Published online Feb 27, 2022. doi: 10.4254/wjh.v14.i2.319
Peer-review started: June 18, 2021
First decision: August 18, 2021
Revised: September 10, 2021
Accepted: January 27, 2022
Article in press: January 27, 2022
Published online: February 27, 2022
Inflammatory bowel diseases (IBD) are associated with various hepatobiliary disorders. They can occur at any moment in the course of the disease or associated with the treatment. The prevalence of liver dysfunction can reach up to 50% in different studies. Nonalcoholic fatty liver disease is considered the most common hepatobiliary complication in IBD, while primary sclerosing cholangitis is the most specific. Management of hepatic manifestations in IBD involves a multidisciplinary approach that includes a high index of suspicion and joint management with hepatologists. The medical confrontation with abnormal liver tests must include an exhaustive study to determine if these patterns can be related to IBD, associated diseases or to the therapies used.
Core Tip: Inflammatory bowel diseases are associated with various hepatobiliary disorders. They can occur at any moment in the course of the disease or associated with the treatment. Although hepatic manifestations are known, they are not always searched for in a directed manner. This review article presents the main hepatobiliary manifestations, including those caused by new therapies (biologics and small molecules). Finally, we propose a management algorithm.
- Citation: Núñez F P, Castro F, Mezzano G, Quera R, Diaz D, Castro L. Hepatobiliary manifestations in inflammatory bowel disease: A practical approach. World J Hepatol 2022; 14(2): 319-337
- URL: https://www.wjgnet.com/1948-5182/full/v14/i2/319.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i2.319
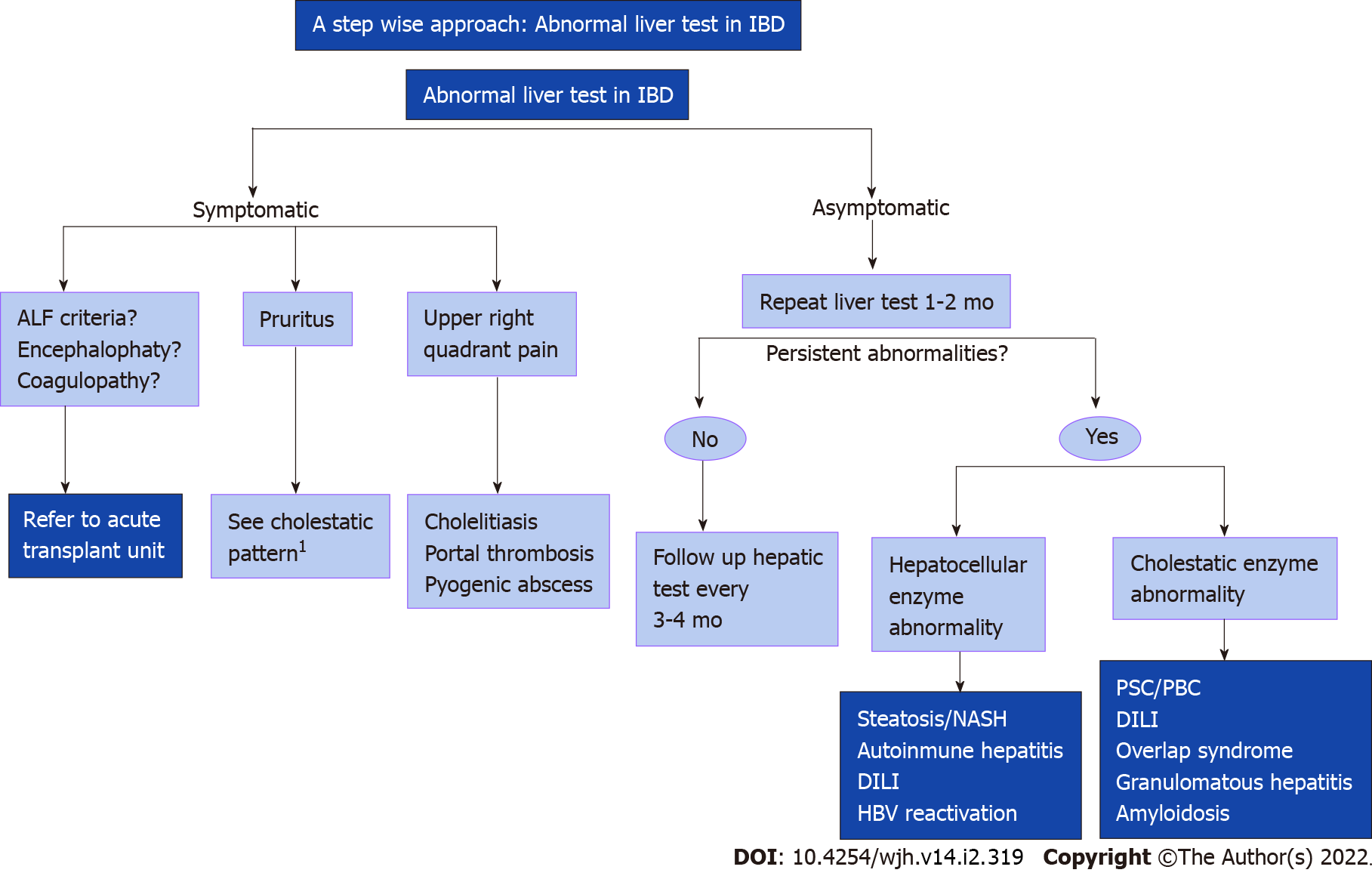
Inflammatory bowel diseases (IBD) are associated with various hepatobiliary disorders. They can occur at any moment in the course of the disease or associated with the treatment. The prevalence of liver dysfunction can reach up to 50% in different studies. Non-alcoholic fatty liver disease (NAFLD) is considered the most common hepatobiliary complication in IBD, while primary sclerosing cholangitis (PSC) is the most specific. Management of hepatic manifestations in IBD involves a multidisciplinary approach that includes a high index of suspicion and joint management with hepatologists. The medical confrontation with abnormal liver tests must include an exhaustive study to determine if these patterns can be related to IBD, associated diseases or to the therapies used (Figure 1).
It is defined as a chronic cholestatic liver disease characterized by intra and/or extra-hepatic bile duct lesions[1]. The incidence in the adult population is 1.1 per 100 thousand inhabitants and the prevalence is between 8.5-13.6 per 100 thousand patients/year[2-4]. PSC is more common in men with a 2:1 ratio and its age of manifestation is usually in the 4th to 5th decade of life. The prevalence of IBD in patients with PSC is 70%-80%, the most common being ulcerative colitis (UC), while only 2%-7% of patients with IBD will develop PSC[5,6]. The prevalence of PSC may increase when using techniques with greater diagnostic sensitivity, improving the survival rate[7]. Lunder et al[8] found 3 times more PSC performing magnetic resonance cholangiopancreatography (MRCP) as screening in all patients with IBD (7.5% vs 2.2%) when comparing only based on the clinical picture or altered liver function tests. The prevalence of PSC among relatives is 100 times higher than that of the general population, this has made it possible to establish, through genomic association studies, the presence of HLA-B* 08 and DR*03 as a risk locus[9]. The pathophysiology of PSC is unknown, nonetheless it is suggested that there is an autoimmune component given its association with the presence of autoantibodies such as: Anti-neutrophil cytoplasmic antibodies (ANCA), anti-nuclear antibodies (ANA) and anti-smooth muscle antibodies (ASMA)[10]. However, this factor has been questioned given that there is a higher prevalence of PSC in men (contrary to most immune-mediated diseases), the limited effectiveness of immunosuppressive drugs, and the lack of a specific autoantibody[10]. One theory is that PSC is a consequence of the sustained inflammatory response as a product of bacterial and viral translocation typical of IBD[8]. These bacterial/viral products would go to the portal system contributing to the exaggerated inflammatory response of the cholangiocyte, which evolves into fibrosis[11,12]. Diagnosis: The most frequent symptoms are abdominal pain, jaundice, itching, and metabolic bone disease, cholangitis and decompensated cirrhosis; however, between 21%-44% of patients may be asymptomatic at the time of diagnosis. Within the laboratory test results, the persistent elevation of alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT) stand out, oftentimes being an incidental finding[13]. The non-invasive evaluation with MRCP allows to optimally evaluate the presence of multifocal segmental stenosis of the intra- and extra-hepatic biliary tree that give the typical beaded appearance; this technique has a sensitivity of 86% and a specificity of 77%, including a sensitivity of 98.9% to detect cholangiocarcinoma (CCA) in this group of patients[14]. Currently, endoscopic retrograde cholangiography has a therapeutic role in cases of suspected dominant biliary tree stenosis. Liver biopsy is used in the event of diagnostic doubts or suspicion of small duct involvement. The classic finding is the presence of “onion skin” periductal fibrosis which leads to ductopenia and cholestasia that can be present in 50% of biopsies as well as the presence of obliterative fibrosing cholangitis, which is found only in 5% of patients[15]. Recently, it was observed that the senescent cholangiocyte (with p16 + immunohistochemical marker) is associated with both clinical and histological severity, so it could represent a new target for prognosis and therapy[16].
UC-PSC: UC represents 80% of IBD cases in patients with PSC, while indeterminate colitis and Crohn disease (CD) constitute the remaining 20%[2]. The clinical course tends to be milder, and the colonic involvement is generally extensive; mild, with greater inflammatory involvement of the ascending colon, mucosa rectal sparing and associated with backwash ileitis[17,18]. Despite having an asymptomatic clinical course, the risk of CRC is significantly higher[19,20].
CD-PSC: Similarly, to UC, the clinical course of CD tends to be more benign, with the predominant phenotype being inflammatory[17]. Rectal involvement is lower compared to UC (20% vs 68%, P = 0.07), with right colonic involvement being more common (50% vs 29% in UC, P = 0.3)[18]. From a prognostic point of view, in an IBD-PSC cohort in England, these diseases association increased risk of death [hazard ratio (HR): 3.2] and CRC (2.4). In that group the CRC was diagnosed a lower median age (59 years vs 69 years without PSC). In patients younger than 40 years at the PSC diagnosis the liver transplantation and PSC-related events were more frequently than in people more than 60 years (75% vs 31%)[21]. Patients with UC had an increased risk of liver disease progression compared with patients with CD (HR: 1.56; P < 0.001) or no IBD (HR: 1.15; P = 0.002)[22].
Small duct disease: Approximately 20%-30% of PSC correspond to small duct involvement. Defined as liver histopathology periductal, concentric fibrosis; fibro-obliterative cholangitis or primary ductular involvement with normal MRCP. Progression towards classic disease is 3%-7% per year[23]. This subtype of the disease is associated with both types of IBD, however there is a higher proportion of CD vs UC (22% vs 6%[24,25]). On the other hand, the prognosis of this group of patients is considerably better than classic PSC, with a greater average liver transplant-free survival. In general, it has a lower risk of developing CCA in comparison with main duct involvement[9].
Immunoglobulin G 4-related sclerosing cholangitis: IBD has been associated with autoimmune pancreatitis (AIP) and immunoglobulin G (IgG)4-related sclerosing cholangitis. This is the biliary manifestation of IgG4-related disease, a systemic fibroinflammatory condition that is characterized by mass lesions and/or strictures with classical histopathological findings in involved organ (salivary glands, retroperitoneum, kidneys, and lymph nodes)[26,27]. It is the most common extrapancreatic manifestation in patients with AIP type 1. Diagnosis is based in histopathological appearances, radiological features, and serological abnormalities. Typically have elevated serum IgG4 levels (> 135 mg/dL) and histopathologic findings (> 10 IgG4-positive plasma cells per high-power field. Diffuse or segmental narrowing of the intrahepatic and/or extrahepatic bile duct associated with thickening of the bile duct wall. Usually presents with obstructive jaundice (70% to 80%), weight loss, and abdominal pain. Age of onset is (50-60)’s, predominately men. 75% PSC-patients have underlying IBD compare to IgG4 cholangitis in which case only 5% develops IBD[28]. IgG4+ plasma cells have been identified in colon tissue from patients with refractory forms of IBD patients. There appears to be association between disease activity and reduced fecal elastase or elevated serum IgG4, although the latter finding was more prevalent in patients with UC[29]. Differential diagnosis with pancreatic or biliary cancers, PSC, secondary sclerosing cholangitis is a challenge. The mainstay of treatment is systemic corticosteroids and most of the time a steroid trail is used to confirm the diagnosis. The response is good in two-thirds of strictures cases, different than PSC patients[30].
Post orthotopic liver transplantation IBD: There are discrepancies regarding the clinical course of post-liver transplant in IBD patients. About 30% may have a more severe course that includes clinical, endoscopic and histological deterioration within 10 years[31]. De novo IBD (that which develops after transplantation) is 10 times more frequent in patients transplanted due to PSC vs the general population, with a risk of 10%-11% at the 5 years mark and 14%-30% at the 10 years mark[31,32]. The use of cyclosporine and azathioprine (AZA) is preferred over tacrolimus given the protective factor during the clinical course[31,32].
It corresponds to a chronic autoimmune cholestatic disease of unknown cause that is histologically presented as a chronic non-suppurative destructive cholangitis[33]. Like all cholestatic diseases, its clinical manifestations range from asymptomatic to itching and fatigue. It is characterized by the presence of anti-mitochondrial antibodies and alteration of GGT and ALP levels[33]. Although its association with several autoimmune diseases such as: Sicca, Sjögren, among others, is known, its association with IBD is anecdotal. Liberal et al[34], described a series of 151 patients with primary biliary cirrhosis (PBC), of which only 6 had concomitant IBD. In all cases, PBC was diagnosed delayed from the IBD. Its association was similar with both UC and CD and its average age of diagnosis was the 5th decade of life. Although it is an infrequent association, it is important to keep it in mind since the use of ursodeoxycholic acid (UDCA) allows normalization of liver function tests and impacts the prognosis of the liver disease.
Autoimmune hepatitis (AIH) has the classic autoimmune disease behavior, being more frequent in women, with positive antibodies and responding to immunosuppressive therapy[35]. The clinical spectrum of autoimmune hepatitis ranges from asymptomatic elevation of liver function tests, passing through acute on chronic liver failure to cirrhosis. There are two types of AIH based in serological autoantibodies. Type 1 is characterized by ANA and/or SMA/anti-actin antibodies. Atypical pANCA-positive is more frequent in type 1 rather than type 2. Type 1 AIH affect mostly adults, have chronic presentation usually, hypergammaglobulinemia and other concomitant disease are autoimmune thyroiditis, rheumatological diseases and inflammatory back pain (IBP). It could present as autoinmmune sclerosing cholangitis (ASC) in children and PSC overlap in adults and remission after drug withdrawal is possible. Type 2 AIH is characterized by antibodies to liver kidney microsome type 1 (anti-LKM1), usually in the absence of ANA and SMA. Affect frequently children under 14 years, with acute onset at presentation. The most common concomitant diseases are autoimmune thyroiditis, diabetes mellitus and vitiligo and rarely presents as ASC or PSC in children and adults respectively. Usually need long term immunosuppressive therapy[36]. The diagnosis consists of the combination of epidemiological factors, serology with antinuclear and ASMA and liver biopsy, which is mandatory for diagnostic and prognostic purposes. The most frequent histological findings are the presence of lobular involvement, plasma cell infiltrate, involvement of the limiting lamina and pseudo rosette formation[37]. Immunosuppressive therapy is the mainstay of treatment, being corticosteroid and thiopurine association the first line of treatment. In resistant cases, drugs such as mycophenolate, calcineurin inhibitors and even anti-tumor necrosis factor (TNF)-α can be used[37]. Its association with IBD is of low prevalence, the most frequent being AIH with UC and to a lesser extent with CD or non-classifiable IBD[38,39]. In the most significant study, out of 105 patients with AIH, only 17 had findings suggestive of UC. Clinically, patients with AIH and IBD, mostly UC, developed AIH at younger age, had a lower remission rate, a higher rate of treatment failure, and progressed more to cirrhosis[40,41].
It seems logical to raise overlaps when clinical, analytical, and imaging findings are shared or overlapped. This way, the presence of PSC/AIH overlap can be considered when there is a MRCP that shows typical findings of PSC but with AIH compatible antibodies with an elevated IgG and a histological finding of interface hepatitis. In the same way, AIH/PSC overlap can be considered when the diagnosis of AIH coincides with pruritus, elevated ALP and GGT, and alterations in the MRCP. The overlap between AIH and PBC is the most frequent in the general population, the Paris criteria are used to guide the diagnosis[42]. There is also an overlap between AIH and PSC and less frequently PBC and PSC[43]. In IBD, there are no established diagnostic criteria, which makes it even more difficult to establish the real incidence of this entity; on the other hand, its appearance is usually sequential over the years[44,9]. The prevalence of this entity is lower than PSC alone and it is more commonly diagnosed in the pediatric and adolescent population[9,45]. Given the autoimmune nature of the disease associated with cholestasia, the concomitant use of immunosuppressive therapy (corticosteroids and thiopurines) and UDCA is recommended, however, there are no randomized studies that support this strategy. The clinical evolution of these patients seems to be similar to those of PSC/IBD without significant differences in its behavior in the few published reports[46].
This is a form of SC with strong autoimmune features with overlap with AIH, virtually all patients have raised IgG levels and autoimmune liver serology identical to AIH type 1, being ANA and/or SMA positive[47]. It was originally described in pediatric patients. In the initial report the patients were mostly men with concomitant IBD[48]. The largest prospective study was done in King’s College Hospital with 55 pediatric patients with definite or probable AIH diagnosis accord to International Autoimmune Hepatitis Group. In that cohort, half of patients with anormal MRCP were classified as ASC, of those 44% had IBD compared to 18% of patients with HAI. In the retrospective largest cohort including 718 patients with PSC, 33% had concomitant AIH[49]. ASC disease in equally frequently in women and men and possession of HLA DRB1*1301 is associated to ASC, while possession of DRB1*0301 confers susceptibility to AIH type 1 and of DRB1*0701 to AIH type 2[50]. Histological differences between AIH and ASC patients included a higher median inflammatory activity index in AIH compared to ASC, and a higher frequency of cholangitis in ASC, but they are quite similar, and the final diagnosis is based in MRCP anormal findings. A clear diagnosis criterion for ASC is lacking. There are no randomized controlled treatment trials for ASC and these patients are treated with therapy based in UDCA and prednisolone ± AZA. In follow up MRCP, disease showed progression in half of ASC with an estimated 10 years transplant-free survival of 65%[51]. In a prospective study in ASC and AIH disease biochemical remission is similar, but the liver transplant rate was higher in ASC than in AIH in a 13 years period[52]. Seems like AIH and ASC are different disease based in different gender distribution, HLA association, IBD association and outcomes. There is some concern whether adult PSC is a late-stage phenotype of ASC and it is necessary long term follow up period to clarify this[47]. There are data suggesting progression of liver disease and post-liver transplantation recurrence of both AIH and ASC are associated with poorly controlled IBD[52,53]. Some studies demonstrated lower PSC recurrence post liver transplantation in patients with colectomy after surgery[54].
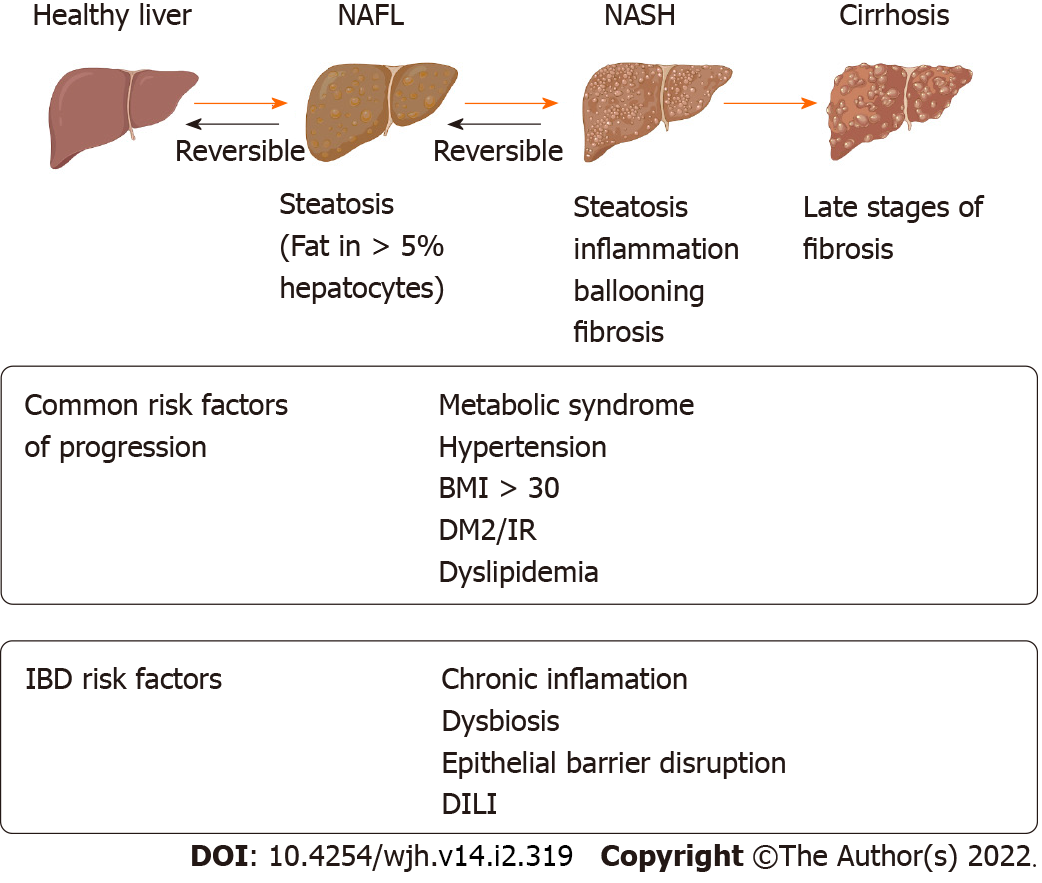
It is characterized by fat storage in > 5% of hepatocytes. Its development is directly related to obesity, insulin resistance and metabolic syndrome (MS), being currently considered the hepatic manifestation of MS. The clinical and histological spectrum is broad, from simple steatosis to steatohepatitis with inflammation nonalcoholic steatohepatitis (NASH), progression into fibrosis, liver cirrhosis, and hepatocellular carcinoma. The diagnosis requires the exclusion of secondary causes, such as daily alcohol intake (> 30 g/men and > 20 g/women) and the use of steatogenic drugs[54]. Demonstration of fat infiltration, either through histology (biopsy) or imaging, is required for diagnosis. Ultrasonography (US) is the most widely used technique, with a sensitivity of 85% [95% confidence interval (CI): 79.5%-88.9%] and a specificity of 94% (95%CI: 87.2%-97%) for the diagnosis of NAFLD[55]. Current data suggests an increase in the prevalence of NAFLD, currently estimated to be 25% globally (95%CI: 10-22-28). It is the leading cause of chronic liver disease in the western world and a growing cause of liver transplantation worldwide[54,56,57]. In IBD patients, it appears to be at least similar to or greater than in the general population and is currently considered the most frequent hepatobiliary manifestation in these patients[54]. The reports are varied with a prevalence ranging between 8%-71%[1]. This heterogeneity depends on the sample size, diagnostic criteria, and design used in the various studies, but also on the origin of the population studied, the year of study, and probably the change in pharmacological therapies in recent decades. A recent meta-analysis that included 19 studies (n = 5620 subjects), in which the diagnosis of NAFLD was based on imaging techniques, liver biopsy or transient liver elastography, reported a prevalence of NAFLD of 27.5% in patients with IBD (95%CI: 20.7%-34.2%)[56], quite similar to that of the general population[2]. The cumulative prevalence was higher in recent studies (2016 to 2018) compared to the cumulative prevalence of previous studies [(33.0%, 95%CI: 22.0%-44.1%) vs (21.3%, 95%CI: 13.1%-29.5%); P = 0.09], which may be related to the increase in obesity and MS in recent years. In turn, in studies of IBD patients with an 18-year follow-up, it showed that 48% of patients with CD and 44% of patients with UC presented NAFLD diagnosis by US. The presence of NAFLD was associated with older age, hypertension, and higher body mass index (BMI) in both groups[58]. Using transient liver elastography, it was observed that 32.8% of patients with IBD met NAFLD criteria and even 12.2% of patients already had liver fibrosis at the time of the study[59]. In patients with IBD, a higher prevalence of NAFLD has been observed in older patients [mean difference (MD) = 8.22; 95%CI: 6.22-10.22], with history of type 2 diabetes mellitus [odd ratio (OR) = 3.85; 95%CI: 2.49-5.95], hypertension (OR = 3.18; 95%CI: 2.36-4.28), obesity (OR = 2.79; 95%CI: 1.73-4.50), insulin resistance (OR = 6.66; 95%CI: 1.28-34.77) , MS (OR = 4.96; 95%CI: 3.05-8.05), chronic kidney disease (OR = 4.83; 95%CI: 1.79-13.04), methotrexate (MTX) treatment (OR = 1.76; 95%CI: 1.02-3.06), history of intestinal surgery (OR = 1.28; 95%CI: 1.02-1.62) and duration of IBD (MD = 5.60; 95%CI: 2.24-8.97)[60]. A recent retrospective study showed that the presence of clinical activity (Harvey Bradshaw Index > 4), history of intestinal resection, endoscopic activity, and the use of AZA would be risk factors with statistical association for NAFLD in patients with CD. In the case of UC, there was an association between NAFLD and endoscopic activity[61]. MS appears not to be the only triggering factor for NAFLD in patients with IBD. The prevalence of MS in patients with coexisting IBD-NAFLD could be lower than in NAFLD patients without IBD[62,63], thus the prevalence of these risk factors such as obesity, hypertension, dyslipidemia and type 2 diabetes was significantly lower in those patients with coexistence of IBD-NAFLD compared with the group of patients who only had NAFLD[64]. Another study that included 232 patients (78 patients with NAFLD-IBD, 154 patients with NAFLD only) showed that the patients with NAFLD-IBD were younger compared to the group of NAFLD patients without IBD, had lower body weight, smaller abdominal circumference and lower prevalence of MS (23.1% vs 56.6% respectively, P < 0.001)[65]. In patients with IBD-NAFLD, the severity of IBD was associated with greater severity of hepatic steatosis as measured by abdominal US[65]. These findings suggest that patients with IBD develop fatty liver disease with fewer metabolic risk factors than the population without IBD and that the severity of IBD could influence the degree of steatosis. This raises the existence of other factors, outside of metabolic ones, that could play a role in the coexistence of both diseases. The degree of chronic inflammation, the role of intestinal barrier disruption, increased intestinal permeability, microbial dysbiosis, immune activation, and drug-induced hepatotoxicity are factors that should be evaluated in directed studies[66]. Thus, the risk factors for NAFLD in IBD could be divided into those related to MS-obesity and those related to IBD itself in Figure 2. The actual prognostic impact of the NAFLD-IBD association is unclear. Steroids, especially higher doses, and longer duration, and immunomodulators used to treat IBD may increase the risk of progression to NAFLD. They increased weight gain and metabolic parameters, although there is no evidence that medications alone are responsible. TNF-α inhibitors could have a protecting roll in IBD patients from developing NAFLD. A systematic review was carried out through October 2017, this did not demonstrate a significant association between medication treatment in IBD and the risk of developed NAFLD. This suggests a complex, multifactorial relationship between IBD and NAFLD[67]. The coexistence of NAFLD-IBD poses a challenge in the management strategies of these patients. The presence of NAFLD and mainly the presence of NASH, can increase the risk drug induced liver injury (DILI), limiting the use of certain immunosuppressive therapies. It has been observed that in patients with IBD on immunosuppressive therapy, those who displayed an elevation of aminotransferases levels had a higher prevalence of NAFLD[68]. Thus, NAFLD could represent a risk factor in patients who require immunosuppressive drugs with hepatotoxic potential. A two-fold increase in mortality was reported in hospitalized patients with IBD and concomitant chronic liver disease (mainly cirrhosis due to NAFLD) compared to those without liver disease (2.7% vs 1.3%, P < 0.01)[69], which suggests that in patients with IBD and risk factors, the existence of NAFLD should be actively sought in order to plan a therapeutic strategy and rule out other differential diagnosis. Treatment of NAFLD should be based on managing metabolic risk factors and lifestyle changes. The objective is to achieve a weight reduction of at least 7%, which has been associated with biochemical and histological improvement[19,69]. Currently there are no specific recommendations for the management of NAFLD in patients with IBD. Control of metabolic risk factors and maintenance of IBD remission should be emphasized. Screening, prevention, and early treatment of NAFLD should be part of the comprehensive management of patients with IBD, especially those with risk factors.
Given the new therapeutic options in IBD, there is a risk that these patients will develop DILI during the management of their disease, requiring a timely evaluation by an hepatologist in case of suspected hepatotoxicity[70]. DILI in a patient with IBD may manifest with elevated alanine aminotransferase (ALT) and aspartate aminotransferase (hepatocellular pattern); ALP and GGT (cholestasis pattern), jaundice (hyperbilirubinemia) or a mixed pattern. This complication can occur acutely, with the development of acute liver failure, autoimmune hepatitis, and reactivation of hepatitis B, and a percentage of these patients may develop chronic damage[71]. Therapies with 5-aminosalicylates, as well as immunomodulators, can cause alterations in liver function tests, these have been widely described in the literature, they are summarized in Table 1[72-78].
| Drug | Prevention | Hepatic injury | Treatment |
| 5-Amino salicilyc acid | Check before start treatment annual check | Drug induced hepatitis; Drug induced cholestasic; Granuloma formation (sulfa) | Stop drug; Follow-up |
| Tiopurines (azathioprine/6MP) | Check before treatment: TPMT and liver test; Check every week in first month, withing 2 wk in 2nd mo, every 3 mo | Drug induced hepatitis; Drug induced cholestasic; Sinusoidal obstruction syndrome; Nodular regenerative hyperplasia; Peliosis hepatitis | Drug induced hepatitis and cholestasic are idyosincratic reactions; More cases in the first three months of treatment; Stop drug |
| Methotrexate | Check before start treatment; Check every 2 wk until 2nd mo; Check every 3 mo; Add folic acid | Fibrosis/cirrhosis; Steatohepatitis | Stop MTX if ALT > 3 times; Despite alcohol; Comsume; Fibroscan |
Sulfasalazine is an anti-inflammatory medication consisting of both 5-aminosalycilic acid and sulfapyridine. The last molecule causes more frequently toxicity, with a characteristic hypersensitivity reaction with hepatitis and withdrawal and steroids therapy could be required[79]. Other clinical manifestations are granulomatous hepatitis, cholestatic liver disease, and rarely acute liver failure. In a study included 4.7 million prescription, acute hepatitis occurred in only 9 patients[80]. Mesalamine is more commonly used in IBD patients and DILI associated is very low, 0%-4% of patients on this drug[81].
Thiopurines, AZA and mercaptopurine (MP), are commonly used in IBD patients and have proven efficacy in maintenance remission. They are used as monotherapy or associated to biological therapy. AZA is transformed into 6MP via glutathione depending process. 6MP undergoes complex three enzymatic transformations to 6-thioguanine nucleotide (6-TGN), the active metabolite of thiopurine drugs. This can take alternative two other pathways, being converted to 6 thiouric acid or being metabolized to 6-methyl mercaptopurine (6MMP) by thiopurine methyltransferase (TPMT). This enzyme has an important role in the balance between 6-TGN and 6MMP. In IBD patients data support 6-TGN and 6MMP to improve clinical response and safety profile of thiopurines respectively. 6MMP is an inactive and potentially hepatotoxic metabolite. The therapeutic use of these drugs may be limited by dose-related or idiosyncratic adverse effects[72]. A subgroup of patients, “hyperme-thylators”, 30%, preferentially produced 6MMP instead of 6-TGN producing treatment resistance and risk of hepatotoxicity. In this group, hepatotoxicity can be reversed by reducing the AZA dose by 75 % and adding xanthine oxidase inhibitors (allopurinol). The steady-state thiopurine metabolite concentrations are generally reached after approximately 4-8 wk of therapy to be measured. Blood levels of 6MMP > 5700 pmol/(8 × 10 red blood cell) are associated with a threefold hepatotoxicity risk[82] but toxicity has also been observed in patients with low 6MMP concentrations. Several factors, such as smoking, obesity, ethnicity, and genetic factors, may influence the response to thiopurine therapy. NAFLD is a risk factor for the development of hepatotoxicity in IBD patients on thiopurines[83]. A multivariable analysis determinate early predictor for the development of hepatotoxicity in patients on stable thiopurine dose demonstrated increased risk with older people (> 50 years), BMI (> 25), gender (male) and 6MMP level > 3615 pmol 1 wk after treatment initiation[84]. These drugs have been reported to induce liver injury in up to 17% of patients[72]. Although the incidence varies depending of the hepatotoxicity definition in different studies. In a study hepatoxicity was define as ALT twice the upper limit normal and the incidence was 2.6% per patient-year[85]. There are three types of liver injury: (1) Hypersensitivity reaction; (2) Acute cholestatic or hepatitis pattern mostly idiosyncratic; and (3) Long term dose dependent endothelial injury involving sinusoidal dilatation, peliosis of the liver, sinusoidal obstruction syndrome and regenerative nodular hyperplasia with portal hypertension as manifestation[86]. Mostly cases the severity is mild and return to normal values even without drug dose modifications. Thiopurine withdrawal could be necessary in less than 4%, when severe cholestasis jaundice, moderate/severe injury without improvement after 50% dose reduction or development of endothelial chronic injuries[85].
MTX is an antagonist of the dihydrofolate reductase enzyme. The initial studies determinate hepatotoxicity risk was accumulative doses dependent but lately a meta-analysis showed that there is no association between the cumulative dose of methotrexate and the development of liver damage[86]. In a study about MTX for IBD, there was a 14.3% incidence of hepatotoxicity (defined as ALT or GGT > 1.5 ULN) that occurred after a median latency of 26 mo. Adverse events were more frequently seen in patients who were not taking concomitant folic acid supplementation, so it is recommended[87]. In a study with 518 patients treated with MTX, 44 patients (8.5%) had FibroScan and/or FibroTest results suggesting severe liver fibrosis. In a multivariate analysis, factors associated with this were the BMI > 28 kg/m2 and high alcohol consumption[79]. So, it is a very important practice to modified these factors in this population of IBD with MTX treatment. Every time an increased in liver labs occurs in patients been treated with MTX, is necessary to rule out other diagnosis. Today we have tools for assessment in a non-invasive way liver fibrosis. Transient elastography allows to evaluate with a good accuracy[88].
Corticosteroids are considered safe drugs regarding to hepatotoxicity. Only one case of effervescent prednisolone form induced hepatitis has been reported associated with IBD[89]. Another new case was reported with use of prednisolone recently. It contains sodic metasulfobenzoate and there is some concern whether itself is the cause of hepatotoxicity[90]. Generally, we assumed steroids are rarely cause of DILI in this setting of patients. We don´t have to forget that steroids could induce or deteriorate NAFLD[91] and could also reactivate viral hepatitis with prolonged use[40].
Anti-TNF: Anti-TNF [infliximab (IFX), adalimumab (ADA), golimumab and certolizumab], these agents have been associated with various adverse events, including alterations in liver function tests[91], with a prevalence of 2.5% (free ammonia > 2.5 times the upper limit of normal) and 16% (ALT > 3 times the upper normal limit) respectively[2]. The latter, generally mild and transient, occurs more frequently after the 2nd to 5th infusion of IFX. Long-standing IBD, use of IFX as monotherapy, increased BMI, and hepatic steatosis have been some of the observed risk factors[92]. Less frequently, cases of autoimmune hepatitis have been described, with the switch to ADA being possible since it is not a class effect[93]. The effects on liver function tests with ADA, golimumab and certolizumab are less frequent[94]. Its discontinuation has been suggested in case of transaminase elevations > 3 times the normal value[95]. Liver function tests should be evaluated at the start of any anti-TNF agent therapy and then routinely monitored every 4 mo[95].
Anti-integrins: Anti-integrins are humanized monoclonal antibodies that block the adhesion and migration of leukocytes from blood vessels to inflamed tissue. DILI due to vedolizumab is uncommon and subsides after the suspension of the biological drug[96,97]. Varies from asymptomatic elevation of transaminase levels to symptomatic hepatocellular or cholestatic involvement[98].
Anti-interleukin 12/23: Ustekinumab is a fully humanized G1 immunoglobulin that binds to the p40 subunit of interleukin (IL)-12/23, which has been shown to be effective in inducing and maintaining remission in patients with CD and moderate to severe UC[99]. The pivotal studies in CD (UNITI-1, UNITI-2 and IM-UNITI) and UC (UNIFI) did not demonstrate an increased risk of hepatotoxicity[100,101], which has been confirmed in subsequent studies[102]. Even though there is no formal recommendation for follow-up, monitoring of liver function tests every 6 mo is suggested[103].
Small molecules Janus-Kinases: Tofacitinib, an inhibitor of type 3, 1 and, to a lesser extent, type 2 Janus-kinases, and tyrosine kinase, has been shown to be effective in inducing and maintaining remission in patients with moderate to severe UC[99]. Subsequent studies have shown no risk of liver damage in patients treated with tofacitinib when compared with placebo[104]. It also seems prudent to monitor liver function tests every 6 mo.
Sphingosine 1-phosphate receptors: Ozanimod a small molecule selective agonist against phosphate-1-sphingosine type 1 and 5 receptors that prevents lymphocyte trafficking to the intestine has recently been approved for moderate-to-severe active UC[105]. The increase in GGT was seen in 5.3% of the patients[106]. Studies carried out on the real world should confirm the safety of this drug.
The combination of two biological agents or one biological with a small molecule aims to produce a synergic effect, thus increasing the probability of achieving remission of intestinal inflammatory activity and of extraintestinal manifestations. Recently, a study with high-risk IBD, refractory to biological therapy and small molecules, demonstrated that a combined strategy does not increase the risk of adverse events (including liver damage)[107]. Will be necessary new studies to confirm it.
Like other immunosuppressive therapies (including the use of corticosteroids in high doses or for prolonged periods of time), anti-TNF, anti-integrin therapy has been associated with hepatitis B virus (HBV) reactivation, especially in patients with hepatitis B surface antigen (HBsAg) positive[107,108]. It is for this reason that every patient must be tested with HBsAg, hepatitis B surface antibody (HBsAb) and the total HBcAb before starting the biological agent, being necessary prophylactic therapy in case of presenting a positive surface antigen[109]. To date, there is no information on cases of HBV reactivation in IBD patients treated with ustekinumab. In the case of tofacitinib, reactivations of the HBV have been observed in patients with rheumatoid arthritis[110], so it is prudent to carry out a control before starting therapy.
The prevalence of hepatitis C virus (HCV) infection in IBD patients seems to be lower than expected, similar to the general population. These results indicate that IBD patients in western European countries should no longer be considered as a risk group for HBV or HCV infection. Numerous case reports indicate that anti-TNF-α therapy in the setting of HCV appears to be safe. However, the long-term effect of anti-TNF-α agents on HCV is not. In particular, while the use of anti-TNFα in non-cirrhotic patients appears safe, it is contraindicated in patients with decompensated cirrhosis[111]. On the other hand, anti-TNF-α drugs seem to reduce inflammation through TNF-α inhibition, playing a role in the pathogenesis of HCV[112]. There are few and small HCV reactivation studies, the HCV reactivation was 8/51 (15.7%) and in 1/10 (10%) HCV RNA positive patients, respectively[113,114]. All cases of reactivation had a very mild course, except for one patient, who died. No recommendations have been proposed for HCV screening prior to starting immunomodulators. However, HCV antibody screening should be routinely performed upon the completion of liver function tests before starting immunosuppressive therapy[115].
Development of PSC in IBD patients had increased risks of CCA (HR, 28.46), hepatocellular carcinoma (HR, 21.00), pancreatic cancer (HR, 5.26), and gallbladder cancer (HR, 9.19)[2]. Patients with PSC with or without IBD are also at increased risk of primary hepatobiliary neoplasia and CCA. Although IBD may be a risk factor for CCA, likely via PSC, it is not clear that IBD confers any added risk for CCA in PSC patients[116]. The lifetime CCA incidence in PSC patients is between 5%-10%, affecting people in the fourth decade of life. It is usually a perihiliar neoplasia (75%) and fibrosis is not necessary for its development. Most of the tumors are diagnosis in the first four years after PSC diagnosis, being more than 50% in the first year since the diagnosis. These are frequently detected in its advanced stages when prognosis is poor[117,118]. The mortality in IBD-PSC patients is higher than IBD patients without PSC, being malignance the main factor. CCA have reduced survival compared to CRC. The survival curve in patients with CRC was similar to the PSC-IBD without CRC or CCA probably related to periodic colonoscopy surveillance that allow early CRC diagnosis[117]. Well known CRC surveillance annually recommendations are established but in the field of CCA there is lack of robust evidence in PSC population surveillance. Although most of clinicians ask for MRCP and CA19-9 marker annually it’s well known the limited specificity in the setting of PSC and the difficulty in image diagnosis in early stages. Risk factors associated with CCA among patients with PSC-IBD patients are not well known and are traying to be find to stratify patients. One recently study of the large cohort showed, in a multivariable model, that duration of IBD was the only independent predictor of increase risk of CCA, with a 33% increase risk per 10 years of IBD. And in the subset patients with colectomy when the indication of surgery was CRC or dysplasia the risk was increased compare those with colectomy for refractory disease (HR, 2.68, 95%CI: 1.01-7.07) after adjustment for disease duration[118]. The pathobiological mechanisms underlying are not clear but the altered bile acids and microbiome environment that exists in IBD may persist after colectomy[119,120]. It seems as colectomy does not modify the increased risk of CCA associated with prolonged IBD, persisting the risk after colectomy. So, in these setting of patients, specific surveillance may be appropriate.
It is a frequent pathology in patients with IBD. In the study by Fousekis et al[58], cholelithiasis was the second hepato-biliary manifestation. Patients with CD have double the risk of developing cholelithiasis in relation to control subjects, while UC show no differences with the control population[121,122]. A prevalence of 11%-34% has been estimated in CD[28]. The underlying pathophysiological mechanism appears to be multifactorial. Patients with CD present gallbladder hypomotility with decreased emptying and gallbladder ectasia[123]. The involvement of the ileum would lead to a reduction in the reabsorption of bile salts, with the consequent alteration in the enterohepatic circulation and secondarily an increase in biliary cholesterol saturation[9]. The risk would also be related to the degree and extent of ileo-caecal involvement and the number of intestinal resections[121,124]. Ileocolonic involvement, with more than 15 years of disease, with frequent outbreaks (> 3), prolonged hospital stays or a number greater than 3 hospitalizations, according to the number of intestinal surgeries or ileal resection (> 30 cm) and total parenteral nutrition requirements have been reported as a risk in CD[121].
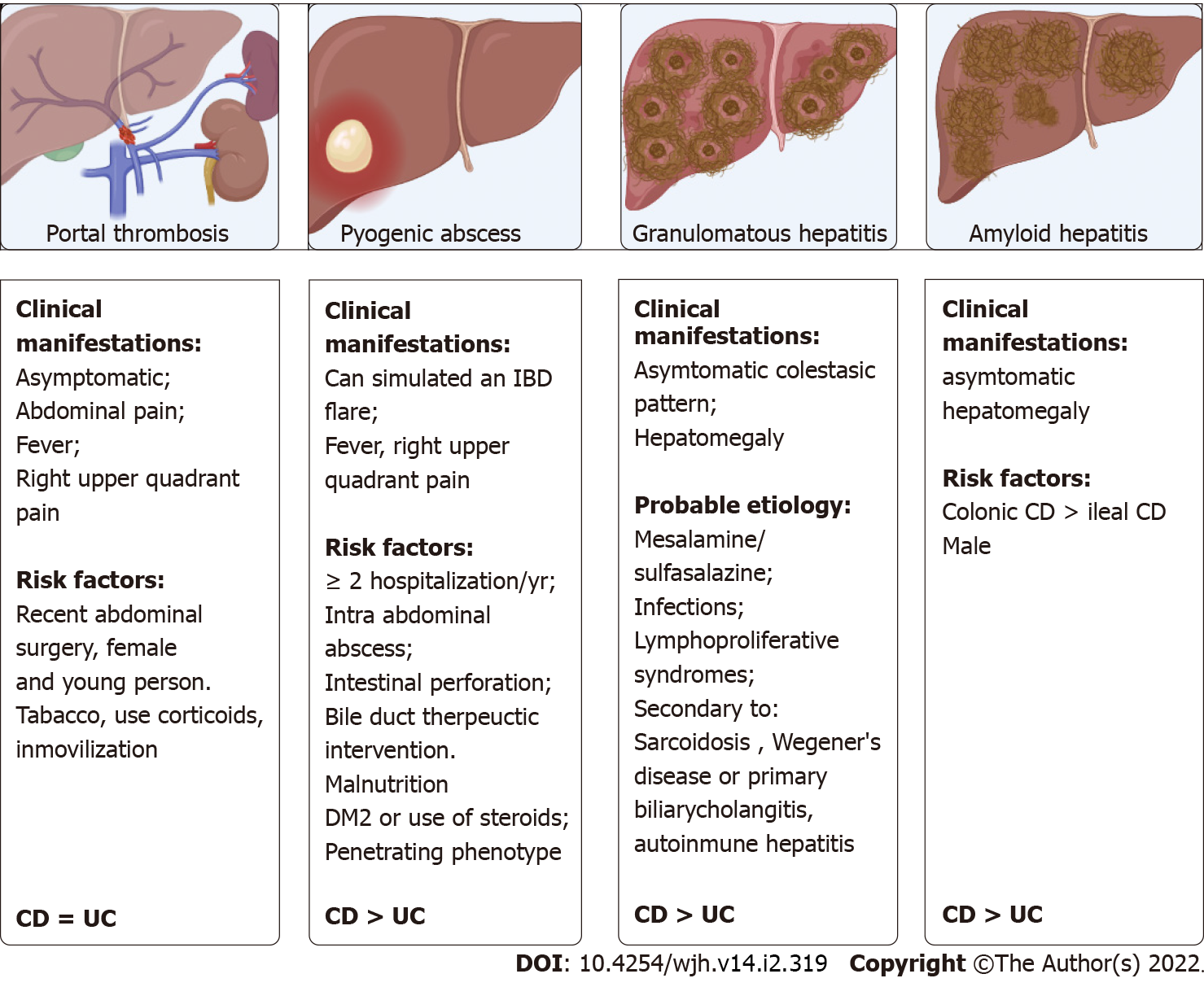
Thromboembolic events are more frequent than in the general population[125]. Prevalence studies indicate that 1.3% of patients can present it, with a mortality of 50%[126]. Porto-mesenteric axis thrombosis is a rare form of venous thrombosis, reporting a prevalence of 0.1%-1.7% in patients with IBD, being found in the postoperative period of digestive surgery or in an imaging study[127-129]. A retrospective multicenter study reported that up to 40% of patients with IBD and porto-mesenteric thrombosis may present an associated prothrombotic factor, the most frequent being hyperhomocysteinemia due to folate and vitamin B12 deficiency[130]. Additionally, patients with IBD may have an imbalance between coagulation factors, with a decrease in the level of antithrombin III and an elevation of factors V-VIII, of the platelet count and of the fibrinogen level, which can finally lead to a prothrombotic state. IBD may itself be a risk factor for thromboembolism[131]. The clinical manifestations and risk factors are summarized in Figure 3[28,130]. The European Crohn's and Colitis Organisation guidelines recommend measures to prevent thromboembolic events during hospitalization or during exacerbation of IBD[132]. In the presence of porto-mesenteric thrombosis, evaluation of acquired and hereditary prothrombotic conditions and early anticoagulant treatment are recommended[132]. Portal hypertension non-cirrhotic intrahepatic portal hypertension (NCIPH) is associated to Schistosomiasis; toxins/drugs (arsenic, vitamin A, AZA, 6-thioguanine), immune disorders (Felty’ syndrome, common variable immunodeficiency disorder) and myeloproliferative syndromes. More recent evidence of associated gut disorders has been described. In a retrospective cohort of NCIPH, three (9%) patients had UC while five of 31 (16%) tested had celiac disease[133]. Microvascular damage described induced by thiopurines are veno-occlusive disease, peliosis hepatis, perisinusoidal fibrosis and nodular regenerative hyperplasia (NRH). This is an uncommon condition characterized by the diffuse transformation of normal hepatic parenchyma into small, regenerative nodules with little to no fibrosis. Vascular flow impairment induces diffuse hepatocyte hyperplasia and nodule formation[134]. The mechanisms of NRH in patients with IBD include immunological and thrombotic factors in addition to external factors such as AZA. The uncontrolled inflammatory response itself in patients with IBD stimulates these factors, which also could cause NRH[135]. The largest case series describing NRH in IBD patients reported a total of 37 cases, between 1994 and 2005, in 11 hospitals. The cumulative risk of NRH could be estimated from the experience in one of center as 0.50% at five years and 1.25% at 10 years and in the multivariate analysis was associated to male sex, stricturing behavior IBD patients treated with AZA[136]. NRH may be detected using biopsies or magnetic resonance imaging (MRI). Recently MRI was proponed as an alternative diagnostic test but the sensitivity and specificity were relatively low[136]. NRH is most often diagnosed after there is evidence of portal hypertension. A low platelet count may be the earliest manifestation of NRH to consider in long-term thiopurine therapy. Generally, the prognosis of NRH is better than that of chronic liver disease and is related to the complications of portal hypertension and the severity of the underlying disease. NRH is probably not reversible, even after stopping the treatment with AZA[134].
They are a rare complication of IBD, with a reported inci-dence greater than the general population (6.72 vs 4.06 per 10000 person-years; spontaneously hypertensive rats = 1.46 (95%CI: 1.01-2.12)[137]. Most of the cases described are in patients with CD[138]. The clinical manifestations and risk factors are summarized in Figure 3[139,140]. The most frequent etiological agents are Streptococcus milleri and Gram-negative anaerobic bacilli[138,141], with a mortality rate close to 38%, with worse results when the diagnosis is late or when there are multiple abscesses of biliary origin[142]. Management does not differ from management in the general population and involves broad spectrum intravenous antibiotics for prolonged periods of time and when necessary, percutaneous, or surgical drainage.
Granulomatous hepatitis is also an infrequent manifestation in patients with IBD, being mainly described in CD. A prevalence of less than 1% has been reported and is characterized by the presence of non-calcified hepatic granulomas demonstrated in the histological study (liver biopsy)[41]. The clinical manifestations and probable etiologies are summarized in Figure 3[143,144]. With a good prognosis, it would improve with the control of the causative agent, such as mesalamine suspension[145].
Secondary amyloidosis consists in the storage of insoluble protein fragments, called amyloid, in various organs. This pathology is infrequent in patients with IBD, with a prevalence of 0.5%, being more frequent in CD with a prevalence that varies between 0.9% and 3%[133]. The clinical manifestations and risk factors are summarized in Figure 3[146]. Treatment is based on controlling intestinal inflammation, thus reducing the release of acute phase reactants, such as amyloid A. Resolution of amyloidosis has been reported after resection of the compromised intestine[147].
Undoubtedly, a multidisciplinary management allows a timely diagnosis of hepatobiliary manifestations that are frequent in both CD and UC (summarized in Table 2). Its diagnosis has prognostic implications, given the risk of progressing to chronic liver disease and its possible association with neoplastic diseases. In regard to new therapies, although they have been classified as safe, there is a risk of alterations in liver function tests, being more frequent with anti-TNF biological agents. However, in all these, either small molecule or biological therapies, it is advisable to carry out a control of liver function tests prior to starting treatment and sequentially according to the type of therapy used, and a HBV screen to avoid risks of reactivation.
| Type of manifestation | Diagnostic | Treatment |
| Cholestasis | ||
| PSC | MRCP | UDCA |
| SDD | Liver biopsy | UDCA |
| IgG4 cholangitis | MRCP + liver biopsy + Ig4 subclass in blood | Glucocorticoids |
| PBC | AMA serology or liver biopsy in some cases | UDCA |
| DILI | Approach based in ruling out others diagnosis and likelihood depending the drug and latency | Withdrawal the drug and steroids in some hypersensibility cases |
| Cholangiocarcinoma | MRCP + CA199 markers | Surgery, chemotherapy, liver transplantation (special cases) |
| Hepatocelular pattern | ||
| NAFLD | Abdominal US, fatty liver indexs, ruling out other diagnosis, liver biopsy in some cases | Change style of life with loose weight, calories restricted diet, exercise and control IBD inflammatory activity |
| AIH | Serology (ANA, ASM, LKM1, IgG, liver biopsy). Simplified autoimmune hepatitis score | Azathioprine ± steroids |
| Chronic viral hepatitis | Serology, non-invasive fibrosis tests | DDA in HCV and long-term antiviral in HBV |
| DILI | Withdrawal the drug and steroids in some hypersensibility cases | |
| Mix pattern | ||
| Overlap/AIC | MRCP + liver biopsy | Azathioprine ± steroids + UDCA |
| DILI | Withdrawal the drug and steroids in some hypersensibility cases | |
| Chronic viral hepatitis | Serological markers | DDA in HCV and long term antiviralin HBV |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Sociedad Chilena de Gastroenterología.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Acharyya BC, Christodoulou D, Damiani G, Kuznietsova H S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Gohlke F, Lohse AW, Dienes HP, Löhr H, Märker-Hermann E, Gerken G, Meyer zum Büschenfelde KH. Evidence for an overlap syndrome of autoimmune hepatitis and primary sclerosing cholangitis. J Hepatol. 1996;24:699-705. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Yarur AJ, Czul F, Levy C. Hepatobiliary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1655-1667. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. Lancet. 2018;391:2547-2559. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Miloh T, Arnon R, Shneider B, Suchy F, Kerkar N. A retrospective single-center review of primary sclerosing cholangitis in children. Clin Gastroenterol Hepatol. 2009;7:239-245. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007;102:1042-1049. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Charatcharoenwitthaya P, Pimentel S, Talwalkar JA, Enders FT, Lindor KD, Krom RA, Wiesner RH. Long-term survival and impact of ursodeoxycholic acid treatment for recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2007;13:1236-1245. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Lunder AK, Hov JR, Borthne A, Gleditsch J, Johannesen G, Tveit K, Viktil E, Henriksen M, Hovde Ø, Huppertz-Hauss G, Høie O, Høivik ML, Monstad I, Solberg IC, Jahnsen J, Karlsen TH, Moum B, Vatn M, Negård A. Prevalence of Sclerosing Cholangitis Detected by Magnetic Resonance Cholangiography in Patients With Long-term Inflammatory Bowel Disease. Gastroenterology. 2016;151:660-669.e4. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Palmela C, Peerani F, Castaneda D, Torres J, Itzkowitz SH. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver. 2018;12:17-29. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Eaton JE, Welle CL, Bakhshi Z, Sheedy SP, Idilman IS, Gores GJ, Rosen CB, Heimbach JK, Taner T, Harnois DM, Lindor KD, LaRusso NF, Gossard AA, Lazaridis KN, Venkatesh SK. Early Cholangiocarcinoma Detection With Magnetic Resonance Imaging Versus Ultrasound in Primary Sclerosing Cholangitis. Hepatology. 2021;73:1868-1881. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Trivedi PJ, Chapman RW. PSC, AIH and overlap syndrome in inflammatory bowel disease. Clin Res Hepatol Gastroenterol. 2012;36:420-436. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Sirpal S, Chandok N. Primary sclerosing cholangitis: diagnostic and management challenges. Clin Exp Gastroenterol. 2017;10:265-273. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis--aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48 Suppl 1:S38-S57. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397-411. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Tabibian JH, Ali AH, Lindor KD. Primary Sclerosing Cholangitis, Part 1: Epidemiology, Etiopathogenesis, Clinical Features, and Treatment. Gastroenterol Hepatol (N Y). 2018;14:293-304. [PubMed] [Cited in This Article: ] |
| 16. | Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200-206. [PubMed] [Cited in This Article: ] |
| 17. | Satiya J, Mousa OY, Gupta K, Trivedi S, Oman SP, Wijarnpreecha K, Harnois DM, Corral JE. Diagnostic yield of magnetic resonance imaging for cholangiocarcinoma in primary sclerosing cholangitis: a meta-analysis. Clin Exp Hepatol. 2020;6:35-41. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | de Vries EM, de Krijger M, Färkkilä M, Arola J, Schirmacher P, Gotthardt D, Goeppert B, Trivedi PJ, Hirschfield GM, Ytting H, Vainer B, Buuren HR, Biermann K, Harms MH, Chazouilleres O, Wendum D, Kemgang AD, Chapman RW, Wang LM, Williamson KD, Gouw AS, Paradis V, Sempoux C, Beuers U, Hübscher SG, Verheij J, Ponsioen CY. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: An international cohort study. Hepatology. 2017;65:907-919. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Cazzagon N, Sarcognato S, Floreani A, Corrà G, De Martin S, Guzzardo V, Russo FP, Guido M. Cholangiocyte senescence in primary sclerosing cholangitis is associated with disease severity and prognosis. JHEP Rep. 2021;3:100286. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA, Marschall HU, Thorburn D, Weersma RK, Fevery J, Mueller T, Chazouillères O, Schulze K, Lazaridis KN, Almer S, Pereira SP, Levy C, Mason A, Naess S, Bowlus CL, Floreani A, Halilbasic E, Yimam KK, Milkiewicz P, Beuers U, Huynh DK, Pares A, Manser CN, Dalekos GN, Eksteen B, Invernizzi P, Berg CP, Kirchner GI, Sarrazin C, Zimmer V, Fabris L, Braun F, Marzioni M, Juran BD, Said K, Rupp C, Jokelainen K, Benito de Valle M, Saffioti F, Cheung A, Trauner M, Schramm C, Chapman RW, Karlsen TH, Schrumpf E, Strassburg CP, Manns MP, Lindor KD, Hirschfield GM, Hansen BE, Boberg KM; International PSC Study Group. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152:1975-1984.e8. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Aranake-Chrisinger J, Dassopoulos T, Yan Y, Nalbantoglu I. Primary sclerosing cholangitis associated colitis: Characterization of clinical, histologic features, and their associations with liver transplantation. World J Gastroenterol. 2020;26:4126-4139. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Trivedi PJ, Crothers H, Mytton J, Bosch S, Iqbal T, Ferguson J, Hirschfield GM. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People With Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology. 2020;159:915-928. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Shah SC, Ten Hove JR, Castaneda D, Palmela C, Mooiweer E, Colombel JF, Harpaz N, Ullman TA, van Bodegraven AA, Jansen JM, Mahmmod N, van der Meulen-de Jong AE, Ponsioen CY, van der Woude CJ, Oldenburg B, Itzkowitz SH, Torres J. High Risk of Advanced Colorectal Neoplasia in Patients With Primary Sclerosing Cholangitis Associated With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2018;16:1106-1113.e3. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Núñez F P, Quera P R, Gomollón F. Primary sclerosing cholangitis and inflammatory bowel disease: Intestine-liver interrelation. Gastroenterol Hepatol. 2019;42:316-325. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Rossi RE, Conte D, Massironi S. Primary sclerosing cholangitis associated with inflammatory bowel disease: an update. Eur J Gastroenterol Hepatol. 2016;28:123-131. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Dastis SN, Latinne D, Sempoux C, Geubel AP. Ulcerative colitis associated with IgG4 cholangitis: similar features in two HLA identical siblings. J Hepatol. 2009;51:601-605. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Culver EL, Barnes E. IgG4-related sclerosing cholangitis. Clin Liver Dis (Hoboken). 2017;10:9-16. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Faria RJ, Clemente CM, Carneiro FP, Santos-Neto L. Can IgG4 Levels Identify the Ulcerative Colitis Subtype of Inflammatory Bowel Disease? Gastroenterology Res. 2015;8:178-185. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Björnsson E, Boberg KM, Cullen S, Fleming K, Clausen OP, Fausa O, Schrumpf E, Chapman RW. Patients with small duct primary sclerosing cholangitis have a favourable long term prognosis. Gut. 2002;51:731-735. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Irshad A, Ackerman S, Sosnouski D, Anis M, Chavin K, Baliga P. A review of sonographic evaluation of renal transplant complications. Curr Probl Diagn Radiol. 2008;37:67-79. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Singh S, Loftus EV Jr, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol. 2013;108:1417-1425. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Fattahi MR, Malek-Hosseini SA, Sivandzadeh GR, Safarpour AR, Bagheri Lankarani K, Taghavi AR, Ejtehadi F. Clinical Course of Ulcerative Colitis After Liver Transplantation in Patients with Concomitant Primary Sclerosing Cholangitis and Ulcerative Colitis. Inflamm Bowel Dis. 2017;23:1160-1167. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Liberal R, Gaspar R, Lopes S, Macedo G. Primary biliary cholangitis in patients with inflammatory bowel disease. Clin Res Hepatol Gastroenterol. 2020;44:e5-e9. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Rocha HC, Vilela EG. Clinical aspects and prognosis of patients with inflammatory bowel disease associated with autoimmune liver diseases. Gastroenterol Hepatol. 2021;. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, Lohse AW, Montano-Loza AJ. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Perdigoto R, Carpenter HA, Czaja AJ. Frequency and significance of chronic ulcerative colitis in severe corticosteroid-treated autoimmune hepatitis. J Hepatol. 1992;14:325-331. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Tan C, Zhou K, Ma W, Attard B, Zhang P, Kuang T. Selective laser melting of high-performance pure tungsten: parameter design, densification behavior and mechanical properties. Sci Technol Adv Mater. 2018;19:370-380. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Bailey JMD, Sreepati GMD, Love JBS, Fischer MMD, Vuppalanchi RMD, Ghabril MMD, Gawrieh SMD, Orman EMD, Chalasani NMD, Lammert CMD. Autoimmune hepatitis with inflammatory bowel disease is distinct and may be more refractory to traditional treatment. Am J Gastroenterol. 2014;109:S149. [Cited in This Article: ] |
| 42. | Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, Mieli-Vergani G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544-553. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Lohse AW, zum Büschenfelde KH, Franz B, Kanzler S, Gerken G, Dienes HP. Characterization of the overlap syndrome of primary biliary cirrhosis (PBC) and autoimmune hepatitis: evidence for it being a hepatitic form of PBC in genetically susceptible individuals. Hepatology. 1999;29:1078-1084. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Algaba A, Guerra I, Ricart E, Iglesias E, Mañosa M, Gisbert JP, Guardiola J, Mínguez M, Castro B, de Francisco R, Nos P, Bertoletti F, Mesonero F, Barrio J, Martín-Arranz MD, Calvet X, García-López S, Sicilia B, Alcaín G, Esteve M, Márquez L, Piqueras M, Jiménez L, Perez-Calle JL, Bujanda L, García-Sepulcre M, Fernández A, Moraleja I, Lorente RH, García-Bosch O, Lambán A, Blázquez I, Rodríguez E, Huguet JM, Lucendo AJ, Almela P, Busquets D, Ramírez de la Piscina P, Pérez M, Domenech E, Bermejo F; Spanish GETECCU Group (ENEIDA Project). Extraintestinal Manifestations in Patients with Inflammatory Bowel Disease: Study Based on the ENEIDA Registry. Dig Dis Sci. 2021;66:2014-2023. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Ballotin VR, Bigarella LG, Riva F, Onzi G, Balbinot RA, Balbinot SS, Soldera J. Primary sclerosing cholangitis and autoimmune hepatitis overlap syndrome associated with inflammatory bowel disease: A case report and systematic review. World J Clin Cases. 2020;8:4075-4093. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Nuñez Figueroa P, Sedano Muñoz R, Quera Pino R, Carrasco- Avino G, O`Brien A, Brahm Barril J. Autoinmune sequential overlap syndrome (autoinmune hepatitis/ primary sclerosing cholangitis) and inflammatory bowel disease: three clinical cases. Rev Esp Enferm Dig 2020; 112(10): 788-791. [DOI] [Cited in This Article: ] |
| 47. | Terziroli Beretta-Piccoli B, Vergani D, Mieli-Vergani G. Autoimmune sclerosing cholangitis: Evidence and open questions. J Autoimmun. 2018;95:15-25. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | el-Shabrawi M, Wilkinson ML, Portmann B, Mieli-Vergani G, Chong SK, Williams R, Mowat AP. Primary sclerosing cholangitis in childhood. Gastroenterology. 1987;92:1226-1235. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Deneau MR, El-Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, Chan A, Cotter J, Doan S, El-Youssef M, Ferrari F, Furuya KN, Gottrand M, Gottrand F, Gupta N, Homan M, Kamath BM, Kim KM, Kolho KL, Konidari A, Koot B, Iorio R, Ledder O, Mack C, Martinez M, Miloh T, Mohan P, O'Cathain N, Papadopoulou A, Ricciuto A, Saubermann L, Sathya P, Shteyer E, Smolka V, Tanaka A, Varier R, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Jensen MK. The natural history of primary sclerosing cholangitis in 781 children: A multicenter, international collaboration. Hepatology. 2017;66:518-527. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Underhill JA, Ma Y, Bogdanos D, Cheeseman P, Mieli-Vergani G, Vergani D. Different immunogenetic background in autoimmune hepatitis type 1, type 2 and autoimmune sclerosing cholangitis. J Hepatol. 2002;36:156. [DOI] [Cited in This Article: ] |
| 51. | Scalori A, Heneghon MA, Hadzic ND, Vergani D, Mieli-Vergani G. Outcome and survival in childhood onset autoimmune sclerosing cholangitis and autoimmune hepatitis; A 13 years follow-up study. Hepatology. 2007;46:555A. [Cited in This Article: ] |
| 52. | Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330-340. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Mieli-Vergani G, Vergani D. Sclerosing Cholangitis in Children and Adolescents. Clin Liver Dis. 2016;20:99-111. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-1090. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Fousekis FS, Katsanos KH, Theopistos VI, Baltayiannis G, Kosmidou M, Glantzounis G, Christou L, Tsianos EV, Christodoulou DK. Hepatobiliary and pancreatic manifestations in inflammatory bowel diseases: a referral center study. BMC Gastroenterol. 2019;19:48. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Karaivazoglou K, Konstantakis C, Tourkochristou E, Assimakopoulos SF, Triantos C. Non-alcoholic fatty liver disease in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2020;32:903-906. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Zou ZY, Shen B, Fan JG. Systematic Review With Meta-analysis: Epidemiology of Nonalcoholic Fatty Liver Disease in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1764-1772. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Hoffmann P, Jung V, Behnisch R, Gauss A. Prevalence and risk factors of nonalcoholic fatty liver disease in patients with inflammatory bowel diseases: A cross-sectional and longitudinal analysis. World J Gastroenterol. 2020;26:7367-7381. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Glassner K, Malaty HM, Abraham BP. Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease Among Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:998-1003. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Sourianarayanane A, Garg G, Smith TH, Butt MI, McCullough AJ, Shen B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e279-e285. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Sartini A, Gitto S, Bianchini M, Verga MC, Di Girolamo M, Bertani A, Del Buono M, Schepis F, Lei B, De Maria N, Villa E. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018;9:87. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Restellini S, Chazouillères O, Frossard JL. Hepatic manifestations of inflammatory bowel diseases. Liver Int. 2017;37:475-489. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Schröder T, Schmidt KJ, Olsen V, Möller S, Mackenroth T, Sina C, Lehnert H, Fellermann K, Büning J. Liver steatosis is a risk factor for hepatotoxicity in patients with inflammatory bowel disease under immunosuppressive treatment. Eur J Gastroenterol Hepatol. 2015;27:698-704. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Nguyen DL, Bechtold ML, Jamal MM. National trends and inpatient outcomes of inflammatory bowel disease patients with concomitant chronic liver disease. Scand J Gastroenterol. 2014;49:1091-1095. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Lapumnuaypol K, Kanjanahattakij N, Pisarcik D, Thongprayoon C, Wijarnpreecha K, Cheungpasitporn W. Effects of inflammatory bowel disease treatment on the risk of nonalcoholic fatty liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:854-860. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Yaccob A, Mari A. Practical clinical approach to the evaluation of hepatobiliary disorders in inflammatory bowel disease. Frontline Gastroenterol. 2019;10:309-315. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Garcia-Cortes M, Robles-Diaz M, Stephens C, Ortega-Alonso A, Lucena MI, Andrade RJ. Drug induced liver injury: an update. Arch Toxicol. 2020;94:3381-3407. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Sehgal P, Colombel JF, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther. 2018;47:1597-1609. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, Gomollón F, García-Planella E, Merino O, Gutiérrez A, Esteve M, Márquez L, Garcia-Sepulcre M, Hinojosa J, Vera I, Muñoz F, Mendoza JL, Cabriada JL, Montoro MA, Barreiro-de Acosta M, Ceña G, Saro C, Aldeguer X, Barrio J, Maté J, Gisbert JP. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404-1410. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Tominaga K, Sugaya T, Tanaka T, Kanazawa M, Iijima M, Irisawa A. Thiopurines: Recent Topics and Their Role in the Treatment of Inflammatory Bowel Diseases. Front Pharmacol. 2020;11:582291. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Lim SZ, Chua EW. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front Pharmacol. 2018;9:1107. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Mottet C, Schoepfer AM, Juillerat P, Cosnes J, Froehlich F, Kessler-Brondolo V, Seibold F, Rogler G, Vavricka SR, Michetti P. Experts Opinion on the Practical Use of Azathioprine and 6-Mercaptopurine in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2733-2747. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Vasudevan A, Parthasarathy N, Con D, Nicolaides S, Apostolov R, Chauhan A, Bishara M, Luber RP, Joshi N, Wan A, Rickard JA, Long T, Connoley D, Sparrow MP, Gibson PR, van Langenberg DR. Thiopurines vs methotrexate: Comparing tolerability and discontinuation rates in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:1174-1184. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Llaó J, Masnou H, Romero C, Bargalló A, Gely C, Mañosa M, Gordillo J, Garcia-Planella E, Domènech E. Noninvasive assessment of liver fibrosis in Crohn's disease patients exposed to methotrexate. Eur J Gastroenterol Hepatol. 2021;33:794-798. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Azzam A, Jiyad Z, O'Beirne J. Is methotrexate hepatotoxicity associated with cumulative dose? Australas J Dermatol. 2021;62:130-140. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Li YC, Shen JD, Lu SF, Zhu LL, Wang BY, Bai M, Xu EP. Transcriptomic analysis reveals the mechanism of sulfasalazine-induced liver injury in mice. Toxicol Lett. 2020;321:12-20. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis. 2010;28:508-518. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Mantzaris GJ. Thiopurines and Methotrexate Use in IBD Patients in a Biologic Era. Curr Treat Options Gastroenterol. 2017;15:84-104. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705-713. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Phillips J, Preskey R, Penfold C, Gordon F, Tyrrell-Price J. Liver steatosis is a risk factor for hepatotoxicity in inflammatory bowel disease patients treated with azathioprine. Eur J Gastroenterol Hepatol. 2020;32:1390-1394. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Wong DR, Coenen MJ, Derijks LJ, Vermeulen SH, van Marrewijk CJ, Klungel OH, Scheffer H, Franke B, Guchelaar HJ, de Jong DJ, Engels LG, Verbeek AL, Hooymans PM; TOPIC Recruitment Team. Early prediction of thiopurine-induced hepatotoxicity in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:391-402. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Gisbert JP, Luna M, González-Lama Y, Pousa ID, Velasco M, Moreno-Otero R, Maté J. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007;13:1106-1114. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Bermejo F, Aguas M, Chaparro M, Domènech E, Echarri A, García-Planella E, Guerra I, Gisbert JP, López-Sanromán A; en representación de GETECCU. Recommendations of the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) on the use of thiopurines in inflammatory bowel disease. Gastroenterol Hepatol. 2018;41:205-221. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Saibeni S, Bollani S, Losco A, Michielan A, Sostegni R, Devani M, Lupinacci G, Pirola L, Cucino C, Meucci G, Basilisco G, D'Incà R, Bruno S. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123-127. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Laharie D, Seneschal J, Schaeverbeke T, Doutre MS, Longy-Boursier M, Pellegrin JL, Chabrun E, Villars S, Zerbib F, de Lédinghen V. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case-control study. J Hepatol. 2010;53:1035-1040. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Labadie JG, Jain M. Noninvasive Tests to Monitor Methotrexate-Induced Liver Injury. Clin Liver Dis (Hoboken). 2019;13:67-71. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Gerolami R, Mambrini P, Barthet M, Jean-Pastor MJ, Salducci J, Grimaud JC. [Acute hepatitis caused by Solupred in a patient with Crohn disease]. Gastroenterol Clin Biol. 1997;21:236-237. [PubMed] [Cited in This Article: ] |
| 92. | Coelho J, Ozenne V, Dray X, Chaput U, Marteau P. Case of prednisolone-induced hepatitis in a patient with ulcerative colitis. Inflamm Bowel Dis. 2013;19:E34-E35. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Matsumoto T, Yamasaki S, Arakawa A, Abe K, Abe H, Kon K, Kobayashi S, Takasaki Y. Exposure to a high total dosage of glucocorticoids produces non-alcoholic steatohepatits. Pathol Int. 2007;57:388-389. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Koller T, Galambosova M, Filakovska S, Kubincova M, Hlavaty T, Toth J, Krajcovicova A, Payer J. Drug-induced liver injury in inflammatory bowel disease: 1-year prospective observational study. World J Gastroenterol. 2017;23:4102-4111. [PubMed] [DOI] [Cited in This Article: ] |
| 95. | Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M, Mariz E, Bernardes M, Lopes J, Carneiro F, Macedo G. Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: A single center report of 8 cases. World J Gastroenterol. 2015;21:7584-7588. [PubMed] [DOI] [Cited in This Article: ] |
| 96. | Grasland A, Sterpu R, Boussoukaya S, Mahe I. Autoimmune hepatitis induced by adalimumab with successful switch to abatacept. Eur J Clin Pharmacol. 2012;68:895-898. [PubMed] [DOI] [Cited in This Article: ] |
| 97. | Ling C, Gavin M, Hanson J, McCarthy DM. Progressive Epigastric Pain with Abnormal Liver Tests in a Patient with Crohn's Disease: Don't DILI Dally. Dig Dis Sci. 2018;63:1751-1755. [PubMed] [DOI] [Cited in This Article: ] |
| 98. | Miehsler W, Novacek G, Wenzl H, Vogelsang H, Knoflach P, Kaser A, Dejaco C, Petritsch W, Kapitan M, Maier H, Graninger W, Tilg H, Reinisch W; Austrian Society of Gastroenterology and Hepatology. A decade of infliximab: The Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4:221-256. [PubMed] [DOI] [Cited in This Article: ] |
| 99. | Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D'Haens G, Panaccione R, Loftus EV Jr, Sankoh S, Fox I, Parikh A, Milch C, Abhyankar B, Feagan BG. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66:839-851. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Loftus EV Jr, Feagan BG, Panaccione R, Colombel JF, Sandborn WJ, Sands BE, Danese S, D'Haens G, Rubin DT, Shafran I, Parfionovas A, Rogers R, Lirio RA, Vermeire S. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:1353-1365. [PubMed] [DOI] [Cited in This Article: ] |
| 101. | Honap S, Sticova E, Theocharidou E, Berry P, Irving PM, Samaan MA, Kotha S. Vedolizumab-Associated Drug-Induced Liver Injury: A Case Series. Inflamm Bowel Dis. 2021;27:e32-e34. [PubMed] [DOI] [Cited in This Article: ] |
| 102. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [PubMed] [DOI] [Cited in This Article: ] |
| 103. | Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, Adedokun OJ, Li K, Peyrin-Biroulet L, Van Assche G, Danese S, Targan S, Abreu MT, Hisamatsu T, Szapary P, Marano C; UNIFI Study Group. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2019;381:1201-1214. [PubMed] [DOI] [Cited in This Article: ] |
| 104. | Honap S, Meade S, Ibraheim H, Irving PM, Jones MP, Samaan MA. Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2021;. [PubMed] [DOI] [Cited in This Article: ] |
| 105. | Barnhill MS, Steinberg JM, Jennings JJ, Lewis JH. Hepatotoxicty of Agents Used in the Management of Inflammatory Bowel Disease: a 2020 Update. Curr Gastroenterol Rep. 2020;22:47. [PubMed] [DOI] [Cited in This Article: ] |
| 106. | Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P; UNITI–IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375:1946-1960. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | D'Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019;12:1756284819848631. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Scott FL, Clemons B, Brooks J, Brahmachary E, Powell R, Dedman H, Desale HG, Timony GA, Martinborough E, Rosen H, Roberts E, Boehm MF, Peach RJ. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1 ) and receptor-5 (S1P5 ) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778-1792. [PubMed] [DOI] [Cited in This Article: ] |
| 109. | Sandborn WJ, Feagan BG, Hanauer S, Vermeire S, Ghosh S, Liu WJ, Petersen A, Charles L, Huang V, Usiskin K, Wolf DC, D'Haens G. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J Crohns Colitis. 2021;15:1120-1129. [PubMed] [DOI] [Cited in This Article: ] |
| 110. | Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-1309. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Ng SC, Hilmi IN, Blake A, Bhayat F, Adsul S, Khan QR, Wu DC. Low Frequency of Opportunistic Infections in Patients Receiving Vedolizumab in Clinical Trials and Post-Marketing Setting. Inflamm Bowel Dis. 2018;24:2431-2441. [PubMed] [DOI] [Cited in This Article: ] |
| 112. | Carroll MB, Forgione MA. Use of tumor necrosis factor alpha inhibitors in hepatitis B surface antigen-positive patients: a literature review and potential mechanisms of action. Clin Rheumatol. 2010;29:1021-1029. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Chen YM, Huang WN, Wu YD, Lin CT, Chen YH, Chen DY, Hsieh TY. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis. 2018;77:780-782. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Sansone S, Guarino M, Castiglione F, Rispo A, Auriemma F, Loperto I, Rea M, Caporaso N, Morisco F. Hepatitis B and C virus reactivation in immunosuppressed patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3516-3524. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Campbell S, Ghosh S. Infliximab therapy for Crohn's disease in the presence of chronic hepatitis C infection. Eur J Gastroenterol Hepatol. 2001;13:191-192. [PubMed] [DOI] [Cited in This Article: ] |
| 116. | Loras C, Gisbert JP, Mínguez M, Merino O, Bujanda L, Saro C, Domenech E, Barrio J, Andreu M, Ordás I, Vida L, Bastida G, González-Huix F, Piqueras M, Ginard D, Calvet X, Gutiérrez A, Abad A, Torres M, Panés J, Chaparro M, Pascual I, Rodriguez-Carballeira M, Fernández-Bañares F, Viver JM, Esteve M; REPENTINA study; GETECCU (Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa) Group. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010;59:1340-1346. [PubMed] [DOI] [Cited in This Article: ] |
| 117. | Morisco F, Castiglione F, Rispo A, Stroffolini T, Sansone S, Vitale R, Guarino M, Biancone L, Caruso A, D'Inca R, Marmo R, Orlando A, Riegler G, Donnarumma L, Camera S, Zorzi F, Renna S, Bove V, Tontini G, Vecchi M, Caporaso N. Effect of immunosuppressive therapy on patients with inflammatory bowel diseases and hepatitis B or C virus infection. J Viral Hepat. 2013;20:200-208. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [PubMed] [DOI] [Cited in This Article: ] |
| 119. | Guerra I, Bujanda L, Castro J, Merino O, Tosca J, Camps B, Gutiérrez A, Gordillo Ábalos J, de Castro L, Iborra M, Carbajo AY, Taxonera C, Rodríguez-Lago I, Mesonero F, de Francisco R, Gómez-Gómez GJ, Chaparro M, Tardillo CA, Rivero M, Algaba A, Martín Arranz E, Cañete F, Vicente R, Sicilia B, Antolín B, Prieto V, Márquez L, Benítez JM, Camo P, Piqueras M, Gargallo CJ, Hinojosa E, Huguet JM, Pérez Calle JL, Van Domselaar M, Rodriguez C, Calvet X, Muñoz-Villafranca C, García-Sepulcre MF, Munoz-Garrido P, Fernández-Clotet A, Gómez Irwin L, Hernández S, Guardiola J, Sempere L, González Muñoza C, Hernández V, Beltrán B, Barrio J, Alba C, Moraleja I, López-Sanromán A, Riestra S, Martínez Montiel P, Garre A, Arranz L, García MJ, Martín Arranz MD, Corsino P, Arias L, Fernández-Salazar L, Fernández-Pordomingo A, Andreu M, Iglesias E, Ber Y, Mena R, Arroyo Villarino MT, Mora M, Ruiz L, López-Serrano P, Blazquez I, Villoria A, Fernández M, Bermejo F, Banales JM, Domènech E, Gisbert JP; Spanish GETECCU group (ENEIDA Project). Clinical Characteristics, Associated Malignancies and Management of Primary Sclerosing Cholangitis in Inflammatory Bowel Disease Patients: A Multicentre Retrospective Cohort Study. J Crohns Colitis. 2019;13:1492-1500. [PubMed] [DOI] [Cited in This Article: ] |
| 120. | Gulamhusein AF, Eaton JE, Tabibian JH, Atkinson EJ, Juran BD, Lazaridis KN. Duration of Inflammatory Bowel Disease Is Associated With Increased Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis and IBD. Am J Gastroenterol. 2016;111:705-711. [PubMed] [DOI] [Cited in This Article: ] |
| 121. | Jahnel J, Fickert P, Hauer AC, Högenauer C, Avian A, Trauner M. Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug Metab Dispos. 2014;42:1423-1431. [PubMed] [DOI] [Cited in This Article: ] |
| 122. | Temel T, Cansu DÜ, Korkmaz C, Kaşifoğlu T, Özakyol A. The long-term effects of anti-TNF-α agents on patients with chronic viral hepatitis C and B infections. Int J Rheum Dis. 2015;18:40-45. [PubMed] [DOI] [Cited in This Article: ] |
| 123. | Parente F, Pastore L, Bargiggia S, Cucino C, Greco S, Molteni M, Ardizzone S, Porro GB, Sampietro GM, Giorgi R, Moretti R, Gallus S. Incidence and risk factors for gallstones in patients with inflammatory bowel disease: a large case-control study. Hepatology. 2007;45:1267-1274. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | Zhang FM, Xu CF, Shan GD, Chen HT, Xu GQ. Is gallstone disease associated with inflammatory bowel diseases? J Dig Dis. 2015;16:634-641. [PubMed] [DOI] [Cited in This Article: ] |
| 125. | Damião AO, Sipahi AM, Vezozzo DP, Gonçalves PL, Fukui P, Laudanna AA. Gallbladder hypokinesia in Crohn's disease. Digestion. 1997;58:458-463. [PubMed] [DOI] [Cited in This Article: ] |
| 126. | Fraquelli M, Losco A, Visentin S, Cesana BM, Pometta R, Colli A, Conte D. Gallstone disease and related risk factors in patients with Crohn disease: analysis of 330 consecutive cases. Arch Intern Med. 2001;161:2201-2204. [PubMed] [DOI] [Cited in This Article: ] |
| 127. | Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657-663. [PubMed] [DOI] [Cited in This Article: ] |
| 128. | Talbot RW, Heppell J, Dozois RR, Beart RW Jr. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140-145. [PubMed] [DOI] [Cited in This Article: ] |
| 129. | Hatoum OA, Spinelli KS, Abu-Hajir M, Attila T, Franco J, Otterson MF, Telford GL, Binion DG. Mesenteric venous thrombosis in inflammatory bowel disease. J Clin Gastroenterol. 2005;39:27-31. [PubMed] [Cited in This Article: ] |
| 130. | Bruining DH, Siddiki HA, Fletcher JG, Tremaine WJ, Sandborn WJ, Loftus EV Jr. Prevalence of penetrating disease and extraintestinal manifestations of Crohn's disease detected with CT enterography. Inflamm Bowel Dis. 2008;14:1701-1706. [PubMed] [DOI] [Cited in This Article: ] |
| 131. | Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, Heller J, Morard I, Lasser L, Langlet P, Denninger MH, Vidaud D, Condat B, Hadengue A, Primignani M, Garcia-Pagan JC, Janssen HL, Valla D; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51:210-218. [PubMed] [DOI] [Cited in This Article: ] |
| 132. | Landman C, Nahon S, Cosnes J, Bouhnik Y, Brixi-Benmansour H, Bouguen G, Colombel JF, Savoye G, Coffin B, Abitbol V, Filippi J, Laharie D, Moreau J, Veyrac M, Allez M, Marteau P; Groupe d'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. Portomesenteric vein thrombosis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:582-589. [PubMed] [DOI] [Cited in This Article: ] |
| 133. | Rojas-Feria M, Castro M, Suárez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013;19:7327-7340. [PubMed] [DOI] [Cited in This Article: ] |
| 134. | Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD, Juillerat P, Karlsen TH, Koutroubakis I, Lakatos PL, Orchard T, Papay P, Raine T, Reinshagen M, Thaci D, Tilg H, Carbonnel F; European Crohn’s and Colitis Organisation. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:239-254. [PubMed] [DOI] [Cited in This Article: ] |
| 135. | Eapen CE, Nightingale P, Hubscher SG, Lane PJ, Plant T, Velissaris D, Elias E. Non-cirrhotic intrahepatic portal hypertension: associated gut diseases and prognostic factors. Dig Dis Sci. 2011;56:227-235. [PubMed] [DOI] [Cited in This Article: ] |
| 136. | Calabrese E, Hanauer SB. Assessment of non-cirrhotic portal hypertension associated with thiopurine therapy in inflammatory bowel disease. J Crohns Colitis. 2011;5:48-53. [PubMed] [DOI] [Cited in This Article: ] |
| 137. | Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013;38:1025-1037. [PubMed] [DOI] [Cited in This Article: ] |
| 138. | Vernier-Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, Cadiot G, Bouhnik Y, De Vos M, Boureille A, Duclos B, Seksik P, Mary JY, Colombel JF. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut. 2007;56:1404-1409. [PubMed] [DOI] [Cited in This Article: ] |
| 139. | Dooremont D, Decaestecker J, De Wulf D, Ghillebert G, Van Vlierberghe H, Van Dorpe J, Baert F. Azathioprine induced serious portal hypertension: a case series of three IBD patients and review of the literature. Acta Gastroenterol Belg. 2013;76:342-346. [PubMed] [Cited in This Article: ] |
| 140. | Lin JN, Lin CL, Lin MC, Lai CH, Lin HH, Kao CH. Pyogenic liver abscess in patients with inflammatory bowel disease: a nationwide cohort study. Liver Int. 2016;36:136-144. [PubMed] [DOI] [Cited in This Article: ] |
| 141. | Margalit M, Elinav H, Ilan Y, Shalit M. Liver abscess in inflammatory bowel disease: report of two cases and review of the literature. J Gastroenterol Hepatol. 2004;19:1338-1342. [PubMed] [DOI] [Cited in This Article: ] |
| 142. | Kapoor S. Pyogenic liver abscesses: a serious but often unrecognized hepatic complication of Crohn's disease. Inflamm Bowel Dis. 2014;20:E1-E2. [PubMed] [DOI] [Cited in This Article: ] |
| 143. | McGreal S, Sayers R, Wurm P, West K. Crohn's disease presenting with pyogenic liver abscess: a case report. Case Rep Gastrointest Med. 2012;2012:762480. [PubMed] [DOI] [Cited in This Article: ] |
| 144. | Braun M, Fraser GM, Kunin M, Salamon F, Tur-Kaspa R. Mesalamine-induced granulomatous hepatitis. Am J Gastroenterol. 1999;94:1973-1974. [PubMed] [DOI] [Cited in This Article: ] |
| 145. | Gizard E, Ford AC, Bronowicki JP, Peyrin-Biroulet L. Systematic review: The epidemiology of the hepatobiliary manifestations in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2014;40:3-15. [PubMed] [DOI] [Cited in This Article: ] |
| 146. | Greenstein AJ, Sachar DB, Panday AK, Dikman SH, Meyers S, Heimann T, Gumaste V, Werther JL, Janowitz HD. Amyloidosis and inflammatory bowel disease. A 50-year experience with 25 patients. Medicine (Baltimore). 1992;71:261-270. [PubMed] [DOI] [Cited in This Article: ] |