensis, and A. totae by having 31 to 34 pored lateral-line scales (vs. more than 34). Astyanax multidens is distinguished from A. totae andA. nicaraguensis by having 20 to 23 branched anal-fin rays (vs. 15 to 18 and 24 to 29, respectively); from A. angustifrons and A. leopoldi by the absence of a dark blotch on the caudal peduncle (vs. presence); and fromA. guaporensis and A. guianensis by having a dark triangular blotch on middle caudal-fin rays (vs. middle caudal-fin rays hyaline).
Description. Morphometrics presented in Table 1. Overall body size small (largest examined specimen 52.8 mm SL). Body compressed, moderately elongate. Greatest body depth slightly anterior to dorsal-fin origin. Dorsal profile of head convex from upper lip to vertical through anterior nostril; straight from that point to tip of supraoccipital spine. Dorsal profile of body slightly convex along predorsal region, straight and posteroventrally inclined along dorsal-fin base, straight to slightly convex from terminus of dorsal-fin base to adipose-fin origin, and concave along caudal peduncle. Ventral profile of head and body convex from tip of lower jaw to anal-fin origin, straight and posterodorsally inclined along anal-fin base, and concave along caudal peduncle.
Jaws equal, mouth terminal. Premaxillary teeth in two rows (Fig. 3). Outer row with 2*(3), 3*(23), 4*(8), or 5*(2) tricuspid teeth. Inner row with 5*(32) or 6 (2) tri- to pentacuspid teeth. Tip of maxilla slightly posterior of vertical through anterior margin of eye, with 5*(21), 6 (9), or 7 (8) uni- to tetracuspid teeth. Dentary with 4* large pentacuspid, one tricuspid and series of 6-10 small uni- to tricuspid teeth. Central median cusp in all teeth longer than lateral cusps. Branchiostegal rays 4. First gill arch with 2 (2) or 3 (2) hypobranchial, 9 (2) or 10 (2) ceratobranchial, 1 intermediate cartilage, and 6 (3) or 7 (1) epibranchial gill rakers. Each gill raker with small denticles on its length.
Scales cycloid, with few radii on posterior border. Lateral line straight to slightly curved ventrally, with 31*(2), 32*(3), 33*(18) or 34 (14) perforated scales from supracleithrum to caudal-fin base. Longitudinal scale rows between dorsal-fin origin and lateral line 5 (33) or 6 (4). Longitudinal scale rows between lateral line and pelvic-fin origin 3*(3), 4*(28) or 5*(6). Predorsal scales 8 (1), 9 (18), or 10* (14) in one series. Row of 5 (8), 6 (8), 7 (9), or 8 (3) scales at base of anteriormost anal-fin rays. Circumpeduncular scale rows 14. Caudal fin with scales along basal one-sixth of upper lobe and basal one-fourth of lower lobe.
Pectoral-fin raysi,11*(2), i,12*(9), i,13 (15), i,14 (7), or i,15 (1). Tip of adpressed fin at pelvic fin-origin or falling slightly short of that point. Pelvic-fin rays i,7*(33) or i,8 (1). Tip of adpressed fin at anal-fin origin or falling slightly short. Supraneurals 3 (1) or 4 (3). Dorsal-fin origin at middle of SL and slightly posterior to vertical through pelvic-fin origin. Dorsal-fin rays ii,9. First unbranched ray about one-half length of second unbranched ray. Base of last dorsal-fin ray slightly anterior to vertical through anal-fin origin. First dorsal-fin pterygiophore inserted behind neural spine of 5thvertebra. Adipose-fin origin at vertical through base of 16thto 18th branched anal-fin rays. Anal-fin rays iv,20 (1), iv,21*(17), iv,22 (12), or iv,23*(4). First anal-fin pterygiophore inserted behind haemal spine of 12th (3) or 13th (1) vertebra. Caudal-fin rays i,9,8,i*. Caudal fin forked, with lobes of similar size. Dorsal procurrent caudal-fin rays 11 (3) or 12 (1); ventral procurrent caudal-fin rays 11 (4). Total vertebrae 32 (4): precaudal vertebrae 15 and caudal vertebrae 17.
Color in alcohol. Overall ground color yellowish. Opercular area silvery. Lips, anterior portion of maxilla, and dorsal portion of head with small dark chromatophores. Larger chromatophores on upper one-half of opercle. Scales of two or three dorsalmost longitudinal scale rows bordered by dark pigmentation. Humeral blotch conspicuous, vertically oriented, from one scale row ventral of, and three scale rows dorsal of lateral line, and over three scales horizontally. Lower half of humeral spot often interrupted by clear horizontal area. Clear area between humeral blotch and posterior pigmented area that fades longitudinally. Stripe extending over four or five longitudinal scale rows dorsal to lateral line, and from vertical through 7th or 8th lateral line scale to caudal peduncle. Dark pigmentation along horizontal septum, approximately from vertical through dorsal-fin origin to caudal peduncle. Clear area anterior to caudal-fin base. Pectoral, pelvic and adipose fins with scattered dark chromatophores. First unbranched dorsal-fin ray with dark chromatophores; remaining rays hyaline. Distal portion of interadial membranes of all dorsal-fin rays with scattered dark chromatophores. Anal fin with scattered small chromatophores. Chromatophores frequently concentrated at proximal and distal portions of interadial membranes. Caudal fin with somewhat and sometimes diffuse triangular blotch on middle rays. Anterior margin of blotch straight; posterior pointed. Caudal-fin lobes hyaline or with scattered dark chromatophores.
Color in life. Coloration in life similar to described above for preserved specimens, except for dorsal portion of iris red, distal portion of anteriormost rays of dorsal, pelvic and anal fins white, adipose fin red, and caudal fin with pale orange blotch immediately dorsal and ventral to dark median blotch (Fig. 4).
Sexual dimorphism. No sexually dimorphic modification was observed.
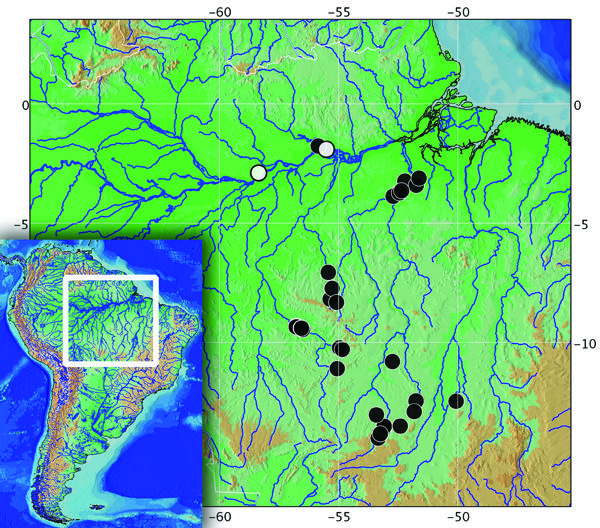
Distribution. Astyanax multidens is known from the rio Amazonas at Silves, State of Amazonas, and Óbidos, State of Pará, and the rios Trombetas, Tapajós, Xingú and Araguaia (Fig. 5).
Distribution of Astyanax multidens in the rio Amazonas basin. White circle represent the type localities.
Remarks. During the Thayer Expedition, James collected 40 specimens of small characids at Óbidos, which were kept in a single jar and catalogued as MCZ 20840. Eigenmann (1908, 1918, 1927) recognized two distinct species among these specimens, electing 5 specimens as paratypes of Hemigrammus microstomusand 35 as paratypes of Astyanax multidens. Later, the 35 syntypes of Astyanax multidens were removed to MCZ 89559 (the five paratypes of Hemigrammus microstomus remained as MCZ 20840). During this process, the 35 syntypes of Astyanax multidens received the informal number MCZ 21064a, in reference to the other syntype from lago Saraca, catalogued as MCZ 21064. Six of the 35 syntype were then exchanged to USNM (USNM 120244). The others 29 syntypes remained catalogued as MCZ 89559. Thus, the syntypes of Astyanax multidens are MCZ 21064 (1), MCZ 89559 (29) and USNM 12044 (6).
Eigenmann (1927) cited a single specimen (MCZ 20826, Fig. 1c) as "probably identical with" the type specimens ofAstyanax multidens. Our examination confirm Eigenmann's (1927) suggestion that the aforementioned specimen is indeed conspecific with the syntypes of Astyanax multidens.
Examinations of the type series of Moenkhausia collettii deposited at the NMW revealed that part of the syntypes from Óbidos (NMW 57382, 5), is conspecific with Astyanax multidens. Astyanax multidenscan be promptly distinguished from Moenkhausia collettiiby having a the middle caudal-fin rays dark (vs. middle caudal-fin rays hyaline), 5 to 7 maxillary teeth (vs. 1 to 3), scales restricted to the base of the caudal fin, along basal one-sixth of upper lobe and basal one-fourth of lower lobe (vs. along one-fourth of the upper lobe and half of the lower lobe) and by the absence of a black line on the anal-fin base (vs. presence). Since the type series of Moenkhausia collettii is composed of more than one species, a lectotype designation of that species is in need. However, this is beyond of the scope of the present contribution, as it should be done in a separate study about the taxonomy and distribution of M. collettii. Noteworthy to say that Steindachner (1882) described the maxilla as edentulous in the original description of M. collettii and also illustrated a specimen with middle caudal-fin rays hyaline, features not present in specimens of Astyanax multindes.
Although Bertoni (1939) and Fowler (1948) mentioned Astyanax multidensfor the upper rio Paraguay, their reports are doubtful since the extensive material analyzed from that drainage do not have any specimens of Astyanax multidens. Géry (1977: 426) also mentioned Astyanax multidens, referring it to his "Astyanax-paucidens group", which also includes other poorly known species such as A. scintillans Myers, 1928, A. guaporensis, A. guianensis, A. paucidens (Ulrey, 1894), A. gracilior Eigenmann, 1908,A. kennedyi Géry, 1964, and A. essequibensisEigenmann, 1909 [=Jupiaba essequibensis].
Material examined. Brazil. ANSP 191047, 5, 34.2-41.8 mm SL; DZSJRP 12546, 5, 32.4-44.1 mm SL; INPA 33987 5, 33.0-44.3 mm SL; MCP 44598, 5, 31.7-43.3 mm SL; MNRJ 37225, 5, 31.8-43.3 mm SL; MZUSP 96606, 75, 27.3-45.3 mm SL, Mato Grosso, Peixoto de Azevedo, rio Peixoto de Azevedo, tributary of rio Teles Pires, rio Tapajós basin, 10°13'14"S 54°58'2"W. MCZ 21064, 1, 29.9 mm SL, syntypes ofAstyanax multidens Eigenmann, 1908, "Silva, Lake Saraca" (=Amazonas, lago Saraca at Silves, 2º53'S 58º21'W]. MCZ 89559, 29; USNM 120244, 6, 26.1-30.6 mm SL, syntypes of Astyanax multidens Eigenmann, 1908, "Óbidos", (=Amazonas, Óbidos, rio Amazonas, 1º52'S 55º30'W). MCZ 20826, 1, 36.3 mm SL, same data as MCZ 89559. MZUSP 5454, 12, 32.5-37.0 mm SL, Pará, Oriximiná, rio Trombetas basin. MZUSP 52228, 27, 24.8-29.9 mm SL, Tocantins, Sandolândia, rio água Fria, rio Araguaia basin. MZUSP 74635, 10, 16.2-24.8 mm SL, Mato Grosso, São José do Xingu, rio Preto, tributary of rio Xingu. MZUSP 91706, 23, 16.2-33.9 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu. MZUSP 91215, 2, 21.9 and 25.8 mm SL, Mato Grosso, Bom Jesus do Araguaia, rio Suiazinho, tributary of rio Suiá-Miçu, rio Xingu basin, 12°27'25"S 51°45'35"W. MZUSP 91226, 1, 20.7 mm SL, Mato Grosso, Canarana, rio Sete de Setembro, tributary of rio Xingu, 13°30'19"S 52°24'57"W. MZUSP 91369, 2, 22.3 and 22.7 mm SL, Mato Grosso, Gaúcha do Norte, rio Curisevo, tributary of rio Xingu, 13°2'5"S 53°25'19"W. MZUSP 91738, 2, 25.0 and 25.6 mm SL, Mato Grosso, Paranatinga, rio Culuene, 13°50'21"S 53°15'13"W. MZUSP 91800, 2, 22.9 and 32.0 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 14°0'32"S 53°20'46"W. MZUSP 91955, 54, 14.9-25.1 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 13°49'S 53°15'W. MZUSP 94171, 3, 20.4-26.8 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 13°50'S 53°15'W. MZUSP 94251, 11, 18.5-23.8 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 13°50'22"S 53°14'59"W. MZUSP 94284, 20, 18.8-24.5 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 13°35'49"S 53°6'35"W. MZUSP 94339, 46, 17.0-27.4 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 13°30'51"S 53°5'49"W. MZUSP 94410, 4, 17.0-17.3 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 13°25'48"S 53°2'24"W. MZUSP 95689, 37, 15.7-26.8 mm SL, Mato Grosso, Paranatinga, ribeirão da Anta, tributary of rio Culuene, rio Xingu basin, 13°30'53"S 53°5'34"W. MZUSP 95954, 2, 30.4 and 31.3 mm SL, Mato Grosso, Itaúba, Teles Pires drainage, tributary of rio Renato, rio Tapajós basin, 11°5'42"S 55°3'54"W. MZUSP 96726, 133, 24.4-39.5 mm SL, 2 cs, 34.4 and 34.8 mm SL, Mato Grosso, Paranaíta, rio Teles Pires, near ferryboat of road MT-416, rio Tapajós basin, 9°27'7"S 56°30'46"W. MZUSP 96727, 88, 25.7-37.7 mm SL, Mato Grosso, Paranaíta, marginal remaining lagoons from mining activities, rio Tapajós basin, 9°25'44"S 56°32'36"W. MZUSP 96730, 26, 31.9-38.9 mm SL, Pará, Jacareacanga, rio Teles Pires downstream of Sete Quedas rapids, rio Tapajós basin, 9°19'1"S 56°46'47"W. MZUSP 96745, 29, 33.1-48.5 mm SL, Mato Grosso, Peixoto de Azevedo, tributary of right margin of rio Peixoto de Azevedo, rio Tapajós basin, 10°17'14"S 54°50'57"W. MZUSP 96907, 43, 19.0-25.9 mm SL, Mato Grosso, Paranatinga, córrego do Lício, tributary of rio Culuene, rio Xingu basin, 13°50'22"S 53°14'59"W. MZUSP 97205, 2, 24.1 and 24.8 mm SL, Pará, Altamira, rio Curuá, tributary of rio Iriri, rio Xingu basin, 8°19'7"S 55°5'23"W. MZUSP 97249, 185, 25.3-52.8 mm SL, 2 cs, 44.2 and 44.3 mm SL, Pará, Novo Progresso, rio Jamanxim, tributary of rio Tapajós, about 30 km from Castelo dos Sonhos, 8°11'4"S 55°21'28"W. MZUSP 97307, 37, 26.6-41.7 mm SL, Pará, Novo Progresso, rio Jamanxim, tributary of rio Tapajós, near village of Mil, 7°43'51"S 55°16'36"W. MZUSP 97407, 13, 22.8-23.5 mm SL, Mato Grosso, Paranatinga, rio Culuene, tributary of rio Xingu, 13°49'46"S 53°15'6"W. MZUSP 97475, 34, 26.1-33.4 mm SL, Pará, Novo Progresso, rio Jamanxim, tributary of rio Tapajós, at beach near city, 7°3'52"S 55°26'28"W. MZUSP 99600, 31, 17.5-35.8 mm SL, Mato Grosso, Paranaíta, left margin of rio Teles Pires, rio Tapajós basin, 9°27'6"S 56°30'44"W. MZUSP 99818, 8, 21.7-30.0 mm SL, Pará, Jacareacanga, rio Teles Pires, downstream of Sete Quedas rapids, rio Tapajós basin, 9°19'56"S 56°46'33"W. MZUSP 99845, 8, 18.6-24.7 mm SL, Pará, Jacareacanga, rio Teles Pires, downstream of Sete Quedas rapids, rio Tapajós basin, 9°18'42"S 56°46'47"W. MZUSP 99960, 16, 22.1-41.0 mm SL, Pará, Jacareacanga, rio Teles Pires, downstream of Sete Quedas rapids, rio Tapajós basin, 9°20'38"S 56°46'42"W. MZUSP 100037, 11, 20.8-31.7 mm SL, Mato Grosso, Paranaíta, rio Teles Pires, upstream of Sete Quedas rapids, rio Tapajós basin, 9°23'53"S 56°34'37"W. MZUSP 100100 (41, 17.9-26.6 mm SL), Pará, Jacareacanga, rio Teles Pires, upstream of Sete Quedas rapids, rio Tapajós basin, 9°24'5"S 56°33'49"W. MZUSP 104495, 9, 18.0-22.9 mm SL, Mato Grosso, ribeirão Cascalheira, rio Turvo, tributary of rio Suiá-Miçu, rio Xingu basin. MZUSP 110938, 2, 26.3 and 28.6 mm SL, Pará, Altamira, rio Iriri, tributary of rio Xingu, at Cachoeira Grande, 3°52'8"S 52°41'52"W. MZUSP 110939, 68, 21.1-31.5 mm SL, Pará, Altamira, rio Xingu, 3°42'32"S 52°27'11"W. MZUSP 110940, 268, 20.5-30.6 mm SL, Pará, Altamira, rio Xingu, 3°38'14"S 52°21'53"W. MZUSP 110941, 1, 30.9 mm SL, Pará, Altamira, rio Xingu, 3°24'39"S 51°44'50"W. MZUSP 110943, 18, 19.8-29.7 mm SL, Pará, Altamira, rio Xingu, 3°14'12"S 52°13'21"W. NMW 57382, 5, syntypes of Tetragonopterus collettii Steindachner, 1882, 27.8-31.0 mm SL, Óbidos.
Discussion
Astyanax multidens does not belong to Mirande's (2010)Astyanax clade for having five to seven maxillary teeth, and thus lacking the unique synapomorphy of that clade (i.e., none or one maxillary teeth). Based on external similarities, Astyanax multidens most closely resembles A. guaporensis andA. guianensis. The latter two species also possess somewhat diamond-shape body, two vertically oriented humeral blotch and no dark blotch on caudal peduncle, five or more teeth on maxilla, and relatively few pored scales on lateral line (33 to 36).
Specimens of Astyanax multidens were collected in sandy beaches in the rio Teles Pires and rio Jamanxim in syntopy with specimens of Jupiaba paranatinga Netto-Ferreira, Zanata, Birindelli & Sousa (Fig. 6a), and in sandy beaches of the rio Xingu in syntopy with Jupiaba cf. essequibensisEigenmann (Fig. 6b). The three aforementioned species are very similar in coloration (e.g., dark marking on dorsal and anal fins), body size and shape. However, Astyanax multidens lack all the synapomorphic features ofJupiaba, including a modified elongate pelvic bone developed as an anteriorly oriented spine (Zanata, 1997), an antipredatory mechanism (Zanataet al., 2009). Therefore, given the external morphological similarity between the three species, their sympatric occurrence, and their distinct phylogenetic position (considering Jupiaba as monophyletic, following Zanata, 1997), we interpret this as a case of Batesian mimicry, comparable to other previously described (Zanata et al., 2009; Zanata & Ohara, 2009).
(a) Jupiaba paranatinga, holotype, MZUSP 100855, 37.4 mm SL, rio Teles Pires, tributary of rio Tapajós, (b) Jupiaba cf. essequibensis, MZUSP 110952, 36.7 mm SL, rio Xingu; both collected in syntopy with Astyanax multidens.
Three cases of Batesian mimicry including species of Jupiaba and a harmless mimetic species were previously reported: Astyanax anteriorEigenmann as a mimic of Jupiaba abramoides (Eigenmann),J. anteroides (Géry) and J. poranga Zanata in the rio Tiquié (rio Negro basin) and rio Teles Pires; Moenkhausia pirauba Zanata, Birindelli & Moreira as a mimic of Jupiaba yarina Zanata and J. apenima Zanata in the rio Xingu, rio Tapajós basins (Zanata et al., 2009); and Moenkhausiasp. from the rio Madeira basin as a mimic of Jupiaba citrinaZanata & Ohara (Zanata & Ohara, 2009). Despite of the high diversity of the family Characidae, these were the first cases of Batesian mimicry described for the small-sized Characidae. Other cases of Batesian mimicry among Neotropical freshwater fishes involve Piaractus brachypomus(Cuvier) and Pygocentrus cariba (Humboldt, 1821) (Mago Leccia, 1978), Corydoras diphyes Axenrot & Kullander andOtocinclus mimulus Axenrot & Kullander, Corydoras garbei Ihering and Otocinclus xakriaba Schaefer,Corydoras paleatus (Jenyns) and Otocinclus flexilis Cope and O. arnoldi Regan (Axenrot & Kullander, 2003; Lehmann et al. 2010), Brachyrhamdia imitator Myers and Corydoras melanistius Regan (Axenrot & Kullander, 2003).
Comparative material.Jupiaba cf. essequibensis. Brazil. Pará. MZUSP 110952, 11, 34.7-37.4 mm SL, Altamira, rio Xingu, 3°24'39"S 51°44'50"W.Jupiaba paranatinga. Brazil. Mato Grosso. MZUSP 100855, holotype, 37.4 mm SL, Paranaíta, rio Teles Pires, rio Tapajós basin. MZUSP 95720, 23, 33.0-37.6 mm SL, Paranaíta, rio Teles Pires, rio Tapajós basin. MZUSP 95956, 13, 31.2-33.0 mm SL, Itaúba, rio Teles Pires, rio Tapajós basin.Astyanax guaporensis. Brazil. Rondônia. FMNH 54709, 38.6 mm SL, holotype of Astyanax guaporensis Eigenmann, 1911, rio Guaporé at Maciel, rio Madeira basin. FMNH 54710, 1, 32.0 mm SL, rio Guaporé at Maciel, rio Madeira basin. MZUSP 77888, 1, 30.7 mm SL, Costa Marques, rio Guaporé, rio Madeira basin. Astyanax guianensis. Brazil.Amapá. MZUSP 104823,16, 37.4-44.4 mm SL, Laranjal do Jari, rio Jari.Guyana. BMNH 1911.10.31.314-8, 5, 32.1-36.5 mm SL, syntypes ofAstyanax guianensis Eigenmann, 1909, Essequibo river at Warraputa. BMNH 1911.10.31.319-20, 2, 41.8 and 43.3 mm SL, syntypes ofAstyanax guianensis Eigenmann, 1909, Essequibo river at Warraputa. Moenkhausia collettii. Brazil.Amapá. MZUSP 73951, 4, 37.7-43.4 mm SL, rio Araguari. Pará. MZUSP 105535, 15, 28.6-36.8 mm SL, Marabá, rio Tocantins, 5º36'47"S 50º26'42"W. Amazonas. NMW 57379, 3, 39.3-50.1 mm SL; NMW 57380, 3, 33.5-48.0 mm SL; NMW 57381, 2, 47.0 and 47.9 mm SL, syntypes of Tetragonopterus collettiiSteindachner, 1882, Hyavari [= rio Javari, tributary of rio Solimões, 4°21'S 70°2'W].
We are grateful to Naércio Menezes (MZUSP) and three anonymous referees for reading and commenting on the manuscript. We thank Francisco Langeani and Roselene Ferreira (DZSJRP), Margarete Lucena (MCP), Lúcia Py-Daniel and Marcelo Rocha (INPA), Marcelo Britto and Paulo Buckup (MNRJ), Mark Sabaj-Pérez (ANSP), Richard Vari and Sandra Raredon (USNM), James Maclaine, Oliver Crimmen and Ralf Britz (BMNH), Karsten Hartel and Andrew Williston (MCZ), Kevin Swagel (FMNH), and Helmut Wellendorf (NMW), for their help with loans and exchanges of specimens. We thank MCZ staff for providing images of syntype specimens (available at: http://mczbase.mcz.harvard.edu/) and Fernando Ferreira and Eduardo Baena for providing Fig. 6 a andb, respectively. For assistance with scanning electron microscopy images we thank Lara Maria Guimarães (Laboratório de Microscopia Eletrônica, MZUSP). For assistance during field trips we thank Alberto Akama, Aléssio Datovo, Alexandre Oliveira, André Netto-Ferreira, Carlos Figueiredo, Cezar Nolasco, Cristiano Moreira, Flávio Lima, Francisco Machado, Henrique Varella, Isabel Landim, Leandro Sousa, Mark Pérez, Nathan Lujan, Osvaldo Oyakawa, and Pedro Hollanda-Carvalho. Most specimens were collected during the Pipe Expedition to Serra do Cachimbo in October 2008 (http://silurus.acnatsci.org/ACSI/field/Pipe_Expedition), funded by All Catfish Species Inventory (NSF DEB-0315963), and during an expedition to the rio Xingu in November 2011, funded by the South American Characiformes Inventory (FAPESP 2011/50282-7, http://www.usp.br/peixes). The authors were financially supported by FAPESP (09/15075-0, MMFM; 10/51250-9, JLOB).
Literature Cited
- Almirón, A. E., J. R. Casciotta, M. de las M. Azpelicueta & M. Loureiro. 2010. Redescription of Astyanax stenohalinus Messner, 1926 (Characiformes: Characidae), a poorly known species from Argentina and Uruguay. Zootaxa, 2434: 60-68.
- Axenrot, T. & S. Kullander. 2003. Corydoras diphyes (Siluriformes: Callichthyidae) and Otocinclus mimulus (Siluriformes: Loricariidae), two new species of catfishes from Paraguay, a case of mimetic association. Ichthyological Exploration of Freshwaters, 14: 249-272.
- Azpelicueta, M. de las M., J. R. Casciotta & A. E. Almirón. 2002. Two new species of the genus Astyanax (Characiformes, Characidae) from the Paraná river basin in Argentina. Revue suisse de Zoologie, 109: 243-259.
- Azpelicueta, M. de las M. & M. Loureiro. 2009. Astyanax laticeps (Teleostei: Characiformes: Characidae) from rivers and streams of Uruguay. Vertebrate Zoology, 59: 3-9.
- Bertaco, V. A. & C. A. S. Lucena. 2006. Two new species of Astyanax (Ostariophysi: Characiformes: Characidae) from eastern Brazil, with a synopsis of the Astyanax scabripinnis species complex. Neotropical Ichthyology, 4: 53-60.
- Bertaco, V. A., F. R. Carvalho & F. C. Jerep. 2010. Astyanax goyacensis (Miranda-Ribeiro, 1944), new combination and Astyanax courensis, new species (Ostariophysi: Characiformes): two Characidae from the upper rio Tocantins basin, Central Brazil. Neotropical Ichthyology, 8: 265-275.
- Bertaco, V. A. & C. A. S. Lucena. 2010. Redescription of Astyanax obscurus (Hensel, 1870) and A.laticeps (Cope, 1894) (Teleostei: Characidae): two valid freshwater species originally described from rivers of southern Brazil. Neotropical Ichthyology, 8: 7-20.
- Bertoni, A. W. 1939. Catálogos sistemáticos de los Vertebrados del Paraguay. Revista de la Sociedad Científica del Paraguay, 4: 1-60.
- Calcagnotto, D., S. A. Schaefer & R. DeSalle. 2005. Relationships among characiform fishes inferred from analysis of nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution, 36: 135-153.
- Eigenmann, C. H. 1908. Preliminary descriptions of new genera and species of tetragonopterid characins (Zoölogical Results of the Thayer Brazilian expedition). Bulletin of the Museum of Comparative Zoology, 52: 91-106.
- Eigenmann, C. H. 1910. Catalogue of the fresh-water fishes of tropical and south temperate America. Pp. 375-511. In: Reports of the Princeton University expeditions to Patagonia 1896-1899. Zoology.
- Eigenmann, C. H. 1917. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43: 1-102.
- Eigenmann, C. H. 1921. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43: 209-310.
- Eigenmann, C. H. 1927. The American Characidae. Memoirs of the Museum of Comparative Zoology, 43: 311-428.
- Eschmeyer, W. N. & Fricke, R. 2012. Catalog of Fishes electronic version (30 Jan 2012). Available from: http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
» Available from: http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Fink, W. L. & S. H. Weitzman. 1974. The so-called cheirodontin fishes of Central America with description of two new species (Pisces, Characidae). Smithsonian Contributions to Zoology, 172: 1-46.
- Fowler, H. W. 1943. Zoological results of the second Bolivian expedition for the Academy of Natural Sciences of Philadelphia, 1936-1937. Part II. Additional new fishes. Notulae Naturae, 120: 1-7.
- Fowler, H. W. 1948. Os peixes de água doce do Brasil. Arquivos de Zoologia do Estado de São Paulo, 204p.
- Garavello, J. C. & F. A. A. Sampaio. 2010. Five new species of genus Astyanax Baird & Girard, 1854 from rio Iguaçu, Paraná, Brazil (Ostariophysi, Characiformes, Characidae). Brazilian Journal of Biology, 70: 847-865.
- Garutti, V. 2003. Revalidação de Astyanax rupununi Fowler, 1914 (Teleostei, Characidae) e descrição de duas espécies novas para o gênero. Papéis Avulsos de Zoologia, São Paulo, 43: 1-9.
- Garutti, V. & F. Langeani. 2009. Redescription of Astyanax goyacensis Eigenmann, 1908 (Ostariophysi: Characiformes: Characidae). Neotropical Ichthyology, 7: 371-376.
- Géry, J. 1977. Characoids of the World. Neptune City, New Jersey, 215p.
- Javonillo, R., L. R. Malabarba, S. H. Weitzman & J. R. Burns. 2010. Relationships among major lineages of characid fishes (Teleostei: Ostariophysi: Characiformes), based on molecular sequence data. Molecular Phylogenetics and Evolution, 54: 498-511.
- Lehmann, P. A. , F. Mayer & R. E. Reis. 2010. Re-validation of Otocinclus arnoldi Regan and reappraisal of Otocinclus phylogeny (Siluriformes: Loricariidae). Neotropical Ichthyology, 8: 57-68.
- Lima, F. C. T., L. R. Malabarba, P. A. Buckup, J. F. P. Silva, R. P. Vari, A. Harold, R. C. Benine, O. Oyakawa, C. S. Pavanelli, N. A. Menezes, C. A. S. Lucena, M. C. S. L. Malabarba, Z. M. S. Lucena, R. E. Reis, F. Langeani, L. Casatti, V. Bertaco, C. Moreira & P. H. F. Lucinda. 2003. Genera Incertae sedis in Characidae. Pp. 106-169. In: Reis, R.E., S. O. Kullander, & C. J. Ferraris-Jr (Eds.). Check List of Freshwater Fishes of South and Central America. Edipucrs, Porto Alegre, 729p.
- Lima, F. C. T., P. A. Buckup, N. A. Menezes, C. A. S. Lucena, Z. M. S. Lucena, M. Toledo-Piza, & A. Zanata. 2007. Família Characidae: Gêneros incertae sedis. Pp. 106-169. In: Buckup, P. A., N. A. Menezes & M. A. Ghazzi (Eds.). Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro, Museu Nacional, 195p.
- Mago Leccia, F. 1978. Los peces de água dulce de Venezuela. Cuadernos Lagoven, Caracas, 141p.
- Marinho, M. M. F. & F. C. T. Lima. 2009. Astyanax ajuricaba: a new species from the Amazon basin in Brazil (Characiformes: Characidae). Neotropical Ichthyology, 7: 169-174.
- Melo, F. A. G. & P. A. Buckup. 2006. Astyanax henseli, a new name for Tetragonopterus aeneus Hensel, 1870 from southern Brazil (Teleostei: Characiformes). Neotropical Ichthyology, 4: 45-52.
- Mirande, J. M., M. de las M. Azpelicueta & G. Aguilera. 2006. Redescription of Astyanax correntinus (Holmberg, 1891) (Teleostei: Characiformes: Characidae), more than one hundred years after original description. Zoologische Abhandlungen, 55: 9-15.
- Mirande, J. M. 2010. Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy. Neotropical Ichthyology, 8: 385-568.
- Nelson, J. S. 2006. Fishes of the World. 4th edition. John Wiley and Sons, New York, 601p.
- Oliveira, C., G. S. Avelino, K. T. Abe, T. C. Mariguela, R. C. Benine, G. Ortí, R. P. Vari & R. M. C. Castro. 2011. Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling. Evolutionary Biology, 11: 275.
- Pavanelli, C. S. & C. A. M. Oliveira. 2009. A redescription of Astyanax gymnodontus (Eigenmann, 1911), new combination, a polymorphic characid fish from the rio Iguaçu basin, Brazil. Neotropical Ichthyology, 7: 569-578.
- Rosen, D. E. 1972. Origin of the characid fish genus Bramocharax and a description of a second, more primitive, species in Guatemala. American Museum Novitates, 500: 1-21.
- Soneira, P., J. Casciotta, A. Almirón, L. Ciotek & P. Giorgis. 2010. Redescription of Astyanax erythropterus (Holmberg, 1891) (Teleostei: Characiformes: Characidae) from La Plata basin in Argentina. Neotropical Ichthyology, 8: 779-785.
- Taylor, W. R. & G. C. van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9:107-119.
- Vari, R. P. & J. C. Howe. 1991. Catalog of type specimens of Recent fishes in the National Museum of Natural History, Smithsonian Institution. 1. Characiformes (Teleostei, Ostariophysi) . Smithsonian Contributions to Zoology, 517: 1-52.
- Weitzman, S. H. & W. L. Fink. 1983. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei, Characidae), with comments on the phylogeny of New World Characiformes. Bulletin of the Museum of Comparative Zoology, 150: 339-395.
- Weitzman, S. H. & L. R. Malabarba. 1998. Perspectives about the phylogeny and classification of the Characidae (Teleostei: Characiformes). Pp. 161-170. In: Malabarba, L.R., R. E. Reis, R.P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Phylogeny and Classification of Neotropical Fishes. Porto Alegre, Edipucrs, 603p.
- Zanata, A. M. 1997. Jupiaba, a new genus of Tetragonopterinae with spine-like pelvic bones (Characidae, Characiformes). Iheringia, Série Zoologia, 83: 99-136.
- Zanata, A. & P. Camelier, 2008. Two new species of Astyanax (Characiformes: Characidae) from upper rio Paraguaçu and rio Itapicuru basins, Chapada Diamantina, Bahia, Brazil. Zootaxa, 1908: 28-40.
- Zanata, A. M. , J. L. O. Birindelli & C. R. Moreira. 2009. New species of Moenkhausia Eigenmann (Characiformes: Characidae) from Rio Xingu and Rio Tapajós basins, Brazil, with comments on a putative case of polymorphic Batesian mimicry. Journal of Fish Biology, 75: 2615-2628.
- Zanata, A. M. & W. M. Ohara. 2009. Jupiaba citrina, a new species from rio Aripuanã, rio Madeira basin, Amazonas and Mato Grosso states, Brazil (Characiformes: Characidae). Neotropical Ichthyology, 7: 513-518.
-
Published March 31, 2013
Publication Dates
-
Publication in this collection
Jan-Mar 2013
History
-
Received
03 Feb 2012 -
Accepted
11 Oct 2012