Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
Bacopa monnieri Extract As a Neuroprotective and Cognitive Enhancement Agent
Bevin J. Nishanth 1, Princy Vijayababu 2, * , and Noble K. Kurian 3
1 Department of Biotechnology, Loyola College, Chennai 600034, India
2 BiogenecodeX, Thiruvananthapuram, Kerala, 695504, India
3 School of Life Sciences, B.S.Abdur Rahman Crescent Institute of Science and Technology, Chennai 600048, India
* Correspondence: princyvijayababu@gmail.com
Received: 14 July 2023
Accepted: 14 August 2023
Published: 27 December 2023
Abstract: Traditional Indian medicine uses the neuroprotective and cognitive-enhancing effects of the spice, Bacopa monnieri, commonly known as Brahmi. This paper provides an overview of the potential neuropharmacological benefits and therapeutic applications of Bacopa monnieri. Bacopa monnieri has therapeutic value due to the inclusion of bioactive compounds, including alkaloids, flavonoids, tannins, and phenolics. Bacoside A, a triterpenoid saponin of the Dammarane family, has been studied most because of its potential to improve memory and cognitive function. Specific brain regions that are affected by bacoside A experience an increase in protein and RNA production, oxidative stress protection, improved cerebral blood flow, and enhanced synaptic activity. By boosting antioxidant defense mechanisms, lowering oxidative stress, and altering neurotransmitter levels, Bacopa monnieri demonstrates neuroprotective effects. By blocking the activity of acetylcholinesterase, lowering the production of β-amyloid plaques, and modifying neurotransmitter levels, it has demonstrated potential for treating neurodegenerative disorders, such as Alzheimer’s and Parkinson’s disease. As a natural neuroprotective and cognitive-improving agent, Bacopa monnieri is generally found to be promising. To completely comprehend its mechanisms of action and assess its long-term toxicity, more studies are necessary. Further research is needed to determine whether Bacopa monnieri can be used as a possible treatment for neurodegenerative diseases.
Keywords:
Bacopa monnieri Brahmi neuropharmacology neuroprotection cognitive enhancement neurodegenerative diseases1. Introduction
The usage of herbal medicines for treating different ailments has been practiced in India since ancient times. It has gained worldwide fame due to its low cost and minimal negative impact on patients [1]. In neurology, these herbal plants play a significant role as drugs for neuropsychiatric disorders. Bacopa monnieri is one such herb, and it is widely used as a nerve tonic to enhance memory and restore damaged brain tissue [2]. It is also called a nootropic herb because it boosts cognition by manipulating hormones, enzymes, and neurotransmitters, including acetylcholine [3]. The existence of certain chemical compounds such as Bacoside A is considered one of the active components responsible for the plant’s potential cognitive-enhancing effects; some of its roles in the brain are shown in Figure 1. Research suggests that bacoside A may have various benefits for brain health and cognitive function. Bioactive chemicals include alkaloids, flavonoids, tannins, and phenolics, with the first three being the most prominent [4].
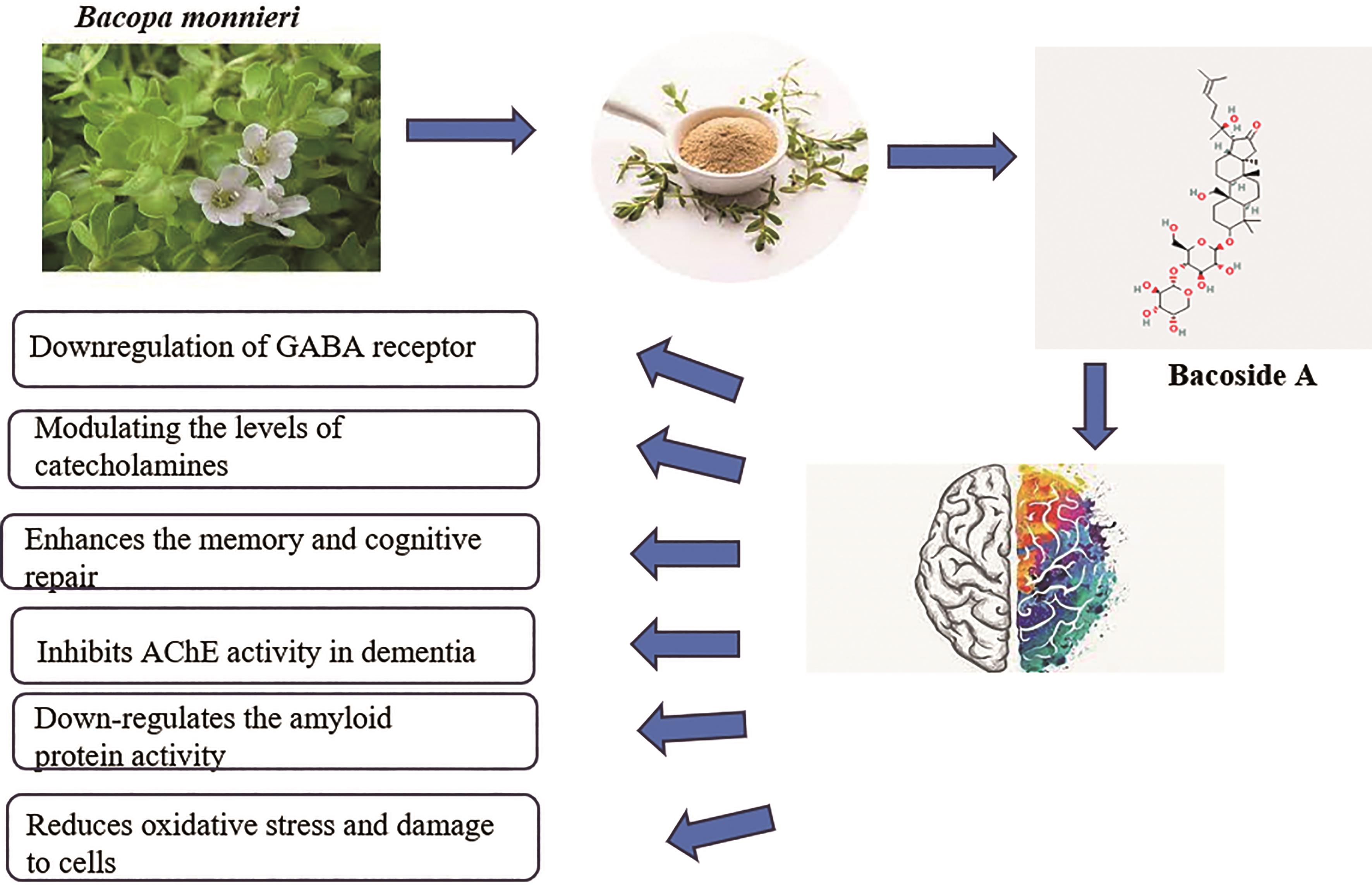
Figure 1. Potential effects of bacoside A on the brain.
2. Taxonomical Classification
The evergreen plant known as Brahmi (Bacopa monnieri Linnaeus.) may be found in damp swampy areas. It has a maximum height of 90 cm, 1 mm thick roots, and a thin, green stem. Its flowers may be either white, purple, or pink; they are typically tiny and have five petals [2,4]. The 146 species of aquatic herbs that make up the genus Bacopa may be found in places as diverse as the southern United States, China, and Nepal.
3. Nootropic Herb
Neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease, are class of chronic conditions affecting the central nervous system. They are characterized by the death of neurons in the brain and spinal cord. Some plants, known as ‘Memory Boosting Plants’, have received increased attention in the pharmaceutical field recently because of their potential to increase brain activity [5,6]. One such plant is the Bacopa monnieri; it was ranked second in an evaluation of the therapeutic value of Indian herbs. This plant has anti-neoplastic, anti-depressant, anti-convulsant and anti-inflammatory properties. It is a chelating agent; it can get rid of any metal that is poisonous to the body [7]. The toxicity of Bacopa monnieri is minimal in the model organism, but its long-term effects have yet to be investigated.
4. Bioactive Compounds in Bacopa monnieri
The whole bacopa plant can be used; it is used to cure various medical conditions including asthma, epilepsy, and neurological diseases. It has been shown to boost cognitive abilities, including learning and memory, and respiratory health; it is often used as a nootropic and digestive aid. Saponins, flavonoids, and alkaloids are only some of the active components found in bacopa. Brahmine was the first alkaloid discovered in bacopa [8], followed by herpestine [9] and nicotine (8%) (Table 1). Bacoside A is a complex compound with neuropharmacological effects; it contains aglycone units derived from jujubogenin [10]. Triterpenoid saponins contain jujubogenin or pseudo-jujubogenin moieties as their glycone units, of which there are 12 recognized members of the bacoside family. Bacoside A is a mixture of four triglycosidic saponins: bacoside A3, bacopaside II, bacopasaponin C, and the jujubogenin isomer of bacosaponin C, bacopaside X (Figure 2); this makes it the most concentrated fraction [11‒13]. Additionally, the composition of bacoside B is made up of the bacopasides N1, N2, IV, and V compounds (Figure 3).
Table 1. Bioactive compounds in Bacopa monnieri.
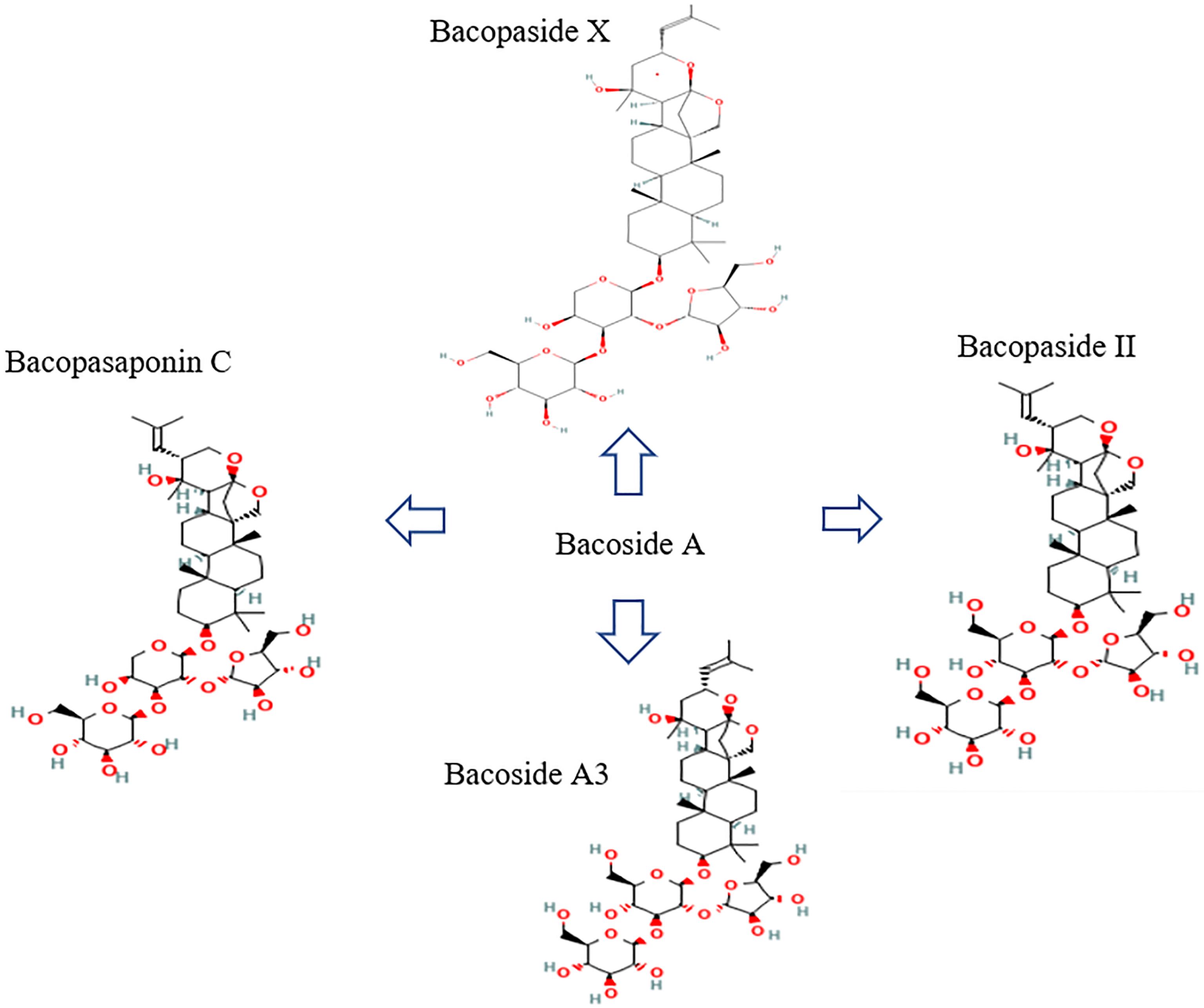
Figure 2. Chemical structure of bacoside A retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov).
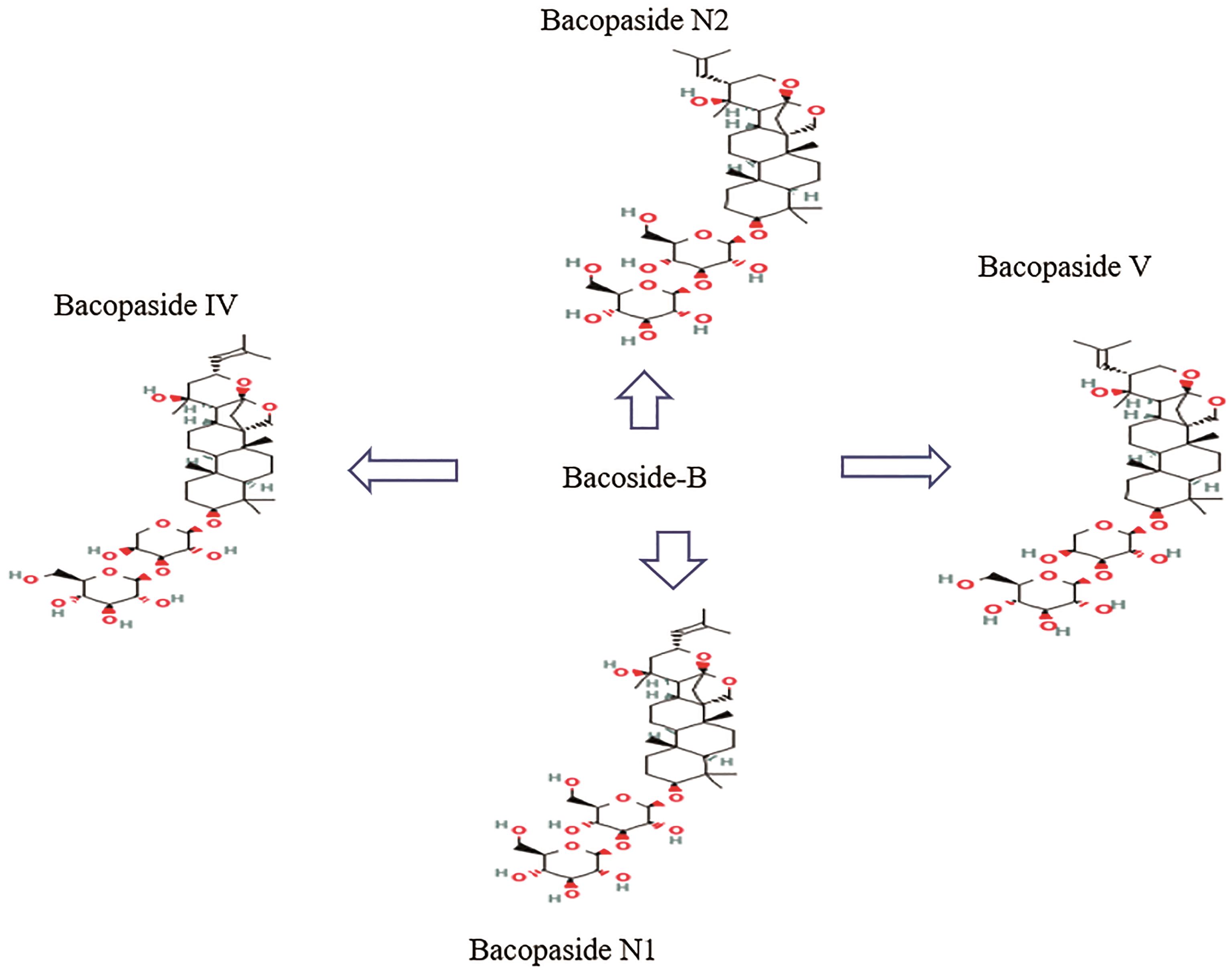
Figure 3. Chemical structure of bacoside B retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov).
Bacosides A and B are often used to improve memory. Bacoside A contains nitric oxide, which facilitates blood flow. Furthermore, these components have anticarcinogenic effects. Other chemicals found in bacopa have also been identified [11,14]. These compounds consist of flavonoids, phenylethanoid glycosides, cucurbitacin, and apigenin. Bacobitacin A‒D and the cytotoxic cucurbitacin E are the four cucurbitacins extracted from Bacopa monnieri. In addition, three phenylethanoid glycosides have been isolated from the plant: monnieriside I, monnieriside III, and plantioside B. Two other saponins and a unique glycoside have been isolated from Bacopa monnieri, and a few further noteworthy saponins have also been discovered and described [15].
5. Neuropharmacological Activity of Bacoside A
The principal constituents are dammarane types of triterpenes saponin known as bacosides; there are two types known as jujubogenin and pseudojujubogenin, and they differ only by the sugar units in the glycosidic chain. The major neuropharmacological compound is bacoside A. These bacosides may enhance protein and RNA production in certain regions of the brain by inducing membrane dephosphorylation. These compounds protect against oxidative stress, age-related cognitive deterioration, memory problems, and cold stress. It increases cerebral blood flow, possesses an antidementia mechanism, improves delayed recall, decreases lipoxygenase activity, and inhibits hydrogen peroxide-induced lipid peroxidation [15‒17]. It enhances the protein kinase activity before the hippocampus region, which in turn repairs damaged neurons; also it supports neuronal synthesis and restoration of synaptic activity [18,19]. Also, bacosides increase memory function through the enzyme Tryptophan Hydroxylase (TPH2) and increases the serotonin transporter (SERT) [20].
6. Neuroprotective Effects of Bacopa monnieri
It may enhance the antioxidant defence systems in the brain by activating specific enzymes and reducing oxidative stress; it can also reduce the levels of harmful metal ions, such as iron and copper, which contribute to oxidative damage in the brain [6]. The bioactive chemicals in Bacopa monnieri may either block the enzyme responsible for degrading the neurotransmitter acetylcholine (acetylcholinesterase) or stimulate the enzyme responsible for producing acetylcholine (choline acetyltransferase). Altering acetylcholine levels may improve cognitive performance. Beta-amyloid, a protein that forms plaques in the brains of Alzheimer’s sufferers, may also be reduced with the use of Bacopa monnieri. Synapses play a crucial role in brain function, and their abundance may be rapidly altered [21‒23]. The gradual breakdown of the structure and function of the nervous system is a hallmark of neurodegenerative illnesses. Cognitive decline, impaired motor control, and decreased general brain health are common consequences of these diseases. Although many variables contribute to neurodegenerative illnesses, some proteins have been singled out as major contributors to certain well-known ailments. For instance, in dementia and amnesia, the protein acetylcholinesterase (AChE) is implicated, while α-synuclein is associated with Parkinson’s disease. Furthermore, β-amyloid is closely linked to Alzheimer’s disease, and VGluT1, VGluT2, and VGluT3 are involved in schizophrenia [12,13]. The glutamate-8 receptor (mGluR8) plays a role in epileptic seizures. Understanding the proteins involved in these disorders is essential for advancing research, developing effective treatments, and ultimately improving the lives of those affected by neurodegenerative diseases. The proteins associated with neurodegenerative diseases are listed in Table 2.
Table 2. Proteins associated with neurodegenerative diseases.
By inhibiting AChE activity, Bacopa monnieri may help prevent the breakdown of acetylcholine and maintain its proper levels in the brain, thus potentially mitigating the neurotoxic effects associated with increased AChE levels induced by the amyloid peptide.
7. Molecular Mechanism of Bacopa monnieri in Disorders Affecting the Brain
7.1. Alzheimer’s Disease
Since cognitive decline is a hallmark of Alzheimer’s disease, research into the cognitive-enhancing effects of Bacopa monnieri is warranted. One important factor in Bacopa monnieri’s pro-cognitive influence is its limited capacity to sustain flagging circuits related to synaptic flexibility. Synaptic pliancy proteins include the NR1 subunit of the N-methyl-D-aspartate (NMDA) receptor, calmodulin-subordinate kinase II, and cyclic adenosine monophosphate-responsive element-binding protein (CREB) [30,31]. Learning and memory rely heavily on a phenomenon called synaptic plasticity.
In addition, Bacopa monnieri has been demonstrated to shield the cholinergic system, which is critical to cognitive processes and is compromised in Alzheimer’s disease. Safeguarding this system may help preserve cognitive abilities. Bacopa monnieri has also demonstrated the potential to improve cerebral blood flow, which is essential for supplying oxygen and nutrients to the brain [32]. Enhanced blood flow can enhance brain function and support cognitive processes.
Positive effects on body weight, learning abilities, memory, and focus have been shown in experiments using AD-induced mice, suggesting that Bacopa monnieri may have cognitive advantages in this animal model. By reducing oxidative stress, neuroinflammation, and neuronal death, it has also been effective in reversing memory impairment in rat models. This is achieved through the restoration of cellular factors involved in antioxidant defence (Nrf2 and HO-1) and glutathione synthesis (GCLC) [33]. Moreover, Bacopa monnieri has demonstrated the ability to reverse amnesia induced by the drug, scopolamine, in mice, as scopolamine is known to impair memory and cognitive function. The role of Brahmi extract in Alzheimer’s disease is shown in Table 3.
Table 3. Effects and role of Bacopa monnieri (Brahmi) in Alzheimer’s disease.
In a 21-day study involving rats, the effects of Bacopa monnieri (Brahmi) supplementation on enzymatic activity were investigated. The review focused on the function of superoxide dismutase (Grass), catalase (Feline), and glutathione peroxidase (GPx) in the prefrontal cortex, hippocampus, and striatum of the brain. The results showed that after EBm formation, the activity of these proteins dramatically increased throughout all three regions of the brain. Despite being a well-known cell reinforcer, an investigation using deprenyl revealed that it only increased protein function in the prefrontal cortex and striatum, and not the hippocampus. These findings demonstrate the potential of Bacopa monnieri as a regulator of enzymatic cell reinforcement systems and suggest its restorative relevance in neurological conditions, like Alzheimer’s disease. It may provide neuroprotective effects against oxidative pressure and contribute to the management of Alzheimer’s disease by enhancing cell reinforcement chemical activity, notably in the vulnerable hippocampus [34].
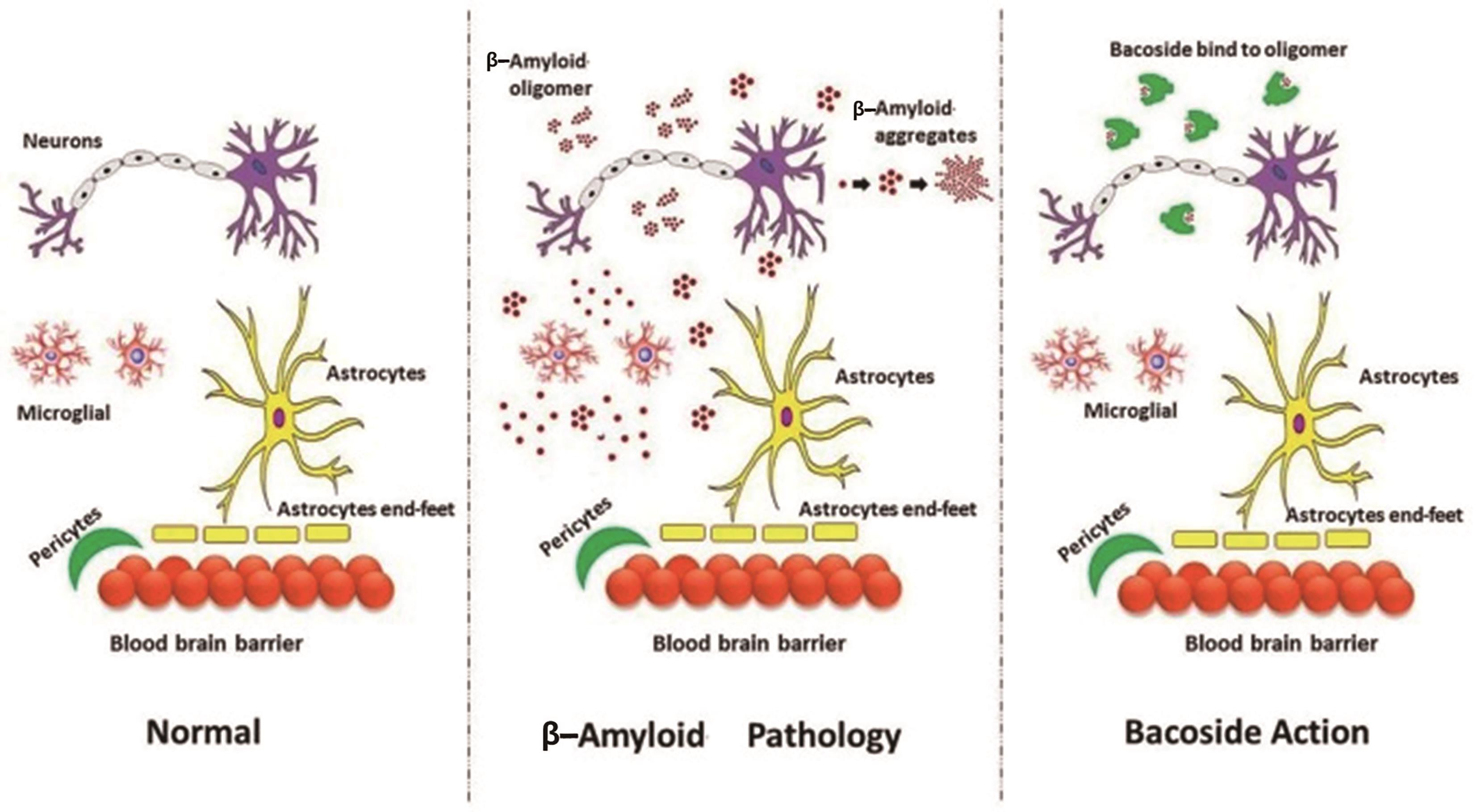
Figure 4. Bacoside’s anti-beta-amyloid mechanism of action [35].
Bacoside A has been studied for its possible therapeutic actions against beta-amyloid. This protein plays a crucial role in neurodegenerative illnesses like Alzheimer’s disease. The effects of bacoside A’s interaction with beta-amyloid on the protein’s structure and function were investigated. The results of the experiments showed that bacoside A has anti-amyloid toxicity characteristics by affecting the beta amyloid’s interactions with cellular membranes. To be more specific, beta-amyloid fibril production was stimulated and peptide interactions with cell membranes were suppressed when the peptide was preincubated with bacoside A. By encouraging fibrillation and altering beta-amyloid’s membrane contacts, our findings imply bacoside A may have a role in reducing amyloid protein toxicity. More research is needed to determine how bacoside A prevents the build-up of amyloid and whether or not it may be used as a treatment for neurodegenerative disorders [24].
7.2. Parkinson’s Disease
Parkinson’s disease is characterized by the build-up of a protein called alpha-synuclein and the subsequent death of dopaminergic neurons. People who experience this have difficulty with cognition and mobility. Bacopa monnieri outperformed levodopa, a major medicine used to treat Parkinson’s disease, in a mouse model of the condition produced by the chemical, rotenone [36,37].
One of the ways Bacopa monnieri exerts its neuroprotective effects is by modulating the levels of catecholamines, which include epinephrine, norepinephrine, dopamine, and serotonin. These neurotransmitters play crucial roles in brain function, and it has been found to regulate their levels in Parkinson’s disease rats [38].
In addition to its effects on neurotransmitter levels, Bacopa monnieri possesses anti-inflammatory and antioxidant properties that are beneficial in Parkinson’s disease. In rats, Bacopa monnieri has been found to suppress the levels of pro-inflammatory cytokines, alpha-synuclein (a protein associated with Parkinson’s disease), and reactive oxygen species (ROS) generation. These actions help reduce neuroinflammation and oxidative stress, which are contributing factors in Parkinson’s disease.
Superoxide dismutase (Grass), catalase (Cat), and glutathione peroxidase (GPx) levels were increased due to Bacopa monnieri, to counteract the lipid peroxidation (fat damage) caused by MPTP. By strengthening the body’s regular cell support mechanisms, it may defend against oxidative damage in Parkinson’s disease. It has been shown to cooperate with the delivery system of particles serving as disease inhibitors, such as glutathione peroxidase (GPx), glutathione superoxide dismutase (Grass), and catalase (Cat). Bacopa monnieri ability to reduce oxidative stress and promote a balanced redox state suggests it might be used to protect cells against ROS and maintain their health. Taken together, these findings suggest that it may be more effective in alleviating the symptoms and pathology associated with Parkinson’s disease, compared to conventional medications like levodopa [39].
Vitamin E (-tocopherol), glutathione (GSH), and L-ascorbic acid (ascorbic corrosive) are three nonenzymatic cancer prevention agents that provide substantial cell insurance. Detoxifying ROS and protecting cells from oxidative damage are the jobs of enzymes, including catalase (Feline), superoxide dismutase (Grass), and glutathione peroxidase (GPx). ROS lead to oxidative pressure by upsetting the redox balance; ROS include hydrogen peroxide (H2O2), hydroxyl free radicals (OH•), superoxide anions (O2•-), and peroxynitrite (ONOO-). Oxidative pressure has a deleterious effect on the lipids, proteins, and DNA [40].
7.3. Epilepsy
Epilepsy, a chronic disorder affecting the central nervous system (CNS), is characterized by impairments in cognitive functions, memory, and learning. This condition is linked to abnormal regulation of central cholinergic neurons, and imbalances in gamma-aminobutyric acid (GABA) and glutamatergic neurons [41]. The impact of Bacopa monnieri extract on hippocampal changes induced by pilocarpine-induced epilepsy in rats was investigated. Notably, the expression of the NMDA receptor subunit NMDA R1 was significantly decreased, affecting glutamate receptor binding within the hippocampus. These results imply that Bacopa monnieri concentrate might have a neuroprotective effect on epilepsy by restoring glutamate receptor modulation in the hippocampus and influencing the NMDA receptor component NMDA R1. These findings lend support to the potential use of Bacopa monnieri as a therapeutic approach to address glutamatergic framework alterations associated with epilepsy [42‒44].
Moreover, Bacopa monnieri was found to influence the activity of acetylcholinesterase, malate dehydrogenase, insulin, and T3 levels in the serum. These adjustments are associated with seizure prevention and diminished peripheral nervous system impairment [45]. Collectively, these investigations propose that Bacopa monnieri and its constituents, especially bacoside A, show promise as potential therapeutic interventions for epilepsy. Their mechanisms involve modulating neurotransmitter systems, safeguarding against neuronal damage, and enhancing peripheral nervous system functionality.
7.4. Neuronal and Glial Plasticity
The brain capacity to change in response to environmental challenges is known as neuronal and glial plasticity [44]. Brain-derived neurotrophic factor (BDNF) is a neurotrophin that has been shown to have important effects on neuronal plasticity and memory formation via its association with the transcription of the Arc gene. A higher risk of developing Alzheimer’s disease is seen in those whose BDNF levels are low.
A key marker of glial plasticity is the glial fibrillary acidic protein (GFAP); it controls astrocyte shape, neuroglial connections, and memory formation pathways. Conditions linked to memory loss have been linked to reduced GFAP expression [46].
The expression of BDNF, arachidonic acid (Arc), and GFAP was significantly reduced in mice when the cognitive-impairing medication scopolamine was administered, according to research by Konar et al. [46]. While GFAP expression did not change much, BDNF expression increased by 1.3-fold and Arc expression by 2-fold in mice treated with EBm (Brahmi extract). The injection of EBm either before or after scopolamine treatment increased plasticity markers in the brain, with BDNF and Arc expression being more strongly affected than GFAP. These results provide strong evidence that EBm significantly contributes to enhancing brain plasticity through many pathways. The impact on GFAP expression, however, seems to be weaker. This study demonstrates that brahmi extract improves neuronal and glial plasticity [47].
8. Therapeutic Importance of Bacopa monnieri
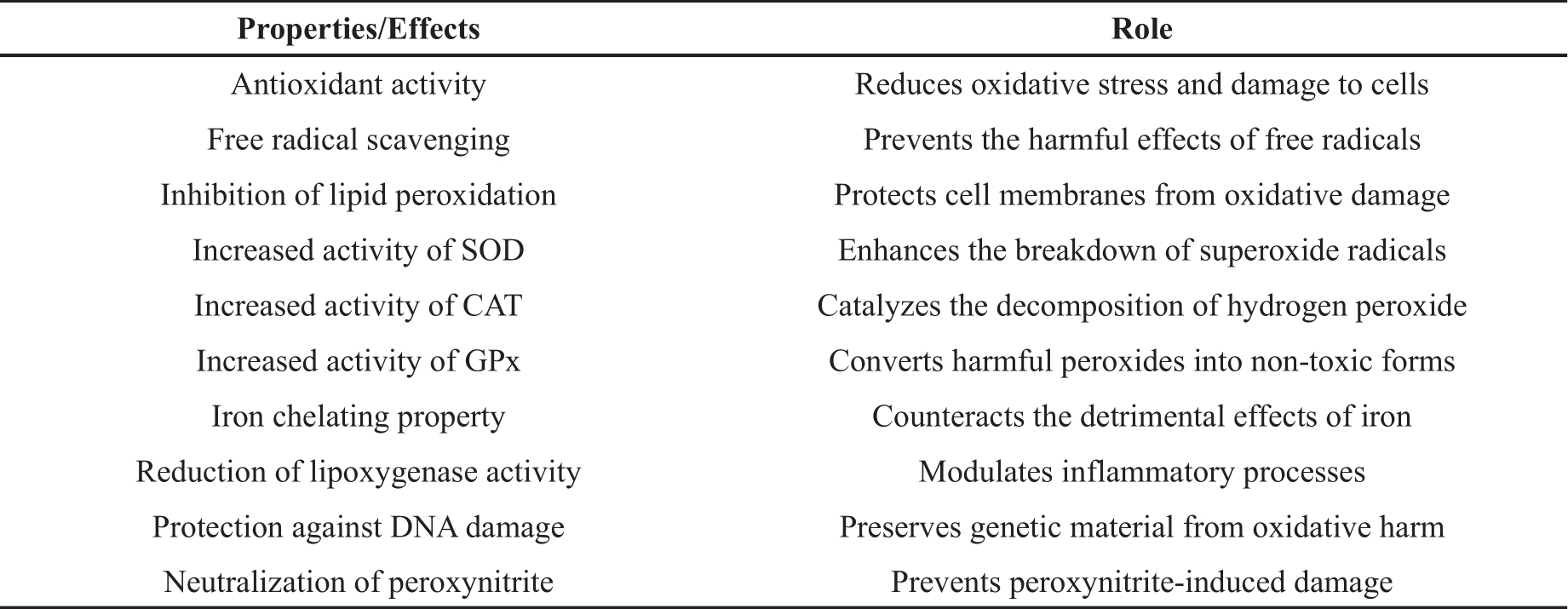
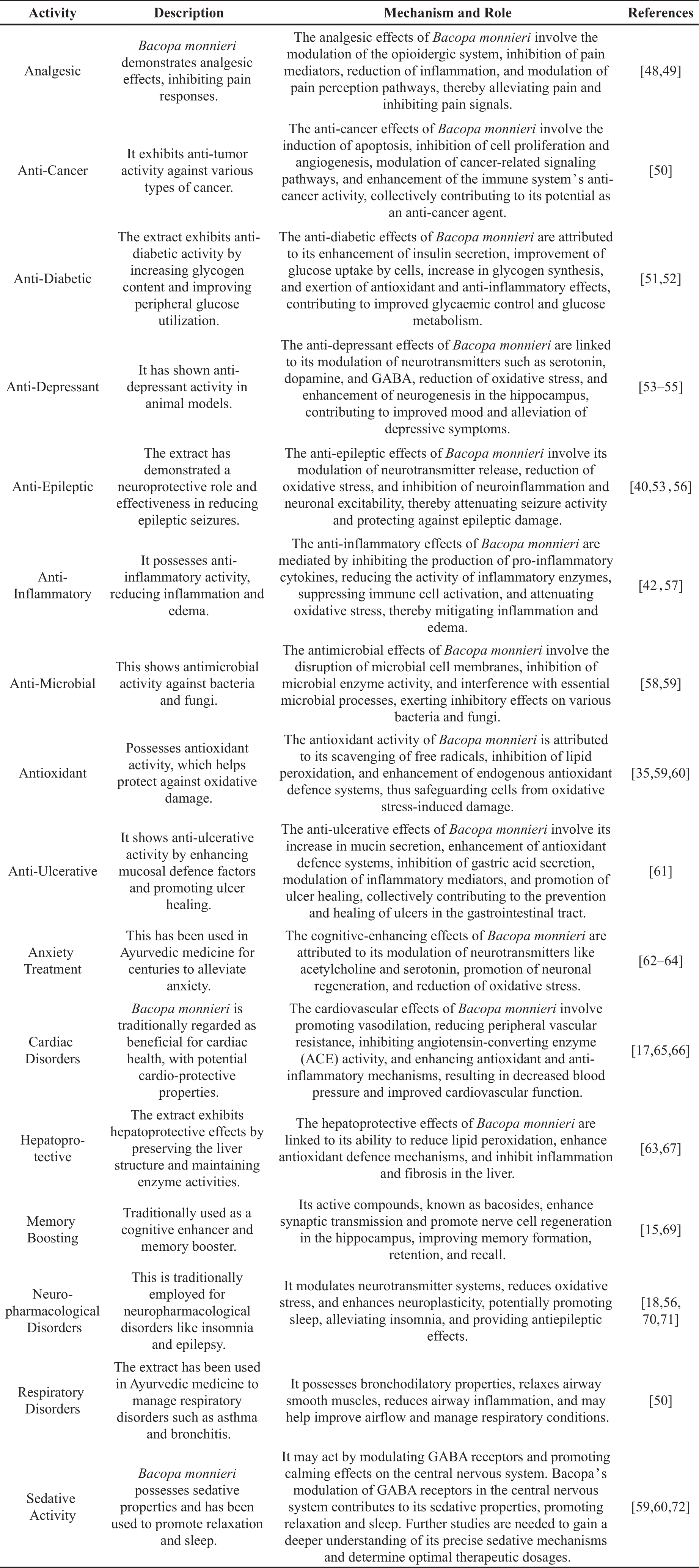
Pharmacological studies have extensively explored the therapeutic potential of brahmi, as indicated in Table 4. These studies have highlighted its efficacy in treating anxiety and enhancing memory. In addition, brahmi has shown promise as a treatment for various sleep and other neuropharmacological conditions. It has also been shown to have anti-cancer, anti-inflammatory, antipyretic, anti-lipid-peroxidative, analgesic, and anti-inflammatory effects.
Table 4. Pharmacological activities and mechanisms of Bacopa monnieri.
9. Future Perspectives
Efforts to comprehensively understand the mechanisms underlying neuroprotective and cognitive-enhancing properties of Bacopa monnieri are ongoing, necessitating further investigation to unlock its full therapeutic potential. Key avenues for future research include conducting extensive long-term toxicity studies to assess its safety over repeated use; robust clinical trials to establish its efficacy in treating neurological conditions and enhancing cognitive function; and delving into the intricate molecular processes through which it confers its benefits. To ensure consistent quality and precise dosing, standardized extraction techniques should be employed for Bacopa monnieri extracts. Exploring its interactions with other herbal extracts or pharmaceuticals could enhance treatment strategies for cognitive decline and neurodegenerative diseases. Innovations in formulation, such as nanoemulsions or encapsulation methods, have the potential to amplify its bioavailability and therapeutic effectiveness. Comparative studies with existing nootropic agents would provide valuable insights into Bacopa monnieri’s relative efficacy and safety profiles. It is important to conduct scientific research to validate the traditional claims associated with Bacopa monnieri and explore its potential as a neuroprotective and cognitive-enhancing agent. Such investigations will pave the way for the development of evidence-based therapeutic interventions for neurological disorders and cognitive impairments.
10. Conclusion
Bacopa monnieri, more often known as brahmi, is a medicinal herb that has shown significant promise in the treatment of cancer, enhancement of cognition, and neuroprotection. It has a long history of use in Ayurvedic medicine for the enhancement of memory, the remediation of neurological disorders, and the promotion of healthy mental processes. Alkaloids, flavonoids, saponins, and triterpenoid saponins, such as bacoside A and bacoside B, are only some of the bioactive chemicals found in Bacopa monnieri, that contribute to the plant’s pharmacological effects. These compounds have been shown to have neuroprotective properties via decreasing oxidative stress, regulating neurotransmitter levels, and increasing cerebral blood flow. Furthermore, recent studies point to Bacopa monnieri as having possible anticancer effects. Purified components from Bacopa monnieri extract have been proven in studies to have specific effectiveness against cancer cells, causing cell death through the manipulation of signaling pathways and cell cycle arrest. Bacopa monnieri has neuroprotective effects through various modes of action, including a barrier to oxidative stress, control of neurotransmitter levels, increased cerebral blood flow, and inhibition of acetylcholinesterase activity. It can protect brain health and improve cognitive performance thanks to these mechanisms. The potential of Bacopa monnieri to treat neurodegenerative diseases has been demonstrated in studies employing several cell lines and animal models. To completely understand the precise mechanisms and particular bioactive substances responsible for Bacopa monnieri’s medicinal actions, additional study is required. To determine its effectiveness, ideal dosage, and long-term safety for treating various neurological disorders and cancers, rigorous clinical studies are required. Further scientific investigation will increase our knowledge of Bacopa monnieri’s therapeutic potential and open the door to its use in contemporary medicine.
Author Contributions: N.B. conceptualized the study, conducted the investigation, curated the data, and prepared the original draft. V.P. provided supervision, participated in investigation and data curation, and contributed to both the original draft and manuscript review. K.N. allocated resources, participated in data curation, and contributed to manuscript review. All authors have read and approved the final version of the manuscript for publication.
Funding: This research received no external funding.
Data Availability Statement: Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Subhose, V.; Srinivas, P.; Narayana, A. Basic principles of pharmaceutical science in Ayurvĕda. Bull. Indian Inst. Hist. Med. Hyderabad 2005, 35, 83‒92.
- Gohil, K.J.; Patel, J.A. A review on Bacopa monniera: current research and future prospects. Int. J. Green Pharm. 2010, 4, 1–9. DOI: https://doi.org/10.4103/0973-8258.62156
- Amin, H.; Sharm,a R. Nootropic efficacy of Satvavajaya Chikitsa and Ayurvedic drug therapy: A comparative clinical exposition. Int. J. Yoga. 2015, 8, 109‒116. DOI: https://doi.org/10.4103/0973-6131.158473
- Rajan, K.E.; Preethi, J.; Singh, H.K.; et al. Molecular and Functional Characterization of Bacopa monniera: A Retrospective Review. Evid. Based Complement. Alternat. Med. 2015, 2015, 945217. DOI: https://doi.org/10.1155/2015/945217
- Barrett, S.C.H.; Strother, J.L. Taxonomy and natural history of Bacopa (Scrophulariaceae) in California. Systemat. Botany 1978, 408‒419. DOI: https://doi.org/10.2307/2418753
- Bhandari, P.; Kumar, N.; Singh, B.; et al. Cucurbitacins from Bacopa monnieri. Phytochemistry 2007, 68, 1248‒5124. DOI: https://doi.org/10.1016/j.phytochem.2007.03.013
- Mohapatra, H.P.; Rath, S.P. In vitro studies of Bacopa monnieri--an important medicinal plant with reference to its biochemical variations. Indian, J. Exp. Biol. 2005, 43, 373‒376.
- Bose, K.C.; Bose, N.K. Observations on the actions and uses of Herpestis monniera. J. Ind. Med. Assoc. 1931, 1, 60.
- Russo, A.; Borrelli, F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine 2005, 12, 305‒317. DOI: https://doi.org/10.1016/j.phymed.2003.12.008
- Vishnupriya, P.; Padma, V.V. A review on the antioxidant and therapeutic potential of Bacopa monnieri. React. Oxygen Spec. 2017, 3, 111‒120. DOI: https://doi.org/10.20455/ros.2017.817
- Elangovan, V.; Govindasamy, S.; Ramamoorthy, N.; et al. In vitro studies on the anticancer activity of Bacopa monnieri. Fitoterapia 1995, 66, 211‒215.
- Jasim, B.; Daya, P.S.; Sreelakshmi, K.S.; et al. Bacopaside N1 biosynthetic potential of endophytic Aspergillus sp. BmF 16 isolated from Bacopa monnieri. 3 Biotech. 2017, 7, 210. DOI: https://doi.org/10.1007/s13205-017-0788-4
- Saini, N.; Oelhafen, S.; Hua, H.; et al. Extended lifespan of Drosophila parkin mutants through sequestration of redox-active metals and enhancement of anti-oxidative pathways. Neurobiol. Dis. 2010, 40, 82‒92. DOI: https://doi.org/10.1016/j.nbd.2010.05.011
- Singh, H.K.; Rastogi, R.P.; Srimal, R.C.; et al. Effect of bacosides A and B on avoidance responses in rats. Phytother. Res. 1988, 2, 70‒75. DOI: https://doi.org/10.1002/ptr.2650020205
- Kamkaew, N.; Scholfield, C.N.; Ingkaninan, K.; et al. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J. Ethnopharmacol. 2011, 137, 790‒795. DOI: https://doi.org/10.1016/j.jep.2011.06.045
- Kumar, S.S.; Saraswathi, P.; Vijayaraghavan, R. Effect of bacopa monniera on cold stress induced neurodegeneration in hippocampus of wistar rats: a histomorphometric study. J. Clin. Diagn. Res. 2015, 9, AF05‒AF07. DOI: https://doi.org/10.7860/JCDR/2015/10199.5423
- Dhanasekaran, M.; Tharakan, B.; Holcomb, L.A.; et al. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother. Res. 2007, 21, 965‒969. DOI: https://doi.org/10.1002/ptr.2195
- Singh, H.K.; Dhawan, B.N. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi). Ind. J. Pharm. 1997, 29, 359.
- Charles, P.D.; Ambigapathy, G.; Geraldine, P.; et al. Bacopa monniera leaf extract up-regulates tryptophan hydroxylase (TPH2) and serotonin transporter (SERT) expression: implications in memory formation. J. Ethnopharmacol. 2011, 134, 55‒61. DOI: https://doi.org/10.1016/j.jep.2010.11.045
- Charoenphon, N.; Anandsongvit, N.; Kosai, P.; et al. Brahmi (Bacopa monnieri): Up-to-date of memory boosting medicinal plant: A review. Ind. J. Agri. Res. 2016, 50, 1‒7. DOI: https://doi.org/10.18805/ijare.v50i1.8582
- Hou, C.C.; Lin, S.J.; Cheng, J.T.; et al. Bacopaside III, bacopasaponin G, and bacopasides A, B, and C from Bacopa monniera. J. Nat. Prod. 2002, 65, 1759‒1763. DOI: https://doi.org/10.1021/np020238w
- Chakravarty, A.K.; Garai, S.; Masuda, K.; et al. Bacopasides III-V: Three new triterpenoid glycosides from Bacopa monniera. Chem. Pharm. Bull. 2003, 51, 215‒217. DOI: https://doi.org/10.1248/cpb.51.215
- Le, X.T.; Nguyet Pham, H.T.; Van Nguyen, T.; et al. Protective effects of Bacopa monnieri on ischemia-induced cognitive deficits in mice: the possible contribution of bacopaside I and underlying mechanism. J. Ethnopharmacol. 2015, 164, 37‒45. DOI: https://doi.org/10.1016/j.jep.2015.01.041
- Jadiya, P.; Khan, A.; Sammi, S.R.; et al. Anti-Parkinsonian effects of Bacopa monnieri: insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2011, 413, 605‒610. DOI: https://doi.org/10.1016/j.bbrc.2011.09.010
- Piyabhan, P.; Wannasiri, S.; Naowaboot, J. Bacopa monnieri (Brahmi) improved novel object recognition task and increased cerebral vesicular glutamate transporter type 3 in sub-chronic phencyclidine rat model of schizophrenia. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1234‒1242. DOI: https://doi.org/10.1111/1440-1681.12658
- Malishev, R.; Shaham-Niv, S.; Nandi, S.; et al. Bacoside-A, an Indian Traditional-Medicine Substance, Inhibits β-Amyloid Cytotoxicity, Fibrillation, and Membrane Interactions. ACS. Chem. Neurosci. 2017, 8, 884‒891. DOI: https://doi.org/10.1021/acschemneuro.6b00438
- Joshi, H.; Parle, M. Brahmi rasayana improves learning and memory in mice. Evid. Based Complement. Alternat. Med. 2006, 3, 79‒85. DOI: https://doi.org/10.1093/ecam/nek014
- Paulose, C.S.; Chathu, F.; Khan, S.R.; et al. Neuroprotective role of Bacopa monnieri extract in epilepsy and effect of glucose supplementation during hypoxia: glutamate receptor gene expression. Neurochem. Res. 2008, 33, 1663‒1671. DOI: https://doi.org/10.1007/s11064-007-9513-8
- Nobili, F.; Vitali, P.; Canfora, M.; et al. Effects of long-term Donepezil therapy on rCBF of Alzheimer’s patients. Clin. Neurophysiol. 2002, 113, 1241‒1248. DOI: https://doi.org/10.1016/S1388-2457(02)00110-4
- Pandey, R.; Gupta, S.; Tandon, S.; et al. Baccoside A suppresses epileptic-like seizure/convulsion in Caenorhabditis elegans. Seizure 2010, 19, 439‒442. DOI: https://doi.org/10.1016/j.seizure.2010.06.005
- Le, X.T.; Pham, H.T.; Do, P.T.; et al. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem. Res. 2013, 38, 2201‒2215. DOI: https://doi.org/10.1007/s11064-013-1129-6
- Kamkaew, N.; Norman Scholfield, C.; Ingkaninan, K.; et al. Bacopa monnieri increases cerebral blood flow in rat independent of blood pressure. Phytother. Res. 2013, 27, 135‒138. DOI: https://doi.org/10.1002/ptr.4685
- Dwivedi, S.; Nagarajan, R.; Hanif, K.; et al. Standardized Extract of Bacopa monniera Attenuates Okadaic Acid Induced Memory Dysfunction in Rats: Effect on Nrf2 Pathway. Evid. Based Complement. Alternat. Med. 2013, 2013, 294501. DOI: https://doi.org/10.1155/2013/294501
- Malishev, R.; Nandi, S.; Kolusheva, S.; et al. Bacoside-A, an anti-amyloid natural substance, inhibits membrane disruption by the amyloidogenic determinant of prion protein through accelerating fibril formation. Biochim. Biophys. Acta 2016, 1858, 2208‒2214. DOI: https://doi.org/10.1016/j.bbamem.2016.06.019
- Abdul Manap, A.S.; Vijayabalan, S.; Madhavan, P.; et al. Bacopa monnieri, a Neuroprotective Lead in Alzheimer Disease: A Review on Its Properties, Mechanisms of Action, and Preclinical and Clinical Studies. Drug Target Insights 2019, 13, 1177392819866412. DOI: https://doi.org/10.1177/1177392819866412
- Swathi, G.; Rajendra, W. Protective role of Bacopa monnieri on induced Parkinson’s disease with particular reference to catecholamine system. Int. J. Pharm. Sci. 2014, 6, 379‒382.
- Singh, B.; Pandey, S.; Rumman, M.; et al. Neuroprotective effects of Bacopa monnieri in Parkinson’s disease model. Metab. Brain Dis. 2020, 35, 517‒525. DOI: https://doi.org/10.1007/s11011-019-00526-w
- Komali, E.; Venkataramaiah, C.; Rajendra, W. Antiepileptic potential of Bacopa monnieri in the rat brain during PTZ-induced epilepsy with reference to cholinergic system and ATPases. J. Tradit. Complement. Med. 2020, 11, 137‒143. DOI: https://doi.org/10.1016/j.jtcme.2020.02.011
- Mathew, J.; Balakrishnan, S.; Antony, S.; et al. Decreased GABA receptor in the cerebral cortex of epileptic rats: effect of Bacopa monnieri and Bacoside-A. J. Biomed. Sci. 2012, 19, 25. DOI: https://doi.org/10.1186/1423-0127-19-25
- Mathew, J.; Gangadharan, G.; Kuruvilla, K.P.; et al. Behavioral deficit and decreased GABA receptor functional regulation in the hippocampus of epileptic rats: effect of Bacopa monnieri. Neurochem. Res. 2011, 36, 7‒16. DOI: https://doi.org/10.1007/s11064-010-0253-9
- Mathew, J.; Paul, J.; Nandhu, M.S.; et al. Bacopa monnieri and Bacoside-A for ameliorating epilepsy associated behavioral deficits. Fitoterapia 2010, 81, 315‒322. DOI: https://doi.org/10.1016/j.fitote.2009.11.005
- McEwen, B.S. Structural plasticity of the adult brain: how animal models help us understand brain changes in depression and systemic disorders related to depression. Dialogues Clin. Neurosci. 2004, 6, 119‒133. DOI: https://doi.org/10.31887/DCNS.2004.6.2/bmcewen
- Zheng, F.; Luo, Y.; Wang, H. Regulation of brain-derived neurotrophic factor-mediated transcription of the immediate early gene Arc by intracellular calcium and calmodulin. J. Neurosci. Res. 2009, 87, 380‒392. DOI: https://doi.org/10.1002/jnr.21863
- Sukumaran, N.P.; Amalraj, A.; Gopi, S. Neuropharmacological and cognitive effects of Bacopa monnieri (L.) Wettst - A review on its mechanistic aspects. Complement. Ther. Med. 2019, 44, 68‒82. DOI: https://doi.org/10.1016/j.ctim.2019.03.016
- Konar, A.; Shah, N.; Singh, R.; et al. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One 2011, 6, e27265. DOI: https://doi.org/10.1371/journal.pone.0027265
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; et al. Bacopa monnieri and Their Bioactive Compounds Inferred Multi-Target Treatment Strategy for Neurological Diseases: A Cheminformatics and System Pharmacology Approach. Biomolecules 2020, 10, 536. DOI: https://doi.org/10.3390/biom10040536
- Lorca, C.; Mulet, M.; Arévalo-Caro, C.; et al. Plant-derived nootropics and human cognition: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 1‒25.
- Fatima, U.; Roy, S.; Ahmad, S.; et al. Investigating neuroprotective roles of Bacopa monnieri extracts: Mechanistic insights and therapeutic implications. Biomed. Pharmacother. 2022, 153, 113469. DOI: https://doi.org/10.1016/j.biopha.2022.113469
- Vohora, S.B.; Khanna, T.; Athar, M.; et al. Analgesic activity of bacosine, a new triterpene isolated from Bacopa monnieri. Fitoterapia 1997, 68, 361-365.
- Bellah, S.F.; Firoj, A.; Rahman, A.A.; et al. Preliminary phytochemical, anti-bacterial, analgesic, anti-diarrhoeal and cytotoxic activity of methanolic extract of polyalthia suberosa leaves. Int. J. Pharm. Sci. Res. 2012, 3, 1322‒1326.
- Ghosh, T.; Maity, T.K.; Singh, J. Antihyperglycemic activity of bacosine, a triterpene from Bacopa monnieri, in alloxan-induced diabetic rats. Planta. Med. 2011, 77, 804‒808. DOI: https://doi.org/10.1055/s-0030-1250600
- Khan, R.; Krishnakumar, A.; Paulose, C.S. Decreased glutamate receptor binding and NMDA R1 gene expression in hippocampus of pilocarpine-induced epileptic rats: Neuroprotective role of Bacopa monnieri extract. Epilepsy Behav. 2008, 12, 54‒60. DOI: https://doi.org/10.1016/j.yebeh.2007.09.021
- Kumar, E.P.; Elshurafa, A.A.; Elango, K.; et al. Cytotoxic and anti -tumour properties of ethanolic extract of bacopa monnieri (L) penn. Anc. Sci. Life 1998, 17, 228‒234.
- Sabina, E.P.; Baskaran, U.L.; Martin, S.J.; et al. Assessment of antidiabetic activity of the traditional indian ayurvedic formulation brahmi gritham in streptozotocin-induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2014, 6, 11.
- Hazra, S.; Banerjee, R.; Das, B.K.; et al. Evaluation of antidepressant activity of Bacopa monnieri in rat: a study in animal model of depression. Drug Discov. 2012, 2, 8‒3.
- Sairam, K.; Dorababu, M.; Goel, R.K.; et al. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine 2002, 9, 207‒211. DOI: https://doi.org/10.1078/0944-7113-00116
- Hossain, H.; Al-Mansur, A.; Akter, S.; et al. Evaluation of anti-inflammatory activity and total tannin content from the leaves of Bacopa monnieri (Linn.). Int. J. Pharm. Sci. Res. 2014, 5, 1246.
- Singh, V.; Jain, N.; Shri, R. Effect of abiotic factors on bacoside A content, acetylcholinesterase inhibitory and antioxidant activities of Bacopa monnieri (L.) Wettst. J. Applied Pharma. Sci. 2021, 11, 63‒70.
- Aguiar, S.; Borowski, T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013, 16, 313‒326. DOI: https://doi.org/10.1089/rej.2013.1431
- Simpson, T.; Pase, M.; Stough, C. Bacopa monnieri as an Antioxidant Therapy to Reduce Oxidative Stress in the Aging Brain. Evid. Based Complement. Alternat. Med. 2015, 2015, 615384. DOI: https://doi.org/10.1155/2015/615384
- Ghosh, S.; Khanam, R.; Acharya Chowdhury, A. The Evolving Roles of Bacopa monnieri as Potential Anti-Cancer Agent: A Review. Nutr. Cancer 2021, 73, 2166‒2176. DOI: https://doi.org/10.1080/01635581.2020.1841248
- Gupta, G.L.; Sharma, L. Bacopa monnieri abrogates alcohol abstinence-induced anxiety-like behavior by regulating biochemical and Gabra1, Gabra4, Gabra5 gene expression of GABAA receptor signaling pathway in rats. Biomed. Pharmacother. 2019, 111, 1417‒1428. DOI: https://doi.org/10.1016/j.biopha.2019.01.048
- Banerjee, S.; Anand, U.; Ghosh, S.; et al. Bacosides from Bacopa monnieri extract: An overview of the effects on neurological disorders. Phytother. Res. 2021, 35, 5668‒5679. DOI: https://doi.org/10.1002/ptr.7203
- Konar, A.; Gautam, A.; Thakur, M.K. Bacopa monniera (CDRI-08) Upregulates the Expression of Neuronal and Glial Plasticity Markers in the Brain of Scopolamine Induced Amnesic Mice. Evid. Based Complement. Alternat. Med. 2015, 2015, 837012. DOI: https://doi.org/10.1155/2015/837012
- Sabina, E.P.; Peter, S.J.; Prathap, S.; et al. A comparison of hepatoprotective activity of Bacoside to Silymarin treatment against a combined Isoniazid and Rifampin-induced hepatotoxicity in female Wistar rats. J. Histotechnol. 2019, 42, 128‒136. DOI: https://doi.org/10.1080/01478885.2019.1638535
- Channa, S.; Dar, A.; Anjum, S.; et al. Atta-Ur-Rahman. Anti-inflammatory activity of Bacopa monniera in rodents. J. Ethnopharmacol. 2006, 104, 286‒289. DOI: https://doi.org/10.1016/j.jep.2005.10.009
- Khan, A.V.; Ahmed, Q.U.; Shukla, I.; et al. Antibacterial efficacy of Bacopa monnieri leaf extracts against pathogenic bacteria. Asian Biomedicine 2010, 4, 651‒655. DOI: https://doi.org/10.2478/abm-2010-0084
- Fazlul, M.K.K.; Deepthi, S.P.; Irfan, M. Antibacterial and antifungal activity of various extracts of Bacopa monnieri. Int. J. Pharm. Res. 2019, 11, 1698–1702
- Prabhakar, S.; Saraf, M.K.; Pandhi, P.; et al. Bacopa monniera exerts antiamnesic effect on diazepam-induced anterograde amnesia in mice. Psychopharmacology 2008, 200, 27‒37. DOI: https://doi.org/10.1007/s00213-007-1049-8
- Gudipati, T.; Srivastava, P.; Bhadauria, R.; et al. Hepatoprotective potential of in vitro Bacopa monnieri (L.) against carbon tetrachloride-induced hepatotoxicity in albino mice. Int. J. Pharm. Biologic. Sci. 2012, 3, 664‒672.
- Fatima, U.; Roy, S.; Ahmad, S.; et al. Pharmacological attributes of Bacopa monnieri extract: Current updates and clinical manifestation. Front. Nutr. 2022, 9, 972379. DOI: https://doi.org/10.3389/fnut.2022.972379
- Bhattacharya, S.K.; Bhattacharya, A.; Kumar, A.; et al. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother. Res. 2000, 14, 174‒179. DOI: https://doi.org/10.1002/(SICI)1099-1573(200005)14:3<174::AID-PTR624>3.0.CO;2-O






