Part 2 - Sphaeromatidae::“Cute As Buttons”
Part 2 - Sphaeromatidae::“Cute As Buttons”
Part 2 - Sphaeromatidae::“Cute As Buttons”
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
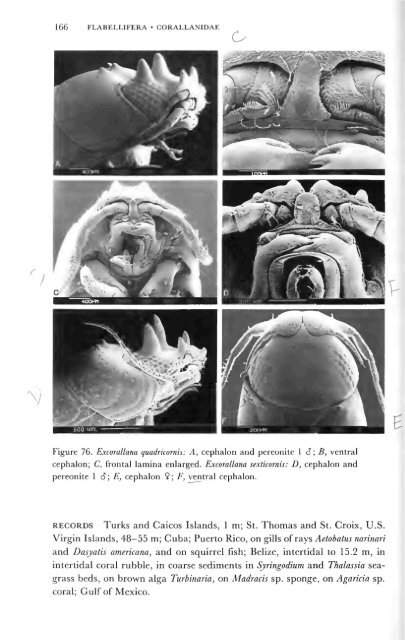
166 FLABELLIFERA • CORALLANIDAE<br />
Figure 76. Excorallana quadricornis: A, cephalon and pereonite 1 6; B, ventral<br />
cephalon; C, frontal lamina enlarged. Excorallana sexticornis: D, cephalon and<br />
o<br />
pereonite 1 8; E, cephalon 9; F, ventral cephalon.<br />
RECORDS Turks and Caicos Islands, 1 m; St. Thomas and St. Croix, U.S.<br />
Virgin Islands, 48—55 m; Cuba; Puerto Rico, on gills of rays Aetobatus narinari<br />
and Dasyatis americana, and on squirrel fish; Belize, intertidal to 15.2 m, in<br />
tertid bble sediments in Syringodium and Thalassia<br />
g r a s s b e d s , o n b r o w n a l g a T u r b i n a r i a , o n M a d r a c i s s p . s p o n g e , o n A g a r i c i a s p<br />
c o r a l ; G u l f o f M e x i c o .
Excorallana warmingii 167<br />
F i g u r e 7 7 . Excorallana tricornis tricornis: A , c e p h a l o n a n d p e r e o n i t e 1 6 ; B , v e n t r a l<br />
c e p h a l o n ; C, p l e o t e l s o n a n d u r o p o d s .<br />
R E M A R K S T h e s u b s p e c i e s E x c o r a l l a n a t r i c o r n i s o c c i d e n t a l i s R i c h a r d s o n , 1 9 0 5 a ,<br />
f r o m s o u t h e r n C a l i f o r n i a , d i f f e r s f r o m t h e G u l f a n d C a r i b b e a n s u b s p e c i e s i n<br />
l a c k i n g a g a p b e t w e e n t h e m a r g i n s o f t h e p l e o t e l s o n i c i n c i s i o n , a n d i n h a v i n g<br />
a r e l a t i v e l y w i d e r u r o p o d a l e x o p o d w h i c h s h o w s a d i s t i n c t l y a s y m m e t r i c a l<br />
a p i c a l n o t c h .<br />
E x c o r a l l a n a w a r m i n g i i ( H a n s e n , 1 8 9 0 )<br />
F i g u r e 7 5 1 , J<br />
D I A G N O S I S ( J 9 . 7 m m , 9 1 2 . 0 m m . C e p h a l o n u n o r n a m e n t e d . E y e s c o n <br />
t i g u o u s , o c c u p y i n g m o s t o f d o r s a l s u r f a c e o f h e a d . P o s t e r i o r m a r g i n s o f<br />
p l e o n i t e s v e r y f a i n t l y t u b e r c u l a t e . F r o n t a l l a m i n a , l e n g t h s l i g h t l y m o r e t h a n<br />
t w i c e b a s a l w i d t h , t a p e r i n g a n t e r i o r l y t o r o u n d e d a p e x . P l e o t e l s o n u n o r n a <br />
m e n t e d e x c e p t f o r t w o f a i n t s u b m e d i a n t u b e r c l e s b a s a l l y ; l a t e r a l i n c i s i o n s<br />
l a c k i n g ; a p e x b r o a d l y r o u n d e d , w i t h f i v e l o w b u t d i s t i n c t m a r g i n a l t e e t h .<br />
*
168 FLABELLIFERA • CORALLAN1DAE<br />
Figure 78. Nalicora rapax: A, 9; B> maxilla 1; C> maxilla 2; D, maxilliped.<br />
RECORDS Bahamas; between Cuba and the Yucatan Pensinsula; Puerto<br />
Rico.<br />
Off Brazil near Rio de Janeiro.<br />
Nalicora Moore, 1901<br />
DIAGNOSIS Maxilla 1 exopod a single strongly falcate distal spine with<br />
knoblike mesial process, and basal caplike convex papilla-bearing structure.<br />
Maxilla 2 of four articles, distal article slender. Maxillipedal palp of five<br />
articles; endite lacking.
Nalicora rapax Moore, 1901<br />
Figure 78<br />
FLABELL1FERA • CYMOTHOIDAE 169<br />
DIAGNOSIS 6 6.9 mm, ovigerous 9 10.0 mm. Eyes well developed. Frontal<br />
lamina basally slender, widening anteriorly, apex subacute. Posterior half of<br />
body bearing numerous scattered stiff setae. Pereonites 4-7 with row of low<br />
rounded tubercles near posterior margin. Posterior margins of pleonites 3—5<br />
faintly tuberculate, more noticeable in 6. Pleotelson wider than long; lateral<br />
margins faintly sinuous; apex rounded.<br />
RECORDS Florida Keys, 55 m; Puerto Rico, 50-150 m; Gulf of Mexico off<br />
Florida, 37-73 m.<br />
Family Cymothoidae Leach, 1818<br />
DIAGNOSIS Antennules and antennae reduced, no clear distinction between<br />
peduncles and flagella. Mandibular palp of three articles. Maxilla 1 with four<br />
terminal spines. Maxilla 2 apically bilobed, armed with several spines. Max-<br />
illipedal palp of two articles, terminal article bearing hooks. All seven pairs of<br />
pereopods prehensile, ending in strongly hooked dactyli. Pleopods lacking<br />
marginal setae in adults.<br />
REMARKS The cymothoids are exclusively ectoparasites on marine, fresh<br />
water, and brackish-water fishes. Most cymothoids occur in shallow water,<br />
mainly in tropical and subtropical areas. The position of attachment on the<br />
host (externally, in the buccal cavity, or in the gill chamber) is usually genus-<br />
or species-specific. The body of gill parasites is often asymmetrical, being<br />
slightly twisted, perhaps an effect of the position on the host. The mouthparts<br />
are highly adapted for the parasitic mode of life, while all seven pairs of<br />
pereopods are strongly prehensile. The posterior pereopods of some genera<br />
have the basal article expanded and carinate, allowing for increased mus<br />
culature. The secretion of anticoagulants in the juvenile stages further aids<br />
the blood-feeding habit. The surface area of the pleopods is often increased<br />
by the development of lobes on the bases or the lamellae, providing an in<br />
creased respiratory ability.<br />
The post-mancal juvenile stages (sometimes referred to as the aegathoid<br />
stage) have large eyes, and highly setose pleopods for active swimming. The<br />
juveniles will attach themselves indiscriminantly to any convenient fish host,<br />
but eventually attach to the preferred host-species. The juvenile then de<br />
velops into a functional male, losing the swimming setae of the pleopods.<br />
Both juveniles and males feed actively, drawing blood from the host fish. The
170 FLABELLIFERA • GYMOTHOIDAE<br />
Key to genera of Cymothoidae<br />
1. Antennule broader and usually longer than antenna; cephalon very<br />
weakly sunk into pcreonite 1 2<br />
Antennule not broader or longer than antenna; cephalon distinctly<br />
immersed in, or not at all immersed in pereonite 1 4<br />
2. Bases of antennules widely separated 3<br />
Bases of antennules contiguous Glossobius<br />
3. Body curved to one side; pleonitc 1 extended laterally more on one side<br />
than on other Mothocya<br />
Body rarely curved to one side; pleonitc 1 extended equally on each<br />
side Renocila<br />
4. Pereonites and coxal plates 4-7 strongly expanded on one side only<br />
Agarna<br />
No pereonites or coxal plates strongly expanded 5<br />
5. Cephalon not immersed in pereonite 1; posterior margin of cephalon<br />
trisinuate 6<br />
Cephalon to some degree immersed in pereonite 1; posterior margin of<br />
cephalon not trisinuate 7<br />
6. Posterolateral angles of pereonites 2-6 not produced; coxal plates short,<br />
rarely reaching posterior margin of their pereonites Anilocra<br />
Posterolateral angles of pereonites 2-6 posteriorly increasingly<br />
produced; coxal plates usually reaching to posterior margin of their<br />
pereonites Nerocila<br />
7. Basal antennular articles expanded and contiguous Ceratothoa<br />
Basal antennular articles expanded but not contiguous, or basal<br />
antennular articles neither expanded nor contiguous 8<br />
8. Basal antennular articles expanded but not contiguous Kuna<br />
Basal antennular articles neither expanded nor contiguous 9<br />
9. Plconal margins continuous with pereonal margins, pleon not abruptly<br />
narrowed, only weakly immersed in pereonite 7 Lironeca<br />
Pleon to some degree narrower than pereon; pleon usually deeply<br />
immersed in pereonite 7 Cymothoa
FLABELLIFERA • CYMOTIIOIDAE 171<br />
male eventually becomes a female (all cymothoids are protandrous) should a<br />
female not already be present. In some species, the female is nonfeeding. In<br />
those species which settle either in the mouth cavity or gill chamber of the<br />
host, integumental pigment is frequently lost, and the eyes become reduced.<br />
Given the highly variable morphology of the cymothoids, in part imposed<br />
by the parasitic mode of life, and the existence of polymorphism and possible<br />
sibling species, the taxonomy of this family demands the examination of large<br />
numbers of specimens. <strong>As</strong> a further aid to identification, Table 3 is provided,<br />
giving host species, parasite, and site of attachment.<br />
TABLE 3. GYMOTHOID PARASITES FROM THE CARIBBEAN AREA, LISTED BY FISH<br />
HOST SPECIES<br />
Fish host Cymothoid parasite Site of attachment<br />
Abudefduf saxatilis<br />
Acanthurus bahianus<br />
Acanthurus chirurgus<br />
Alutera schoepji<br />
Anchoa lamprotaenia<br />
Apogon lachneri<br />
Apogon maculatus<br />
Apogon townsendi<br />
Ariusfelis<br />
<strong>As</strong>trapogon stellatus<br />
Batrachoides surinamensis<br />
Caranx hippos<br />
Caranx latus<br />
Caranx ruber<br />
Caranx sp.<br />
Chaetodipterus faber<br />
Chaetodon capistratus<br />
Chaetodon ocellatus<br />
Chaetodon sedentarius<br />
Chaetodon striatus<br />
Anilocra abudefduji<br />
Kuna insularis<br />
Anilocra acanthuri<br />
Anilocra acanthuri<br />
Nerocila acuminata<br />
Lironeca tenuistylis<br />
Mothocya bohlkeorum<br />
Renocila colini<br />
Renocila colini<br />
Nerocila acuminata<br />
Mothocya bohlkeorum<br />
Nerocila acuminata<br />
Cymothoa oestrum<br />
Cymothoa oestrum<br />
Cymothoa oestrum<br />
Cymothoa oestrum<br />
Nerocila acuminata<br />
Anilocra chaetodontis<br />
Anilocra chaetodontis<br />
Anilocra chaetodontis<br />
Anilocra chaetodontis<br />
beneath eye<br />
gill chamber<br />
9 at base of pectoral fin;<br />
immature on or near<br />
pectoral or pelvic fin<br />
9 at base of pectoral fin;<br />
immature on or near<br />
pectoral or pelvic fin<br />
on or at base of fin<br />
posterior to pectoral fin<br />
in gill chamber<br />
next to dorsal fin<br />
next to dorsal fin<br />
on or at base of fin<br />
in gill chamber<br />
on or at base of fin<br />
inside mouth<br />
inside mouth<br />
inside mouth<br />
inside mouth<br />
on or at base of fin<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
(continued)
172<br />
TABLE 3. {Continued)<br />
Fish host Cymothoid parasite Site of attachment<br />
Chilomycterus schoepfi<br />
Chromis cyaneus<br />
Chromis multilineatus<br />
Cynoscion nebulosus<br />
Cynoscion sp.<br />
Epinephelus cruentatus<br />
Epinephelus Julvus<br />
Epinephelus guttatus<br />
Epinephelus itajara<br />
Epinephelus sp.<br />
Exocoetus spp.<br />
Genes rhombeus<br />
Haemulon aurolineatum<br />
Haemulon carbonarium<br />
Haemulon chrysargyreum<br />
Haemulon Jlavolineatum<br />
Haemulon macrostomum<br />
Haemulon plumieri<br />
Haemulon sciurus<br />
Nerocila acuminata<br />
Anilocra chromis<br />
Anilocra chromis<br />
Cymothoa excisa<br />
Cymothoa oestrum<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Nerocila acuminata<br />
Cymothoa oestrum<br />
Glossobius impressus<br />
Lironeca redmanni<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Hemirhamphus brasiliensis Glossobius hemirhamphi<br />
Hirundichthys speculifer<br />
Holacanthus tricolor<br />
Holocentrus ascensionis<br />
Glossobius impressus<br />
Anilocra holacanthi<br />
Anilocra holocentri<br />
Hyporhamphus unifasciatus Mothocya nana<br />
Leiostomus xanthurus<br />
Lepiosteus spatula<br />
Lutjanus analis<br />
Lutjanus mahogoni<br />
Lutjanus synagris<br />
Megalops atlanticus<br />
Monacanthus ciliatus<br />
Mugil cephalus<br />
Myripristis jacobus<br />
Ocyurus chrysurus<br />
Orthopristis chrysoptera<br />
Nerocila acuminata<br />
Cymothoa excisa<br />
Lironeca redmanni<br />
Nerocila acuminata<br />
Cymothoa excisa<br />
Cymothoa excisa<br />
Cymothoa excisa<br />
Cymothoa oestrum<br />
Nerocila acuminata<br />
Nerocila acuminata<br />
Anilocra myripristi<br />
Cymothoa excisa<br />
Cymothoa excisa<br />
on or at base of fin<br />
beneath eye<br />
beneath eye<br />
inside mouth<br />
inside mouth<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
on or at base of fin<br />
on or at base of fin<br />
inside mouth<br />
in gill chamber<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
beneath eye<br />
inside mouth<br />
inside mouth<br />
beneath eye<br />
? between eyes, 8 and<br />
immature beneath<br />
eye<br />
in gill chamber<br />
on or at base of fin<br />
inside mouth<br />
in gill chamber<br />
on at base of fin<br />
inside mouth<br />
inside mouth<br />
inside mouth<br />
inside mouth<br />
on or at base of fin<br />
on or at base of fin<br />
9 between eyes, imma<br />
ture beneath eye<br />
inside mouth<br />
inside mouth
Agarna cumulus 173<br />
Fish host Cymothoid parasite Site of attachment<br />
Orthopristis ruber<br />
Paranthias Jurcifer<br />
Phaeoptyx conklini<br />
Phaeoptyx pigmentaria<br />
Pogonias cromis<br />
Pomacentrus partitas<br />
Priacanthus arenatus<br />
Scomberomorus cavalla<br />
Scomberomorus maculatus<br />
Scomberomorus regalis<br />
Selar crumenophthalmus<br />
Serranus tigrinus<br />
Sphoeroides maculatus<br />
Synodus foetens<br />
Anilocra haemuli<br />
Anilocra haemuli<br />
Mothocya bohlkeorum<br />
Mothocya bohlkeorum<br />
Nerocila acuminata<br />
Anilocra partiti<br />
Cymothoa oestrum<br />
Lironeca redmanni<br />
Lironeca redmanni<br />
Lironeca redmanni<br />
Cymothoa oestrum<br />
Renocila bowmani<br />
Renocila waldneri<br />
Nerocila acuminata<br />
Cymothoa excisa<br />
Agarna Schioedte and Meinert, 1883<br />
beneath eye<br />
beneath eye<br />
in gill chamber<br />
in gill chamber<br />
on or at base of fin<br />
beneath eye<br />
inside nouth<br />
in gill chamber<br />
in gill chamber<br />
in gill chamber<br />
inside mouth<br />
next to dorsal fin<br />
next to dorsal fin<br />
on or at base of fin<br />
inside mouth<br />
DIAGNOSIS Cephalon with posterior margin not trilobed; immersed in per-<br />
eonite 1. Antennular bases contiguous. Pereonites 4-7 on one side flattened<br />
and expanded; coxal plates of pereopods 4-7 also expanded and flattened<br />
but generally hidden by lateral expansion of pereonites. Bases of posterior<br />
three pereopods with well-formed carinae. Pleonites 1 and 2 immersed in<br />
pereonite 7; pleonites 2-5 with free fingerlike lateral margins.<br />
Agarna cumulus (Haller, 1880)<br />
Figure 79<br />
DIAGNOSIS 9 18 mm. Eyes present, indistinct. Pereon strongly "humped"<br />
dorsally. Uropod about { fo length of pleotelson; uropodal exopod slightly<br />
longer, and twice width of endopod. Pleotelson triangular, length 3 A basal<br />
width, apex rounded.<br />
RECORDS No host recorded: Key West, Florida.
174 FLABELLIFERA • GYMOTHOIDAE<br />
Figure 79. Agarna cumulus: A, 9, dorsal view; By 9, ventral view, coxal plates<br />
stippled.<br />
Anilocra Leach, 1818<br />
DIAGNOSIS Cephalon usually narrowed anteriorly to triangular apex folded<br />
ventrally between bases of antennules; posterior margin trilobed; not im<br />
mersed, or only weakly immersed in pereonite 1. Coxal plates small, com<br />
pact, not reaching level of posterior margin of their respective pereonites.<br />
Pereopods increasing in length posteriorly, pereopod 7 often markedly longer<br />
than 6. Pleon not immersed or only slightly immersed in pereonite 7.<br />
Pleopods 3—5 often formed into deep pockets or pleats. Uropods often ex<br />
tending beyond pleotelsonic apex.<br />
REMARKS Williams and Williams (1981) have provided a comprehensive<br />
treatment of this genus and nine of its species in the West Indies. Table 1 in<br />
this latter paper provides characters for separating these nine species. This<br />
table also indicates that for each species, the site of attachment of the adult to<br />
the host fish is specific, with six species attaching under the eye of the host.
Key to species of Anilocra<br />
Anilocra abudefdufi 175<br />
1. Pereopods 2-4 with swelling on outer margin of dactylus 2<br />
Pereopods 2-4 lacking swelling on outer margin of dactylus 5<br />
2. Body axis distorted by more than 10° holacanthi<br />
Body axis distorted by less than 5° 3<br />
3. Dactylus of pereopod 7 longer than propodus partiti<br />
Dactylus of pereopod 7 shorter than propodus 4<br />
4. Posteroventral angle of pereonite 7 overlapping pleonite 1 only<br />
Posteroventral angle of pereonite 7 overlapping pleonites 1 and 2<br />
abudefdufi<br />
chaetodontis<br />
5. Posteroventral angle of pereonite 7 produced 6<br />
Posteroventral angle of pereonite 7 not produced 7<br />
6. Uropod reaching posterior margin of pleotelson myripristis<br />
Uropod not reaching posterior margin of pleotelson haemuli<br />
7. Posteroventral angle of pereonite 7 overlapping pleonite 1 . . . . holocentri<br />
Posteroventral angle of pereonite 7 not overlapping pleonite 1 8<br />
8. Uropod reaching posterior margin of pleotelson acanthuri<br />
Uropod not reaching posterior margin of pleotelson chromis<br />
Anilocra abudefdufi Williams and Williams, 1981<br />
Figure 80A-C<br />
DIAGNOSIS Ovigerous 9 19.0-31.0 mm, 6 7.0-8.5 mm. Pereopods 2-4<br />
with swelling on outer margin of dactylus. Posteroventral angle of pereonite 6<br />
slightly produced, of pereonite 7 more produced, overlapping pleonite 1.<br />
Uropodal endopod variable, not reaching, to extending well beyond, apex of<br />
exopod. Color: upper lateral half to three-fourths of dorsal surface of 9 when<br />
attached to host is dark brown; rest of dorsal surface light brown to yellow.<br />
Attaching beneath eye of host.
176 FLABELLIFERA • GYMOTHOIDAE<br />
Figure 80. Anilocra abudefdufi: A, 9, lateral view; B, pereopod 3; C, pereopod 7.<br />
Anilocra acanthuri: D, pleotelson and uropods. Anilocra chaetodontis: E, 9, lateral<br />
view. Anilocra chromis: F, pleotelson and uropods.<br />
Anilocra acanthuri Williams and Williams, 1981<br />
Figure 80D<br />
DIAGNOSIS Ovigerous 9 29.0-40.0 mm, 6 4.0-8.0 mm. Pereopods 2-4<br />
without swelling on outer margin of dactylus. Posteroventral angles of per-<br />
eonites not produced. Uropod not reaching posterior margin of pleotelson.<br />
Endopod of uropod variable, not reaching, to extending well beyond, apex of<br />
exopod. Color: dorsal surface of 9 black to lead gray, ventral surface gray.<br />
Attaching under pectoral fin of host.<br />
RECORDS Doctorfish Acanthurus chirurgus: Florida Keys; Bahamas; Puerto<br />
Rico; U.S. Virgin Islands. Ocean surgeon Acanthurus bahianus: Florida Keys;<br />
Bahamas; Cuba; Jamaica; Dominican Republic; Puerto Rico; U.S. Virgin<br />
Islands.
Anilocra chaetodontis Williams and Williams, 1981<br />
Figure 80E<br />
Anilocra haemuli 177<br />
DIAGNOSIS Ovigerous 9 18-28 mm, 6 4-5 mm. Pereopods 2-4 with swell<br />
ing on outer margin of dactylus, Posteroventral angles of pereonites 4—7 be<br />
coming progressively produced, that of pereonite 7 overlapping pleonite 2.<br />
Uropod not reaching posterior margin of pleotelson; uropodal endopod ex<br />
tending beyond apex of exopod. Pleotelson as wide as long to slightly wider<br />
than long. Color: dorsal surface of 9 black to lead gray, ventral surface gray.<br />
Attaching beneath eye of host.<br />
RECORDS Foureye butterflyfish Chaetodon capistratus: Bahamas; Puerto Rico;<br />
British and U.S. Virgin Islands. Banded butterflyfish Chaetodon striatus:<br />
Bahamas; Puerto Rico; British Virgin Islands. Spotfin butterflyfish Chaetodon<br />
ocellatus: Bahamas; Puerto Rico; U.S. Virgin Islands. Reef butterflyfish<br />
Chaetodon sedentarius: Puerto Rico.<br />
Anilocra chromis Williams and Williams, 1981<br />
Figure 80F<br />
DIAGNOSIS Ovigerous 9 16-28 mm, 8 4-9 mm. Pereopods 2-4 lacking<br />
swelling on outer margin of dactylus. Posteroventral angles of pereonites not<br />
produced. Uropod extending beyond posterior margin of pleotelson; uropo<br />
dal endopod not reaching beyond exopod. Color: upper lateral one-fourth to<br />
two-thirds of dorsal surface of 9 when attached is dark gray, shading to off-<br />
white lower lateral area. Attaching beneath eye of host.<br />
RECORDS Brown chromis Chromis multilineatus: Puerto Rico; British and<br />
U.S. Virgin Islands. Blue chromis Chromis cyaneus: Bahamas; Dominican Re<br />
public. No host recorded: Anguilla.<br />
Anilocra haemuli Williams and Williams, 1981<br />
Figure 81A,B<br />
DIAGNOSIS Ovigerous 9 21-40 mm, 6 7 mm. Body axis distorted less than<br />
5°. Pereopods 2—4 lacking swelling on outer margin of dactylus. Postero<br />
ventral angle of pereonites 6 and 7 produced, latter overlapping pleonite 1.<br />
Uropod not reaching posterior margin of pleotelson; uropodal endopod<br />
reaching beyond apex of exopod. Color: dorsal surface of 9 yellow to light<br />
brown. Attaching beneath eye of host.
178 FLABELLIFERA • GYMOTHOIDAE<br />
Figure 81. Anilocra haemuli: A, 9, dorsal view; B, 9, lateral view. Anilocra<br />
holacanthi: C, 9. Anilocra holocentri: D, 9. Anilocra myripristis: E, pleotelson and<br />
uropods. Anilocra partiti: F, 9; G, pereopod 7.<br />
RECORDS French grunt Haemulon Jlavolineatum: Florida Keys; Puerto Rico;<br />
British and U.S. Virgin Islands. Tomtate Haemulon aurolineatum: Jamaica;<br />
Puerto Rico. Smallmouth grunt Haemulon chrysargyreum: Puerto Rico; U.S.<br />
Virgin Islands. Caesar grunt Haemulon carbonarium: Puerto Rico; U.S. Virgin
Anilocra holocentri 179<br />
Islands. Spanish grunt Haemulon macrostomum: Puerto Rico. White grunt<br />
Haemulon plumieri: Florida Keys; Yucatan Peninsula. Bluestriped grunt<br />
Haemulon sciurus: Florida Keys. Cora cora Orthopristis ruber: Margarita Island,<br />
Venezuela. Coney Epinephelus Julvus: Bahamas; Dominican Republic; Puerto<br />
Rico; U.S. Virgin Islands; Guadeloupe. Red hind Epinephelus guttatus: Puerto<br />
Rico; British and U.S. Virgin Islands. Graysby Epinephelus cruentatus:<br />
Bahamas; Dominican Republic; U.S. Virgin Islands. Creole-fish Paranthias<br />
jurcifer: Dominican Republic; Puerto Rico; Colombia. No host recorded:<br />
Cuba; Jamaica; Dominica; Barbados; Venezuela; Brazil.<br />
Anilocra holacanthi Williams and Williams, 1981<br />
Figure 81C<br />
DIAGNOSIS Ovigerous 9 21-33 mm, 6 4-7 mm. Body axis distorted by<br />
more than 10°. Pereopods 2-4 with swelling on outer margin of dactylus.<br />
Posteroventral angles of pereonites 5—7 progressively more produced, that of<br />
pereonite 7 overlapping pleonite 1. Uropod not reaching posterior margin of<br />
pleotelson; uropodal endopod reaching beyond apex of exopod. Color: dorsal<br />
surface of 9 black to lead gray. Attaching beneath eye of host.<br />
RECORDS Rock beauty Holacanthus tricolor: Bahamas; Jamaica; Dominican<br />
Republic; Puerto Rico; British and U.S. Virgin Islands.<br />
Anilocra holocentri Williams and Williams, 1981<br />
Figure 81D<br />
DIAGNOSIS Ovigerous 9 32-46 mm, 6 5-9 mm. Body axis distorted less<br />
than 5°. Pereopods 2—4 lacking swelling on outer margin of dactylus.<br />
Posteroventral angle of pereonite 7 produced, overlapping pleonite 1.<br />
Uropod not reaching posterior margin of pleotelson; uropodal endopod<br />
reaching beyond apex of exopod. Color: dorsal surface of 9 dark brown,<br />
ventral surface light brown. 9 attaching between eyes of host; 6 or transi<br />
tional stage beneath eye.<br />
RECORDS Squirrelfish Holocentrus ascensionis: Puerto Rico; U.S. Virgin<br />
Islands.<br />
No host recorded: Patagonia, Straits of Magellan.
180 FLABELLIFERA • CYMOTHOIDAE<br />
Anilocra myripristis Williams and Williams, 1981<br />
Figure 81E<br />
DIAGNOSIS Ovigerous 9 29-40 mm, 6 6-7 mm. Body axis distorted less<br />
than 5°. Pereopods 2-4 lacking swellings on outer margin of dactylus.<br />
Posteroventral angle of pereonites 6 and 7 produced, latter overlapping<br />
pleonite 1. Uropod reaching beyond posterior margin of pleotelson; uropodal<br />
endopod reaching beyond apex of exopod. Color: dorsal surface of 9 light<br />
reddish brown, ventral surface yellow. 9 attaching between eyes of host;<br />
immature or transitional forms sometimes beneath eve.<br />
4<br />
RECORDS Blackbar soldierfish Myripristis jacobus: Bahamas; Dominican Re<br />
public; Puerto Rico.<br />
Anilocra partiti Williams and Williams, 1981<br />
Figure 81F,G<br />
DIAGNOSIS Ovigerous 9 12-16 mm, transitional 7.6-9.0 mm. Body axis<br />
distorted less than 5°. Pereopods 2-4 with swelling on outer margin of dac<br />
tylus. Pereopod 7 with dactylus longer than propodus. Posteroventral angle<br />
of pereonite 7 produced, overlapping pleonite 1. Uropod not reaching pos<br />
terior margin of pleotelson; uropodal endopod not reaching apex of exopod.<br />
Color: dorsal surface black to slate gray. Attaching beneath eye of host.<br />
RECORDS Bicolor damselfish Pomacentrus partitas: Jamaica.<br />
Ceratothoa Dana, 1852<br />
DIAGNOSIS Cephalon more or less immersed in pereonite 1, posterior mar<br />
gin not trisinuate. Bases of antennules expanded, contiguous. Coxal plates<br />
compact; anterior plates not extending beyond posterior margins of their<br />
respective pereonites; posterior coxal plates may or may not be produced<br />
beyond the posterior margins of the pereonites. Anterior pleonites narrowed,<br />
immersed in pereonite 7, Copulatory stylet lacking on pleopod 2 of 6 of some<br />
species.<br />
Ceratothoa deplanata Bovallius, 1885<br />
Figure 82A<br />
DIAGNOSIS 9 18 mm. Cephalon subtriangular, anterior margin rounded.<br />
Pereopods 4—7 with strongly carinate bases. Uropod reaching or extending
Ceratothoa deplanata 181<br />
Figure 82. A, Ceratothoa deplanata (from Bovallius, 1885); B, Cymothoa caraibica; C,<br />
Cymothoa excisa; D, Cymothoa oestrum.<br />
slightly beyond posterior margin of pleotelson; rami subequal in length and<br />
width. Pleotelson basally wider than long, posterior margin broadly<br />
rounded. Color: bright yellow.<br />
RECORDS Haiti, host not recorded.
182 FLABELUFERA • GYMOTHOIDAE<br />
Cymothoa Fabricius, 1793<br />
DIAGNOSIS Body usually not distorted. Cephalon with posterior margin not<br />
trilobed; more or less immersed in pereonite 1; latter with anterolateral cor<br />
ners produced to embrace cephalon. Bases of antennules not expanded, well<br />
separated. Anterior coxal plates not reaching posterior borders of their re<br />
spective pereonites, posterior coxal plates nearly reaching or extending be<br />
yond posterior borders of pereonites. Pleon narrower than, and immersed in<br />
pereonite 7. Pleonites increasing in length and width posteriorly.<br />
Key to species of Cymothoa<br />
1. Anterolateral angles of pereonite 1 reaching to half length of cephalon<br />
or less; eyes or traces of eyes present 2<br />
Anterolateral angles of pereonite 1 broad, reaching to anterior margin<br />
of cephalon; eyes absent oestrum<br />
2. Anterolateral angles of pereonite 1 narrow, subacute excisa<br />
Anterolateral angles of pereonite 1 broad, rounded caraibica<br />
Cymothoa caraibica Bovallius, 1885<br />
Figure 82B<br />
DIAGNOSIS 9 17 mm, 6 12-16 mm. Anterior margin of cephalon broadly<br />
rounded. Eyes large, distinct. Broadly rounded anterolateral angles of per<br />
eonite 1 reaching to about midlength of cephalon. Bases of pereopods 4—7<br />
with strong, rounded carina. Uropodal rami subequal in length, equal to<br />
peduncle in length. Pleotelson width about twice length, posterolateral mar<br />
gin broadly rounded.<br />
RECORDS Puerto Rico; Gulf of Mexico.<br />
Cymothoa excisa Perty, 1833<br />
Figure 82C<br />
DIAGNOSIS Ovigerous 9 20-24 mm. Anterior margin of cephalon in dorsal<br />
view truncate to slightly excavate; eyes small, indistinct. Anterolateral angles<br />
of pereonite 1 narrowly rounded to subacute, reaching anteriorly to about<br />
midlength of cephalon. Pereopods 4—7 with high rounded carina on basis.
Glossobius 183<br />
Uropods hardly reaching halfway along lateral margin of pleotelson; exopod<br />
slightly longer than endopod. Pleotelson about twice wider than long;<br />
broadly rounded and somewhat bilobed.<br />
RECORDS Yellowtail snapper Ocyurus chrysurus: Yucatan Peninsula, Mexico;<br />
Carrie Bow Cay, Belize; Margarita Island, Venezuela; Panama. Mutton<br />
snapper Lutjanus analis: Yucatan Peninsula, Mexico; Panama. Lane snapper<br />
Lutjanus synagris: Panama. Mahogany snapper Lutjanus mahogoni: Panama.<br />
Pigfish Orthopristis chrysoptera: Florida, Gulf of Mexico. Spot Leiostomus<br />
xanthurus: Texas, Gulf of Mexico. Spotted seatrout Cynoscion nebulosus: Texas,<br />
Gulf of Mexico. Inshore lizardfish Synodusfoetens: Texas, Gulf of Mexico. No<br />
host recorded: Massachusetts; South Carolina; Georgia; Florida Keys;<br />
Bahamas; Cuba; Trinidad; Brazil.<br />
Cymothoa oestrum (Linnaeus, 1793)<br />
Figure 82D<br />
DIAGNOSIS Ovigerous 9 38 mm. Cephalon in dorsal view with ante<br />
rolateral angles rounded, anterior margin slightly excavate; eyes absent. An<br />
terolateral angles of pereonite 1 expanded, broadly rounded, reaching to<br />
level of anterior margin of cephalon. Pereonites 4—7 with high rounded<br />
carina on basis. Uropod reaching posteriorly beyond midlength of<br />
pleotelson; exopod slightly longer than endopod. Pleotelson length slightly<br />
more than half basal width.<br />
RECORDS Bigeye scad Selar crumenophthalmus: Bermuda; U.S. Virgin Is<br />
lands. Bigeye Priacanthus arenatus: Bermuda. Bar jack Caranx ruber: Florida<br />
Keys; Carrie Bow Cay, Belize. Horse-eye jack Caranx latus: Bahamas; Bar<br />
bados. Crevalle jack Caranx hippos: Venezuela. Jack Caranx sp.: Jamaica; Cu<br />
rasao. Hind Epinephelus sp.: Grenada. Parrotfish: Jamaica. Seatrout Cynoscion<br />
sp.: Panama. Tarpon Megalops atlantica: Texas, Gulf of Mexico. No host re<br />
corded: Honduras; Haiti.<br />
Glossobius Schioedte and Meinert, 1883<br />
DIAGNOSIS Cephalon not immersed in pereonite 1; excavate on either side<br />
in anterior half, forming broad and anteriorly rounded median area; anten<br />
nae fitting into excavate areas. Bases of antennules contiguous, expanded.<br />
Antennules broader and longer than antennae. Bases of pereopods 4—7 with<br />
posterior margin expanded and flattened. Pleonites 1-3 immersed in per<br />
eonite 7.
184 FLABELLIFERA • CYMOTHOIDAE<br />
Key to species of Glossobius<br />
1. Coxal plates of perconites 1 and 2 anteroventrally protruding impressus<br />
Coxal plates of pereonites 1 and 2 close to body, not protruding<br />
Glossobius hemiramphi Williams and Williams, 1985a<br />
Figure 83A<br />
hemirhamphi<br />
DIAGNOSIS Ovigerous 9 27 mm. Eyes small but distinct. Cephalon pointed<br />
anteriorly. Fused coxa of pereonite 1 and free coxa of pereonite 2 carinate but<br />
not protruding. Coxa of pereonite 7 semicircular in dorsal view. Pleotelson<br />
with middorsal length more than half basal width; lateral margins somewhat<br />
tapered; posterior margin variable, sinuate or excavate. Uropods reaching to<br />
or slightly beyond posterior pleotelsonic margin; rami subequal in length,<br />
exopod slightly broader than endopod.<br />
RECORDS Ballyhoo Hemiramphus brasiliensis; Puerto Rico.<br />
Glossobius impressus (Say, 1818)<br />
Figure 83B<br />
DIAGNOSIS Ovigerous 9 33 mm. Eyes small but distinct. Cephalon<br />
rounded anteriorly. Fused coxal plate of pereonite 1 and distinct coxal plate<br />
of pereonite 2 protruding strongly in oblique anteroventral direction. Uropod<br />
reaching to posterior half of pleotelson; exopod shorter and narrower than<br />
endopod. Pleotelson basal width twice length, posteriorly broadly bilobed.<br />
Attaching inside mouth of host.<br />
RECORDS Flyingfish Exocoetus spp.: Rio de Janeiro, Brazil; North Atlantic,<br />
especially in the Gulf Stream.<br />
Mirrorwing flyingfish Hirundichthys speculifer: North Atlantic. No host rec<br />
ord: Senegal, West Africa.<br />
Kuna Williams and Williams, 1986<br />
DIAGNOSIS Cephalon somewhat immersed in pereonite 1. Anterior margin<br />
of pereonite 1 not trisinuate. Number of articles in antennules and antennae
Kuna 185<br />
Figure 83. A, Glossobius hemiramphi; B, Glossobius impressus; C, Kuna insularis; D,<br />
Lironeca redmani; E, Lironeca tenuistylis.<br />
reduced. Antennule somewhat expanded; basal article expanded but not<br />
contiguous. Copulatory stylet present on pleopods 1-3 in 6. Pleonites dor-<br />
sally strongly convex, not immersed in pereonite 7.
186 FLABELLIFERA • GYMOTHOIDAE<br />
Kuna insularis (Williams and Williams, 1985b)<br />
Figure 83C<br />
DIAGNOSIS Ovigerous 9 11.1-17.2 mm, 6 4.2-8.7 mm, transitional 9.6-<br />
9.8 mm. Antennules and antennae consisting of four articles each. Uropods<br />
short, not reaching posterior margin of pleotelson. Clavate eopulatory stylet<br />
present on pleopods 1—3 in 6. Pleotelson basally broader than long, pos<br />
terior margin broadly rounded.<br />
RECORDS Sergeant major Abudefdufsaxatilis: Carrie Bow Cay, Belize; Cura<br />
sao; Panama.<br />
Lironeca Leach, 1818<br />
DIAGNOSIS Cephalon weakly to deeply immersed in pereonite 1; posterior<br />
border rarely trisinuate. Bases of antennules not expanded, well separated.<br />
Posterior pereopods with carinae on bases in 6, carinae present or absent in<br />
9. Pleonites subequal in width; pleonites 1 and 2 rarely narrowed and<br />
weakly to moderately immersed in pereonite 7. Pleopods highly folded, and<br />
with lamellar or digitiform accessory gills in some species.<br />
Key to species of Lironeca<br />
1. Uropodal endopod about twice longer than wide; pleon somewhat<br />
immersed in pereon redmanni<br />
Uropodal endopod about three times longer than wide; pleon barely<br />
immersed in pereon tenuistylis<br />
Lironeca redmanni Leach, 1818<br />
Figure 83D<br />
DIAGNOSIS Ovigerous 9 19.5-25.0 mm. Cephalon barely immersed in per<br />
eonite 1. Pleon somewhat immersed in pereon, but lateral margins of pleonite<br />
1 free. Pleotelson basally wider than long. Uropodal rami reaching well be<br />
yond posterior margin of pleotelson; exopod longer than endopod, both rami<br />
somewhat broad, endopod about twice longer than wide. Attaching to gills of<br />
host.
Mothocya 187<br />
RECORDS New Jersey to Florida; gills of kingfish, Jamaica; Cuba; St.<br />
Christopher; Spanish mackerel Scomberomorus maculatus and cero Scomberomorus<br />
regalisy Puerto Rico; king mackerel Scomberomorus cavalla, Colombia; Genes rho-<br />
mbeus, Panama; spot Leiostomus xanthurus, Gulf of Mexico.<br />
Brazil.<br />
Lironeca tenuistylis (Richardson, 1912b)<br />
Figure 83E<br />
DIAGNOSIS 9 13 mm. Cephalon barely immersed in pereonite 1. Uropodal<br />
rami reaching beyond rounded posterior margin of pleotelson; exopod longer<br />
than endopod; endopod slender, about three times longer than wide. Pleonite<br />
1 barely immersed in pereonite 7. Pleotelson basally wider than long. Attach<br />
ing to host between pectoral and anal fin.<br />
RECORDS Longnose anchovy Anchoa lamprotaenia: Panama.<br />
Mothocya Costa, 1851<br />
DIAGNOSIS Cephalon more or less immersed in pereonite 1. Bases of anten-<br />
nules widely separated; antennules longer and more robust than antennae.<br />
Coxae nearly reaching or extending beyond posterior margin of respective<br />
pereonites. Pleon somewhat immersed in pereonite 7. Uropodal exopod<br />
longer than endopod.<br />
REMARKS Bruce (1986b) revised the genus Mothocya, The species of Moth<br />
ocya are almost entirely gill parasites on the fish families Hemiramphidae,<br />
Apogonidae, Belonidae, and Atherinidae.<br />
Key to species of Mothocya<br />
1. Cephalon anteriorly narrowed, slightly immersed in pereonite 1;<br />
pleotelson subrectangular bohlkeorum<br />
Cephalon anteriorly broad, deeply immersed in pereonite 1; pleotelson<br />
subtriangular nana
188 FLABELLIFERA • GYMOTHOIDAE<br />
Mothocya bohlkeorum Williams and Williams, 1982<br />
Figure 84B<br />
DIAGNOSIS Ovigerous 9 7.6-8.5 mm, 6 3.7 mm. Cephalon anteriorly nar<br />
rowed in dorsal view, ventrally flexed, broadly rounded; slightly immersed in<br />
pereonite 1. Pleotelson subrectangular. Uropods extending slightly beyond<br />
posterior margin of pleotelson; exopod only slightly longer than endopod. 9<br />
lateral lobes of pleopodal peduncles not developed. Endopods of pleopods 3-<br />
5 with small proximomedial lobe.<br />
RECORDS Whitestar cardinalfish Apogon lachneri: Puerto Rico. Dusky car-<br />
dinalfish Phaeoptyx pigmentaria: Bahamas. Freckled cardinalfish Phaeoptyx con-<br />
klini: Florida Keys; Bahamas. Conchfish <strong>As</strong>trapogon stellatus: Leeward<br />
Islands.<br />
Mothocya nana (Schioedte and Meinert, 1884)<br />
Figure 84A<br />
DIAGNOSIS Ovigerous 9 11.0-17.0 mm, 6 7.9-8.3 mm. Cephalon deeply<br />
immersed in pereonite 1; rostrum anteroventrally narrowly rounded. Uropo-<br />
dal exopod markedly longer than endopod. Pleotelson broad, with posterior<br />
margin rounded sufficiently to give appearance of being subtriangular.<br />
RECORDS Halfbeak Hyporhamphus unifasciatus: Chesapeake Bay, Maryland;<br />
Georgia; Florida; Colon, Panama. Halfbeak Hemiramphus bermudensis:<br />
Bermuda.<br />
Nerocila Leach, 1818<br />
DIAGNOSIS Body generally more depressed than in most cymothoid genera,<br />
rarely curved. Cephalon with anterior margin convex, narrowly rounded, or<br />
concave; not, or only slightly, immersed in pereonite 1. Pereonite 1 anterior<br />
margin trisinuate. Posterolateral angles of pereonites weakly to strongly pro<br />
duced, increasing in length posteriorly. Coxal plates prominent, usually al<br />
most reaching or extending to posterior margin of their respective pereonites.<br />
Juveniles and 6 usually with spines on posterior pereopods; 9 lacking these<br />
spines. Pleon not immersed in pereonite 7. Pleonites subequal in length;<br />
pleonites 1 and 2 usually produced posterolateral^. Pleopods typically with<br />
small lamellar accessory gills; pleopods 3-5 folded into deep pockets or<br />
pleats. Uropods usually extending beyond pleotelsonic apex.
Figure 84. A, Mothocya nana; By Mothocy<br />
acuminata; D, Nerocila acuminata f. aster.<br />
B
190 FLABELLIFERA • CYMOTHOIDAE<br />
Nerocila acuminata Schioedte and Meinert, 1881<br />
DIAGNOSIS Ovigerous 9 16.2-19.0 mm. Cephalon with anterior margin<br />
convex. Posterolateral angles of all, or of posterior pereonites only, produced<br />
into acute or subacute angles.<br />
RECORDS Striped burrfish Chilomycterus schoepfi: Texas, Gulf of Mexico.<br />
Northern puffer Sphoeroides maculatus: New York. Striped mullet Mugil<br />
cephalus: Texas, Gulf of Mexico. Jewfish Epinephelus itajara: Texas, Gulf of<br />
Mexico. Hogfish: Bermuda. Alligator gar Lepisosteus spatula: Louisiana, Gulf<br />
of Mexico. Hardhead catfish Ariusfelis: Texas, Gulf of Mexico. Sawfish: Flor<br />
ida (Atlantic). Black drum Pogonias cromis: Texas, Gulf of Mexico. Orange<br />
filefish Alutera schoepfi: Texas, Gulf of Mexico. Toadfish Batrachoides sur-<br />
inamensis: Colon, Panama. Spot Leiostomus xanthurus: Florida, Gulf of Mexico.<br />
Spadefish Chaetodipterus faber: Florida, Gulf of Mexico; Virginia. Fringed fil<br />
efish Monacanthus ciliatus: Florida, Gulf of Mexico. No host recorded: Mas<br />
sachusetts; Florida Keys; Florida, Gulf of Mexico. Louisiana, Gulf of Mex<br />
ico. Texas, Gulf of Mexico.<br />
REMARKS Brusca (1981) has shown that this highly variable species occurs<br />
on both sides of the Isthmus of Panama, in two relatively distinct forms.<br />
Intergrades between the two forms do occur but are uncommon. Brusca<br />
(1981:159) also lists all the host-records for this species in the eastern Pacific.<br />
Nerocila acuminata Schioedte and Meinert, 1881, forma acuminata<br />
Figure 84C<br />
DIAGNOSIS Cephalon width equal to or greater than length; frontal margin<br />
narrowly rounded. Posterolateral angles of anterior pereonites weakly pro<br />
duced, rounded to subacute; of posterior pereonites more strongly produced,<br />
subacute to acute. Coxal plates 3—7, 4—7, or 5—7 with acute posterolateral<br />
angles; coxae rarely reaching beyond posterior margins of their respective<br />
pereonites.<br />
Nerocila acuminata Schioedte and Meinert, 1881, forma aster<br />
Figure 84D<br />
DIAGNOSIS Cephalon always wider than long; anterior margin broadly<br />
rounded. Posterolateral angles of all pereonites strongly produced, acute, all<br />
reaching well beyond posterior margins of their respective pereonites. Coxal<br />
plates 2—7 strongly produced with acute posterior angles.
Renocila Miers, 1880<br />
Renocila colini 191<br />
DIAGNOSIS Body rarely curved. Cephalon anteriorly weakly to distinctly<br />
truncate. Antennular bases well separated. Antennules and antennae some<br />
what flattened, antennules usually broader and longer than antennae. Per-<br />
eonites 5—7 with posterolateral corners more or less strongly produced.<br />
Pleonites not laterally incised.<br />
REMARKS Williams and Williams (1980) provide a key to nine species of<br />
Renocila.<br />
Key to species of Renocila<br />
1. Posteroventral angle of pereonite 7 reaching pleonite 1 colini<br />
Posteroventral angle of pereonite 7 reaching beyond pleonite 1 2<br />
2. Dorsal surface of body brown; posteroventral angle of pereonite 7<br />
reaching pleonite 2 waldneri<br />
Dorsal surface of body black; posteroventral angle of pereonite 7<br />
reaching pleonite 3 bowmani<br />
Renocila bowmani Williams and Williams, 1980<br />
Figure 85A<br />
DIAGNOSIS 9 18.0 mm, 6 11.5 mm. Posteroventral angles of pereonites 5—<br />
7 produced, that of pereonite 7 overlapping pleonites 1-3. Pereopods 1—3<br />
lacking swelling on dactylus. Pereopods 6-7 subequal in length. Uropodal<br />
exopod longer than endopod. Pleotelson length 3 A basal width. Color: dorsal<br />
surface of body and appendages uniform black. Attached to dorsum of body<br />
close to dorsal fin.<br />
RECORDS Harlequin bass Serranus tigrinus: Dominican Republic.<br />
Renocila colini Williams and Williams, 1980<br />
Figure 85B,C<br />
DIAGNOSIS Ovigerous 9 12.0-17.5 mm, 6 7.5-13.0 mm. Pereonites 5-7<br />
with posteroventral angle produced, that of pereonite 7 overlapping pleonite<br />
1 only. Pereopods 1—3 lacking swelling on dactyli; pereopods 6—7 subequal in<br />
length. Uropod reaching beyond pleotelson, endopod more than half length
192 FLABELLIFERA • CYMOTHOIDAE<br />
c<br />
Figure 85. A, Renocila bowmani. Renocila colini: B, 9; C, d. D> Renocila waldneri.<br />
of exopod. Pleotelson l /i to 72 wider than long, with slight rounded apex<br />
Color: dorsal surface of body and appendages uniformly yellowish brown<br />
Attached to dorsum of body, close to dorsal fin.
FLABELLIFERA • L1MNOR11DAE 193<br />
RECORDS Flamefish Apogon maculatus: Puerto Rico. Belted cardinalfish Ap-<br />
ogon townsendi: Puerto Rico.<br />
Renocila waldneri Williams and Williams, 1980<br />
Figure 85D<br />
DIAGNOSIS Ovigerous 9 15.3-19.3 mm, 6 5.0-10.8 mm. Posteroventral<br />
angle of pereonite 5 moderately produced, of pereonites 6-7 more strongly<br />
produced, that of pereonite 7 overlapping pleonites 1 and 2. Pereopods 1-3<br />
without swelling on dactyli. Pereopods 6 and 7 subequal in length. Uropodal<br />
exopod slightly longer than endopod. Pleotelson basally wider than long;<br />
posterior margin broadly and evenly rounded. Color: dorsal surface of body<br />
uniform brown; appendages yellowish brown. Attached to dorsum of body<br />
close to dorsal fin.<br />
RECORDS Harlequin bass Serranus tigrinus: Dominican Republic.<br />
Family Limnoriidae Harger, 1879<br />
DIAGNOSIS Body ovate in cross section, often becoming more setose posteri<br />
orly. Cephalon subspherical, freely articulating with pereonite 1; eyes lateral.<br />
Antennules and antennae well separated at bases. Mandible with strong in<br />
cisor; lacking molar and well-defined lacinia mobilis, but with species-<br />
distinctive lacinioid bristle or seta; palp usually of three articles. Maxillipe-<br />
dal palp of five articles; endite well developed. Coxae present on pereonites<br />
2-7. Pleon consisting of five free pleonites plus pleotelson; latter subcircular,<br />
set obliquely to axis of body, usually with anterolateral crests. Uropod with<br />
strong protopod inserted ventrolaterally.<br />
Key to genera of Limnoriidae<br />
1. Uropodal rami very unequal 2<br />
Uropodal rami subequal Paralimnoria<br />
2. Mandibular incisors possessing rasp and file Limnoria<br />
Mandibular incisors lacking rasp and file Phycolimnoria
194 FLABELLIFERA • LIMNORIIDAE<br />
REMARKS This family includes a number of species that are of considerable<br />
economic importance. Given that species of Limnoria are wood borers,<br />
wooden structures such as wharf pilings that are immersed in sea water and<br />
even in water of reduced salinity are vulnerable to attack by these gribbles.<br />
Prolonged exposure can lead to weakening and eventual collapse of these<br />
structures (see Ray, 1959). Even creosote-treated wood is not fully protected;<br />
Limnoria tuberculata will bore into such wood to where the creosote has not<br />
penetrated.<br />
The isopods rasp at the wood fibres with the rasp and file structures of the<br />
mandibles, usually following the grain of the wood. With this boring activity,<br />
saprophytic fungi and bacteria invade the wood and assist in the breakdown<br />
process. Limnoria lack cellulase-secreting microflora in their gut, but proba<br />
bly secrete a cellulase themselves (Boyle and Mitchell, 1978). It is also prob<br />
able that the fungi and bacteria, the latter often densely aggregated on the<br />
setae of the isopod, form part of the animals' diet. In the natural environ<br />
ment, Limnoria perform an important role in the breakdown of dead wood,<br />
especially in mangrove areas.<br />
Sexual dimorphism of the pleotelson does occur in some species. This as<br />
pect of the morphology, however, has hardly been investigated.<br />
Limnoria Leach, 1814<br />
DIAGNOSIS Antennular flagellum of four articles. Antennal flagellum of<br />
three to five articles. Incisor of right mandible equipped with filelike struc<br />
ture on upper surface; incisor of left mandible with rasplike structure. Rami<br />
of pleopod 5 lacking marginal setae. Uropodal exopod much shorter than<br />
endopod, bearing terminal claw. Pleotelson smooth, or variously ornamented<br />
with tubercles and ridges.<br />
Limnoria indica Becker and Kampf, 1958<br />
Figure 86A,B<br />
DIAGNOSIS 6 3.0 mm, ovigerous 9 3.0 mm. Pleonite 5 with submedian pair<br />
of strong rounded ridges, converging slightly posteriorly. Pleotelson basally<br />
with two pairs of submedian tubercles and pair of lateral tubercles.<br />
RECORDS Cozumel, Mexico; Man o'War Cay, Belize.<br />
India; Hong Kong; Philippines; east coast of Australia.
Key to species of Limnoria<br />
Limnoria insulae 195<br />
1. Dorsal surface of pleotelson lacking prominent tubercles, ridges, or<br />
carinae (L. simulata may appear to lack ornamentation; in this<br />
species the tubercles are very small) 2<br />
Dorsal surface of pleotelson bearing tubercles, ridges, or carinae .... 3<br />
2. Pleotelson flat; pleonite 5 with broadly rounded middorsal ridge<br />
Pleotelson cup shaped; pleonite 5 with strong narrowly rounded<br />
platycauda<br />
middorsal ridge insulae<br />
3. Pleotelson with basal tubercles but lacking ridges 4<br />
Pleotelson with ridges but lacking freestanding tubercles 7<br />
4. 6 pleotelson with single strong middorsal tubercle unicornis<br />
Pleotelson with more than one basal tubercle 5<br />
5. Pleotelson with three basal tubercles tuberculata<br />
Pleotelson with more than three basal tubercles 6<br />
6. Pleotelson with four basal tubercles in line (difficult to detect) simulata<br />
6 pleotelson with six basal tubercles indica<br />
7. Pleotelson with single middorsal longitudinal ridge multipunctata<br />
Pleotelson with two rounded basal ridges 8<br />
8. Pleonite 5 with strong Y-shaped ridge Pfeffe<br />
Pleonite 5 with two posteriorly converging ridges saseboensis<br />
Limnoria insulae Menzies, 1957<br />
Figure 86C<br />
DIAGNOSIS 6 3.0 mm, ovigerous 9 3.4 mm. Pleonite 5 with strong middor<br />
sal ridge. Pleotelson cup shaped, lateral crests extended anteromesially, sep<br />
arated basally by distinct gap; posterior margin and lateral crests not<br />
tuberculate.<br />
RECORDS Twin Cays, Belize.
196 FLABELLIFERA • LIMNORIIDAE<br />
200HM<br />
Figure 86. Limnoria indica: A, pi 6;B, pi 9. Limnoria insulae: C<br />
pleotelson. Limnoria multipunctata: D, pleotelson in oblique-lateral view<br />
L i m n o r i a m u l t i p u n c t a t a M e n z i e s , 1 9 5 7<br />
F i g u r e s 8 6 D ; 8 7 A<br />
D I A G N O S I S 6 2 . 8 m m , o v i g e r o u s 9 3 . 0 m m . P l e o n i t e 5 d o r s a l l y s m o o t h .<br />
P l e o t e l s o n w i t h m i d d o r s a l l o n g i t u d i n a l r o u n d e d r i d g e b e a r i n g s e v e r a l
Limnoria multipunctata 197<br />
Figure 87. Limnoria multipunctata: A, pleotelson; Limnoria pfefferi: B} pleotelson<br />
Limnoria platycauda: C, pleotelson; Limnoria saseboensis: D> pleotelson; Limnoria<br />
simulata: E, pleotelson; Limnoria tuberculata: F, pleotelson.<br />
b u t t o n - s h a p e d t u b e r c l e s i n p o s t e r i o r h a l f ; p o s t e r i o r m a r g i n a n d l a t e r a l c r e s t s<br />
t u b e r c u l a t e .<br />
R E C O R D S P u e r t o R i c o ; J a m a i c a ; T w i n C a y s , B e l i z e<br />
J a p a n ; K a i I s l a n d s , S o u t h P a c i f i c .
198 FLABELLIFERA • LIMNORIIDAE<br />
Limnoria pfefferi Stebbing, 1904<br />
Figure 87B<br />
DIAGNOSIS 8 3.8 mm, ovigerous 9 4.0 mm. Pleonite 5 with conspicuous<br />
middorsal Y-shaped carina. Pleotelson basally with pair of submedian<br />
rounded ridges; lateral crests lacking tubercles.<br />
RECORDS Florida Keys; Bahamas; Puerto Rico; U.S. Virgin Islands; Twin<br />
Cays and Man o'War Cay, Belize; Yucatan Peninsula, Mexico.<br />
Minikoi Atoll and Aldabra Atoll, Indian Ocean; Philippines; New Guinea;<br />
Panama.<br />
Limnoria platycauda Menzies, 1957<br />
Figure 87C<br />
DIAGNOSIS 6 2.5 mm, ovigerous 9 2.6 mm. Pleonite 5 with broad middor<br />
sal longitudinal rounded ridge. Pleotelson lacking dorsal ornamentation;<br />
posterior margin and lateral crests bearing tubercles.<br />
RECORDS Cuba; Puerto Rico to Curasao; Cozumel, Mexico; Twin Cays<br />
and Man o'War Cay, Belize.<br />
Aldabra Atoll, Indian Ocean.<br />
Limnoria saseboensis Menzies, 1957<br />
Figure 87D<br />
DIAGNOSIS 6 3.5 mm. Pleonite 5 with submedian pair of ridges, converging<br />
slightly posteriorly. Pleotelson basally with submedian pair of anteriorly tu-<br />
berculate ridges; posterior margin and lateral crests tuberculate.<br />
RECORDS Miami, Florida.<br />
Japan; Fiji.<br />
Limnoria simulata Menzies, 1957<br />
Figure 87E<br />
DIAGNOSIS 6 3.8 mm, ovigerous 9 4.0 mm. Pleonite 5 with obscure me<br />
dian longitudinal groove. Pleotelson basally with submedian pair of tubercles<br />
and small lateral tubercles, latter often difficult to detect; lateral crests<br />
tuberculate.<br />
RECORDS Florida Keys; U.S. Virgin Islands; Gulf of Mexico.
Limnoria tuberculata Sowinsky, 1884<br />
Figure 87F<br />
Paralimnoria andrewsi 199<br />
DIAGNOSIS 6 2.8 mm, ovigerous 9 3.0 mm. Pleonite 5 with two anterior<br />
tubercles, one middorsal posterior tubercle, area between tubercles de<br />
pressed. Pleotelson basally with middorsal tubercle, followed by pair of sub-<br />
median tubercles, all three tubercles having short obscure carina; posterior<br />
margin and lateral crests tuberculate.<br />
RECORDS Rhode Island to Venezuela; Cuba; Man o'War Cay, Belize; Gulf<br />
of Mexico.<br />
Uruguay; West Africa; Mediterranean; Black Sea; India; Hong Kong;<br />
Hawaii; Australia; California.<br />
REMARKS This species has frequently been recorded under the name Lim<br />
noria tripunctata Menzies, 1951a.<br />
Limnoria unicornis Menzies, 1957<br />
Figure 88A,B<br />
DIAGNOSIS 6 2.6 mm, ovigerous 9 2.6 mm. Mandibular palp of one arti<br />
cle. Pleonite 5 with somewhat obscure Y-shaped ridge middorsally.<br />
Pleotelson in 6 with strong basal slightly curved middorsal tubercle; lateral<br />
crests lacking tubercles.<br />
RECORDS Bahamas; Man o'War Cay and Twin Cays, Belize.<br />
Caroline Islands; Palau; Society Islands.<br />
Paralimnoria Menzies, 1957<br />
DIAGNOSIS Antennular flagellum of five articles. Antennal flagellum of five<br />
or six articles. Mandibular incisor with rasp and file. Pleopod 5, rami bearing<br />
marginal setae. Uropodal rami subequal in length, each with clawlike apex.<br />
Paralimnoria andrewsi (Caiman, 1910)<br />
Figure 88C,D<br />
DIAGNOSIS 6 2.6 mm, 9 2.6 mm. Pleonite 5 with or without triangular<br />
middorsal depressed area. Pleotelson with basal submedian pair of tubercles<br />
either obscurely or strongly carinate; lateral crest tubercles of variable<br />
strength.
200 FLABELLIFERA • LIMNORIIDAE<br />
Figure 88. Limnoria unicornis: A, pleotelson, 8; B, pleon, 6*, in lateral view.<br />
Paralimnoria andrewsi: C, p l e o n i t e 5 a n d p l e o t e l s o n ; D , u r o p o d . Phycolimnoria clarkae:<br />
E , p l e o n i t e 5 a n d p l e o t e l s o n ; F , u r o p o d a n d p l e o t e l s o n i n l a t e r a l v i e w .<br />
R E C O R D S F l o r i d a K e y s ; P u e r t o R i c o ; T w i n C a y s , B e l i z e ; C u r a g a o<br />
C h r i s t m a s I s l a n d s , I n d i a n O c e a n ; S a m o a ; H a w a i i ; J a p a n .<br />
R E M A R K S M e n z i e s ( 1 9 5 7 ) d i s c u s s e s t h r e e f o r m s o f t h i s s p e c i e s : F o r m a typ-<br />
i c a , w h i c h l a c k s a c e n t r a l d e p r e s s e d a r e a d o r s a l l y o n p l e o n i t e 5 a n d h a s a p a i r<br />
o f s u b m e d i a n o b s c u r e l y c a r i n a t e t u b e r c l e s o n t h e p l e o t e l s o n ; F o r m a A , w h i c h<br />
h a s a t r i a n g u l a r d e p r e s s e d a r e a d o r s a l l y o n p l e o n i t e 5 a n d a p a i r o f s u b m e -
FLABELLIFERA • SEROLIDAE 201<br />
dian tubercles supported by strong carinae on the pleotelson; Forma B5 hav<br />
ing a triangular depressed area dorsally on pleonite 5 and an obscurely cari-<br />
nate pair of tubercles on the pleotelson. Given that at least two of these forms<br />
have been recorded occurring together, it would seem that this is merely a<br />
highly variable species.<br />
Phycolimnoria Menzies, 1957<br />
DIAGNOSIS Mandibular incisor lacking rasp and file. Uropodal rami une<br />
qual, exopod longer than endopod, latter usually with clawlike apex.<br />
REMARKS Most species of Phycolimnoria are algal borers, frequently encoun<br />
tered in the holdfasts of brown algae such as Macrocystis, Laminaria, and<br />
Sargassum, The one species recorded from the Caribbean, P. clarkae, however,<br />
has only been taken from decaying wood.<br />
Phycolimnoria clarkae Kensley and Schotte, 1987<br />
Figure 88E,F<br />
DIAGNOSIS 6 4.3 mm, ovigerous 9 3.3-4.4 mm. Uropodal exopod less<br />
than half length of endopod, straight, tipped with short squat claw. Pleonite<br />
5 with broad raised middorsal region having irregular bumps. Pleotelson<br />
wider than long, with two rounded submedian ridges basally, becoming ob<br />
solete posteriorly.<br />
RECORDS Bahamas; Twin Cays, Belize.<br />
Aldabra Atoll, Indian Ocean.<br />
Family Serolidae Dana, 1852<br />
DIAGNOSIS Body dorsoventrally depressed. Eyes present or absent.<br />
Cephalon fused with pereonite 1 dorsally. Mandible bearing palp. Maxillipe-<br />
dal palp of one to four articles. Pereonites 2—4 with coxae demarked; per-<br />
eonites 5 and 6 with coxae not demarked; pereonite 7 narrow, lacking free<br />
lateral margins. Pereopod 1 in 6 and 9 subchelate, pereopod 2 subchelate or<br />
ambulatory in 6, ambulatory in 9. Pleonites 1 and 2 free, articulated, re<br />
mainder of pleonites fused with telson. Pleopods 1—3 small, natatory;<br />
pleopods 4 and 5 large, operculate. Uropods lateral, biramous.<br />
REMARKS The serolids reach their greatest diversity (and their greatest size<br />
of up to 80 mm in length) in the southern oceans, with few species extending
202 FLABELLIFERA • SPHAEROMATIDAE<br />
into the subtropics and tropics. The deep- and abyssal-dwelling species usu<br />
ally lack eyes. The animals are epibenthic, living in the upper few centime<br />
ters of the bottom sediment, where they are scavengers and carnivores.<br />
Serolis Leach, 1818<br />
DIAGNOSIS Body markedly dorsoventrally flattened. Coxal plates produced<br />
laterally. Mandible having lacinia mobilis and single spine. Maxillipedal<br />
palp of three articles (rarely two to four). Pereopod 2 exhibiting sexual di<br />
morphism, subchelate in cJ, ambulatory in 9. Pleopods 1-3, peduncles elon<br />
gate, rami subelliptical. Pleopod 3, exopod uniarticulate.<br />
Serolis mgrayi Menzies and Frankenberg, 1966<br />
Figure 89<br />
DIAGNOSIS S 4.5 mm, ovigerous 9 4.7 mm. Eyes present. Cephalon with<br />
two middorsal tubercles. Pereonites 2—4 each with faint rounded tubercle<br />
just mesial to coxal suture. Pereon and pleon with faint middorsal longitudi<br />
nal carina bearing small blunt tubercle on posterior margin of each segment.<br />
Pleonites 1 and 2 with lateral margins not contributing to body outline, over<br />
lapped by pereonite 6. Pleotelson broadly triangular, with lateral carina in<br />
anterior half; apex truncate. Uropodal rami reaching to or slightly beyond<br />
pleotelsonic apex.<br />
RECORDS Off North Carolina, 18-34 m; off South Carolina, 22 m; off<br />
Georgia, 18-47 m; Florida Keys, 18-88 m; Trinidad; Venezuela, 95 m; Flor<br />
ida, Gulf of Mexico, 11-88 m.<br />
Family <strong>Sphaeromatidae</strong> H. Milne Edwards, 1840<br />
DIAGNOSIS Antennular peduncle of three articles, antennal peduncle of five<br />
articles. Mandible stout, lacinia mobilis and molar usually well developed,<br />
palp of three articles. Maxillipedal palp of five articles. Mouthparts in some<br />
genera metamorphosed and somewhat reduced in ovigerous 9 . Pleon of five<br />
partially or completely fused pleonites, often indicated by lateral sutures,<br />
plus dorsally convex and sometimes inflated pleotelson. Uropods lateral, ex<br />
opod free if present, endopod fused with sympod. Sexual dimorphism often<br />
marked, especially in pleotelsonal structure. Animal often capable of con<br />
globating or folding over. Young brooded in internal pouches or anterior or<br />
posterior pockets; oostegites variable in number, if present.
FLABELLIFERA • SPHAEROMATIDAE 203<br />
Figure 89. Serolis mgrayi: A, 6; B, pereopod 1; C, pereopod 2, 8.<br />
REMARKS Right into the 1980s this family was routinely divided into three<br />
groups, based on the structure of the two posterior pairs of pleopods:<br />
Platybranchiatae—pleopods 4 and 5 with both rami membranous and lack<br />
ing branchial pleats; Hemibranchiatae—pleopods 4 and 5 with branchial<br />
pleats on endopods only; Eubranchiatae—pleopods 4 and 5 with branchial<br />
pleats on both rami. These three "groups" were recognized formally as sub<br />
families by Hurley and Jansen (1977) but the names were not based on con-
204 FLABELL1FERA • SPHAEROMATIDAE<br />
tained genera and were replaced with current subfamily names by Bowman<br />
(1981) and Iverson (1982), the latter providing diagnoses for all five sub<br />
families. Four of these are represented in the Caribbean area; the fifth, the<br />
Tecticipitinac, contains only the single primarily Pacific genus Tecticeps.<br />
While the subfamilial status now appears to be resolved, many of the gen<br />
era still require unambiguous diagnoses. The work of Harrison (1984) on the<br />
structure of the female broodpouch, with its various components of<br />
oostegites, internal pouches, and anterior and posterior pockets (Figure 90),<br />
along with the metamorphosis of the female mouthparts (see Figure 96) has<br />
helped enormously to standardize the genera. Nevertheless, these features of<br />
the female remain unknown in several genera. Further, with this stabilization<br />
based on females, many problems of incorrect generic designation have been<br />
uncovered. In this work, Harrison's generic diagnoses are followed as far as<br />
possible. Where uncertainty exists, this is indicated. In some cases, we may<br />
still be unaware of existing problems: future work will without doubt result in<br />
the shifting of species to different genera, as well as in the creation of new<br />
genera.<br />
Key to subfamilies of <strong>Sphaeromatidae</strong><br />
1. Pereopod 1 prehensile in both sexes; pereopod 2 prehensile only in 6<br />
Ancininae<br />
Pereopods 1 and 2 ambulatory 2<br />
2. Pleopods 4 and 5 lacking branchial pleats Cassidininae<br />
Pleopods 4 and 5 with branchial pleats on endopods 3<br />
3. Pleopods 4 and 5 with branchial pleats on both rami . . . Dynameninae<br />
Pleopods 4 and 5 with branchial pleats on endopods only<br />
Subfamily Ancininae Tattersall, 1905<br />
Sphaeromatinae<br />
DIAGNOSIS Body markedly dorsoventrally depressed. Cephalon fused me<br />
dially with pereonite 1. Pereopod 1 prehensile in 8 and 9. Pereopod 2 pre<br />
hensile in 6 only. Pleopods 4 and 5 similar, lacking branchial pleats.<br />
Uropods uniramous.
internal pouch<br />
Ancinus belizensis 205<br />
Figure 90. Diagrammatic representation of 9 sphaeromatid, showing marsupial<br />
structures (adapted from Harrison, 1984).<br />
Ancinus H. Milne Edwards, 1840<br />
DIAGNOSIS Eyes dorsal. 9 mouthparts not metamorphosed. Mandibular<br />
molar absent; palp of three articles. Maxilla 1 of single ramus, endite rudi<br />
mentary. Maxilla 2 of two rami. 9 with oostegites absent; brood held in two<br />
opposing pockets, opening as narrow ventral slit between pereopods 4. Pleon<br />
consisting of short anterior pleonite with free lateral margin, plus broadly<br />
triangular pleotelson. Pleopod 1 uniramous, endopod absent. Pleopod 2 op-<br />
erculiform. Pleopod 3, exopod of single article. Uropod lacking exopod, sym-<br />
pod not laterally expanded.<br />
Key to species of Ancinus<br />
1. Pleotelson as long as basal width, apex narrowly rounded . . . brasiliensis<br />
Pleotelson with basal width greater than length, apex subtruncate<br />
Ancinus belizensis Kensley and Schotte, 1987<br />
Figure 91A-C<br />
belizensis<br />
DIAGNOSIS 8 4.1 mm, 9 2.8 mm. Body oval, about twice longer than wide.<br />
Dorsal integument strongly pitted. Antennular flagellum of 12 articles; an-
206 FLABELLIFERA • SPHAEROMATIDAE<br />
Figure 91. Ancinus belizensis: A, 9; B, pereopod 1 cJ; C, pereopod 2 8. Ancinus<br />
braziliensis: D, adult (from Glynn and Glynn, 1974).<br />
tennal flagellum of 10 articles. 8 pereopod 2, dactylus strongly curved,<br />
reaching to proximal lobe of propodus. Pleopod 2 about 2.5 times longer than<br />
basal width.<br />
RECORDS Carlson Point, Belize, in seagrass flats, 0.5 m.<br />
Ancinus brasiliensis Lemos de Castro, 1959<br />
Figure 91D<br />
DIAGNOSIS 8 7.0 mm, 9 6.0 mm. Body about twice longer than wide. Dor<br />
sal integument smooth. Antennular flagellum of 17 articles; antennal<br />
flagellum of 10 articles. 8 pereopod 2, dactylus strongly curved, reaching to<br />
midlength of posterior margin of carpus. Pleopod 2 almost three times longer<br />
than basal width.
Cass idin idea 207<br />
RECORDS Brazilian coast from Rio de Janeiro northward, 1.5 m; Costa<br />
Rica, Panama; shallow infratidal below sandy beaches.<br />
REMARKS Glynn and Glynn (1974) discussed color polymorphism in this<br />
species.<br />
Subfamily Cassidininae Iverson, 1982<br />
DIAGNOSIS Cephalon not medially fused with pereonite 1. Pereopod 1 am<br />
bulatory. Pleopods 4 and 5, both rami lacking transverse pleats, outer rami<br />
unsegmented. Pleopod 5, outer ramus with low subapical squamiferous pro<br />
tuberances. Pleotelsonic apex entire. Uropods with exopods reduced.<br />
REMARKS The genus Dies has twice been recorded from the Caribbean: D.<br />
arndti Ortiz and Lalana, 1980, from Cuba, and D. barnardi Carvacho, 1977,<br />
from Guadeloupe. This genus is distinguished from Cassidinidea solely on the<br />
basis of the penial structure: biramous in Cassidinidea, uniramous in Dies.<br />
Harrison (1984) has pointed out that the separation of these two genera has<br />
not been satisfactorily resolved. The penis of neither the Cuban nor the<br />
Guadeloupan species has been illustrated, but the whole-animal illustrations<br />
of both look suspiciously like Cassidinidea ovalis. Examination of material of D.<br />
barnardi from the Paris Museum supports the view that this species was based<br />
on immature material of C. ovalis. Neither of the so-called species oi Dies are<br />
dealt with in this work, both being regarded as junior synonyms of C. ovalis.<br />
Key to genera of Cassidininae<br />
1. Frontal lamina visible dorsally between antennular bases; two basal<br />
articles of antennular peduncle not expanded Cassinidinea<br />
Frontal lamina not visible between antennular bases; two basal articles<br />
of antennular peduncle broadly expanded Paraleptosphaeroma<br />
Cassidinidea Hansen, 1905b<br />
DIAGNOSIS Body strongly dorsoventrally depressed. Eyes dorsal, situated<br />
at posterolateral corners of cephalon. Latter somewhat sunken into pereonite<br />
1. Frontal lamina expanded, visible dorsally between antennular bases. An<br />
tenna directed laterally. Pleon consisting of one free pleonite having short<br />
free lateral margin, plus broadly triangular pleotelson. Uropodal endopod
208 FLABELL1FERA • SPHAEROMATIDAE<br />
well developed, fused with sympod; exopod markedly reduced. Penial rami<br />
elongate, separate. 9 mouthparts not metamorphosed. Oostegites absent.<br />
Brood housed in pouch formed by opposing pockets overhanging ventrum,<br />
opening by slit between fourth pereopods.<br />
Key to species of Cassidinidea<br />
1. Posterior margin of pleotelson truncate ovalis<br />
Posterior margin of pleotelson rounded mosaica<br />
Cassidinidea mosaica Kensley and Schotte, 1987<br />
Figure 92A<br />
DIAGNOSIS 6 1.8 mm, ovigerous 9 1.6 mm. Body twice longer than wide.<br />
Dorsal integument bearing close-packed flattened tubercles. Pleotelson tri<br />
angular, with posterior margin narrowly rounded, dorsally convex, basally<br />
inflated.<br />
RECORDS Carrie Bow Cay, Belize, 1.5-10 m; in silty sand and rubble be<br />
tween patch reefs and coral buttresses.<br />
Cassidinidea ovalis (Say, 1818)<br />
Figure 92B-E<br />
DIAGNOSIS 6 and 9 3.6 mm. Body width slightly less than half length.<br />
Dorsal integument smooth. Pleotelson with raised anteromesial area, but<br />
lacking sculpture; posterior margin truncate.<br />
RECORDS New Jersey to Florida, in marsh mud and among dead leaves, 0—<br />
1 m; Trinidad; Belize; Panama; Dominica; Louisiana and Vera Cruz, Gulf of<br />
Mexico. Known from waters of less than l%o to 35%o.<br />
Paraleptosphaeroma Buss and Iverson, 1981<br />
DIAGNOSIS Body oval in outline, entire circumference with transparent<br />
flange of fused setae on two expanded basal articles of antennule, on per-<br />
conites, pleonite 1, and uropods. Expanded basal articles of antennules con-
Paraleptosphaeroma 209<br />
Figure 92. Cassidinidea mosaica: A, 6. Cassidinidea ovalis: B, 6] C, pereopod 1; Dy<br />
pleopod 4; E, pleopod 5. Paraleptosphaeroma glynni: Fy 6.<br />
tiguous in midline. Single articulated pleonite with short free lateral margin.<br />
Uropodal sympod and endopod fused; exopod articulated, much shorter<br />
than fused endopod.
210 FLABELLIFERA • SPHAEROMATIDAE<br />
Paraleptosphaeroma glynni Buss and Iverson, 1981<br />
Figure 92F<br />
DIAGNOSIS 6 2.58 mm, ovigerous 9 2.38 mm. Pleotelson basally broad,<br />
tapering to notched posterior margin. Fused uropodal endopod and sympod<br />
of each side almost touching posterior to pleotelsonic apex.<br />
RECORDS Portsmouth, Dominica, intertidal rock pools.<br />
Punta Paitilla, Pacific Panama.<br />
REMARKS Buss and Iverson (1981) demonstrated that this species displays<br />
sequential protogynous hermaphroditism, and that the change from female<br />
to male seems to be mediated by social conditions, especially the proportion<br />
of males to females. The principal food source for this species was shown to<br />
be abascan bryozoans.<br />
Key to genera of Dynameninae<br />
1. Pleotelson very similar in both sexes 2<br />
Pleotelson showing marked sexual dimorphism 3<br />
2. Cephalon and pleotelson smooth, lacking ridges Ischyromene<br />
Pleotelson and cephalon with ridges Cerceis<br />
3. Uropods lamellar in both sexes 4<br />
Uropods lamellar in 9, endopod reduced, exopod elongate-cylindrical<br />
in 6 5<br />
4. Ovigerous 9 lacking oostegites;
Subfamily Dynameninae Bowman, 1981<br />
Discerceis 211<br />
DIAGNOSIS Cephalon not fused with pereonite 1. Pereopods 1 and 2 am<br />
bulatory. Pleopods 4 and 5, both rami having branchial pleats. Pleopod 4,<br />
exopod unjointed, usually lacking setae, endopod with few setae at most.<br />
Pleotelsonic apex often with terminal notch or foramen, especially in
212 FLABELLIFERA • SPHAEROMATIDAE<br />
Figure 93. "Cerceis" carinata: A, 6. Discerceis linguicauda: B, d. Dynamenella<br />
acutitelson: C, 9; D, pleon 8. Dynamenella angulata: E, 9.<br />
internal pouches (number unknown). Pockets absent. 6 with uropodal endo-<br />
pod and sympod fused, very short; exopod elongate, cylindrical.
Discerceis linguicauda (Richardson, 1901)<br />
Figure 93B<br />
Dynamenella 213<br />
DIAGNOSIS 6 7.2 mm. Dorsal integument, especially of posterior half, with<br />
numerous scattered granular tubercles. Uropodal endopod and sympod<br />
fused, very short, exopod elongate, subcylindrical and slightly bowed. Ante<br />
rior half of pleotelson inflated, with three elongate rounded ridges (each com<br />
posed of two contiguous tubercles) ending posteriorly in subacute tubercle;<br />
posterior margin trilobed, median lobe broadly rounded, with subacute tu<br />
bercle at base, lateral lobe truncate, well separated from median lobe. Head<br />
and pereonite 1 not fused. Frontal lamina visible dorsally between antennal<br />
bases. Penes short, separate. Copulatory stylet basally relatively broad, dis-<br />
tally broadly rounded.<br />
RECORDS Cape Catoche, Yucatan, Mexico, 48-50 m.<br />
REMARKS This species is known only from the four male syntypes.<br />
Dynamenella Hansen, 1905b<br />
DIAGNOSIS Species exhibiting obvious sexual dimorphism. Both sexes lack<br />
ing processes on pereon and pleon, Uropodal rami lamellar. Exopod of<br />
pleopod 3 with or without articulation. 9: Mouthparts not metamorphosed.<br />
Broodpouch lacking oostegites, but formed by two opposing ventral pockets<br />
opening in midline between fourth pereopods. Apex of pleotelson with notch,<br />
Key to species of Dynamenella<br />
1. 6 with pleotelsonic foramen 2<br />
6 lacking foramen but with notch, or appearing entire; 9 pleotelson<br />
with faint notch visible acutitelson<br />
2. 6 with four strong pleotelsonic ridges; 9 with subcircular pleotelsonic<br />
foramen quadrilirata<br />
6 lacking pleotelsonic ridges; 9 with posterior margin of pleotelson<br />
entire perforata
214 FLABELLIFERA • SPHAEROMATIDAE<br />
groove, or foramen. 6: Penes basally fused, rami long, tapering. Copulatory<br />
stylet proximally broad, tapering to acute tip, reaching to or just beyond<br />
apex of endopod. Uropods broader than in $. Posterior pleotelson with dor-<br />
sally directed foramen connected to apex by narrow slit.<br />
REMARKS The species described by Richardson (1901) as Dynamene angulata<br />
from No Name Key, Florida, and referred to by some authors as a Dyna-<br />
menella, while figured here (Figure 93E), is not included in the present key.<br />
The species is known only from immature females; correct generic placement<br />
is thus not possible.<br />
Dynamenella acutitelson Menzies and Glynn, 1968<br />
Figure 93C,D<br />
DIAGNOSIS 6 3.5 mm, 9 2.3 mm. 6: Pereonites 4-6 with transverse ridge<br />
over dorsum, ridge interrupted to form short median section. Pleotelson with<br />
two submedian and two lateral rounded tubercles basally, two submedian,<br />
poorly defined ridges in central area; posterior margin tapering in dorsal<br />
view, with slit eitherjust visible or appearing entire. In lateral view, posterior<br />
pleotelson seen to be laterally compressed, forming narrow groove.<br />
RECORDS Puerto Rico, intertidal rocks and algae.<br />
REMARKS Menzies and Glynn (1968) described this species with two vari<br />
eties, the holotype as D. acutitelson var. typica, and 11 paratypes as D. acu<br />
titelson var. glabrothorax. The major difference between these varieties lay in<br />
the presence of transverse ridges on pereonites 4—6 in typica and their absence<br />
xnglabrothorax. The holotype, however, at 3.5 mm, would seem to be a mature<br />
male, while all the paratypes are smaller. The differences described by Men<br />
zies and Glynn (1968) may thus be due to immaturity. <strong>As</strong> further compara<br />
tive material is lacking, these varieties (or whatever their true status) are not<br />
recognized here.<br />
Menzies and Glynn (1968, fig. 30a) illustrate D. acutitelson var. glabrothorax<br />
as having scattered tiny granules over the dorsal integument. These were not<br />
seen when the type material was reexamined.<br />
Harrison and Holdich (1982) placed this species in Paradella, based on the<br />
literature. However, the penes for both varieties are shown as short and sepa<br />
rate, as in Ischyromene. Again, until further mature males and ovigerous<br />
females are seen, the generic placement of this species must remain in doubt.
Dynamenella perforata (Moore, 1901)<br />
Figure 94A,B<br />
Geocerceis barbarae 215<br />
DIAGNOSIS 6 3.2 mm, 9 2.6 mm. 6: Pleon bearing two low rounded sub-<br />
median "mounds." Pleotelson with strongly convex anterior two-thirds, with<br />
T-shaped foramen. Pleon and pleotelson with numerous scattered small tu<br />
bercles. Uropodal rami broadly ovate, outer margins crenulate. 9:<br />
Pleotelson broadly rounded in dorsal view, posterior margin entire. Inner<br />
uropodal ramus distally subacute.<br />
RECORDS Bermuda to Puerto Rico, intertidal coral rubble and algae, and<br />
under chiton Acanthopleura granulate; Dominican Republic; Cuba.<br />
Dynamenella quadrilirata Kensley, 1984<br />
Figure 94C-H<br />
DIAGNOSIS 6 2.6 mm, 9 2.5 mm. 6: Two low rounded submedian tuber<br />
cles on last pleonite. Anterior half of pleotelson inflated, with four rounded<br />
longitudinal ridges; posterior half tapered, somewhat dorsally flexed, with<br />
cordate foramen. Uropodal rami distally rounded, outer margins crenulate<br />
to dentate. 9: Lacking pleonal tubercles. Pleotelson inflated, unornamented,<br />
posterior margin forming subcircular foramen.<br />
RECORDS Carrie Bow Cay, and Twin Cays, Belize; intertidal to 3 m.<br />
Geocerceis Menzies and Glynn, 1968<br />
DIAGNOSIS Ovigerous 9 with mouthparts metamorphosed. Broodpouch<br />
with three pairs of oostegites, on pereonites 2—4, just overlapping in midline.<br />
Brood held in internal pouches (number unknown). Pockets absent. Uropo<br />
dal rami lamellar, shorter than pleotelson. 6 uropodal endopod fused with<br />
sympod, very short; exopod elongate, club shaped. Pleopod 2 with copula-<br />
tory stylet articulating distally on endopod.<br />
Geocerceis barbarae Menzies and Glynn, 1968<br />
Figure 95A-C<br />
DIAGNOSIS 8 3.3 mm, 9 2.5 mm. Pleopod 3 exopod of single article. Pleon<br />
with two elongate sutures reaching lateral pleon margin. 6: Frontal lamina<br />
expanded into ventrally directed beaklike process. Penes separate, relatively
216 FLABELLIFERA • SPHAEROMATIDAE<br />
Figure 94. Dynamenella perforata: A, 6; B> pleon 9. Dynamenella quadrilirata: C, 6;<br />
D, pleon 9; E, pleon
Ischyromene 217<br />
Figure 95. Geocerceis barbarae: A, 8; B, pleon 9; C,
218 FLABELLIFERA • SPHAEROMATIDAE<br />
nounced. Uropodal rami lamellar. 8 pleopod 2 with copulatory stylet<br />
basally narrow, reaching to or just beyond distal margin of endopod.<br />
Ischyromene barnardi (Menzies and Glynn, 1968)<br />
Figure 95D<br />
DIAGNOSIS 6 4.5 mm, 9 3.7 mm. Both sexes lacking processes on pereon<br />
and pleon. Accessory unguis of pereopods often bifid. Pleopod 3, exopod of<br />
single article. Uropodal rami lamellar. 6: Pereonite 7, posterior margin<br />
bilobed. Penes short, separate to base.<br />
RECORDS Puerto Rico, intertidal.<br />
Paracerceis Hansen, 1905b<br />
DIAGNOSIS Pleopod 3 exopod with transverse suture in distal half. Pleon<br />
with two long sutures reaching to posterolateral margin. 6: Penial rami<br />
short, separate. Pleotelson with basal area strongly vaulted; deep posterior<br />
notch sometimes having denticles on inner margins, and/or median tooth at<br />
base of notch. Uropodal endopod short, fused with sympod; exopod elongate,<br />
club shaped. 9: Mouthparts metamorphosed. Mandible fused with<br />
cephalon. Broodpouch of four pairs of oostegites, three posterior pairs over<br />
lapping. Brood retained in internal pouches. Uropodal rami subequal, lamel<br />
lar. Pleon usually less ornamented than in
Paracerceis caudata 219<br />
3. 6, pleotelsonic notch deep, margins usually with two teeth on each<br />
side; strong median tubercle on anterior pleotelson bluntly bifid; 9,<br />
pleotelson with one or two rounded median tubercles and 2 smaller<br />
tubercles on each side caudata<br />
6, pleotelsonic notch shallow, with tiny lateral denticles; median<br />
tubercle of pleotelson conical, acute; 9, pleotelson with three large<br />
conical acute tubercles and several smaller scattered tubercles in<br />
anterior half cohenae<br />
Paracerceis caudata (Say, 1818)<br />
Figure 96<br />
DIAGNOSIS 6 8.1 mm, 9 6.4 mm. 8: Pleotelson with blunt median bifid<br />
tubercle, with two smaller tubercles on each side. Pleotelsonic notch usually<br />
with two strong denticles on each margin, basal median tooth lacking.<br />
Uropodal exopod reaching well beyond pleotelson, slightly bowed, with 2—4<br />
setose bumps on outer margin. 9: Pleonite 5 with three low tubercles.<br />
Pleotelsonic apex broadly rounded in dorsal view, with two rounded median<br />
tubercles and two smaller tubercles on each side. Uropodal rami subequal,<br />
lamellar, outer distal angle of each acute.<br />
RECORDS Bermuda; New Jersey to Florida Keys; Yucatan to Venezuela;<br />
Turks and Caicos Islands; Cuba; Puerto Rico; Bahamas; Jamaica; Haiti; St.<br />
Maartens, 0.2-127 m; St. Lucia; Gulf of Mexico. Found in the following<br />
algae: Caulerpa, Halimeda, Turbinaria> Amphiroa, Laurencia, Dictyota; between<br />
sponges and tunicates on red mangrove roots; in coral rubble; in spur and<br />
groove zone of reefs, lagoon, back reef, seagrass flats, and fringing<br />
mangroves.<br />
REMARKS Menzies and Glynn (1968:55, fig. 22f) named and figured P.<br />
caudata var. brevipes from Puerto Rico. This variant was characterized as hav<br />
ing the margins of the pleotelsonic notch lacking denticles. Given the con<br />
siderable variation in ornamentation in this species, we feel that no validity<br />
can be given to the name "brevipes."<br />
This is the commonest sphaeromatid in the Caribbean, and it has very<br />
broad ecological requirements, being found in a wide range of habitats and<br />
depths.
220 FLABELL1FERA • SPHAEROMATIDAE<br />
Figure 96. Paracerceis caudata: A, 8,B, pleon 9; C, mandible 6] D, maxilla 1 6; E,<br />
maxilla 2 6; F, maxilliped 6; G> mandible 9; Hy maxilla 19;/, maxilla 1 9; J,<br />
maxilliped 9.<br />
Paracerceis cohenae Kensley, 1984<br />
Figure 97A,B<br />
DIAGNOSIS 6 10.0 mm, 9 7.9 mm. 6: Pereonites each with median tuber<br />
cle and several smaller lateral tubercles near posterior margin of somite.<br />
Pleonite 5 with large median conical tubercle. Anterior two-thirds of
Paracerceis glynni 221<br />
pleotelson inflated, faintly tripartite, with strong median conical tubercle;<br />
notch in posterior margin shallow, with low median tooth and tiny lateral<br />
denticles; posterolateral margins finely dentate. Uropodal exopod cylindri<br />
cal, distally denticulate, six to seven times longer than basal width. 9: Per-<br />
eon and pleon much as in 6 y but pleotelsonic notch shallower and<br />
posterolateral margins not denticulate. Uropodal rami subequal, lamellar,<br />
exopod with distolateral angle acute.<br />
RECORDS Carrie Bow Cay, Belize, 15-16 m. Only known from sponge<br />
Callispongia plicifera growing on outer reef slope.<br />
Paracerceis edithae Boone, 1930<br />
Figure 97C-E<br />
DIAGNOSIS 6 4.0 mm, 9 3.1 mm. 6: Posterior three pereonites and<br />
pleonites each with irregular row of small tubercles near posterior margin,<br />
densely setulose tubercles becoming spinose more posteriorly. Pleotelson<br />
with strong median conical tooth in anterior half, flanked by convex spinose<br />
mound. Pleotelsonic notch deep, with elongate median basal tooth bearing<br />
strong acute tooth at its base. Lobes of posterior pleotelsonic margin broad,<br />
flattened, margins denticulate. Uropodal exopod tuberculate, tapering, api-<br />
cally acute. 9: Integument much less tuberculate-spinose than in 6. Imma<br />
ture 9, posterior margin of pleotelson with faintly rounded median lobe. In<br />
mature 9, posterior margin distinctly trilobed. Uropodal rami subequal,<br />
lamellar, distally rounded, with tiny distolateral spine on exopod.<br />
RECORDS Bahamas, 60-66 m, in vase sponge; Haiti; Puerto Rico, 20—25 m.<br />
Paracerceis glynni Kensley, 1984<br />
Figure 97F,G<br />
DIAGNOSIS 6 6.4 mm, 9 5.2 mm. 6: Integument becoming strongly setose<br />
and tuberculate posteriorly from about pereonite 5. Posterior margin of inf<br />
lated anterior area of pleotelson bearing strong median conical tubercle and<br />
smaller acute lateral tubercle, with low swelling beneath each lateral tuber<br />
cle. Posterior notch deep, narrow, with small basal median tooth, lobes form<br />
ing notch tricuspid, outer cusps recurved dorsally. Uropodal exopod fairly<br />
straight, cylindrical, apically acute. 9: Body far less setose and tuberculate<br />
than 6. Pleotelson with strongly inflated anterior area having very faint mid-<br />
dorsal tubercle; notch well marked, formed by triangular lobes of
222<br />
FLABELLIFERA • SPHAEROMATIDAE<br />
E<br />
Figure 97. Paracerceis cohenae: A, 6\ B, pleotelson, 9. Paracerceis edithae: C,<br />
pleotelson, 9; D, mature 6] E, immature S. Paracerceis glynni: F, 8\ G} pleotelson,<br />
9. Paracerceis nuttingi: H, 9.<br />
pleotelsonic margin. Uropodal rami subequal, flattened, endopod with distal<br />
margin faintly trituberculate; exopod with few distal tubercles.<br />
RECORDS Alligator Light, Florida, 11 m; Carrie Bow Cay, Belize, 11-15.2<br />
B<br />
G
Paradella 223<br />
m, from green alga Halimeda sp. on forereef, and from sponge Aphysina<br />
jistularis.<br />
Paracerceis nuttingi (Boone, 1921)<br />
Figure 97H<br />
RECORDS Barbados; Puerto Rico, 1.5 m, from Cymodocea seagrass, and coral<br />
rubble and sponges.<br />
REMARKS The types of this species from Barbados consist only of females<br />
(total length 4.1 mm). Menzies and Glynn (1968) record an immature male<br />
i<br />
from Puerto Rico with an incipient pleotelsonic notch. This specimen, how<br />
ever, still has the subequal lamellar uropodal rami. The mature male, with<br />
the characteristically reduced uropodal endopod and cylindrical exopod, is<br />
unknown. The possibility exists that this is not a true Paracerceis.<br />
Paradella Harrison and Holdich, 1982<br />
DIAGNOSIS Marked<br />
ual dimorphism. Accessory unguis of pereopods simple, not bifid. Pleopod 3<br />
Key to species of Paradella<br />
1. Pereonite 7 with projecting bilobed flange; pleon and pleotelson finely<br />
but distinctly granulate 2<br />
Pleon and pleotelson smooth 3<br />
2. 8 with pleotelsonic foramen distinctly heart shaped, with median<br />
point; four submedian tubercles of pleotelson in 8 and 9 somewhat<br />
elongate; 9 pleotelson posteriorly narrowed, slit visible dorsally<br />
8 with pleotelsonic foramen wider than long, but lacking median<br />
point; four submedian tubercles of pleotelson in 8 small, rounded,<br />
obscure in 9; 9 pleotelson posteriorly truncate, slit not visible<br />
dianae<br />
dorsally plicatura<br />
3. Tubercles on pleotelson in 8 and 9 small to obscure; 8 with<br />
pleotelsonic foramen subcircular quadripunctata<br />
Tubercles on pleotelson broadly rounded mounds in 8 and 9; 8 with<br />
pleotelsonic foramen wider than long tumidicauda
224 FLABELL1FERA • SPHAEROMAT1DAE<br />
exopod with articulation. Uropodal rami lamellar. 9: Mouthparts not meta<br />
morphosed. One pair of oostegites arising from pereonite 4, short, not reach<br />
ing midline. Brood held in pouch formed by two opposing pockets covering<br />
entire ventrum, opening by transverse slit between 4th pereopods.
Paradella quadripunctata 225<br />
Figure 98. Paradella dianae: A, 8; B, pleopod 2 8; C, pleon 9. Paradella plicatura:<br />
Dy 8; E, pleon 9. Paradella quadripunctata: F, 8; G, pleon 9. Paradella tumidicauda;<br />
H, pleon 9 (from Glynn, 1970); /,
226 FLABELL1FERA • SPHAEROMAT1DAE<br />
Paradella tumidicauda (Glynn, 1970)<br />
Figure 98H,I<br />
DIAGNOSIS 8 6.7 mm, 9 6.5 mm. 6: Last pleonite with two submedian<br />
swellings. Pleotelson with four submedian broadly rounded swellinglike tu<br />
bercles and two pairs of lateral tubercles. Foramen wider than long, posterior<br />
contiguous borders of foramen each bearing rounded swelling. 9: Pleotelson<br />
with four submedian swellinglike tubercles, sometimes with two obscure lat<br />
eral tubercles; posterior slit not visible dorsally, area surrounding slit<br />
swollen, horseshoe shaped.<br />
RECORDS Margarita Island, Venezuela, from among intertidal barnacles.<br />
Subfamily Sphaeromatinae H. Milne Edwards, 1840<br />
DIAGNOSIS Cephalon not fused with pereonite 1. Pereopods 1 and 2 am<br />
bulatory. Pleopods 4 and 5, endopods having branchial pleats, exopods un-<br />
pleated, membranous, of two articles. Uropods biramous.<br />
Key to genera of Sphaeromatinae<br />
1. Uropodal exopod with outer margin serrate Sphaeroma<br />
Uropodal exopod with outer margin entire or faintly crenulate 2<br />
2.
Cymodoce ruetzleri 227<br />
Pleopod 5, exopod of two articles, distal article with apex and internal mar<br />
gin covered with fine teeth, anterior surface with long distally toothed boss;<br />
proximal article with two small toothed bosses at internodistal angle. 6:<br />
Maxillipedal palp articles 2—4 bearing setigerous lobes. Penial rami elongate,<br />
separate. Pleon usually more tuberculate than in 9. Uropodal exopod lamel<br />
lar, shorter than endopod. 9: Mouthparts metamorphosed. Broodpouch<br />
formed by four pairs of oostegites arising from pereonites 1-4, overlapping in<br />
midline. Brood housed in five pairs of internal pouches.<br />
"Cymodoce" barrerae (Boone, 1918)<br />
Figure 99A,B<br />
DIAGNOSIS 9 7.5 mm. 9: Body dorsally strongly vaulted, unornamented.<br />
Frontal lamina distally broadly rounded, lateral shoulders rounded.<br />
Mouthparts not metamorphosed. Pleotelson anteriorly strongly inflated with<br />
barest indication of two submedian swellings; posterior margin trilobed, with<br />
median lobe strong, narrowly rounded, outer lobes much smaller and ventral<br />
to median lobe. Uropodal endopod distally obliquely truncate; exopod dis<br />
tally acute.<br />
RECORDS Cabanas, Cuba.<br />
REMARKS This species is known only from the nonovigerous female<br />
holotype. Loyola e Silva (1960) placed the species in Cymodoce, based on a<br />
female specimen from Brazil. <strong>As</strong> the mouthparts are not metamorphosed,<br />
this does not agree with the present concept of Cymodoce, but with neither<br />
ovigerous females nor males available, the correct generic placement cannot<br />
be determined.<br />
Cymodoce ruetzleri Kensley, 1984<br />
Figure 99C-G<br />
DIAGNOSIS 6 5.0 mm, 9 4.2 mm. 6: Integument with numerous small<br />
tubercles, becoming densely setose posteriorly. Pleonite 4, posterior margin<br />
broadly bilobed. Pleotelson bearing pair of strong conical tubercles with<br />
acute tips, each tubercle flanked by low rounded tubercle; apex trilobed,<br />
outer lobes triangular, acute, sharp spine at base of incision, median lobe<br />
apically blunt. Uropodal exopod apically acute, oval in cross section, endo<br />
pod and sympod fused, somewhat flattened, apex triangular with strong<br />
tooth. 9: Pleotelson with two conical apically acute tubercles, apex barely<br />
notched, with short rounded lobe slightly offset from posterior margin. Both
228 FLABELLIFERA • SPHAEROMATIDAE<br />
A<br />
Figure 99. "Qymodoce" barrerae: A, $; B, frontal lamina. Cymodoce ruetzleri: C, pleopod 4; G, pleopod 5.<br />
uropodal rami flattened; exopod with tiny apical tooth, endopod distally<br />
truncate-rounded, with small mediodistal tooth.<br />
RECORDS Carrie Bow Cay, Belize, 0.5-13 m; in algal clumps, reef crest<br />
rubble, and seagrass flats.<br />
E
Exosphaeroma Stebbing, 1900<br />
Exosphaeroma alba 229<br />
DIAGNOSIS Maxillipedal palp articles 2-4 produced medially into lobes.<br />
Pereonites 6 and 7 dorsally unarmed. Pleopod 3, exopod biarticulate. 6:<br />
Penes short, separate. Copulatory stylet of pleopod 2 elongate, slender.<br />
Pleotclson lacking strong apical notch. 9: Mouthparts not metamorphosed.<br />
Broodpouch of three pairs of oostegites on pereonites 2-4; oostegites short,<br />
not reaching midline. Brood held in four pairs of internal pouches.<br />
Key to species of Exosphaeroma<br />
1. Pleotelson with posterior margin entire, evenly convex 2<br />
Pleotelson with posterior margin faintly notched or trilobed 3<br />
2. Frontal lamina with length less than 1.5 times greatest width diminuta<br />
Frontal lamina with length almost two times greatest width<br />
productatelson<br />
3. Pleotelson with posterior margin faintly trilobed, and with three low<br />
rounded tubercles anteriorly yucatanum<br />
Pleotelson with posterior margin faintly notched 4<br />
4. Pleotelson posteriorly broadly notched; two rounded submedian<br />
tubercles on inflated midregion antillense<br />
Pleotelson with faint narrow notch posteriorly; lacking dorsal tubercles<br />
Exosphaeroma alba Menzies and Glynn, 1968<br />
Figure 100A-C<br />
DIAGNOSIS 8 2.0 mm, 9 2.3 mm. Frontal lamina anteriorly broadly<br />
rounded, basally slightly wider than midlength. Pleotelson similar in 8 and<br />
9; anterodorsally inflated and unornamented, posteriorly tapering to slight<br />
median notch, seen in dorsal view. Uropodal rami distally shallowly serrate,<br />
exopod 2.5 times longer than wide.<br />
RECORDS Puerto Rico, intertidal to 0.5 m; in algae on rocks, and under<br />
Chiton tuberculatus and C. marmoratus.<br />
alba
230 FLABELLIFERA • SPHAEROMATIDAE<br />
Figure 100. Exosphaeroma alba: A; B, frontal lamina; Cy uropod. Exosphaeroma<br />
antillense: D; E, frontal lamina; F, uropod. Exosphaeroma diminuta: G; H, frontal<br />
lamina; /, uropod. Exosphaeroma productatelson: J; K> frontal lamina; L, uropod.<br />
Exosphaeroma yucatanum: My pleon (from Richardson, 1905).<br />
Exosphaeroma antillense Richardson, 1912d<br />
Figure 100D,F<br />
DIAGNOSIS 9 5.0 mm. Frontal lamina anteriorly tapering to subacute apex.<br />
Pleotelson with two broadly subconical submedian tubercles on inflated an-
Exosphaeroma yucatanum 231<br />
terior area; posterior margin subtruncate to very faintly emarginate. Uropo<br />
dal exopod distally crenulate, length slightly more than twice greatest width;<br />
endopod with faint distal notch.<br />
RECORDS Montego Bay, Jamaica.<br />
REMARKS The single ovigerous female holotype is the only known specimen<br />
of this species. The overlapping oostegites suggest that this may not be an<br />
Exosphaeroma.<br />
Exosphaeroma diminuta Menzies and Frankenberg, 1966<br />
Figure 100G-I<br />
DIAGNOSIS 8 2.2 mm. Frontal lamina widest at midlength, anteriorly<br />
truncate-rounded. Pleotelson with posterior margin broadly rounded.<br />
Uropodal rami not quite reaching pleotelsonic apex; exopod margin distally<br />
crenulate.<br />
RECORDS Chesapeake Bay to Florida; Venezuela; sand dwelling, intertidal<br />
and shallow subtidal.<br />
Exosphaeroma productatelson Menzies and Glynn, 1968<br />
Figure 100J-L<br />
DIAGNOSIS 6 2.5 mm, 9 1.5 mm. Sexes essentially similar. Frontal lamina<br />
widest at midlength, where slight shoulder apparent, anteriorly broadly<br />
rounded, 1.6 times longer than wide. Pleotelson unornamented, anteriorly<br />
inflated, posterior margin entire, evenly convex. Uropodal exopod distally<br />
shallowly serrate, almost four times longer than wide; endopod wider than<br />
exopod. Broad lateral patches of pigment on pleotelson in both sexes.<br />
RECORDS Puerto Rico, intertidal to 0,5 m, in algae on rocks; Texas, Gulf of<br />
Mexico.<br />
Exosphaeroma yucatanum (Richardson, 1901)<br />
Figure 100M<br />
DIAGNOSIS Frontal lamina anteriorly tapering from widest point to sub<br />
acute apex, proximally narrower than at midlength. Pleotelson posteriorly<br />
obscurely trilobed, median lobe narrowly rounded, longest; three low<br />
rounded tubercles on pleotelson in anterior region.
232 FLABELL1FERA • SPHAEROMAT1DAE<br />
RECORDS Cape Catoche, Yucatan, Mexico, 48 m.<br />
REMARKS This species was described from a single specimen which has<br />
since been lost. The true generic placement of this species is thus undeter<br />
mined and full description awaits the finding of more material.<br />
Harrieta Kensley, 1987c<br />
DIAGNOSIS 9 with mouthparts metamorphosed. Broodpouch of three pairs<br />
of oostegites on pereonites 2—4, overlapping in midline; brood held in five<br />
pairs of internal pouches. Uropodal rami subequal, lamellar in 9, exopod<br />
twice length of endopod and oval in cross section in 8. Pleopod 2 in 6 with<br />
copulatory stylet articulating basally on endopod, curved, barely reaching<br />
apex of endopod. Penes basally fused, rami slender, elongate, tapering.<br />
Harrieta faxoni (Richardson, 1905)<br />
Figure 101 A,B<br />
DIAGNOSIS 6 6.0 mm, 9 6.5 mm. 6: Frontal lamina with broad slightly<br />
convex anterior margin. Two low rounded submedian tubercles on cephalon<br />
near posterior margin. Two rounded submedian tubercles on last pleonite.<br />
Pleotelson anteriorly inflated with two submedian tubercles; posterior mar<br />
gin trilobed. 9: Essentially similar to 69 but posterior margin of pleotelson<br />
less markedly trilobed, with median lobe longer, and uropodal rami subequal<br />
in length.<br />
RECORDS Florida to Texas, Gulf of Mexico, intertidal and subtidal in<br />
Thalassia, Halodule, and Syringodium seagrass beds, in salinities of 7%o to 36%o.<br />
Sphaeroma Bosc, 1802<br />
DIAGNOSIS Maxillipedal palp with three distal articles poorly developed,<br />
lacking lobes; fringe of robust plumose setae with swollen bases on internal<br />
margin of endite; distal margin of endite with simple setae. Pereopods 1—3<br />
with plumose setae on ischium and merus. Posterior margin of pleotelson<br />
entire, similar in 6 and 9. Pleopod 3, exopod uniarticulate. Uropodal ex<br />
opod with outer margin serrate. Able to conglobate. 6: Penes short,<br />
rounded, separate. Pleopod 2, copulatory stylet articulating basally on endo<br />
pod, slender, reaching well beyond rami. 9: Mouthparts not meta-
Figure 101. Harrieta faxoni: A, 6; B, pie<br />
Sphaeroma terebrans; E, Sphaeroma walkeri.<br />
B<br />
E
234 FLABELL1FERA • SPHAEROMAT1DAE<br />
morphosed. Three pairs of overlapping oostegites arising from pereonites 2-<br />
4 (but S. terebrans has anterior pair rudimentary).<br />
REMARKS The genus Sphaeroma is one of the few sphaeromatids in which the<br />
number of oostegites varies, from the diagnostic three pairs, through two<br />
normal pairs (as in S. terebrans), to having the oostegites completely absent<br />
(as in S. annandalei).<br />
Jacobs (1987) has provided a useful reevaluation of the European, Medi<br />
terranean, and northwest African species of Sphaeroma and related genera.<br />
Key to species of Sphaeroma<br />
1. Pleotelson posteriorly bluntly triangular, with 4 strong anterior<br />
tubercles<br />
terebrans<br />
Pleotelson posteriorly broadly rounded 2<br />
2. Pleotelson dorsally smooth or with few low tubercles quadridentata<br />
Pleotelson dorsally with numerous strong tubercules walkeri<br />
Sphaeroma quadridentata Say, 1818<br />
Figure 101C<br />
DIAGNOSIS 6 11.0 mm, 9 8.0 mm. Pleotelson anteriorly inflated, some<br />
times with few low rounded tubercles, posteriorly flattened to concave; pos<br />
terior margin entire, broadly rounded.<br />
RECORDS New England to Florida; Gulf of Mexico, intertidal to 1 m, often<br />
in pilings and partially submerged dead tree trunks, and commonly associ<br />
ated with barnacles.<br />
Sphaeroma terebrans Bate, 1866<br />
Figure 10 ID<br />
DIAGNOSIS 6 10.0 mm, 9 11.5 mm. Pereonite 7 with pair of submedian<br />
and pair of lateral tubercles. Dorsal pleon densely tuberculate. Posterior<br />
pleonite with pair of submedian acute tubercles. Pleotelson anteriorly with<br />
submedian pair and lateral pair of tubercles, posteriorly rounded-triangular.
FLABELLIFERA • TRIDENTELLIDAE 235<br />
RECORDS Virginia to Florida; Belize; Cuba; Venezuela to Brazil; Gulf of<br />
Mexico.<br />
Nigeria, east coast of southern Africa, India, Sri Lanka, Thailand, Indo<br />
nesia, Philippines, Australia.<br />
REMARKS There is no agreement on whether this species is synonymous<br />
with S. destructor Richardson, 1897. This latter (if distinct) bores into wood<br />
pilings in estuarine waters, while S. terebrans is found in the prop roots of the<br />
red mangrove tree, Rhizophora mangle. In this habitat, the isopods are inter<br />
preted either as being destructive agents (e.g., Rehm and Humm, 1973) or as<br />
promoting increased root growth (Simberloff et al., 1978). It is unlikely that<br />
the bored wood itself is a source of food for the isopods; rather, as with the<br />
genus Limnoria, the food is probably detritus or fungi and bacteria growing on<br />
the wood fragments in the burrows or on the setae of the appendages.<br />
Sphaeroma walkeri Stebbing, 1905<br />
Figure 101E<br />
DIAGNOSIS 6 9.5 mm, 9 10.0 mm. Pereonites 3—7 with transverse row of<br />
large rounded tubercles. Last pleonite with row of prominent tubercles and<br />
smaller scattered tubercles laterally. Pleotelson anteriorly inflated, posteri<br />
orly concave and cuplike, with four irregular longitudinal rows of large tu<br />
bercles plus many small scattered tubercles. Posterior margin rounded, en<br />
tire to irregularly crenulate. Uropodal endopod with several rounded<br />
tubercles on dorsal surface; exopod with row of smaller tubercles on ventral<br />
surface.<br />
RECORDS Probably pan-tropical. Florida to Puerto Rico, intertidal.<br />
Family Tridentellidae Bruce, 1984<br />
DIAGNOSIS Eyes well developed. Pereonites 2—7 with distinct coxae. Pleon<br />
consisting of five free pleonites plus pleotelson. Mandible with acute incisor;<br />
lacinia mobilis absent; molar present; palp of three articles. Maxilla 1, outer<br />
ramus styliform with three to five strong terminal spines, and several short<br />
recurved subapical spines. Maxilla 2 uniramous, biarticulate, bearing small<br />
sometimes tridentate spines or scales distally. Maxillipedal palp of five arti<br />
cles; endite slender, lamellar, usually lacking coupling hooks.
236 FLABELLIFERA • TRIDENTELLIDAE<br />
Tridentella Richardson, 1905<br />
DIAGNOSIS Body dorsally often bearing spines, tubercles, or carinae, more<br />
developed in 8 than in 9. Frontal lamina narrow, pentagonal. Antcnnular<br />
peduncle of three articles; antennal peduncle of five articles. Mandibular<br />
molar weakly sclerotized. Pereopods 1-3 weakly prehensile; pereopods 4-7<br />
ambulatory. Copulatory stylet of pleopod 2 rodlike, arising proximally on<br />
mesial margin of endopod. Pleopod 5 endopod lacking marginal setae.<br />
REMARKS Delaney and Brusca (1985) provide useful taxonomic and dis<br />
tributional comments on the family Tridentellidae.<br />
Tridentella virginiana (Richardson, 1900b)<br />
Figure 102<br />
DIAGNOSIS 8 9.5 mm, ovigerous 9 9.5-11.0 mm. Cephalon and pereon<br />
dorsally smooth, pleon minutely granular. Uropodal rami with distal mar<br />
gins faintly dentate, apically narrowly rounded, endopod wider and slightly<br />
longer than exopod. Pleotelson basally wider than middorsal length; pos<br />
terior margin broadly rounded to subtruncate.<br />
RECORDS Nova Scotia to Florida; off Georgia, 550 m; Gulf Stream off Key<br />
West, 220 m.<br />
Suborder Gnathiidea Leach, 1814<br />
DIAGNOSIS Eyes usually well developed, rarely on short lateral processes,<br />
occasionally absent. Mandibles in 8 greatly enlarged, projecting anteriorly<br />
from cephalon, not used in feeding. Mandibles lacking in 9. Mouthparts of<br />
praniza larva styliform, with acute mandibles projecting anteriorly (see Fig<br />
ure 103D). Pereopod 1 modified, forming second pair of broad opercular<br />
maxillipeds covering mouthparts, referred to as pylopods. Pereopods 2—6<br />
ambulatory. Pereonite 7 reduced, lacking pereopod. Pleonites separate, nar<br />
rower than pereon. Uropods lateral, rami lamellar, forming tailfan with<br />
telson. Praniza larva with pereonites 4-6 enlarged, sometimes inflated. 9<br />
with pereonites 4-6 greatly inflated, forming broodpouch for internally<br />
brooded eggs (see Figure 103E).<br />
REMARKS The gnathiideans are entirely marine, most described species<br />
being from shallow waters. The males and females are frequently found in<br />
association with sponges and do not feed. The praniza larva is an efficient<br />
swimmer and has been recorded from shallow-water plankton, but is more
GNATHIIDEA 237<br />
Figure 102. Tridentella virginiana: A, 9; B, pereopod 1; C, maxilla 1; D, mandible;<br />
Ey maxilliped; F, maxilla 2.<br />
frequently encountered as a fish parasite, the favored site for sucking the<br />
host's blood being in the nares. Upton (1987a, 1987b) has shed light on the<br />
unusual life history of at least one gnathiid genus, Paragnathia.<br />
The taxonomy of the Gnathiidae is based almost entirely on males, the<br />
praniza and females of most species being remarkably similar.
238 GNATHIIDEA • GNATHIIDAE<br />
Family Gnathiidae Harger, 1879<br />
DIAGNOSIS <strong>As</strong> for the suborder Gnathiidea.<br />
Gnathia Leach, 1814<br />
DIAGNOSIS In addition to features mentioned in diagnosis of suborder: Eyes<br />
present in most species. Pylopod with two small articles distal to broad oper<br />
cular article 2, terminal article minute.<br />
Key to species of Gnathia (6 only)<br />
1. Anterior margin of cephalon with medial process or slightly convex . . 2<br />
Anterior margin of cephalon concave or lacking medial process 9<br />
2. Anterior margin of cephalon broadly triangular, projecting, with small<br />
lateral teeth triospathiona<br />
Anterior margin of cephalon not triangular and projecting 3<br />
3. Cephalon and two free anterior pereonites dorsally granular 4<br />
Cephalon and two free anterior pereonites smooth 5<br />
4. Lobe of outer margin of mandible notched; pereonite 5 twice wider<br />
than middorsal length velosa<br />
Lobe of outer margin of mandible rounded; pereonite 5 1.5 times wider<br />
than middorsal length 6<br />
5. Anterior margin of cephalon with distinct medial process virginalis<br />
Anterior margin of cephalon barely convex, lacking medial process<br />
rat hi<br />
6. Inner proximal lobe of mandible distinct 7<br />
Inner proximal lobe of mandible indistinct samariensis<br />
7. Inner proximal lobe of mandible entire 8<br />
Inner proximal lobe of mandible with rounded toothlike marginal<br />
structures Johanna<br />
8. Pereonites 3-5 poorly defined puertoricensis<br />
Pereonites 3-5 clearly defined magdalenensis<br />
9. Anterior margin of cephalon concave, lacking projections gonzalezi<br />
Anterior margin of cephalon with four projections beethoveni
Gnathia beethoveni Paul and Menzies, 1971<br />
Figure 103 A<br />
Gnathia magdalenensis 239<br />
DIAGNOSIS 6 3.0 mm. Anterior margin of cephalon with two low tubercles<br />
flanking shallow medial notch plus slightly larger pair of lateral tubercles.<br />
Cephalon lacking dorsal tubercles. Pereonite 5 1.5 times wider than middor-<br />
sal length. Uropodal endopods reaching beyond telsonic apex.<br />
RECORDS Off Venezuela, 95 m. Colombia.<br />
Gnathia gonzalezi Miiller, 1988<br />
Figure 103B<br />
DIAGNOSIS 6 2.0 mm. Body smooth. Anterior margin of cephalon concave.<br />
Pereonites 3—5 distinct; pereonite 5 2.5 times wider than middorsal length.<br />
Cutting margin of mandible with four or five low rounded teeth.<br />
RECORDS Colombia, 30 m.<br />
Gnathia Johanna Monod, 1926<br />
Figure 103C<br />
DIAGNOSIS 8 2.1 mm. Anterior margin of cephalon medially convex be<br />
tween pair of submedian tubercles. Pereonites 4 and 5 poorly separated.<br />
Proximomedial lobe of mandible having four or five rounded crenulations,<br />
with tiny seta between adjacent crenulations.<br />
RECORDS U.S. Virgin Islands, 29-46 m; Colombia.<br />
Gnathia magdalenensis Miiller, 1988<br />
Figure 103D<br />
DIAGNOSIS 6 3.0 mm. Anterior margin of cephalon with three tubercles,<br />
median tubercle slightly shorter than submedian pair. Cephalon with few<br />
scattered low granulations dorsally. Pereonite 5 about 1.5 times wider than<br />
middorsal length. Proximomedial lobe of mandible entire.<br />
RECORDS Carrie Bow Cay, Belize, intertidal; Colombia, 18 m.
Figure 103. Gnathia beethhoveni: A, 6. Gnathia gonzalezi: B, 6 (after Miiller, 1988).<br />
Gnathia Johanna: C,
Gnathia puertoricensis Menzies and Glynn, 1968<br />
Figure 103E-G<br />
Gnathia triospathiona 241<br />
DIAGNOSIS 6 3.0 mm, ovigerous $ 1.8 mm. Anterior margin of cephalon<br />
having three tubercles between mandibular bases, medial tubercle narrower<br />
than submedian pair. Dorsal integument finely granular, with coarser gran<br />
ules mediodorsal to eye. Pereonites 4 and 5 indistinctly separated. Mandible<br />
lacking proximomedial lobe.<br />
RECORDS Carrie Bow Cay, Belize, intertidal to 2 m; Puerto Rico, intertidal;<br />
Cuba.<br />
Gnathia rathi Kensley, 1984<br />
Figure 104 A<br />
DIAGNOSIS 6 1.9 mm, ovigerous 9 2.2 mm. Frontal margin faintly convex<br />
to straight between single low lateral tubercle mesial to mandibular bases.<br />
Dorsal integument of cephalon and anterior two free pereonites coarsely<br />
granular. Lateral margins of telson faintly denticulate. Pereonites 4 and 5<br />
poorly separated.<br />
RECORDS Carrie Bow Cay, Belize, 1-36 m.<br />
Gnathia samariensis Miiller, 1988<br />
Figure 104B<br />
DIAGNOSIS 6 2.0 mm. Anterior margin of cephalon with three tubercles,<br />
median tubercle slightly shorter than submedian pair; dorsal integument<br />
smooth. Pereonites 4 and 5 well differentiated; pereonite 5 about 2.2 times<br />
wider than middorsal length. Mandible lacking proximomedial lobe.<br />
RECORDS Colombia.<br />
Gnathia triospathiona Boone, 1918<br />
Figure 104C<br />
DIAGNOSIS 6 8.8 mm. Anterior margin of cephalon with broad-based tri<br />
angular projection bearing three low teeth; deep V-shaped depression pos<br />
terior to anterior margin, with low flanking granulations.<br />
RECORDS Off Key West, in Gulf Stream, 218 m.
Figure 104. Gnathia rathi: A, 6; Gnathia samariensis: B, 6 (after Miiller, 1988);<br />
Gnathia triospathiona; C, 6; Gnathia virginalis: D, 6; Gnathia velosa: E, 6 (after<br />
Miiller, 1988).<br />
E
Gnathia velosa Muller, 1988<br />
Figure 104E<br />
MIGROGERBERIDEA • MIGROGERBERIDAE 243<br />
DIAGNOSIS 6 1.5 mm. Anterior margin of cephalon with three tubercles,<br />
median tubercles slightly shorter and narrower than submedian pair. Dorsal<br />
integument of cephalon and anterior three pereonites granular. Pereonite 5<br />
about 2.5 times wider than middorsal length. Lateral lobe of mandible<br />
notched.<br />
RECORDS Colombia.<br />
Gnathia virginalis Monod, 1926<br />
Figure 104D<br />
DIAGNOSIS 6 2.2 mm. Anterior margin of cephalon with three tubercles,<br />
median tubercles slightly longer than submedian pair. Dorsal integument of<br />
cephalon and anterior three pereonites granular. Pereonite 5 about 1.7 times<br />
wider than middorsal length. Lateral lobe of mandible rounded.<br />
RECORDS U.S. Virgin Islands, 29 m; Colombia.<br />
Suborder Microcerberidea Lang, 1961<br />
DIAGNOSIS Cephalon free. Mandibles with reduced palp, or lacking palp.<br />
Maxillipedal palp of five articles. Pereon of seven free segments. Pereopod 1<br />
subchelate; pereopods 2—7 ambulatory. Pleon of two free pleonites plus<br />
pleotelson. Pleopod 1 in 6 usually absent. Pleopod 2 modified for copulation.<br />
Pleopod 3 uniramous, opercular. Pleopod 4 biramous. Pleopod 5 reduced.<br />
Uropods usually uniramous or biramous.<br />
Family Microcerberidae Karaman, 1933b<br />
DIAGNOSIS Eyes absent. Body elongate, slender. Antennular peduncle of<br />
three articles; antennal peduncle of six to eight articles. Mandibular palp of<br />
single article; molar reduced to single stout fringed spine. Maxilla 2 reduced<br />
to single ramus bearing two distal fringed lobes. Pereopods 2—7 ambulatory,<br />
dactyli biunguiculate.<br />
REMARKS The species of the Microcerberidae are all very small (less than 2<br />
mm total length) and are most often found in interstitial habitats. They have<br />
been recorded from marine, brackish, and freshwater environments.
244 MICROCERBERIDEA • MICROCERBERIDAE<br />
The microcerbcrideans were often classified with the Anthuridea, mainly<br />
because of the similarity in body shape. Wagele (1983) however, has con<br />
vincingly demonstrated the asellotan affinities of the group.<br />
Key to genera of Microcerberidae<br />
1. MaxilHpedal palp articles 2 and 3 enlarged; basis of pereopods lacking<br />
spinous process Yvesia<br />
MaxilHpedal palp articles 2 and 3 not markedly enlarged; basis of<br />
pereopods with spinous process Microcerberus<br />
Microcerberus Karaman, 1933b<br />
DIAGNOSIS MaxilHpedal palp articles 2 and 3 not enlarged. Articles 2 and 3<br />
of antennal peduncle with spinous process. Basis of pereopods with spinous<br />
process. Propodus of pereopod 2 with two denticulate proximal spines.<br />
Microcerberus syrticus Kensley, 1984<br />
Figure 105A-E<br />
DIAGNOSIS (5 1.1 mm, 9 1.1 mm. Tergal lobes of pereonites 2—4 rounded.<br />
Apical lobe of 6 pleopod 2 acute.<br />
RECORDS Carrie Bow Cay, Belize, interstitial in intertidal sand bar.<br />
REMARKS In addition to M. syrticus, six species of Microcerberus have been<br />
recorded from the Caribbean area: M. littoralis Chappuis and Delamare De-<br />
boutteville, 1956, from the Bahamas; M. minutus Coineau and Botosaneanu,<br />
1973, from Cuba; M. mirabilis Chappuis and Delamare Deboutteville, 1956,<br />
from the Bahamas; M. nunezi Coineau and Botosaneanu, 1973, from Cuba;<br />
M. renaudi Chappuis and Delamare Deboutteville, 1956, from the Bahamas;<br />
M. simplex Coineau and Botosaneanu, 1973, from Cuba. The reader is re<br />
ferred to the original descriptions for separation of the species.
\<br />
Figure 105. Microcerberus syrticus: A, 8; B, pereopod 1; C, maxilliped; D, pereopod<br />
2; Ey pleopod 2 8. Yvesia striata (from Coineau and Botosaneanu, 1973): F9<br />
maxilliped; G, pereopod 1; H, pereopod 2.<br />
G
246 MICROCERBER1DEA • ONISCIDEA<br />
Yvesia Coineau and Botosaneanu, 1973<br />
Yvesia striata Coineau and Botosaneanu, 1973<br />
Figure 105F-H<br />
DIAGNOSIS 9 1.6 mm. Antennal peduncular articles 2 and 3 smooth, lack<br />
ing spinous processes. Maxillipedal palp articles 2 and 3 enlarged. Bases of<br />
pereopods unarmed, lacking spinous processes. Propodus of percopod 1 with<br />
single smooth proximal spine. Body having longitudinal ventrolateral striae.<br />
RECORDS Oriente, Cuba, interstitial on beach.<br />
Suborder Oniscidea Latreille, 1803<br />
DIAGNOSIS Compound eyes usually present. Antennules usually very short.<br />
Antennae with 4- or 5-articulate peduncle; flagellum varying from few arti<br />
cles to multiarticulate. Mandibular palp present. Distal articles of maxillipe<br />
dal palp often reduced. Coxae of pereonites 1—7 usually distinct, expanded.<br />
Pleopods respiratory, often with pseudotrachea; 6 with pleopod 2, and<br />
sometimes pleopod 1 as well, modified for copulation. Uropods terminal or<br />
subterminal with terete rami, or ventral and opercular, with reduced rami.<br />
REMARKS The Oniscidea includes all the isopods that have successfully in<br />
vaded the terrestrial environment. While still in some degree reliant on exter<br />
nal moisture, their morphological and behavioral adaptations have allowed<br />
them to live in almost all terrestrial habitats, from hot, dry deserts, through<br />
tropical rainforests and grasslands, to cold-temperate niches. Several forms<br />
have successfully inveigled themselves into termite or ant colonies, where<br />
with varying degrees of morphological adaptations they take advantage of<br />
the security of these habitats. A small number of species have evolved to live<br />
in more constantly wet habitats. Several species may be found in the marine<br />
intertidal, either living in and under piles of decomposing litter along the<br />
high-tide line, digging into beach sand, or sheltering in the damp cracks and<br />
crevices of rocky shores. A few may also be found in mangrove swamps.<br />
A breakdown of families, genera, and species is not provided for this sub<br />
order, but those few species that are commonly encountered in intertidal<br />
habitats are dealt with individually. Schultz (1974, 1984) records several<br />
oniscidean isopods from the Caribbean area.
Key to genera and species of littoral Oniscidea<br />
ONISCIDEA 247<br />
1. At least one uropodal ramus reaching well beyond outline of body ... 5<br />
Uropodal rami very short, not reaching beyond outline of body 2<br />
2. Uropods ventral, not visible in dorsal view 3 (Tylos)<br />
Uropods visible in dorsal view Armadilloniscus ninae<br />
3. Ventral extensions of pleonite 5 meeting in midline Tylos niveus<br />
Ventral extensions of pleonite 5 not meeting in midline 4<br />
4. Ventral extensions of pleonite 5 very short, obsolete Tylos wegeneri<br />
Ventral extensions of pleonite 5 well separated Tylos marcuzzii<br />
Ventral extensions of pleonite 5 just falling short of meeting in midline<br />
Tylos latreillei<br />
5. Uropodal rami both elongate, subequal 6 (Ligia)<br />
Uropodal rami very unequal in length 8<br />
6. Propodus of 6 pereopod 1 with distal rounded lobe Ligia exotica<br />
Propodus of 6 pereopod 1 lacking rounded lobe 7<br />
7. Apex of 6 pleopod 2 club shaped Ligia olfersii<br />
Apex of 6 pleopod 2 bifid Ligia baudiniana<br />
8. Antennal flagellum of two articles Rhyscotus texensis<br />
Antennal flagellum of three articles 9 (Vandeloscia)<br />
9. Endopod of 6 pleopod 1 with large scalelike subapical process<br />
Endopod of 6 pleopod 1 with small scalelike subapical process<br />
Armadilloniscus Ul'yanin, 1875<br />
Armadilloniscus ninae Schultz, 1984<br />
Figure 106A<br />
Vandeloscia riedli<br />
Vandeloscia culebrae<br />
- - - - - ,<br />
DIAGNOSIS 8 3.2 mm, 9 4.1 mm. Uropodal sympod expanded to form part<br />
of body outline; rami set mesial to expanded base, with exopod half length of<br />
endopod.<br />
RECORDS Ambergris Cay, Belize; under damp objects along beach drift<br />
line.
Figure 106. A, Armadilloniscus ninae. Ligia baudiniana: B; C} 6 pleopod 2 endopod.<br />
Ligia exotica: D, dactylus and propodus of pereopod 1; E, 6 pleopod 2 endopod.<br />
Ligia olfersii: F, 6 pleopod 2 endopod. G, Rhyscotus texensis. Tylos latreillei: H,<br />
ventral pleon. Tylos marcuzzi: I, ventral pleon (from Schultz, 1984). Tylos niveus: J,<br />
lateral view; K} ventral pleon. Tylos wegeneri: L} ventral pleon. Vandeloscia culebrae:<br />
M, apex of pleopod 1 endopod. N, Vandeloscia riedli.
Ligia Fabricius, 1798<br />
Ligia baudiniana H. Milne Edwards, 1840<br />
Figure 106B,C<br />
Rhyscotus texensis 249<br />
DIAGNOSIS 6 and 9 up to 22 mm. Antennal flagellum elongate, multiar-<br />
ticulate. Apex of 6 pleopod 2 bifid, with lateral lobe longer and more slender<br />
than mesial lobe. Uropods inserted terminally on pleotelson; sympods<br />
elongate-cylindrical; rami slender, elongate, subequal.<br />
RECORDS Bermuda; Bahamas; U.S. Virgin Islands; Antigua; Carrie Bow<br />
Cay, Belize; Bonaire; Aruba; Trinidad; Tobago; Gulf of Mexico.<br />
REMARKS <strong>As</strong> is typical in the genus Ligia, this species may be seen on rocks<br />
i<br />
and sea walls, as well as piles of drift debris at low tide. When disturbed, they<br />
run rapidly, to shelter in damp crevices and hollows.<br />
Ligia exotica Roux, 1828<br />
Figure 106D,E<br />
DIAGNOSIS 6 28.5 mm, ovigerous 9 32.0 mm. Propodus of 8 pereopod 1<br />
with rounded lobe on inner distal surface. Apex of 6 pleopod 2 club shaped,<br />
convoluted.<br />
RECORDS New Jersey to Uruguay; Indo-Pacific.<br />
Ligia olfersii Brandt, 1833<br />
Figure 106F<br />
DIAGNOSIS 6 20.0 mm, ovigerous 9 24.0 mm. Apex of 6 pleopod 2 simple,<br />
club shaped.<br />
RECORDS South Florida to Rio de Janeiro, Brazil; Texas, Gulf of Mexico.<br />
Rhyscotus Budde-Lund, 1885<br />
Rhyscotus texensis (Richardson, 1905)<br />
Figure 106G<br />
DIAGNOSIS 6 and 9 6.0 mm. Antennal flagellum of two unequal articles.<br />
Uropodal endopod at least twice length of exopod, inserted distally on base,<br />
exopod inserted distally on base. Pleotelson broadly triangular.<br />
RECORDS Carrie Bow Cay, Belize; Texas, Gulf of Mexico.
250 ONISCIDEA<br />
Tylos Latreille, 1826<br />
Tylos latreillei Audouin, 1826<br />
Figure 106H<br />
DIAGNOSIS 6 12.8 mm, 9 13.0 mm. Ventral extensions of pleonite 5 not<br />
meeting in midline.<br />
RECORDS Bermuda; Cuba; Puerto Rico; Honduras.<br />
Mediterranean.<br />
Tylos marcuzzii Soika, 1954<br />
Figure 1061<br />
DIAGNOSIS 6 6.6 mm. Antennal flagellum of four articles. Ventral exten<br />
sions of pleonite 5 well separated.<br />
RECORDS Florida Keys; Bahamas; Leeward Islands; Ambergris Cay, Be<br />
lize; under debris on sand beach drift line.<br />
Tylos niveus Budde-Lund, 1885<br />
Figure 106J,K<br />
DIAGNOSIS 8 11.0 mm., 9 12.0 mm. Antennal flagellum of four articles.<br />
Ventral extensions of pleonite 5 expanded, medially contiguous.<br />
RECORDS Bahamas; Florida Keys; Cuba; Dominica; Lesser Antilles; Bo<br />
naire; Curasao, under piles of decaying mangrove leaves at beach drift line;<br />
Carrie Bow Cay, Ambergris Cay, Belize, under deep piles of dead plant ma<br />
terial on beach drift line; Tobago; Panama.<br />
Rio de Janeiro, Brazil.<br />
Tylos wegeneri Vandel, 1952<br />
Figure 106L<br />
DIAGNOSIS 6 10.5 mm, 9 15 mm. Antennal flagellum of three articles.<br />
Ventral extensions of pleonites short or nearly absent. Pleonite 5 lacking free<br />
lateral margins.<br />
RECORDS Tobago; Venezuela, under decaying beach debris on drift line;<br />
Trinidad.
Vandeloscia Roman, 1977<br />
Vandeloscia culebrae (Moore, 1901)<br />
Figure 106M<br />
VALVIFERA 251<br />
DIAGNOSIS 8 5.0 mm, 9 6.1 mm. Tiny lateral tubercles present on per<br />
eonites. Endopod of pleopod 1 in 8 with small scalelike subapical process on<br />
laterally folded tip.<br />
RECORDS Florida Keys; U.S. Virgin Islands; Puerto Rico; under decaying<br />
plant material, especially Thalassia testudinea accumulated along beach drift<br />
line,<br />
Vandeloscia riedli (Strouhal, 1966)<br />
Figure 106N<br />
DIAGNOSIS 8 5.9 mm, 9 6.0 mm. Tiny obsolete tubercles present on all<br />
pereonites. Endopod of 8 pleopod 1 with large scalelike subapical process on<br />
laterally folded tip.<br />
RECORDS Yucatan Peninsula, Mexico; Ambergris Cay, Belize; Barbuda;<br />
Venezuela; Brazil.<br />
Gulf of Aqaba; Red Sea; northeastern coast of Africa; Madagascar; Bay of<br />
Bengal; St. Helena Is.<br />
Suborder Valvifera Sars, 1882<br />
DIAGNOSIS Pereopodal coxae, in addition to usual dorsal coxal plates, ex<br />
panded ventrally to form plates. Penes situated ventrally on articulation be<br />
tween pereon and pleon, or on pleonite 1. Pleonites and pleotelson variously<br />
fused. Uropods forming operculum covering over pleopods.<br />
Key to families of Valvifera<br />
1. Body often geniculate, flexed between pereonites 4 and 5; anterior<br />
pereopods setose for feeding, posterior pereopods ambulatory<br />
Arcturidae<br />
Body never geniculate; all pereopods ambulatory Idoteidae
252 VALV1FERA • ARGTUR1DAE<br />
REMARKS Of the six families in the suborder, only two have been recorded<br />
in the Caribbean area, the Idoteidae and the Arcturidae.<br />
Family Arcturidae Sars, 1897<br />
DIAGNOSIS Pereonite 1 either distinct, or completely or imcompletely fused<br />
with cephalon. Anterior four pairs of pereopods directed anteriorly, usually<br />
strongly setose; posterior three pairs of pereopods ambulatory, used for cling<br />
ing to substrate. Body often bent between pereonites 4 and 5. Uropods usu<br />
ally biramous, with minute endopod concealed by larger exopod. Pleonites<br />
variously fused with pleotelson. Sexual dimorphism often marked.<br />
REMARKS Menzies and Kruczynski (1983) described three species of<br />
arcturids from the west coast of Florida, in depths of 55—73 m: Arcturella<br />
spinata, Arcturella bispinata, and Edwinjoycea horologium. These species are not<br />
covered here.<br />
Key to genera of Arcturidae<br />
1. Pereonite 1 not fused with cephalon; at least one free pleonite<br />
Pereonite 1 fused with cephalon; pleonites fused with pleotelson<br />
<strong>As</strong>tacilla Cordiner, 1793<br />
Thermarcturus<br />
<strong>As</strong>tacilla<br />
DIAGNOSIS Antennae at least half length of body. Pereopod 1 with strong<br />
terminal claw on dactylus. Pereopods 2—4 lacking dactyli. Endopod of<br />
Key to species of <strong>As</strong>tacilla<br />
1. Body integument lacking ornamentation cymodocea<br />
Body integument with spines or tubercles 2<br />
2. Pereonite 4 in 6 and 9 with strong middorsal tubercle; pairs of spines<br />
lacking on pereonites regina<br />
Pereonite 4 lacking strong middorsal tubercle; pairs of spines on all<br />
pereonites lasallae
<strong>As</strong>tacilla regina 253<br />
pleopod 1 6 with median notch and three specialized setae; pleopod 2 copu-<br />
latory stylet apically trifid. Pereonite 4 considerably longer than preceeding<br />
or following pereonite.<br />
<strong>As</strong>tacilla cymodocea Menzies and Glynn, 1968<br />
Figure 107A,B<br />
DIAGNOSIS 6 6.4 mm, ovigerous 9 9.0 mm. Body cylindrical, ovigerous 9<br />
with pereonite 4 somewhat bulged, 6 with pereonite 4 elongate-cylindrical.<br />
Shallow groove marking fusion between cephalon and pereonite 1. Pleonites<br />
fused with pleotelson, with two incomplete shallow dorsal grooves marking<br />
lines of fusion anteriorly. Pleotelson lacking any shoulders, posteriorly tape<br />
red to narrowly rounded apex.<br />
RECORDS Florida Keys; Puerto Rico, 1.5 m, on Cymodocea sp. seagrass; Car<br />
rie Bow Cay, Belize, 1—2 m, on Syringodium filiforme seagrass.<br />
REMARKS In life, A. cymodocea is bright green, blending in with its preferred<br />
substrate of seagrasses.<br />
<strong>As</strong>tacilla lasallae Paul and Menzies, 1971<br />
Figure 107C<br />
DIAGNOSIS 9 3.5 mm. Cephalon with large rounded area bearing pair of<br />
spines; all pereonites and two anterior fused pleonites bearing pair of short<br />
submedian spines. Pleotelson with strong anterior shoulder, posteriorly tri<br />
angular, tapering sharply to narrowly rounded apex.<br />
RECORD Off Venezuela, 95 m.<br />
REMARKS This species is known only from the small female holotype, and<br />
until a mature male and ovigerous female are found, it cannot be confidently<br />
diagnosed.<br />
<strong>As</strong>tacilla regina Kensley, 1984<br />
Figure 107D-G<br />
DIAGNOSIS 6 6.5 mm, ovigerous 9 7.1 mm. Body strongly tuberculate,<br />
many tubercles acute. Cephalon with two submedian pairs of acute tuber<br />
cles; fused pereonite 1 and pereonites 2 and 3 each with single middorsal<br />
acute tubercle. Pereonite 4 with strong middorsal tubercle situated in ante-
254 VALVIFERA • ARGTURIDAE<br />
Figure 107. <strong>As</strong>tacilla cymodocea: A, 8; By 9. <strong>As</strong>tacilla lasallae: C, 6. <strong>As</strong>tacilla regina:<br />
D, 6\ E, 9; F, pereopod 4; G, pereopod 1. Thermarcturus venezuelensis: H, 9 (from<br />
Paul and Menzies, 1971).<br />
rior half. Pleotelson with strong lateral shoulder in anterior half, second<br />
shoulder in posterior half, then tapering to rounded apex.<br />
RECORDS Carrie Bow Cay, Belize, on forereef slope, 27—36 m; Barbados,<br />
100—400 m; St. Lucia, 2—3 m, associated with crinoids.
Thermarcturus Paul and Menzies, 1971<br />
VALVIFERA • IDOTEIDAE 255<br />
DIAGNOSIS Pereonite 1 not fused with cephalon. Pereonite 4 subequal in<br />
length to pereonite 3, not markedly elongate. Pereopods 2—4 having dactyli<br />
but lacking elongate setae. Body cylindrical, flexed between pereonites 4 and<br />
5. Pleon consisting of two free pleonites plus pleotelson.<br />
Thermarcturus venezuelensis Paul and Menzies, 1971<br />
Figure 107H<br />
DIAGNOSIS 9 4.5 mm. Cephalon, all pereonites, and anterior two pleonites<br />
each with submedian pair of dorsal tubercles, those on pereonites 2 and 3<br />
broad and expanded. Pleonite 2 with pair of bulbous lateral swellings, pos<br />
terior margin triangular. Pleotelson with lateral shoulder anteriorly, posteri<br />
orly triangular.<br />
RECORDS Off Venezuela, 95 m.<br />
REMARKS Only the holotype (which seems to be lost) is known of this spe<br />
cies. Considerable uncertainty exists regarding some of the features.<br />
Family Idoteidae Fabricius, 1798<br />
Subfamily Idoteinae Dana, 1852<br />
DIAGNOSIS Flagellum of antenna either multiarticulate; clavate, i.e., with<br />
large basal articles and with or without one to four reduced distal articles; or<br />
Key to genera of Idoteinae<br />
1. Antennal flagellum multiarticulate Idotea<br />
Antennal flagellum clavate 2<br />
2. Pereopod 4 reduced, considerably smaller than pereopods 3 or 5 .... 3<br />
Pereopod 4 not reduced, of similar size to pereopods 3 and 5<br />
Erichsonella<br />
3. Pleon consisting of three complete and one incomplete pleonites plus<br />
pleotelson Cleantioides<br />
Pleon consisting of two complete and two incomplete pleonites plus<br />
pleotelson Miratidotea
256 VALVIFERA • IDOTEIDAE<br />
vestigial. Maxillipedal palp consisting of five or fewer articles. Uropods uni-<br />
ramous or biramous, rami usually much smaller than sympod. Pleonites<br />
variously fused with pleotelson; number of fused pleonites often indicated by<br />
lateral sutures or furrows.<br />
REMARKS Brusca (1984) has reviewed the phylogeny, evolution, and bio-<br />
geography of the subfamily Idoteinae, the only one of the five subfamilies<br />
recorded from the Caribbean.<br />
Cleantioides Kensley and Kaufman, 1978<br />
DIAGNOSIS Antennal flagellum a single clavate article. Maxillipedal palp of<br />
four or five articles. Pereopod 4 somewhat reduced. Uropod uniramous.<br />
Pleon consisting of three complete and one incomplete pleonites plus<br />
pleotelson.<br />
Cleantioides planicauda (Benedict, 1899)<br />
Figure 108A<br />
DIAGNOSIS Ovigerous 9 5.5 mm. Body parallel sided. Maxillipedal palp of<br />
five articles. Pleotelson posteriorly broadly rounded, with obliquely truncate<br />
subcircular dorsal area in posterior half.<br />
RECORDS Maryland to Florida; Puerto Rico; Panama; Louisiana, Gulf of<br />
Mexico, intertidal to 44 m; often in hollow stems and roots of seagrasses, and<br />
tubes of the polychaete Diopatra cuprea.<br />
Oaxaca, Pacific Mexico.<br />
REMARKS Cleantioides planicauda has been recorded only once in the eastern<br />
Pacific, where it occurs with the more common C. occidentalis (Richardson,<br />
1899).<br />
Key to species of Erichsonella<br />
1. Pereonites with dorsal spines 2<br />
Pereonites lacking dorsal spines attenuata<br />
2. Pereonites 1—4 with middorsal and lateral spines floridana<br />
Pereonites 1-4 with middorsal spines only Jiliformis
Erichsonella 257<br />
Figure 108. Ay Cleantioides planicauda; B, Erichsonella attenuata 8\ C, Erichsonella<br />
filiformis 6\ Dy Erichsonella Jloridana 9; E, Idotea balthica 6] F, Idotea metallica $; G,<br />
Miratidotea bruscai $.<br />
Erichsonella Richardson, 1901<br />
DIAGNOSIS Antennal flagellum clavate. Maxillipedal palp of four articles.<br />
Uropod uniramous. Pleonites completely fused with pleotelson.<br />
REMARKS Pires (1984) reviewed the genus Erichsonella and did not recog<br />
nize the subspecies E. filiformis tropicalis Menzies and Glynn, 1968.
258 VALVIFERA • IDOTEIDAE<br />
Erichsonella attenuata (Harger, 1873)<br />
Figure 108B<br />
DIAGNOSIS 6 11.4 mm, ovigerous 9 12.0 mm. Body dorsally smooth.<br />
Cephalon lacking middorsal elevation. Antcnnulc reaching only slightly be<br />
yond antennal peduncular article 2. Pleotelson with slight marginal bulge in<br />
anterior half, indicating ventrolateral articulation of uropod.<br />
RECORDS Connecticut to Miami; Florida, Mississippi, Texas, Gulf of Mex<br />
ico; intertidal to 2 m, usually associated with submerged seagrass and algal<br />
beds.<br />
REMARKS While not recorded in the Florida Keys, this species does reach<br />
Miami, and continues into the Gulf of Mexico.<br />
Erichsonella filifor mis (Say, 1818)<br />
Figure 108C<br />
DIAGNOSIS 6 10.5 mm, ovigerous 9 8.2 mm. Body dorsally with bifid tu<br />
bercle on cephalon, and low rounded middorsal tubercle on pereonites. An-<br />
tennule reaching midlength of antennal peduncular article 3. Basis of per-<br />
eopods 2—7 with larges tubercles. Pleotelson with distinct lateral shoulder in<br />
anterior half.<br />
RECORDS Connecticut to Florida, shallow infratidal to 55 m; Bahamas;<br />
Turks and Caicos Islands; Puerto Rico; Quintana Roo, Yucatan Peninsula,<br />
Mexico, 60-109 m; Florida and Texas, Gulf of Mexico.<br />
Brazil.<br />
Erichsonella jloridana Richardson, 1901<br />
Figure 108D<br />
DIAGNOSIS Ovigerous 9 10.0 mm. Antennule reaching distal end of anten<br />
nal peduncular article 3. Cephalon with strong trifid tubercle. Pereonites 1—7<br />
each with posteriorly directed spine near posterior margin; pereonites 1—4<br />
each with lateral spine. Basis of pereopods 2-7 smooth.<br />
RECORDS Florida Keys, intertidal to 2 m; Florida, Gulf of Mexico, interti<br />
dal mud flats.
Idotea Fabricius, 1798<br />
Miratidotea 259<br />
DIAGNOSIS Antennal flagellum multiarticulate. Maxillipedal palp of four or<br />
five articles. Uropod uniramous. Pleon consisting of two complete and one<br />
incomplete pleonites plus pleotelson.<br />
Key to species of Idotea<br />
1. Posterior margin of pleotelson truncate metallica<br />
Posterior margin of pleotelson with distinct median lobe balthica<br />
Idotea balthica (Pallas, 1772)<br />
Figure 108E<br />
DIAGNOSIS 6 24.5 mm, ovigerous 9 13.2-23.5 mm. Anterior margin of<br />
cephalon concave. Cephalon dorsally smooth. Pereonites evenly convex,<br />
smooth. Posterior margin of pleotelson with rounded median lobe.<br />
RECORDS Worldwide in tropical to cold-temperate waters, often on floating<br />
seaweed, from surface to 357 m.<br />
Idotea metallica Bosc, 1802<br />
Figure 108F<br />
DIAGNOSIS 6 30.0 mm, ovigerous 9 22.2 mm. Cephalon with sinuous fur<br />
row in posterior half. Pereonites 2—4 laterally with rounded convex area close<br />
to coxae. Posterior margin of pleotelson truncate.<br />
RECORDS Worldwide in tropical to cold-temperate waters, often on floating<br />
seaweed, from surface to 200 m.<br />
Miratidotea Kensley, 1987a<br />
DIAGNOSIS Antennal flagellum of single clavate article. Maxillipedal palp<br />
of four articles. Uropod uniramous. Pleon consisting of two complete and two<br />
incomplete pleonites plus pleotelson.
260 VALVIFERA • IDOTEIDAE<br />
Miratidotea bruscai Kensley, 1987a<br />
Figure 108G<br />
DIAGNOSIS Ovigerous 9 13.0 mm. Body parallel sided. Maxillipedal palp<br />
of four articles, terminal article very short. Pereopods 1—3 increasing in<br />
length posteriorly, pereopod 4 reduced, shorter than pereopod 5, and with<br />
dactylus spinelike, pereopods 5—7 increasing in length. Pleotelson consisting<br />
of two complete and two incomplete pleonites plus pleotelson; latter with<br />
broadly rounded posterior margin, and with bifid median process situated<br />
dorsal to posterior oblique-concave area.<br />
RECORDS Carrie Bow Cay, Belize, 1.5 m, in hollow root-internodes of sea-<br />
grass Syringodium filiforme.
Zoogeography<br />
FAUNAL PROVINCES<br />
The area under discussion has been divided into several faunal regions or<br />
provinces, of which the Caribbean, West Indian, and Brazilian are the major<br />
ones (Briggs, 1974). The extent and boundaries of the provinces have been<br />
variously defined depending on the group of organisms under discussion.<br />
Inevitably, zones of overlap exist, but for the purposes of this discussion, the<br />
following rough limits have been used.<br />
Brazilian Province: This province stretches from Cape Frio near Rio de<br />
Janeiro in Brazil to the mouth of the Orinoco River in Venezuela. The out<br />
flow of freshwater from the major rivers of this region has probably contrib<br />
uted to the isolation of the Brazilian coral reefs and their associated fauna<br />
from those of the Caribbean. This isolation is demonstrated by the consider<br />
able endemism of the Brazilian reef fauna and that of the Caribbean reef<br />
fauna, with very few species being common to both.<br />
Caribbean Province: This province has two components, a northern part<br />
in peninsular Florida, that stretches from around Cape Kennedy on the east<br />
coast to Tampa or Sanibel Island on the west coast, and a southern compo<br />
nent that runs from the mouth of the Orinoco River to around Cabo Rojo or<br />
Tampico on the gulf coast of Mexico. The northern Gulf of Mexico is ex<br />
cluded from this province and is characterized as being warm-temperate,<br />
rather than subtropical (Briggs, 1974:66).<br />
West Indian Province: This includes all the islands of the West Indian<br />
chain, the Bahamas, and the isolated outrider, Bermuda. The West Indian<br />
Province closely approaches the Caribbean Province in the Yucatan Penin<br />
sula to the north, and between Grenada and Trinidad in the south. There is<br />
also some indication of the isolating effect on the Bahamas of the Florida<br />
Current through the Straits of Florida.<br />
It has been suggested, on the basis of the molluscan fauna, that a relict of<br />
the Neogene Gatunian Province exists around northern Venezuela and Col<br />
ombia (Petuch, 1982). While several isopod species have been recorded only<br />
from this area, these are all described in a single paper that covers a very<br />
small part of this region (Paul and Menzies, 1971). There is as yet too little<br />
evidence to explore the idea of this relict fauna further.<br />
261
262 ZOOGEOGRAPHY<br />
ANALYSIS OF THE ISOPOD FAUNA<br />
In the following discussion, the West Indian and Caribbean provinces are<br />
treated as one, the isopod faunas offering little evidence to warrant a separate<br />
treatment of each.<br />
It is a truism that for any discussion of the zoogeography of an area to have<br />
meaning, the true extent of the fauna must be known. With the area under<br />
review, collecting effort has been uneven, and the true faunal composition of<br />
many regions is still incompletely known. Obviously, any conclusions based<br />
on such incomplete data are approximate and subject to revision. Neverthe<br />
less, certain general patterns or trends emerge when the present isopod fauna<br />
is broken down into its components.<br />
The deepwater isopod fauna of the Caribbean (i.e., from deeper than 200<br />
m) has barely been explored, and little is to be gained from discussing the<br />
relatively few species known. A list of these deeper dwelling species is in<br />
cluded (Table 4).<br />
Although about 280 shallow-water species are covered by this work, cer<br />
tain categories of species must be excluded, for various reasons, before anal<br />
ysis can be attempted. Such excluded groups include the species of Oniscidea<br />
(being essentially terrestrial forms and not part of the marine regime); the<br />
cymothoid species and the species of Aegidae (being fish parasites for at least<br />
part of their life history, and whose distribution is complicated by the dis<br />
tribution and mobility of the hosts); the limnoriids (being wood-borers whose<br />
distribution is more a function of the distribution of floating wood); and the<br />
true cave species (which have a history more reflective of the geological his<br />
tory of the area than of the marine regime). The epicarideans have a distribu<br />
tion somewhat complicated by the distribution of their crustacean hosts and<br />
their pelagic epicaridean and cryptoniscan larvae. Nevertheless, the decapod<br />
hosts of the great majority of species covered here are Caribbean endemics,<br />
and inclusion of the epicarideans changes very little the overall patterns of<br />
distribution, as demonstrated by the two figures provided (Figure 109). After<br />
making these exclusions there remain about 166 species (218 with the epi<br />
carideans) that can be broken down into the following components (figures<br />
in brackets include epicarideans):<br />
1. True Caribbean/Bahamian species—124 species, 74.8% [147, 67.5%].<br />
These are the species recorded only from the Caribbean and the Bahamas.<br />
The term endemic is avoided, as too little is known of the actual distribution<br />
of many species. Of these species, 86 [87] have been recorded from a single<br />
locality.<br />
2. Species occurring south of the discussion area, and extending into<br />
Brazil—5 species, 3.0% [9, 4.1%]. These low numbers indicate that the
TABLE 4. CARIBBEAN ISOPODS RECORDED FROM DEPTHS GREATER THAN 200 M<br />
SUBORDER ANTHURIDEA<br />
Family Paranthuridae<br />
Neoanthura coeca Menzies, 1956b. South of Jamaica, 1244 m<br />
SUBORDER ASELLOTA<br />
Family Dendrotiidae<br />
Dendrotion hanseni Menzies, 1956b. South of Jamaica, 1244 m<br />
Family Desmosomatidae<br />
Desmosoma magnispina Menzies, 1962a. Bay of Panama, 1906 m<br />
Family Echinothambematidae<br />
Echinothambema ophiuroides Menzies, 1956a. North of Puerto Rico Trench,<br />
5104-5122 m<br />
Family Eurycopidae<br />
Acanthocope spinosissima Menzies, 1956b. South of Jamaica, 1224 m<br />
Storthyngura pulchra caribbea (Benedict, 1901). Off Windward Islands, 1256 m<br />
Storthyngura snanoi Menzies, 1962a. Colombia abyssal plain, 4071 m<br />
Family Haploniscidae<br />
Antennuloniscus dirneroceras (Barnard, 1920). North of Puerto Rico Trench,<br />
5440-5410 m; South Atlantic off South and West Africa, 1400-3921 m;<br />
off Argentina, 5843 m<br />
Haploniscus unicornis Menzies, 1956a. North of Puerto Rico Trench, 5104-<br />
5122 m<br />
Hydroniscus quadrifrons Menzies, 1962a. North of Puerto Rico Trench, 5271-<br />
5684 m<br />
Family Ischnomesidae<br />
Haplomesus tropicalis Menzies, 1962a. Colombia abyssal plain, 4071 m; off<br />
South Africa, 2526 m; Mediterranean<br />
Heteromesus bifurcatus Menzies, 1962a. Colombia abyssal plain, 4071 m<br />
Ischnomesus armatus Hansen, 1916. North of Puerto Rico Trench, 5494-5477<br />
m; Davis Straits, 2702 m<br />
Ischnomesus caribbicus Menzies, 1962a. Off Panama, 1714 m<br />
Ischnomesus multispinis Menzies, 1962a. Off Panama, 975 m<br />
Family Janiridae<br />
Abyssianira dentifrons Menzies, 1956a. North of Puerto Rico Trench, 5104-<br />
5122 m; off Argentina, 5024-5293 m; off southwest Africa, 4588 m<br />
Ianirella caribbica Menzies, 1956b. South of Jamaica, 1244 m<br />
Ianirella vemae Menzies, 1956a. Near Puerto Rico Trench, 5104-5122 m<br />
Spinianirella serrata Kensley and Heard, 1985. Off Puerto Rico, 350 m<br />
Family Macrostylidae<br />
Macrostylis caribbicus Menzies, 1962a. Off Colombia, 2875-2941 m<br />
Macrostylis minutus Menzies, 1962a. North of Puerto Rico Trench, 5163-<br />
5494<br />
{continued)
264 ZOOGEOGRAPHY<br />
TABLE 4. {Continued)<br />
Macrostylis setifer Menzies, 1962a. North of Puerto Rico Trench, 5477-<br />
5494 m<br />
Macrostylis vemae Menzies, 1962a. North of Puerto Rico Trench, 5410—<br />
5684 m<br />
Family Mesosignidae<br />
Mesosignum kohleri Menzies, 1962a. Colombia abyssal plain, 2868-4076 m<br />
Family Nannoniscidae<br />
Nannoniscus camayae Menzies, 1962a. Off Panama, 1714 m<br />
SUBORDER GNATH11DEA<br />
Family Gnathiidae<br />
Akidognathia poteriophora Monod, 1926. OffU. S. Virgin Islands, 914 m.<br />
SUBORDER VALVIFERA<br />
Family Arcturidae<br />
Antarcturus annaoides Menzies, 1956b. South of Jamaica, 1244 m<br />
Arcturus caribbaeus Richardson, 1901. OfTAves Island, 1360 m<br />
Arcturus purpureus Beddard, 1886. Off Leeward Islands, 900 m<br />
Note: Records from deep water around Bermuda are not included.<br />
great area of mixed-salinity waters resulting from the outflow of the Orinoco,<br />
Amazon, Tocantins, and Parnaiba rivers form an effective barrier to the<br />
movement of shallow-water isopod species.<br />
3. Species having an amphi-Panamic distribution—7 species, 4.2% [8,<br />
3.7%] (Table 5). In spite of the history of immergence and emergence of the<br />
Isthmus of Panama, this very small amphi-Panamic component in the Carib<br />
bean isopod fauna suggests that most of this fauna has evolved since the last<br />
emergence of the late Pliocene. Given the limited mobility of most isopod<br />
species, the Panama Canal seems to have played a minimal role in contribut<br />
ing to this component.<br />
4. Species occurring outside of the western Atlantic (but excluding the<br />
amphi-Panamic species)—3 species, 1.8% [7, 3.2%].<br />
5. The role of the Gulf of Mexico isopod fauna (see Clark and Robertson,<br />
1982) in the composition of the Caribbean/Bahamian is complex and diffi<br />
cult to analyze. One hundred and thirteen species of shallow-water isopods<br />
have been recorded from the Gulf of Mexico (Table 6). This number would<br />
indicate that many species remain to be recorded in this region. Of these 113<br />
species, 61 (54%) have also been reported from the Caribbean region. It is<br />
therefore possible that there exists a true Gulf of Mexico fauna, whose evolu<br />
tion was perhaps spurred by the relative isolation and reduction of the Gulf
Car<br />
Car<br />
Sout<br />
South 3.0% %<br />
Panam 4.2%<br />
Epicaridea excluded<br />
Panam 3.7%<br />
Out 3.2%<br />
North<br />
Epicaridea included<br />
5.5%<br />
ZOOGEOGRAPHY 265<br />
GoM/N/Car 7.2%<br />
oM/Car 5.4%<br />
3.6%<br />
GoM/N/Car 9.6%<br />
/Car 6.4%<br />
Figure 109. Relative proportions of the zoogeographic components of the<br />
Caribbean isopod fauna, with and without the parasitic Epicaridea. Car,<br />
Caribbean; Out, extra-western Atlantic; GoM/Car, Gulf of Mexico-Caribbean;<br />
GoM/N/Car, Gulf of Mexico-Northern-Caribbean; North, northern; Panam, amphi-<br />
Panamic; South, southern.<br />
during a low-water stand (100 m below present sea level) during the<br />
Pleistocene. A significant proportion (about 27 species, 28%) of the Gulf of<br />
Mexico isopods are known from the eastern coast of the United States north
266 ZOOGEOGRAPHY<br />
TABLE 5. SPECIES OF ISOPODS OCCURRING ON BOTH SIDES OF THE ISTHMUS<br />
OF PANAMA<br />
*Aega deshaysiana (H. Milne Edwards,<br />
1840)<br />
Anopsilana browni (Van Name, 1936)<br />
Cleantioides planicauda (Benedict,<br />
1899)<br />
Excirolana braiiliensis Richardson,<br />
1912<br />
Excorallana tricornis (Hansen, 1890)<br />
*Nerocila acuminata Schioedte and<br />
Meinert, 1881<br />
* fish parasite or fish predator<br />
of Cape Kennedy, which would indicate a significant cooler-water compo<br />
nent. What proportion of originally Gulf species have spread into the Carib<br />
bean, and what proportion of Caribbean and temperate east coast species<br />
have entered the Gulf, cannot yet be assessed, given our incomplete knowl<br />
edge of the Gulf fauna. Because of this unresolved situation, three categories<br />
of species have been separated: species ranging from north of Cape Kennedy<br />
into the Caribbean—6, 3.6% [12, 5.5%]; species occurring in the Gulf of<br />
Mexico and the Caribbean—9, 5.4% [14, 6.4%]; species occurring north of<br />
Cape Kennedy, in the Gulf, and in the Caribbean—12, 7.2% [21, 9.6%] The<br />
conclusion that the fauna of the Gulf of Mexico contains an endemic compo<br />
nent, a Caribbean component, and a warm-temperate component was also<br />
reached by Topp and HofT (1972), in an analysis of the pleuronectiform<br />
fishes of the Gulf.<br />
THE BAHAMAS<br />
The Florida Current flowing through the Straits of Florida has been sug<br />
gested as a factor in reducing the movement of shallow-water fauna between<br />
peninsular Florida and the Florida Keys on the west and the Bahamas on the<br />
east (Briggs, 1974). Comparison of the number of isopod species on either<br />
side of the Straits of Florida (13 from the Bahamas, 50 from southern penin<br />
sular Florida and the Florida Keys) supports this view. Of the 13 species<br />
from the Bahamas, only four arc "endemic," three of these being interstitial<br />
microcerberideans.<br />
Paradella dianae (Menzies, 1962b)<br />
Paraleptosphaeroma glynni Buss and<br />
Iverson, 1981<br />
Probopyrus pandalicola (Packard,<br />
1879)<br />
*Rocinela oculata Harger, 1883<br />
*Rocinela signata Schioedte and<br />
Meinert, 1879<br />
Uromunna reynoldsi Frankenberg and<br />
Menzies, 1966
TABLE 6. ISOPOD SPECIES OCCURRING IN THE GULF OF MEXICO<br />
SUBORDER ANTHURIDEA<br />
*Accalathura crenulata (Richardson,<br />
1901)<br />
*Amakusantkura magnified (Menzies<br />
and Frankenberg, 1966)<br />
Cyathura polita (Stimpson, 1855)<br />
Horoloanthura irpex Menzies and<br />
Frankenberg, 1966<br />
Kupellonura formosa (Menzies and<br />
Frankenberg, 1966)<br />
*Mesanthura Jloridensis Menzies and<br />
Kruczynski, 1983<br />
Mesanthura hopkinsi Hooker, 1985<br />
Mesanthura pulchra Barnard,<br />
1925<br />
Paranthura Jloridensis Menzies and<br />
Kruczynski, 1983<br />
Ptilanthura tricarina Menzies and<br />
Frankenberg, 1966<br />
Skuphonura lindae Menzies and<br />
Kruczynski, 1983<br />
*Xenanthura brevitelson Barnard,<br />
1925<br />
SUBORDER ASELLOTA<br />
Carpias Jloridensis Menzies and<br />
Kruczynski, 1983<br />
Gnathostenetrioides pugio Hooker,<br />
1985<br />
*Joeropsis coralicola Schultz and<br />
McCloskey, 1967<br />
*Joeropsis rathbunae Richardson,<br />
1902<br />
Mexicope kensleyi Hooker, 1985<br />
Munnogonium wilsoni Hooker, 1985<br />
*Pleurocope Jloridensis Hooker,<br />
1985<br />
*Santia milleri (Menzies and Glynn,<br />
1968)<br />
*Stenetrium stebbingi Richardson,<br />
1902<br />
ZOOGEOGRAPHY 267<br />
Uromunna hayesi Robertson, 1978<br />
*Uromunna reynoldsi Frankenberg<br />
and Menzies, 1966<br />
SUBORDER EPICARIDEA<br />
Allodiplophryxus Jloridanus Markham,<br />
1985<br />
*Aporobopyrina anomala Markham,<br />
1973<br />
*Azygopleon schmitti (Pearse, 1932)<br />
*Bopyrina abbreviata Richardson,<br />
1904<br />
*Bopyrione synalphei Bourdon and<br />
Markham, 1980<br />
*Cancricepon choprae (Nierstrasz and<br />
Brender a Brandis, 1925)<br />
Dactylokepon sulcipes Adkison, 1982<br />
Eophryxus subcaudalis (Hay, 1917)<br />
*Gigantione mortenseni Adkison,<br />
1984b<br />
Gigantione uberlackerae Adkison,<br />
1984b<br />
*Hemiarthrus synalphei (Pearse,<br />
1950)<br />
Hyperphrixus castrensis Markham,<br />
1985<br />
*Munidion longipedis Markham,<br />
1975a<br />
Ovobopyrus alphezemiotes Markham,<br />
1985<br />
Parabopyrella mortenseni (Nierstrasz<br />
and Brender a Brandis, 1929)<br />
*Parabopyrella richardsonae<br />
(Nierstrasz and Brender a<br />
Brandis, 1929)<br />
Parabopyriscus stellatus Markham,<br />
1985<br />
*Probopyria alphei (Richardson,<br />
1900b)<br />
Probopyrinella heardi Adkison,<br />
1984a<br />
{continued)
268<br />
TABLE 6. (Continued)<br />
*Probopyrinella latreuticola (Gissler,<br />
1882)<br />
Prodajus cf. bigelowiensis Schultz and<br />
Allen, 1982<br />
Pseudione cognata Markham, 1985<br />
Pseudione upogebiae Hay, 1917<br />
*Schizobopyrina urocaridis (Richardson,<br />
1904)<br />
*Stegophryxus hyptius Thompson, 1902<br />
*Synsynella choprae (Pearse, 1932)<br />
*Synsynella deformans Hay, 1917<br />
Synsynella Integra Bourdon, 1981<br />
* Urobopyrus processae Richardson, 1904<br />
SUBORDER FLABELLIFERA<br />
*Aega deshaysiana (H. Milne Edwards,<br />
1840)<br />
*Aega ecarinata Richardson, 1898<br />
Aega incisa Schioedte and Meinert,<br />
1879<br />
Alcirona krebsii Hansen, 1890<br />
Ancinus depressus (Say, 1818)<br />
Anilocra acuta Richardson, 1910<br />
Anilocra laticauda H. Milne Edwards,<br />
1840<br />
*Bathynomus giganteus A. Milne<br />
Edwards, 1879<br />
*Cassidinidea ovalis (Say, 1818)<br />
Ceratothoa transversa (Richardson,<br />
1900b)<br />
Cirolana borealis Lilljeborg, 1851<br />
*Cirolana obtruncata Richardson, 1901<br />
* Cirolana parva Hansen, 1890<br />
Conilera cylindracea (Montagu, 1804)<br />
*Cymothoa caraibica Bovallius, 1885<br />
*Cymothoa excisa Perty, 1833<br />
*Cymothoa oestrum (Linnaeus, 1793)<br />
*Cerceis carinata Glynn, 1970<br />
*Eurydice convexa Richardson, 1900b<br />
Eurydice littoralis (Moore, 1901)<br />
*Eurydice piperata Menzies and<br />
Frankenberg, 1966<br />
*Excirolana braziliensis Richardson,<br />
1912a<br />
*Excirolana mayana (Ives, 1891)<br />
^Excorallana antillensis (Hansen,<br />
1890)<br />
Excorallana mexicana Richardson,<br />
1905a<br />
*Excorallana tricornis (Hansen,<br />
1890)<br />
*Harrieta faxoni (Richardson,<br />
1905a)<br />
*Limnoria tuberculata Sowinsky,<br />
1884<br />
Lironeca ovalis (Say, 1818)<br />
* Lironeca redmanni Leach, 1818<br />
Lironeca texana Pearse, 1952<br />
Lironeca tropicalis Menzies and<br />
Kruczynski, 1983<br />
*Nalicora rapax Moore, 1901<br />
*Nerocila acuminata Schioedte and<br />
Meinert, 1881<br />
Olencira praegustator (Latrobe, 1802)<br />
*Paracerceis caudata (Say, 1818)<br />
*Paradella dianae (Menzies, 1962b)<br />
Paradynamene benjamensis<br />
Richardson, 1905<br />
*Politolana polita (Stimpson, 1853)<br />
*Rocinela insularis Schioedte and<br />
Meinert, 1879<br />
*Rocinela oculata Harger, 1883<br />
*Rocinela signata Schioedte and<br />
Meinert, 1879<br />
*Serolis mgrayi Menzies and<br />
Frankenberg, 1966<br />
*Sphaeroma quadridentata Say, 1818<br />
*Sphaeroma terebrans Bate, 1866<br />
SUBORDER GNATHIIDEA<br />
Gnathia Jloridensis Menzies and<br />
Kruczynski, 1983<br />
SUBORDER MICROCERBERIDEA<br />
Microcerberus mexicanus Pennak, 1958
SUBORDER VALVIFERA<br />
Antarcturus jloridanus (Richardson,<br />
1900b)<br />
Arcturella bispinata Menzies and<br />
Kruczynski, 1983<br />
Arcturella spinata Menzies and<br />
Kruczynski, 1983<br />
<strong>As</strong>tacilla lauffi Menzies and<br />
Frankenberg, 1966<br />
Chiridotea excavata Harper,<br />
1974<br />
Cleantioides planicauda (Benedict,<br />
1899)<br />
species also occurring in the Caribbean<br />
ZOOGEOGRAPHY 269<br />
Edotea lyonsi (Menzies and<br />
Kruczynski, 1983)<br />
Edotea montosa (Stimpson, 1853)<br />
Edwinjoycea horologium Menzies and<br />
Kruczynski, 1983<br />
*Erichsonella attenuata (Harger,<br />
1873)<br />
* Erichsonella filiformis (Say, 1818)<br />
* Erichsonella Jloridana Benedict,<br />
1901<br />
Erichsonella isabelensis Menzies,<br />
1951b<br />
*Idotea metallica Bosc, 1802<br />
Note: Records for the Gulf of Mexico have been assembled from published<br />
BERMUDA<br />
literature; in most cases, actual material has not been examined.<br />
Twenty-nine species of isopods have been recorded from Bermuda (Table 7).<br />
Of these, nine are endemics (three being cave forms). The remaining 20<br />
species have all been recorded from the Caribbean region, indicating a strong<br />
subtropical connection, in spite of the relatively high latitude (32°15'N). Al<br />
though Bermuda is of Eocene or Oligocene age, the tropical fauna was prob<br />
ably decimated by the low temperatures of the last Pleistocene glaciation<br />
(Briggs, 1974:76).<br />
GAVE ISOPODS<br />
With the expanding efforts of cave divers, more and more true stygobiont<br />
forms are being found. Concurrently, discussion of the origin of cave fauna<br />
has spurred several theories, all invoking the geological history of the Carib<br />
bean area.<br />
Among the isopods, cave forms have been found in four suborders, the<br />
<strong>As</strong>ellota, Anthuridea, Flabellifera, and Microcerberidea. Two valuable dis<br />
cussions on the origin of cave crustaceans may be found in Stock (1986) and<br />
Wagele (1985).<br />
The only true cave asellote, Atlantasellus cavernicolus Sket, was collected<br />
from Bermuda.
270 ZOOGEOGRAPHY<br />
TABLE 7. ISOPOD SPECIES OCCURRING AT BERMUDA<br />
Alcirona krebsi Hansen, 1890<br />
*Anthomuda stenotelson Schultz, 1979<br />
*Apanthura harringtortiensis Wagele,<br />
1981<br />
*Arubolana aruboides (Bowman and<br />
Iliffc, 1983)<br />
*Atlantasellus cavernicolus Sket, 1979<br />
Bopyrissa wolffi Markham, 1978<br />
Cancricepon choprae (Nierstrasz and<br />
Brender a Brandis, 1925)<br />
Carpias bermudensis Richardson, 1902<br />
*Carpias minutus (Richardson, 1902)<br />
*Colanthura tenuis Richardson, 1902<br />
Colopisthus parvus Richardson, 1902<br />
* Curassanthura bermudensis Wagele,<br />
1985<br />
Dynamenella perforata (Moore, 1901)<br />
Eurydice per sonata Kensley, 1987b<br />
* recorded only from Bermuda<br />
Excorallana quadricornis (Hansen, 1890)<br />
Joeropsis rathbunae Richardson, 1902<br />
Leidya bimini Pearse, 1951<br />
Paracerceis caudata (Say, 1818)<br />
Paranthura infundibulata Richardson,<br />
1902<br />
Pendanthura tanaiformis Menzies and<br />
Glynn, 1968<br />
Para theIges piriformis Markham, 1972b<br />
Parathelges tumidipes Markham, 1972b<br />
Probopyrinella latreuticola (Gissler,<br />
1882)<br />
Pseudione affinis (Sars, 1882)<br />
*Stegias clibanarii Richardson, 1904<br />
Stenetrium stebbingi Richardson, 1902<br />
Stenobermuda acutirostrata Schultz, 1979<br />
Synsynella choprae (Pearse, 1932)<br />
Synsynella deformans Hay, 1917<br />
The anthuridean cave representatives are found in two families: the genus<br />
Curassanthura Kensley in the Paranthuridae, and the genus Cyathura subgenus<br />
Stygocyathura Botosaneanu and Stock in the Anthuridae (see Figure 110).<br />
Three species of Curassanthura are known, one each from Curagao, Ber<br />
muda, and Lanzarote in the Canary Islands. Curassanthura halma Kensley,<br />
from Curagao, is an interstitial form found in hypersaline waters. Curas<br />
santhura bermudensis Wagele was found in water of about 26%o salinity. The<br />
Lanzarote species, C. canadensis Wagele, came from seawater in a lava cave.<br />
Wagele (1985) suggests that this amphi-Atlantic distribution of Curassanthura<br />
is the result of plate tectonics separating an ancestral hypogean progenitor<br />
that had a Tethyan distribution.<br />
The genus Cyathura has representatives in the sea, in estuarine-brackish<br />
habitats, and in freshwater caves, and is found in the Atlantic, Indian, and<br />
Pacific oceans. This widespread distribution suggests a very long history for<br />
the genus. Using the morphology of the male copulatory stylet, Wagele<br />
(1985) suggests that marine ancestors, having a Tethyan distribution, en<br />
tered freshwater interstitial habitats. The series of regressions of sea level<br />
forms.<br />
serv
Figure 110. Map showing distribution of cave anthurideans.
* cave cirolanids<br />
Figure 111. Map showing distrib
ZOOGEOGRAPHY 273<br />
The flabelliferan family Cirolanidae contains five stygobiont genera in the<br />
Caribbean: Anopsilana, Arubolana, Bahalana, Creaseriella, and Haptolana (Figure<br />
111). Six other genera are known from the North American continent:<br />
Antrolana, Cirolanidesy Mexilana, Speocirolana, Sphaerolana, and Troglocirolana, all<br />
of which, except Antrolana from the Appalachian Valley of Virginia, occur in<br />
Mexico and Texas (see Notenboom, 1981). A few of these forms occur in<br />
brackish water, but most are found in freshwater of caves. Cave cirolanids<br />
are also known from Palau, North and East Africa, Madagascar, Bulgaria,<br />
Greece, Jugoslavia, Israel, France, and Spain. This widespread distribution<br />
again suggests a Tethyan marine origin, with dispersal and isolation due to<br />
sea regressions.<br />
The suborder Microcerberidea and the asellotan family Microparasellidae<br />
contain almost entirely interstitial forms, although few occur in caves. At<br />
least two genera, Microcerberus and Angliera, have very widespread distribu<br />
tions and are known from marine, brackish-water, and freshwater habitats,<br />
and may well have a history similar to that of Cyathura.
Appendix<br />
Since the manuscript of this work was completed and sent to press, a few<br />
papers have appeared either describing new species, mentioning new<br />
records for the Caribbean and associated areas, or instituting a major<br />
new taxon. It was thought useful to include these, if only in an appendix,<br />
to make the work as current as possible. The relevant taxa are listed<br />
alphabetically, with the full citation given below.<br />
Antheluridae Poore and Lew Ton, 1988<br />
Poore, G. C. B., and H. M. Lew Ton. 1988. Antheluridae, a new<br />
family of Crustacea (Isopoda: Anthuridea) with new species from<br />
Australia. Journal of Natural History 22:489—506.<br />
Within the geographical area covered by this work, only Anthomuda<br />
belongs to this new family.<br />
Aporobopyrus collardi Adkison, 1988<br />
Adkison, D. L. 1988. Pseudione parviramus and Aporobopyrus collardi,<br />
two new species of Bopyridae (Isopoda: Epicaridea) from the Gulf<br />
of Mexico. Proceedings of the Biological Society of Washington<br />
101(3):576-584.<br />
Booralana tricarinata Camp and Heard, 1988<br />
Camp, D. K., and R. W. Heard. 1988. Booralana tricarinata, a new<br />
species of isopod from the western Atlantic Ocean (Crustacea: Iso<br />
poda: Cirolanidae). Proceedings of the Biological Society of Washington<br />
101 (3):603-613.<br />
Originally recorded from the outer shelf and upper slope off the<br />
Little Bahama Bank and the Antilles Islands in 110-610 m, this<br />
species has since been recorded off Haiti in 620 m.<br />
Bythognathia yucatanensis Camp, 1988<br />
Camp, D. K. 1988. Bythognathia yucatanensis, new genus, new species,<br />
from abyssal depths in the Caribbean Sea, with a list of gnathiid<br />
275
276 APPENDIX<br />
species described since 1926 (Isopoda: Gnathiidae). Journal of Crust<br />
acean Biology 8(4):668-678.<br />
Edotea samariensis Muller, 1988<br />
Muller, H. G. 1988. Idoteidae aus N-Kolumbien mit Beschreibung<br />
von Edotea samariensis n. sp. (Crustacea: Isopoda: Valvifera).<br />
Senckenbergiana biologica 68(4/6):407-412.<br />
Gnathia Johanna Monod, 1926<br />
Miiller, H. G. 1988. Redescription of Gnathia Johanna, 1926 (Iso<br />
poda) from St. John, Virgin Islands. Bulletin Zoologisch Museum, Uni-<br />
versiteit van Amsterdam 11(15): 129—133.<br />
Phycolimnoria bacescui Ortiz and Lalana, 1988<br />
Ortiz, M., and R. Lalana. 1988. Una nueva especie del genero Phy<br />
colimnoria (Isopoda, Limnoriidae) de aguas cubanas. Revista de Inves<br />
tigations Marinas, La Habana 9(2): 37—42.<br />
Pseudione parviramus Adkison, 1988<br />
Adkison, D. L. 1988. Pseudione parviramus and Aporobopyrus collardi,<br />
two new species of Bopyridae (Isopoda: Epicaridea) from the Gulf<br />
of Mexico. Proceedings of the Biological Society of Washington<br />
101(3):576-584.
Literature Cited<br />
Adkison, D. L. 1982. Description of Dactylokepon sulcipes n. sp. (Crustacea: Isopoda:<br />
Bopyridae) and notes on D. caribaeus. Proceedings of the Biological Society of Washington<br />
95(4):702-708.<br />
. 1984a. Probopyrinella heardi n. sp. (Isopoda: Bopyridae) a branchial parasite of the<br />
hippolytid shrimp Latreutes parvulus (Decapoda: Caridea). Proceedings of the Biological Society of<br />
Washington 97(3):550-554.<br />
. 1984b. Two new species of Gigantione Kossmann (Isopoda: Epicaridea: Bopyridae)<br />
from the western North Atlantic. Proceedings of the Biological Society of Washington 97(4):761-<br />
772.<br />
Adkison, D. L., and Heard, R. W. 1978. Description of a new genus and species of<br />
Pseudioninae (Isopoda: Bopyridae) parasite of the hermit crab Pagurus annulipes (Stimpson)<br />
from North Carolina. Proceedings of the Biological Society of Washington 91 (2):408—417-<br />
Amar, R. 1957. Gnathostenetroides laodicense nov. gen. nov. sp. Type nouveau d'<strong>As</strong>ellota et<br />
classification des isopodes asellotes. Bulletin de I'lnstitut Oceanographique 1100:1 — 10.<br />
Argano, R. 1971. Cyathura sbordonii, nuova specie cavernicola del Messico sudorientale.<br />
Diagnosi preliminare (Crustacea, Isopoda, Anthuridae). Fragmenta Entomologica 7(4):303-<br />
304.<br />
Audouin, V. 1826. Explication Sommaire des Planches de Crustaces de PEgypte et de la<br />
Syrie. In J.-C. Savigny, Description de VEgypte ou Recueil des Observations et des Recherches qui ont<br />
etefaites en Egypte pendant {'Expedition de sa Majeste VEmpereur Napoleon le Grand. Histoire<br />
Naturelle, vol. 1, pp. 77-98. Paris: L'Imprimerie Imperiale.<br />
Barnard, K. H. 1914. Contributions to the crustacean fauna of South Africa. 3. Additions to<br />
the marine Isopoda, with notes on some previously incompletely known species. Annals of<br />
the South African Museum 10(1 l):325a-442.<br />
. 1920. Contributions to the crustacean fauna of South Africa. 6. Further additions to<br />
the list of marine Isopoda. Annals of the South African Museum 17(5):319—438.<br />
. 1925. A revision of the family Anthuridae (Crustacea Isopoda), with remarks on<br />
certain morphological peculiarities. Journal of the Linnaean Society of London, Zoology 36:109—<br />
160.<br />
Bate, C. S. 1866. Carcinological gleanings, 2. Annals and Magazine of Natural History (3)17:24-<br />
31.<br />
Bate, C. S., and J. O. Westwood. 1868. A History of the British Sessile-eyed Crustacea. Ivi + 536<br />
pp. London: John van Voorst.<br />
Becker, G., and W.-D. Kampf. 1958. Funde der holzzerstorenden Isopodengattung Limnoria<br />
an der Festland Indiens und Neubgeschreibung von Limnoria indica. Zeitschrifi fir Angewandte<br />
Zoologie 45:1—9.<br />
277
278 LITERATURE CITED<br />
Beddard, F. E. 1886. Report on the Isopoda collected by H. M. S. Challenger during the<br />
years 1873-76. <strong>Part</strong> 2. Reports of the Voyage of the Challenger 17:1-178.<br />
Benedict, J. E. 1899. [Cleantis planicauda Benedict, new species.] In Richardson, H. 1899. Key<br />
to the isopods of the Pacific coast of North America, with descriptions of twenty-two new<br />
species. Proceedings of the United States National Museum 21:815-869.<br />
. 1901. In Richardson, H. 1901. Key to the isopods of the Atlantic coast of North<br />
America with descriptions of new and little known species. Proceedings of the United States<br />
National Museum 23:493-579.<br />
Bliss, D. E., editor-in-chief. 1982-1985. The Biology of the Crustacea, vols. 1-10. New York:<br />
Academic Press.<br />
Bonnier, J. 1900. Contribution a l'etude des Epicarides. Les Bopyridae. Travaux de la Station<br />
Zoologique de Wimereux 8:1—396.<br />
Boone, P. L. 1918. Description often new isopods. Proceedings of the United States National<br />
Museum 54:591-604.<br />
. 1921. Report on the Tanidacea and Isopoda, collected by the Barbados-Antigua<br />
Expedition from the University of Iowa in 1918. University of Iowa Studies in Natural History<br />
9(5):91-98.<br />
Boone, L. 1927. Crustacea from tropical east American seas. Bulletin of the Bingham<br />
Oceanographic Collection 1(2): 1-147.<br />
. 1930. New decapod and isopod crustaceans from Gonave Bay, Haiti. Zoologica, New<br />
York 12(4):41-53.<br />
Bosc, L. A. G. 1802. Histoire Naturelle des Crustaces. In G. L. L. de BufTon, Histoire Naturelle<br />
de Buffon classee...d''apres le system de Linne...par R. R. Castel'...nouvelle edition. Paris.<br />
Botosaneanu, L. 1983. First record of an anthurid isopod, Cyathura univam sp. n., on the<br />
South American continent. Bijdragen tot de Dierkunde 53(2):247-254.<br />
Botosaneanu, L., N. L. Bruce, and J. Notenboom. 1986. Isopoda: Cirolanidae. In L.<br />
Botosaneanu, ed., Stygofauna Mundi, pp. 412—422. Leiden: E.J. Brill/Dr. W. Backhuys.<br />
Botosaneanu, L., and J. H. Stock. 1979. Arubolana imula n. gen., n. sp., the first hypogean<br />
cirolanid isopod crustacean found in the Lesser Antilles. Bijdragen tot de Dierkunde<br />
49(2):227-233.<br />
. 1982. Les Cyathura stygobies (Isopoda, Anthuridea) et surtout celles des Grandes et<br />
des Petites Antilles. Bijdragen tot de Dierkunde 52(1):13—42.<br />
Bourdon, R. 1972. Sur quelques Bopyridae (Crustacea, Isopoda) parasites de Galatheides.<br />
Bulletin du Museum national d'Histoire naturelle (3)66, zool. 52:817-838.<br />
. 1981. Remarques sur le genre Synsynella Hay, avec description de S* integra n. sp.<br />
(Crustacea, Epicaridea, Bopyridae). Bulletin du Museum national d'Histoire naturelle, Paris<br />
(4)3(A4):1143-1162.<br />
Bourdon, R., and J. C. Markham. 1980. A new genus and species of bopyrid isopod infesting<br />
alpheid shrimps of the genus Synalpheus in the western Atlantic Ocean. Zoologische<br />
Mededelingen 55( 19):221-230.<br />
Bovallius, C. 1885. New or imperfectly known Isopoda. Bihang till Kongliga Svenska Vetenskaps-<br />
Akademiens Handlingar 10(ll):l-32.<br />
Bowman, T. E. 1956. Una especie nueva de Bopyrella (Crustacea: Isopoda) de Los Roques,
LITERATURE CITED 279<br />
Venezuela. Novedades Cientijicas, Contribuciones Ocasionales del Museo de Historia Natural La<br />
Salle, Caracas, Venezuela (Zoologica) 19:1—4.<br />
. 1965. Cyathura specus, a new cave isopod from Cuba. Studies on the Fauna of Curacao and<br />
other Caribbean Islands 85:88-97.<br />
. 1966. Haptolana trichostomay a new genus and species of troglobitic cirolanid isopod<br />
from Cuba. International Journal of Speleology 2:105—108.<br />
. 1981. Thermosphaeroma milleri and T. smithi, new sphaeromatid isopod crustaceans<br />
from hot springs in Chihuahua, Mexico, with a review of the genus. Journal of Crustacean<br />
Biology 1(1):105-122.<br />
. 1987. Bahalana mayana, a new troglobitic cirolanid isopod from Cozumel Island and<br />
the Yucatan Peninsula, Mexico. Proceedings of the Biological Society of Washington 100(3):659-<br />
663.<br />
Bowman, T. E., and R. Franz. 1982. Anopsilana crenata, a new troglobitic cirolanid isopod<br />
from Grand Cayman Island, Caribbean Sea. Proceedings of the Biological Society of Washington<br />
95(3):522-529.<br />
Bowman, T. E., and T. M. Iliffe. 1983. Bermudalana aruboides, a new genus and species of<br />
troglobitic Isopoda (Cirolanidae) from marine caves on Bermuda. Proceedings of the<br />
Biological Society of Washington 96(2):291-300.<br />
Bowman, T. E., and B. F. Morris. 1979. Carpias Richardson, 1902, a senior synonym<br />
of Bagatus Nobili, 1906, and the validity of Carpias minutus (Richardson, 1902)<br />
(Isopoda: <strong>As</strong>ellota: Janiridae). Proceedings of the Biological Society of Washington 92(3):650-<br />
657.<br />
Boyle, P., and R. Mitchell. 1978. Absence of microorganisms in crustacean digestive tracts.<br />
Science 200(4346): 1157-1159.<br />
Brandt, J. F. 1833. Conspectus monographiae Crustaceorum Oniscodorum Latreillii. Bulletin<br />
de la Societe Imperiale des Naturalistes de Moscou 6:171 — 193.<br />
Briggs, J. C. 1974. Marine Zoogeography. 475 pp. New York: McGraw-Hill Book Company.<br />
Bruce, N. L. 1981. Cirolanidae (Crustacea: Isopoda) of Australia: Diagnoses of Cirolana<br />
Leach, Metacirolana Nierstrasz, Neocirolana Hale, Anopsilana Paulian & Deboutteville, and<br />
three new genera—Natatolana, Politolana, and Cartetolana. Australian Journal of Marine and<br />
Freshwater Research 32:945-966.<br />
. 1984. A new family for the isopod crustacean genus Tridentella Richardson, 1905,<br />
with description of a new species from Fiji. Zoological Journal of the Linnean Society 80:447-<br />
455.<br />
. 1985. Calyptolana hancocki, a new genus and species of marine isopod (Cirolanidae)<br />
from Aruba, Netherlands Antilles, with a synopsis of Cirolanidae known from the<br />
Caribbean and Gulf of Mexico. Journal of Crustacean Biology 5(4):707-716.<br />
. 1986a. Cirolanidae (Crustacea: Isopoda) of Australia. Records of the Australian<br />
Museum, supplement 6:1-239.<br />
. 1986b. Revision of the isopod crustacean genus Mothocya Costa, in Hope, 1851<br />
(Cymothoidae: Flabellifera), parasitic on marine fishes. Journal of Natural History<br />
20(5):1089-1192.<br />
Brusca, R. C. 1981. A monograph on the Isopoda Cymothoidae (Crustacea) of the eastern<br />
Pacific. Zoological Journal of the Linnean Society 73(2): 117—199.
280 LITERATURE CITED<br />
. 1983. A monograph on the isopod family Aegidae in the tropical Eastern Pacific I.<br />
The genus Aega. Allan Hancock Monographs in Marine Biology 12:1-39.<br />
. 1984. Phylogeny, evolution and biogeography of the marine isopod Subfamily<br />
Idoteinae (Crustacea: Isopoda: Idoteidae). Transactions of the San Diego Society of Natural<br />
History 20(7):99-134.<br />
Budde-Lund, G. 1885. Crustacea Isopoda Terrestria per Familias et Genera et Species. 319 pp.<br />
Copenhagen.<br />
Buss, L. W., and E. VV. Iverson. 1981. A new genus and species of <strong>Sphaeromatidae</strong><br />
(Crustacea: Isopoda) with experiments and observations on its reproductive biology,<br />
interspecific interactions and color polymorphisms. Postilla 184:1-23.<br />
Caiman, \V. T. 1904. On the classification of the Crustacea Malacostraca. Annals and<br />
Magazine of Natural History (7)13:144-158.<br />
. 1910. On two new species of wood-boring Crustacea from Christmas Island. Annals<br />
and Magazine of Natural History (8)5:181-186.<br />
Carpenter, J. H. 1981. Bahalana geracei n. gen., n. sp., a troglobitic marine cirolanid isopod<br />
from Lighthouse Cave, San Salvador Island, Bahamas. Bijdragen tot de Dierkunde 51 (2):259—<br />
267.<br />
Carpenter, J. H., and G.J. Magniez. 1982. Deux asellotes stygobies des Indes Occidentales:<br />
Neostenetroides stocki n. gen., n. sp., et Stenetrium sp. Bijdragen tot de Dierkunde 52(2):200-206.<br />
Carvacho, A. 1977. Isopodes de la Mangrove de la Guadeloupe, Antilles Franchises. Studies<br />
on the Fauna of Curasao and other Caribbean Islands 54( 174): 1—24.<br />
Chappuis, P.-A., and C. Delamare Deboutteville. 1955. Recherches sur les Crustaces<br />
souterrains. 7. Les isopodes psammiques de la Mediterranee. Archives de Zoologie<br />
Experimentale et Generale 91 (1): 103—138.<br />
. 1956. Etudes sur la faune interstitielle des iles Bahamas recoltee par Madame<br />
Renaud-Debyser. 1. Copepodes et Isopodes. Vie et Milieu 7(3):373-396.<br />
Clark, S. T., and P. B. Robertson. 1982. Shallow water marine isopods of Texas. Contributions<br />
to Marine Science 25(l):45-59.<br />
Coineau, N., and L. Botosaneanu. 1973. Isopodes interstitiels de Cuba. Resultats des expeditions<br />
bxospeologiques cubano-roumaines a Cuba 1:191—222.<br />
Cordiner, C. 1793. Remarkable ruins and romantic prospects, of North Britain. With ancient monuments<br />
and singular subjects of natural history. 96 plates with letterpress. London: Mazell.<br />
Costa, A. 1851. Caratteeri di alcuni de'generi e specie nouve segnete nel presente catalogo. In<br />
F. W. Hope, Catalogo dei crostacei Italiani e di molti altri de Mediterranean pp. 41-48. Naples.<br />
Coutiere, H. 1908. Sur le Synalpheion Giardi, n. gen., n. sp., entoniscien parasite d'une<br />
Synalphee. Comptes Rendus Hebdomadaires des Seances de VAcademie des Sciences, Paris 146:1333-<br />
1335.<br />
Creaser, E. P. 1936. Crustaceans from Yucatan. Carnegie Institution of Washington Publication<br />
457:117-132.<br />
Dana, J. D. 1852. On the classification of the Crustacea Choristopoda or Tetradecapoda.<br />
American Journal of Science (2)14:297-316.<br />
Delaney, P.M. 1984. Isopods of the genus Excorallana Stebbing, 1904 from the Gulf of<br />
California, Mexico (Crustacea, Isopoda, Corallanidae). Bulletin of Marine Science 34(1): 1—20.
LITERATURE CITED 281<br />
Delaney, P. M., and R. C. Brusca. 1985. Two new species of Tridentella Richardson, 1905<br />
(Isopoda: Flabellifera: Tridentellidae) from California, with a rediagnosis and comments<br />
on the family, and a key to the genera of Tridentellidae and Corallanidae. Journal of<br />
Crustacean Biology 5(4):728-742.<br />
Fabricius, J. C. 1793. Volume 2 in Entomologia systematica emendata et aucta...adjectis synonymis,<br />
locis, observationibus, descriptionibus, 4 vols., 1792-1794. Copenhagen.<br />
. 1798. Entomologia systematica emendata et aucta...adjectis synonymis, locis, obervationibus,<br />
descriptionibus. Supplementum. 1798. Copenhagen.<br />
Frankenberg, D., and R.J. Menzies. 1966. A new species ofasellote marine isopod, Munna<br />
(Uromunna) reynoldsi (Crustacea: Isopoda). Bulletin of Marine Science 16(2):200-208.<br />
Fresi, E., and U. Schieke. 1972. Pleurocope dasyura Walker, 1901, and the Pleurocopidae new<br />
family (Isopoda, <strong>As</strong>ellota). Crustaceana, Supplement 3:207-213.<br />
George, R. Y., and J.-O. Stromberg. 1968. Some new species and new records of marine<br />
isopods from San Juan Archipelago, Washington, U.S.A. Crustaceana 14(3):225-254.<br />
Gissler, C. F. 1882. Bopyroides latreuticola, a new species of isopod crustacean parasitic on a<br />
gulfweed shrimp. American Naturalist 16:591-594.<br />
Glynn, P. W. 1970. A systematic study of the <strong>Sphaeromatidae</strong> (Crustacea: Isopoda) of Isla<br />
Margarita, Venezuela, with descriptions of three new species. Memoria de la Sociedad de<br />
Ciencias Naturales La Salle 30:1-48.<br />
Glynn P. W., D. M. Dexter, and T. E. Bowman. 1975. Excirolana braziliensis, a Pan-American<br />
sand beach isopod: taxonomic status, zonation and distribution. Journal of Zoology, London<br />
175:509-521.<br />
Glynn, P. W., and C. S. Glynn. 1974. On the Systematics on Ancinus (Isopoda,<br />
<strong>Sphaeromatidae</strong>), with the description of a new species from the tropical eastern Pacific.<br />
Pacific Science 28(4) :401-422.<br />
Grobben, C. 1892. Zur Kenntniss der Staumbaumer und der Systems der Crustaceen.<br />
Sitzungsberichte der kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche<br />
Classe, IVien 101 (1 &2) 1:237-274.<br />
Haller, G. 1880. Ueber einige neue Cymothoinen. Archiv fur Naturgeschichte, Berlin 46(1)375-<br />
395.<br />
Hansen, H.J. 1890. Cirolanidae et familiae nonnullae propinquae Musei Hauniensis. Et<br />
Bidrag til Kundskaben om nogle Familier af isopode Krebsdyr. Kongelige Danske<br />
Videnskabernes Selskabs Shifter, 6te Raekke, Naturvidenskabelig og mathematisk Afdeling 3:239-426.<br />
. 1904. On the morphology and classification of the <strong>As</strong>ellota-group of crustaceans,<br />
with descriptions of the genus Stenetrium Hasw. and its species. Proceedings of the Zoological<br />
Society of London, 1904, 2:302-331.<br />
. 1905a. On the morphology and classification of the <strong>As</strong>ellota-group of Crustacea,<br />
with descriptions of the genus Stenetrium Haswell, and its species. Proceedings of the Zoological<br />
Society of London for 1904 2(2):302-331.<br />
. 1905b. On the propagation, structure, and classification of the family Sphaeromidae.<br />
Quarterly Journal of Microscopical Science 49(1):69—135.<br />
. 1916. Crustacea Malacostraca 3. Danish Ingo If Expedition 3(5): 1—262.<br />
Harger, O. 1873. [Erichsonia attenuata.] In A. E. Verrill, S. I. Smith, and O. Harger,
282 LITERATURE CITED<br />
Catalogue of the Marine invertebrate animals of the southern coast of New England, and<br />
adjacent waters, p. 276. In A. E. Verrill and S. I. Smith, Report upon the invertebrate<br />
animals of Vineyard Sound and adjacent waters, with an account of the physical features<br />
of the region. In S. F. Baird, Report of Professor S. F. Baird, Commissioner of Fish and Fisheries,<br />
on the condition of the sea-fisheries of the south coast of New England in 1871 and 1872. 478 pp.<br />
Washington, D.C.: Government Printing Office.<br />
. 1879. Notes on New England Isopoda. Proceedings of the United States National Museum<br />
No.75:157-165.<br />
. 1883. Reports on the results of dredging, under the supervision of Alexander<br />
Agassiz, on the east coast of the United States, during the summer of 1880, by the U.S.<br />
Coast Survey Steamer "Blake," Commander J. R. Bartlett, U.S.N., commanding. Bulletin<br />
of the Museum of Comparative Zoology at Harvard College 11 (4):91 —104.<br />
Harper, D. E., Jr. 1974. Chiridotea excavata n. sp. (Crustacea, Isopoda) from marine waters of<br />
Texas. Contributions to Marine Sciences, Texas A & M University 18:229-239.<br />
Harrison, K. 1984. The morphology of the sphaeromatid brood pouch (Crustacea: Isopoda:<br />
<strong>Sphaeromatidae</strong>). Zoological Journal of the Linnean Society 82:363-407.<br />
Harrison, K., and D. M. Holdich. 1982. New eubranchiate sphaeromatid isopods from<br />
Queensland waters. Memoirs of the Queensland Museum 20(3):421-446.<br />
Hartnoll, R. G. 1966. A new entoniscid from Jamaica (Isopoda, Epicaridea). Crustaceana<br />
ll(l):45-52.<br />
Haswell, W. A. 1881. On some new Australian marine Isopoda. <strong>Part</strong> 1. Proceedings of the<br />
Linnean Society of New South Wales 5:476-478.<br />
. 1884. On a new crustacean found inhabiting the tubes of Vermilia (Serpulidae).<br />
Proceedings of the Linnean Society of New South Wales 9(3):676-680.<br />
Hay, W. P. 1903. On a small collection of crustaceans from the island of Cuba. Proceedings of<br />
the United States National Museum 26:429-435.<br />
. 1917. A new genus and three new species of parasitic isopod crustaceans. Proceedings<br />
of the United States National Museum 51:569-574.<br />
Hooker, A. 1985. New species of Isopoda from the Florida Middlegrounds (Crustacea:<br />
Peracarida). Proceedings of the Biological Society of Washington 98(l):255-280.<br />
Hurley, D. E., and K. P. Jansen. 1977. The marine fauna of New Zealand: Family<br />
<strong>Sphaeromatidae</strong> (Crustacea Isopoda: Flabellifera). New Zealand Oceanographic Institute<br />
Memoir 63:1095.<br />
Iverson, E. W. 1982. Revision of the isopod family <strong>Sphaeromatidae</strong> (Crustacea: Isopoda:<br />
Flabellifera) I. Subfamily names with diagnoses and key. Journal of Crustacean Biology<br />
2(2):248-254.<br />
Ives, J. E. 1891. Crustacea from the northern coast of Yucatan, the harbor of Vera Cruz, the<br />
west coast of Florida and the Bermuda Islands. Proceedings of the Academy of Natural Sciences<br />
of Philadelphia, 1891:176-207.<br />
Jacobs, B.J. M. 1987. A taxonomic revision of the European, Mediterranean and NW.<br />
African species generally placed in Sphaeroma Bosc, 1802 (Isopoda: Flabellifera:<br />
<strong>Sphaeromatidae</strong>). Zoologische Verhandelingen 238:1-71.<br />
Kaestner, A. 1967. Invertebrate Zoology, vol. 3, 523 pp. New York: Interscience Publisher.<br />
[Translated and adapted from the second German edition by H. W. Levi and<br />
L. R. Levi.]
LITERATURE CITED 283<br />
Karaman, S. 1933a. Neue Isopoden aus unterirdischen Gewassern Jugoslawiens. Zoologischer<br />
Anzeiger 102( 1/2): 16-22.<br />
. 1933b. Microcerberus stygius, der dritte Isopod aus dem Grundwasser von Skopje,<br />
Jugoslawien. Zoologischer Anzeiger 102(5/6): 165—169.<br />
. 1934. Beitrage zur Kenntnis des Isopoden-Familie Microparasellidae. Mitteilungen<br />
uber Hohlen- und Karstforschung 1934:42-44.<br />
Kensley, B. 1978. Five new genera of anthurid isopod crustaceans. Proceedings of the Biological<br />
Society of Washington 91 (3):775-792.<br />
. 1980. Records of anthurids from Florida, Central America, and South America<br />
(Crustacea: Isopoda: Anthuridae). Proceedings of the Biological Society of Washington 93(3):725-<br />
742.<br />
. 1981. Amsterdam Expeditions to the West Indian Islands, Report 10. Curassanthura<br />
halma, a new genus and species of interstitial isopod from Curasao, West Indies<br />
(Crustacea: Isopoda: Paranthuridae). Bijdragen tot de Dierkunde 51(1): 131-134.<br />
. 1982. Anthuridea (Crustacea: Isopoda) of Carrie Bow Cay, Belize. In K. Rutzler<br />
and I. G. Macintyre, eds., The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay,<br />
Belize, 1: Structure and Communities, pp. 321-352. Smithsonian Contributions to Marine<br />
Sciences 12, 539 pp.<br />
. 1983. The role of isopod crustaceans in the reef crest community at Carrie Bow Cay,<br />
Belize. Marine Ecology 5(l):29-44.<br />
. 1984. The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize, III: New<br />
marine Isopoda. Smithsonian Contributions to Marine Sciences 24, iv + 81 pp.<br />
. 1987a. Further records of marine isopods from the Caribbean. Proceedings of the<br />
Biological Society of Washington 100(3):559-577.<br />
. 1987b. A re-evaluation of the Systematics of K. H. Barnard's Review of anthuridean<br />
isopods. Steenstrupia 13(3):101 —139-<br />
. 1987c. Harrieta, a new genus for Cymodoce faxoni (Richardson) (Crustacea: Isopoda:<br />
<strong>Sphaeromatidae</strong>). Proceedings of the Biological Society of Washington 100(4):<br />
Kensley, B., and R. Heard. 1985. A new species of the genus Spinianirella Menzies<br />
(Crustacea: Isopoda: Janiridae) from the western Atlantic. Proceedings of the Biological Society<br />
of Washington 98(3):682-686.<br />
Kensley, B., and H. W. Kaufman. 1978. Cleantioides, a new idoteid isopod genus from Baja<br />
California and Panama. Proceedings of the Biological Society of Washington 91(3):658-665.<br />
Kensley, B., and M. Schotte. 1987. New records of isopod Crustacea from the Caribbean,<br />
the Florida Keys, and the Bahamas. Proceedings of the Biological Society of Washington<br />
100(l):216-247.<br />
Kensley, B., and P. Snelgrove. 1987. Records of marine isopod crustaceans associated with<br />
the coral Madracis mirabilis from Barbados. Proceedings of the Biological Society of Washington<br />
100(1):186-197.<br />
Koehler, R. 1885. Description d'un Isopode nouveau, le Joeropsis brevicomis. Annates des Sciences<br />
Naturelles (Paris), Zoologie (6)19:1-7.<br />
Krayer, H. 1839. Munna, en ny kraebsdyrslaegt. Naturhistorisk Tidsskrifl, Kjebenhavn 2:612—<br />
616.<br />
Kussakin, O. G. 1967. Fauna of Isopoda and Tanaidacea in the coastal zones of the
284 LITERATURE CITED<br />
Antarctic and Subantarctic waters. Biological Reports of the Soviet Antarctic Expedition (1955-<br />
1958) 3:220-389. [English translation by the Israel Program for Scientific Translations,<br />
Jerusalem, 1968.]<br />
Lang, K. 1961. Contributions to the knowledge of the genus Microcerberus Karaman<br />
(Crustacea Isopoda) with a description of a new species from the central Californian coast.<br />
Arkivfdr Zoologi (2)13(22):493-510.<br />
Latreille, P. A. 1802. Histoire Naturelle des Crustaces et des Insectes. In Volume 3 of G. L.<br />
L. de BufTon, 1802-1805, Histoire Naturelle, nouvelle edition, accompagnee des notes. Ouvrage redige<br />
par C. S. Sonnini, 14 vols. Paris.<br />
. 1803. Histoire Naturelle des Crustaces et des Insectes. In Volume 5 of G. L. L. de<br />
Buffon, 1802-1805. Histoire Naturelle, nouvelle edition, accompagnee des notes. Ouvrage redige par<br />
C. S. Sonnini. 14 vols. Paris<br />
. 1806. Genera Crustaceorum et Insectorum secundum ordinum naturalem in Familias disposita,<br />
iconibus exemplisque plurimis explicata, vol. 1, 280 pp. Paris: Amand Koenig.<br />
. 1817. Les Crustaces, les Arachnides, et les Insectes. In G. L. C. F. D. Cuvier, Le<br />
Regne Animal, distribue d'apres son organisation, pour servrir de base a I'histoire naturelle des animaux<br />
et d y introduction a Vanatomie comparee, vol. 3. Paris.<br />
. 1826. Explication sommaire des planches (Mollusques, Annelides, Crustace's, Arachnides,<br />
Insectes, Echinoderms, Zoophytes, <strong>As</strong>cidies, Polypes, Hydrophytes, Oiseaux) dont les dessins ont ete<br />
fournis par M. J. C. Savigny. Description de VEgypte, ou recueil des observations et des recherches qui<br />
ont etefaites en Egypte pendant ^expedition de Varmee Francaise (1798-1801). Paris.<br />
. 1831. Cours d'Entomologique, ou de Vhistoire naturelle des Crustaces, des Arachnides, des<br />
Alyriapodes, et des Insectes. 568 pp. Paris.<br />
Latrobe, B. H. 1802. A drawing and description of the Clupea tyrannus and Oniscus praegustator.<br />
Transactions of the American Philosophical Society 5:77—81.<br />
Leach, W. E. 1814. Crustaceology. In Brewster's Edinburgh Encyclopedia, vol. 7, pp. 383-439.<br />
. 1815. A tabular view of the external characters of four classes of animals, which<br />
Linne arranged under Insecta; with the description of the genera comprising three of these<br />
classes into order, etc., and descriptions of several new genera and species. Transactions of<br />
the Linnean Society of London 2:306—400.<br />
. 1818. [Rocinela, Livoneca redmanni.J In Volume 5 of F. Cuvier, ed., 1816—1830,<br />
Dictionnaire des Sciences naturelles. Paris & Strasbourg.<br />
Leidy, J. 1855. Contributions towards a knowledge of the marine invertebrate fauna of the<br />
coasts of Rhode Island and New Jersey. Journal of the Academy of Natural Sciences of<br />
Philadelphia 3:135-152.<br />
Lemos de Castro, A. 1959. Descrigao de uma nova especie do genero "Ancinus" Milne<br />
Edwards (Isopoda, Sphaeromidae). Revista Brasileira de Biologia 19(2):215—218.<br />
Lilljeborg, W. 1851. Norger Crustacear. Ofoersigt af Kongliga Vetenskapsakademiens Forhandlingar,<br />
Stockholm 8:19-25.<br />
Linnaeus, C. 1793. In J. C. Fabricius, 1792-1794. Entomologia systematica emendata et<br />
aucta...adjectis synonymis, locis, observationibus, descriptionibus, 4 vols. Copenhagen.<br />
Loyola e Silva, J. de. 1960. <strong>Sphaeromatidae</strong> do Litoral Brasileiro (Isopoda—Crustacea).<br />
Boletim da Universidade do Parana, Zoologia 4:1 — 182.<br />
Markham, J. C. 1972a. Two new genera of western Atlantic abdominally parasitizing
LITERATURE CITED 285<br />
Bopyridae (Isopoda, Epicaridea), with a proposed new name for their subfamily.<br />
Crustaceana, Supplement 3:39-56.<br />
. 1972b. Four new species of Parathelges Bonnier, 1900 (Isopoda, Bopyridae), the first<br />
record of the genus from the western Atlantic. Crustaceana, Supplement 3:57-78.<br />
. 1973. Six new species of bopyrid isopods parasitic on galatheid crabs of the genus<br />
Munida in the Western Atlantic. Bulletin of Marine Science 23(3) :613-648.<br />
. 1974. A new species of Pleurocrypta (Isopoda, Bopyridae), the first known from the<br />
western Atlantic. Crustaceana 26(3):267-272.<br />
. 1975a. A review of the bopyrid isopod genus Munidion Hansen, 1897, parasitic on<br />
galatheid crabs in the Atlantic and Pacific oceans. Bulletin of Marine Science 25(3):422-441.<br />
. 1975b. Bopyrid isopods infesting porcellanid crabs in the northwestern Atlantic.<br />
Crustaceana 28(3):257-270.<br />
. 1975c. New records of two species of parasitic isopods of the bopyrid subfamily<br />
Ioninae in the western Atlantic. Crustaceana 29(l):55-67.<br />
. 1975d. Two new species of <strong>As</strong>ymmetrione (Isopoda, Bopyridae) from the Western<br />
Atlantic. Crustaceana 29(3):255-265.<br />
. 1977. Description of new western Atlantic species ofArgeia Dana with a proposed<br />
new subfamily for this and related genera (Crustacea Isopoda, Bopyridae). Zoologische<br />
Mededelingen 52(9): 107-123.<br />
. 1978. Bopyrid isopods parasitizing hermit crabs in the northwestern Atlantic Ocean.<br />
Bulletin of Marine Science 28(1): 102-117.<br />
. 1985. A review of the bopyrid isopods infesting caridean shrimps in the<br />
northwestern Atlantic Ocean, with special reference to those collected during the<br />
Hourglass cruises in the Gulf of Mexico, Memoirs of the Hourglass Cruises 7(3): 1 ~ 156.<br />
Menzies, R.J. 1951a. A new species of Limnoria (Crustacea: Isopoda) from Southern<br />
California. Bulletin of the Southern California Academy of Sciences 50(2) :86—88.<br />
. 1951b. A new subspecies of marine isopod from Texas. Proceedings of the United States<br />
National Museum 101:575-579.<br />
. 1956a. New abyssal tropical Atlantic isopods, with observations on their biology.<br />
American Museum Novitates 1798:1 — 16.<br />
. 1956b. New bathyal Isopoda from the Caribbean with observations on their<br />
nutrition. Breviora 63:1-10.<br />
. 1957. The marine borer family Limnoriidae (Crustacea, Isopoda). Bulletin of Marine<br />
Science of the Gulf and Caribbean 7(2):101-200.<br />
1:79-206.<br />
. 1962a. The isopods of abyssal depths in the Atlantic Ocean. Vema Research Series<br />
. 1962b. The marine isopod fauna of Bahia de San Quintin, Baja California, Mexico.<br />
Pacific Naturalist 3(11):337-348.<br />
. 1962c. The zoogeography, ecology, and Systematics of the Chilean marine isopods.<br />
Lunds Universitets Arsskrift, N.F. Avd. 2, 57(11):1 —162.<br />
Menzies, R. J., and D. Frankenberg. 1966. Handbook on the Common Marine Isopod Crustacea of<br />
Georgia. University of Georgia Press, Athens, Georgia. 93 pp.<br />
Menzies, R. J., and P. W. Glynn. 1968. The common marine isopod Crustacea of Puerto
286 LITERATURE CITED<br />
Rico: A handbook for marine biologists. Studies on the Fauna of Curasao and other Caribbean<br />
Islands 27(104):1-133.<br />
Menzies, R. J., and W. L. Kruczynski. 1983. Isopod Crustacea (Exclusive of Epicaridea).<br />
Memoirs of the Hourglass Cruises 6(1): 1 — 126.<br />
Miers, E.J. 1880. On a collection of Crustacea from the Malaysian region. <strong>Part</strong> 4.<br />
Penaeidea, Stomatopoda, Isopoda, Suctoria, and Xiphosura. Annals and Magazine of Natural<br />
History (5)5:457.<br />
Miller, M. A. 1941. The isopod Crustacea of the Hawaiian Islands, II. <strong>As</strong>ellota. Occasional<br />
Papers of the Bernice P. Bishop Museum, Honolulu, Hawaii 16( 13):305—320.<br />
Milne Edwards, A. 1879. Sur un isopode gigantesque, des grandes profondeurs de la mer.<br />
Comptes Rendus Hebdomadaires des Seances de VAcademie des Sciences 88:21-23.<br />
Milne Edwards, H. 1840. Histoire Naturelle des Crustaces, comprenant ranatomie, la physiologie et la<br />
classification de ces animaux, vol. 3. Paris.<br />
Monod, T. 1926. Les Gnathiidae. Essai monographique (morphologie, biologie,<br />
systematique). Memoires de la Societe des Sciences Naturelles du Maroc 13:1—667.<br />
Montagu, G. 1804. Description of several marine animals (Cancer rhomboidalis, C.<br />
maxillaris, C. phasma, C. palmatus, Oniscus hirsutus, etc) found on the south coast of<br />
Devonshire. Transactions of the Linnean Society, London 7:61—85.<br />
Moore, H. F. 1901. Report on Porto Rican Isopoda. U.S. Fish Commission Bulletin for 1900<br />
2:161-176.<br />
Moreira, P. S. 1972. Species of Eurydice (Isopoda, Flabellifera) from southern Brazil. Boletim<br />
do Instituto Paulista de Oceanografico, Sao Paula 21:69-91.<br />
Miiller, H.-G. 1988. The genus Gnathia Leach (Isopoda) from the Santa-Marta area,<br />
northern Colombia, with a review of Gnathiidae from the Caribbean Sea and Gulf of<br />
Mexico. Bijdragen tot de Dierkunde 58(1 ):88-104.<br />
Negoescu, I. 1979. Cyathura cubana sp. n. (Isopoda, Anthuridea) from the Caribbean Sea<br />
(Cuban waters). Travaux du Museum d'Histoire naturelle Grigore Antipa 20:157-164.<br />
Negoescu Vladescu, I. 1983. A study of genus Cyathura from the Cuban freshwaters with the<br />
description of a new cave species: C. orghidani (Isopoda, Anthuridae). Resultats des expeditions<br />
biospeologiques cubano-roumaines a Cuba 4:39—45.<br />
Nierstrasz, H. F. 1931. Die Isopoden der Siboga-Expedition. III. Isopoda Genuina II.<br />
Flabellifera. Siboga Expedition Monographie 32c: 123-232.<br />
Nierstrasz, H. F., and G. A. Brender a Brandis, 1925. Bijdrage tot de kennis der fauna van<br />
Curasao. Epicaridea. Bijdragen tot de Dierkunde 24:1—8.<br />
. 1929. Papers from Dr. Th. Mortensen's Pacific Expedition 1914—16. 48. Epicaridea<br />
1. Videnskabelige Meddelelsers fra Dansk Naturhistorisk Forening i Kjebenhavn 87:1-44.<br />
. 1931. Papers from Dr. Th. Mortensen's Pacific Expedition 1914-16. 57. Epicaridea<br />
2. Videnskabelige Meddelelsers fra Dansk Naturhistorisk Forening i Kjebenhavn 91:147-225.<br />
Nordenstam, A. 1933. Marine Isopoda of the families Serolidae, Idotheidae,<br />
Pseudidotheidae, Arcturidae, Parasellidae and Stenetriidae mainly from the South<br />
Atlantic. Further Zoological Results of the Swedish Antarctic Expedition 1901-1903 3(1): 1—284.<br />
Norman, A. M., and T. R. R. Stebbing. 1886. Crustacea Isopoda of the 'Lightning',<br />
'Porcupine', and 'Valorous' Expeditions, <strong>Part</strong> 1. Transactions of the Zoological Society of London<br />
12(4):119-133.
LITERATURE CITED 287<br />
Notenboom, J. 1981. Amsterdam Expeditions to the West Indies Islands, report 12. Some<br />
new hypogean cirolanid isopod crustaceans from Haiti and Mayaguana (Bahamas).<br />
Bijdragen tot de Dierkunde 51(2):313—331.<br />
. 1984. Arubolana parvioculata n. sp. (Isopoda, Cirolanidae) from the interstitial of an<br />
intermittent river in Jamaica, with notes on A. imula Botosaneanu & Stock and A. aruboides<br />
(Bowman & IlifTe). Bijdragen tot de Dierkunde 54(1 ):51—65.<br />
Nunomura, N. 1977. Marine Isopoda from Amakusa, Kyushu (I). Publications from the<br />
Amakusa Marine Biological Laboratory 4:71-90.<br />
Ortiz, M., and R. Lalana. 1980. Una nueva especie de isopodo (Crustacea, Isopoda), de los<br />
manglares de la costa sur de Cuba. Revista Investigaciones Marinas 1(2-3): 160-174.<br />
Ortiz, M., R. Lalana, and O. Gomez. 1987. Lista de especies y bibliografia de los isopodos<br />
(Crustacea, Peracarida) de Cuba. Revista de Investigaciones Marinas 8(3):29-37.<br />
Packard, A. S. 1879. Zoology for Students and General Readers. 719 pp. New York: Henry Holt & Co.<br />
Pallas, P. S. 1772. In fasc. 9, Spicilegia Zoologica (quibus novae...et obscurae animalium<br />
species...illustrantur). 2 vols. (1767-) 1774-1780. Berlin.<br />
Paul, A. Z., and R.J. Menzies. 1971. Sub-tidal isopods of the Fosa de Cariaco, Venezuela,<br />
with descriptions of two new genera and twelve new species. Boletin de Instituto Universidade<br />
Oriente 10(l):29-48.<br />
Paulian, R., and C. Delamare Deboutteville. 1956. Un cirolanide cavernicole a Madagascar<br />
(Isopode). Memoires de VInstitut Scientifique de Madagascar (A)9:85-88.<br />
Pearse, A. S. 1932. New bopyrid isopod crustaceans from Dry Tortugas, Florida. Proceedings<br />
of the United States National Museum 81(1): 1-6.<br />
. 1950. Bopyrid isopods from the coast of North Carolina. Journal of the Elisha Mitchell<br />
Scientific Society 66:41-43.<br />
. 1951. Parasitic Crustacea from Bimini, Bahamas. Proceedings of the United States<br />
National Museum 101(3280):341-372.<br />
. 1952. Parasitic Crustacea from the Texas coast. Publications of the Institute of Marine<br />
Sciences, University of Texas 2(2):5-42.<br />
Pearse, A. S., and H. A. Walker. 1939. Two new parasitic isopods from the eastern coast of<br />
North America. Proceedings of the United States National Museum 87:19-23.<br />
Pennak, R. W. 1958. A new micro-isopod from a Mexican marine beach. Transactions of the<br />
American Microscopical Society 77:298-303.<br />
Pennant, T. 1777. British Zoology. 4th Edition, vol. 4.<br />
Perty, J. A. 1833. In Delectus animalium articulatorum quae in itinere per Brasiliam annis 1817-<br />
20„.collegerunt..J. B. de Spix...et...C. F. P. de Martius, digessit, descripsit, pingenda curavit M.<br />
Perty, vol. 3. Monaco.<br />
Petuch, E.J. 1982. Geographical heterochrony: contemporaneous coexistence of Neogene and<br />
Recent molluscan faunas in the Americas. Palaeogeography, Palaeoclimatology, Palaeoecology<br />
37:277-312.<br />
Pires, A. M. S. 1980. Revalidation and redescription of the genus Carpias Richardson, 1902<br />
(Isopoda, <strong>As</strong>ellota). Crustaceana 39(1):95-103.<br />
. 1981. Carpias harrietae (Isopoda, <strong>As</strong>ellota), a new species from Florida. Crustaceana<br />
40(2):206-212.
288 LITERATURE CITED<br />
. 1982. Taxonomic revision of Bagatus (Isopoda, <strong>As</strong>ellota) with a discussion of<br />
ontogenetic polymorphism in males. Journal of Natural History 16(2):227—259.<br />
. 1984. Taxonomic revision and phylogeny of the genus Erichsonella with a discussion<br />
on Ronalea (Isopoda, Valvifera). Journal of Natural History 18(5):665-683.<br />
Poore, G. C. B. 1984. Redefinition of Munna and Uromunna (Crustacea: Isopoda: Munnidae),<br />
with descriptions of five species from coastal Victoria. Proceedings of the Royal Society of<br />
Victoria 96(2):61-81.<br />
Poore, G. C. B., and Lew Ton, H. M. 1988. Amakusanthura and Apanthura (Crustacea:<br />
Isopoda: Anthuridae) with new species from tropical Australia. Memoirs of the Museum of<br />
Victoria 49:107-147.<br />
Racovitza, E. G. 1908. Ischyromene Lacazei n.g., n. sp. Isopode mediterraneen de la famille des<br />
Spheromides (Note preliminaire). Archives de Zoologie Experimental et Generale (4)9, notes et<br />
revue 3:LX-LXIV.<br />
Rafinesque-Schmaltz, C. S. 1815. Analyse de la Nature ou Tableau de UUnivers et des Corps<br />
Organises. 224 pp. Palerma.<br />
Ray, D. L. 1959 (Editor). Marine boring and fouling organisms. 536 pp. Seattle: University of<br />
Washington Press.<br />
Rehm, A., and H.J. Humm. 1973. Sphaeroma terebrans: A threat to the mangroves of<br />
southwestern Florida. Science 182:173-174.<br />
Richardson, H. 1897. Description of a new species of Sphaeroma. Proceedings of the Biological<br />
Society of Washington 11:105-107.<br />
. 1898. Description of four new species of Rocinela with a synopsis of the genus.<br />
Proceedings of the American Philosophical Society 37(157):8— 17.<br />
. 1899. Key to the isopods of the Pacific coast of North America, with descriptions of<br />
twenty-two new species. Proceedings of the United States National Museum 21:815-869.<br />
. 1900a. Results of the Branner-Agassiz Expedition to Brazil 2. The isopod Crustacea.<br />
Proceedings of the Washington Academy of Sciences 2:157-159.<br />
. 1900b. Synopses of North American invertebrates. 7. The Isopoda. American<br />
Naturalist 34:207-230.<br />
. 1901. Key to the isopods of the Atlantic coast of North America with descriptions of<br />
new and little known species. Proceedings of the United States National Museum 23:493-579.<br />
. 1902. The marine and terrestrial isopods of the Bermudas, with descriptions of new<br />
genera and species. Transactions of the Connecticut Academy of Sciences 11:277-310.<br />
. 1904. Contributions to the natural history of the Isopoda. Proceedings of the United<br />
States National Museum 27:1-89.<br />
. 1905. A monograph on the isopods of North America. Bulletin of the United States<br />
National Museum 54, liii + 727 pp.<br />
. 1910. Description of a new species of Anilocra from the Atlantic coast of North<br />
America. Proceedings of the United States National Museum 39:137-138.<br />
. 1912a. Descriptions of a new genus of isopod crustaceans, and of two new species<br />
from South America. Proceedings of the United States National Museum 43:201-204.<br />
. 1912b. Description of a new isopod crustacean belonging to the genus Livoneca from<br />
the Atlantic coast of Panama. Proceedings of the United States National Museum 42:173-174.
LITERATURE CITED 289<br />
. 1912c. Marine and terrestrial isopods from Jamaica. Proceedings of the United States<br />
National Museum 42:187-194.<br />
Rioja, E. 1953. Estudios carcinologicos. XXX. Observaciones sobre los cirolanidos<br />
cavernicolas de Mexico (Crustaceos, Isopodos). Anales del Instituto de Biologia, Mexico<br />
24(1):147-170.<br />
Robertson, P. B. 1978. A new species of asellote marine isopod Munna (Uromunna) hayesi<br />
(Crustacea: Isopoda) from Texas. Contributions in Marine Science 21(1 ):39—46.<br />
Robins, C. R., R. M. Bailey, C. E. Bond, J. R. Brooker, E. A. Lachner, R. N. Lea, and W.<br />
B. Scott. 1980. A list of common and scientific names of fishes from the United States and<br />
Canada (4th edition). American Fisheries Society, Special Publication 12:1-174.<br />
Roman, M.-L. 1977. Les oniscoides halophiles de Madagascar (Isopoda, Oniscoidea).<br />
Beaufortia 26:107-152.<br />
Roux, P. 1828. Crustaces de la Mediterranee et de son littoral, decrits et lithographies par<br />
iui-meme. Annates des Sciences Naturelles 16: plate 13.<br />
Sars, G. O. 1882. Oversigt af Norges Crustacea. Forhandlinger i Videnskabsselskabet i Kristiania<br />
1882 18:1-124.<br />
. 1897. An account of the Crustacea of Norway, vol. 2, pts. 3-8. Isopoda. 103 pp. Bergen.<br />
. 1899. An account of the Crustacea of Norway, vol. 2, pts. 13-14. Isopoda. 270 pp. Bergen.<br />
Say, T. 1818. An account of the Crustacea of the United States. Journal of the Academy of<br />
Natural Sciences of Philadelphia 1(2):393-401, 423-433.<br />
Schioedte, J. C, and F. Meinert. 1879. Symbolae ad Monographiam Cymothoarum<br />
Crustaceorum Isopodum Familiae 1. Aegidae. Naturhistorisk Tidsskrifl (3)12:321-414.<br />
. 1881. Symbolae ad Monographiam Cymothoarum Crustaceorum Isopodum<br />
Familiae 2. Anilocridae. Naturhistorisk Tidsskrifl (3)13:1 — 166.<br />
. 1883. Symbolae ad Monographiam Cymothoarum Crustaceorum Isopodum<br />
Familiae 3. Saophridae. 4. Ceratothoinae. Naturhistorisk Tidsskrifl (3)13:281—378.<br />
. 1884. Symbolae ad Monographiam Cymothoarum Crustaceorum Isopodum<br />
Familiae 4. Cymothoidae. Trib. II. Cymothoinae. Trib. III. Livonecinae. Naturhistorisk<br />
Tidsskrifl (3)14:221-454.<br />
Schram, F. R. 1986. Crustacea, xiv + 606 pp. New York, Oxford: Oxford University Press.<br />
Schultz, G. A. 1969. How to know the marine isopod crustaceans, vii + 359 pp. Dubuque, Iowa:<br />
Wm. C. Brown Co.<br />
. 1974. Terrestrial isopod crustaceans (Oniscoidea) mainly from the West Indies and<br />
adjacent regions. I. Tylos and Ligia. Studies on the Fauna of Curaqao and other Caribbean Islands<br />
45(149):162-173.<br />
. 1977. Anthurids from the west coast of North America, including a new species and<br />
three new genera (Crustacea, Isopoda). Proceedings of the Biological Society of Washington<br />
90(4):839-848.<br />
. 1979. A new <strong>As</strong>ellota (Stenetriidae) and two, one new, Anthuridea (Anthuridae)<br />
from Bermuda (Crustacea, Isopoda). Proceedings of the Biological Society of Washington<br />
91(4):904-911.<br />
. 1984. Three new and five other species of Oniscoidea from Belize, Central America<br />
(Crustacea: Isopoda). Journal of Natural History 18:3-14.
290 LITERATURE CITED<br />
Schultz, G. A., and D. M. Allen. 1982. Prodajus bigelowiensis, new species (Isopoda:<br />
Epicaridea: Dajidae) parasite of Mysidopsis bigelowi (Mysidacea) from coastal New Jersey,<br />
with observations on infestation. Journal of Crustacean Biology 2(2):296-302.<br />
Schultz, G. A., and L. R. McCloskey. 1967. Isopod crustaceans from the coral Oculina<br />
arbuscula Verrill. Journal of the Elisha Mitchell Scientific Society 83(2): 103—113.<br />
SimberlolT, D., B.J. Brown, and S. Lowrie. 1978. Isopod and insect root borers may benefit<br />
Florida mangroves. Science 201:630-632.<br />
Sivertsen, E., and L. B. Holthuis. 1980. The marine isopod Crustacea of the Tristan da<br />
Cunha Archipelago. Gunneria 35:1-128.<br />
Sket, B. 1979. Atlantasellus cavemicolus n. gen., n. sp. (Isopoda <strong>As</strong>ellota, Atlantasellidae n.<br />
fam.) from Bermuda. Bioloski Vestnik, Ljubljana 7(2): 175— 183.<br />
Soika, A. G. 1954. Studi di Ecologia e Biogeografia 12. Ecologia, Sistematica, Biogeografia<br />
ed Evoluzione del Tylos latreillei Auct. (Isop. Tylidae). Bollettino del Museo Civico di Storia<br />
Naturale di Venecia 7:63-83.<br />
Sowinsky, V. K. 1884. Contribution to the crustacean fauna of the Black Sea. Memoires de la<br />
Societe des Naturalistes de Kieff7:225-288. [In Russian.]<br />
Stebbing, T. R. R. 1900. On Crustacea brought by Dr. Willey from the South Seas. Willey's<br />
Zoological Results 5:605-690.<br />
. 1904. Marine Crustaceans. 12. Isopoda, with description of a new genus. In J. S.<br />
Gardiner, The Fauna and Geography of the Maldive and Laccadive Archipelagoes, being the account of<br />
the work carried on and of the collections made by an expedition during the years 1899 and 1900, vol. 2,<br />
pp. 699-720. Cambridge: Cambridge University Press.<br />
. 1905. Report to the Government of Ceylon on the pearl oyster fisheries of the Gulf<br />
of Manaar. Report on the Isopoda collected by Professor Herdman, at Ceylon, in 1902.<br />
Ceylon Pearl Oyster Fisheries, 1905, supplementary reports 23:1-64.<br />
Stimpson, W. 1853. Synopsis of the marine Invertebrata of Grand Manan, or the region<br />
about the Bay of Fundy, New Brunswick. Smithsonian Contributions to Knowledge 6:39-44.<br />
. 1855. Descriptions of some new marine Invertebrata. Proceedings of the Academy of<br />
Natural Sciences of Philadelphia 7:385-394.<br />
Stock, J. H. 1977. Microparasellidae (Isopoda, <strong>As</strong>ellota) from Bonaire. Studies on the fauna of<br />
Curacao and other Caribbean islands 51(168) :69—91.<br />
. 1986. Two new amphipod crustaceans of the genus Bahadzia from 'blue holes' in the<br />
Bahamas and some remarks on the origin of the insular stygofaunas of the Atlantic. Journal<br />
of Natural History (4)20:921-933.<br />
Stone, I., and R. W. Heard. 1989. Excorallana delaneyi n. sp. (Crustacea: Isopoda:<br />
Excorallanidae) from the northeastern Gulf of Mexico, with observations on adult<br />
characters and sexual dimorphism in related species of Excorallana Stebbing, 1904. Gulf<br />
Research Reports) 8(2):199-211.<br />
Stork, H. A. 1940. A new fresh-water isopod from Curasao. Studies on the Fauna of Curacao,<br />
Aruba, Bonaire and the Venezuelan Islands 10:147-150.<br />
Strouhal, H. 1966. Eine neue Halophile Stenophiloscia aus den Rotmeergebiete (Isop. terr.).<br />
Annalen die Naturhistorischen Museums, Wien 69:323-333.<br />
Tattersall, W. M. 1905. The marine fauna of the coast of Ireland. <strong>Part</strong> 5. Isopoda. Scientific<br />
Investigations for 1904, Fisheries Branch, Ireland 2:1-90.
LITERATURE CITED 291<br />
Thompson, M. T. 1902. A new isopod parasitic on the hermit crab. U. S. Fish Commission<br />
Bulletin for 1901, pp. 53-56.<br />
Topp, R. W., and F. H. HofT, Jr. 1972. Flatfishes (Pleuronectiformes). Memoirs of the<br />
Hourglass Cruises 4(2): 1-135.<br />
Ul'yanin, B. N. 1875. [Crustacea of Turkestan]. Imperatorskoe Obshchestvo Lyubitelei<br />
Estestvoznaniya Antropologhii i Etnoghrafii, vol. 2, pt. 6. Moscow. [In Russian.]<br />
Upton, N. P. D. 1987a. <strong>As</strong>ynchronous male and female life cycles in the sexually dimorphic,<br />
harem-forming isopod Paragnatkia formica (Crustacea: Isopoda). Journal of Zoology, London<br />
212:677-690.<br />
. 1987b. Gregarious larval settlement within a restricted intertidal zone and sex<br />
differences in subsequent mortality in the polygynous saltmarsh isopod Paragnatkia formica<br />
(Crustacea: Isopoda). Journal of the Marine Biological <strong>As</strong>sociation of the United Kingdom<br />
67(3):663-678.<br />
Vandel, A. 1952. Etude des isopodes terrestres recoltes au Venezuela par le Dr. G.<br />
Marcuzzi, suivie de considerations sur le peuplement du Continent de Gondwana. Memorie<br />
del Museo Civico di Storia Naturale di Verona 3:59-203.<br />
Vanhoffen, E. 1914. Die Isopoden der Deutschen Siidpolar-Expedition 1901-1903. Deutsche<br />
Sudpolar-Expedition 1901-1903, 25 (Zoologie) 7:447-598.<br />
Van Name, W. G. 1936. The American land and fresh-water isopod Crustacea. Bulletin of the<br />
American Museum of Natural History 71:1—535.<br />
Veillet, A. 1945. Recherches sur le parasitisme des crabes et des Galathees par les<br />
Rhizocephales et les Epicarides. Annales de Vlnstitut oceanographique, Paris, new series<br />
22(4):193-341.<br />
Wagele, J.-W. 1979. Morphologische Studien an Eisothistos mit Beschreibung von drei neuen<br />
Arten (Crustacea, Isopoda, Anthuridea). Mitteilungen aus dem Zoologiscken Museum der<br />
Universitat Kiel 1(2): 1-19.<br />
. 1981. Zur Phylogenie der Anthuridea (Crustacea, Isopoda). Mit Beitragen zur<br />
Lebensweise, Morphologie, Anatomie und Taxonomie. Zoologica 132:1-127.<br />
. 1982. On Apanthuretta lathridia n. sp. (Crustacea, Isopoda, Anthuridea) from Cuba<br />
Bijdragen tot de Dierkunde 52(l):43-48.<br />
. 1983. On the origin of the Microcerberidae (Crustacea: Isopoda). Zeitschrift fur<br />
zoologische Systematik und Evolutionsforschung 21 (4):249—262.<br />
. 1985. New west Atlantic localities for the stygobiont paranthurid Curassanthura<br />
(Crustacea, Isopoda, Anthuridea) with description of C. bermudensis n. sp. Bijdragen tot de<br />
Dierkunde 55(2):324-330.<br />
Walker, A. O. 1901. Contributions to the Malacostracan fauna of the Mediterranean. Journal<br />
of the Linnean Society of London, Zoology 28:290-307.<br />
Waterman, T. H., ed. 1960. The Physiology of Crustacea, vols. 1, 2, 670, 681 pp. New York &<br />
London: Academic Press.<br />
Williams, E. H., and L. B. Williams. 1980. Four new species of Renocila (Isopoda:<br />
Cymothoidae), the first reported from the New World. Proceedings of the Biological Society of<br />
Washington 93(3):573-592.<br />
. 1982. Mothocya bohlkeorum, new species (Isopoda: Cymothoidae) from West Indian<br />
cardinalfishes (Apogonidae). Journal of Crustacean Biology 2(4):570-577.
292 LITERATURE CITED<br />
. 1985a. A new cymothoid isopod, Glossobius hemiramphi, from the mouth of the<br />
ballyhoo, Hemiramphus brasiliensis (Linnaeus) (Exocoetidae), in the Caribbean Sea.<br />
Crustaceana 48(2): 147-152.<br />
. 1985b. Cuna insularis n. gen. and n. sp. (Isopoda: Cymothoidae) from the gill<br />
chamber of the sergeant major Abudefduf saxatilis (Linnaeus) (Osteichthyes) in the West<br />
Indies. Journal of Parasitology 71 (2):209-214.<br />
. 1986. Kuna Nomen Novum for Cuna Williams and Williams, 1985, preoccupied by Cuna<br />
Hedley, 1902. Journal of Parasitology 72(6):879.<br />
Williams, L. B., and E. H. Williams. 1981. Nine new species of A nilocra (Crustacea: Isopoda:<br />
Cymothoidae) external parasites of West Indian coral reef fishes. Proceedings of the Biological<br />
Society of Washington 94(4): 1005-1047.<br />
Wilson, G. D. F. 1987. The road to the Janiroidea: Comparative morphology and evolution<br />
of the asellote isopod crustaceans. Zeitschrifi fir Zfiologische Systematik und Evolutionsforschung<br />
25(4):257-280.
Index<br />
abbreviata, Bopyrina, 110, 267<br />
Abudefduf saxatilis, 171, 175, 186<br />
abudefdufi, Anilocra, 171, 175<br />
Abyssianira dentifrons, 263<br />
Acanthocope spinosissima, 263<br />
acanthopkora, Domecia, 111<br />
Acanthopleura granulata, 215<br />
acanthura, Anopsilana, 124, 125<br />
acanthuri, Anilocra, 171, 175, 176<br />
Acanthurus<br />
bahianus, 171, 176<br />
chirurgus, 171, 176<br />
acanthurus, Macrobrachium, 112<br />
crenulata, 64, 65, 267<br />
setosa, 64, 65<br />
Achelion occidentalism 110<br />
acuminata, Nerocila, 171, 172, 173, 190, 266<br />
268<br />
forma acuminata, 190<br />
forma &yter, 190<br />
acuta, Anilocra, 268<br />
acutirostrata, Stenobermuda, 106, 270<br />
acutirostris, Processa, 113<br />
acutitelson, Dynamenella, 213, 214<br />
ilagfl, 116, 117<br />
antillensiSy 117<br />
incisa, 268<br />
-4i?£fl (Aega), 116, 117<br />
deshaysiana, 117, 266, 268<br />
ecarinata, 117, 268<br />
-dtfgfl (Rhamphion), 116, 117<br />
^wtata, 117, 119<br />
tenuipes, 117, 119<br />
Aegidae, 115, 116, 262<br />
Aetobatus narinari, 166<br />
Pseudione, 113, 270<br />
Upogebia, 112<br />
Agaricia, 88, 166<br />
agaricicola, Metacirolana, 153, 154<br />
A£
294 INDEX<br />
angulata<br />
Dynamene, 214<br />
Dynamenella, 214<br />
angustifrons, Hexapanopeus, 111<br />
Anilocra, 170, 174, 175<br />
abudefdufi, 171, 175<br />
acanthuri, 171, 175, 176<br />
acuta, 268<br />
chaetodontis, 171, 175, 177<br />
fAnwiw, 172, 175, 177<br />
haemuli, 172, 173, 175, 177<br />
kolacantki, 172, 175, 179<br />
holocentri, 175, 179<br />
laticauda, 268<br />
myripristis, 172, 175, 180<br />
/wr/i/i, 173, 175, 180<br />
annandalei, Sphaeroma, 234<br />
annaoides, Antarcturus, 264<br />
annulicornis, Pandalus, 113<br />
annulipes, Pagurus, 113<br />
anomala, Aporobopyrina, 110, 267<br />
anops, Creaseriella, 137<br />
Anopsilana, 124, 273<br />
acanthura, 124, 125<br />
ArMWH, 124, 125, 266<br />
owiata, 124, 125<br />
cubensis, 124, 126<br />
;m, 124, 127<br />
radicicola, 124, 127<br />
annaoides, 264<br />
jloridanus, 269<br />
Antennuloniscus dimeroceras, 263<br />
Antheluridae, 275<br />
Anthomuda, 17, 23, 291<br />
stenotehon, 23, 270<br />
Anthuridae, 16, 270<br />
Anthuridea, 2, 14, 15, 16, 244, 263, 269<br />
antiguai, Plesionika, 113<br />
antillense, Exosphaeroma, 229, 230<br />
antillensis<br />
Aega, 117<br />
Dromidia, 111<br />
Excorallana, 161, 162, 268<br />
Paranthura, 69<br />
Antrolana, 273<br />
Apanthura? 17, 25<br />
cracenta, 25<br />
cram, 25, 26<br />
harringtoniensis3 25, 26, 270<br />
Apanthuroides? 17, 26<br />
millae, 27<br />
Aphysina fistulariSy 223<br />
lachneri, 171, 188<br />
maculatus, 171, 193<br />
townsendi, 171, 193<br />
Apogonidae, 187<br />
Aporobopyrina, 110<br />
anomala, 110, 267<br />
Aporobopyrus, 110<br />
collardi, 275<br />
curtatus, 110<br />
arbuscula, Oculina, 88<br />
Archosargus probatocephalus, 122<br />
Arcturella, 252<br />
bispinata, 252, 269<br />
#wiflte, 252, 269<br />
Arcturidae, 251, 252, 264<br />
caribbaeus, 264<br />
purpureus, 264<br />
arenatus, Priacanthus, 173, 183<br />
Argeia, 110<br />
atlantica, 110<br />
Argeiinae, 107<br />
Ariusfelis, 171, 190<br />
Armadilloniscus; 247<br />
m'/zai, 247<br />
Iscfinomesus, 263<br />
Petrolisthes, 110<br />
armillatuSy Alpheus, 112<br />
arndti, Dies, 207<br />
aruboidesy Arubolana, 144, 145, 270<br />
Arubolana, 144, 273<br />
aruboides, 144, 145, 270<br />
/ma/a, 144, 145<br />
parvioculata, 144, 145<br />
ascensionis, Holocentrus, 172, 179<br />
<strong>As</strong>elloidea, 75<br />
<strong>As</strong>ellota, 15, 15, 73, 263, 267, 269<br />
<strong>As</strong>tacilla, 252<br />
cymodocea, 252, 253<br />
/ajtf//
Atlantasellidae, 75<br />
Atlantasellus, 75<br />
cavernicolus, 75, 269, 270<br />
atlantica<br />
Argeia, 110<br />
Megalops, 172, 183<br />
attenuata, Erichsonella, 256, 258, 269<br />
aurolineatum, Haemulon, 172, 178<br />
Azygopleon, 110<br />
schmitti, 110, 267<br />
bacescui, Phycolimnoria, 276<br />
Bagatus, 83<br />
Bahalana, 124, 127, 128, 273<br />
cardiopus, 128<br />
geracei, 128<br />
mayana, 128<br />
bahianus, Acanthurus, 171, 176<br />
bajonado, Calamus, 122<br />
Balanopleon, 110<br />
tortuganus, 110<br />
Balistes vetula, 122<br />
balthica, Idotea, 259<br />
barbadensis, Micropanope, 111<br />
barbarae, Geogerceis, 215<br />
6anzar
296 INDEX<br />
Cancrion carolinus, 111<br />
capistratus, Chaetodon, 171, 177<br />
caraibica, Cymothoa, 182, 268<br />
Caranx, 122, 171<br />
hippos, 171, 183<br />
latus, 171, 183<br />
ruber, 171, 183<br />
carbonarium, Haemulon, 172, 178<br />
carcinus, Macrobrachium, 112<br />
cardiopus, Bahalana, 128<br />
caribaeus, Dactylokepon, 111<br />
caribbaeus, Arcturus, 264<br />
caribbea, Storthyngura pulchra, 263<br />
Ianirella, 263<br />
Malacanthura, 45<br />
Ischnomesus, 263<br />
Macrostylis, 263<br />
caribea, Uromunna, 94<br />
carinata, 'Cerceis,' 211, 268<br />
carolii, Metaphrixus, 111<br />
carolinense, Tozeuma, 112<br />
carolinus, Cancrion, 111<br />
Gzr/u
Colopisthus, 144, 146<br />
parvus, 147, 270<br />
confixa, Cortezura, 31<br />
Conilera cylindracea, 268<br />
Conilerinae, 123, 139<br />
conklini, Phaeoptyx, 173, 188<br />
constricta, Munida, 112<br />
convexa, Eurydice, 147, 148, 149, 268<br />
coralicolay Joeropsis, 88, 267<br />
Corallanidae, 115, 157<br />
corallicola, Minyanthura, 53<br />
corallinus, Pylopagurus, 112<br />
Cortezura, 17, 29<br />
confixa, 31<br />
penascoensis, 31<br />
cracenta, Apanthura, 25<br />
crassa, Virganthura, 73<br />
Creaseriella, 124, 137, 273<br />
anops, 137<br />
crenata, Anopsilana, 124, 125<br />
crenulata, Accalathura, 64, 65, 267<br />
crenulitelson, Cirolana, 132, 133<br />
cromis, Pogonias, 173, 190<br />
cruciaria, <strong>As</strong>talione, 110<br />
cruris, Apanthura, 25, 26<br />
cruentatus, Epinephelus, 172, 179<br />
crumenophthalmus, Selar, 173, 183<br />
Cryptoniscoidea, 107<br />
Crytoniscidae, 107, 109<br />
cubana, Cyathura (Cyathura), 33<br />
cubense, Munidion, 111<br />
Anopsilana, 124, 126<br />
tfocme/a, 119, 120<br />
cuborientalis, Cyathura (Stygocyathura), 34, 35<br />
culebrae, Vandeloscia, 247, 251<br />
cumanensis, Malacanthura, 45<br />
cumulus, Agarna, 173<br />
cuprea, Diopatra, 256<br />
curacaoensis, Hippolyte, 110<br />
Curassanthura, 64, 67, 270<br />
bermudensis, 67, 270<br />
canariensis, 270<br />
Afl/mfl, 67, 68, 270<br />
curassavica, Cyathura (Stygocyathura), 35<br />
o/rn, Haliophasma, 41<br />
curtatus, Aporobopyrus, 110<br />
cuvieri, Galeocerdo, 122<br />
cyaneus, Chromis, 172, 177<br />
Cyathura, 17, 31, 270, 273<br />
/>o/z7a? 267<br />
(Cyathura), 31<br />
cubana, 33<br />
(Stygocyathura), 31, 33, 35, 270<br />
cuborientalis, 34, 35<br />
curassavica, 35<br />
hummelincki, 35<br />
motasi, 35, 36<br />
orghidani, 35, 36<br />
parapotamica, 35, 36<br />
salpiscinalis, 35, 38<br />
sbordonii, 35, 38<br />
specus, 35, 38<br />
univam, 35, 38<br />
Cyclograpsus interger, 111<br />
cylindracea, Conilera, 268<br />
Cymodoce, 226, 227<br />
barrerae, 227<br />
ruetzleri, 227<br />
Cymodocea, 223, 253<br />
cymododea, <strong>As</strong>tacilla, 252, 253<br />
Cymothoa, 170, 172, 182<br />
caraibica, 182, 268<br />
«OM, 172, 173, 182, 268<br />
INDEX 297<br />
wrfrem, 171, 172, 173, 182, 183, 268<br />
Cymothoidae, 115, 169, 170<br />
Cynoscion, 172, 183<br />
nebulosus, 172, 183<br />
Dactylokepon<br />
caribaeus, 111<br />
sulcipes, 267<br />
Dajidae, 107<br />
Dardanus jucosus, 1122<br />
Dasyatis americana, 122, 166<br />
decorata, Mesanthura, 53<br />
deformans, Synsynella, 110, 113, 268, 270<br />
delaneyi, Excorallana, 160, 161<br />
Dendrotiidae, 263<br />
Dendrotion hanseni, 263<br />
dentata, Aega, 117, 119<br />
dentifrons, Abyssianira, 263<br />
deplanata, Ceratothoa, 180<br />
depressa, Panoplax, 111<br />
depressus, Ancinus, 268<br />
deshaysiana, Aega, 117, 266, 268<br />
Desmosoma magnispina, 263<br />
Desmosomatidae, 263<br />
destructor, Sphaeroma, 235<br />
desultor, <strong>As</strong>ymmetrione, 110<br />
rfifl/M*, Paradella, 224, 266, 268<br />
Dicropleon<br />
periclimenis, 111<br />
Dictyota, 219<br />
Z)r>j, 207<br />
flnw/rf, 207<br />
barnardi, 207
298 INDEX<br />
dimeroceras, Antennuloniscus, 263<br />
diminuta, Exosphaeroma, 229, 231<br />
diogenes, Petrochirus, 110<br />
Diopatra cuprea, 256<br />
Diplophryxus, 111<br />
Discerceis, 210, 211<br />
linguicauda, 213<br />
dispar, Paraliomera, 111<br />
distorta, Leidya, 111<br />
Domecia<br />
acanthophora, 111<br />
hispida, 111<br />
Dromidia antillensis, 111<br />
dubitans, Angliera, 91<br />
Dynamene angulata, 214<br />
Dynamenella, 210, 213, 214, 224<br />
acutitelson, 213, 214<br />
var. glabrothorax, 214<br />
var. typica, 214<br />
angulata, 214<br />
perforata, 213, 215, 270<br />
quadrilirata, 213, 215<br />
Dynameninae, 204, 210, 211<br />
ecarinata, Aega, 117, 268<br />
Echinothambema ophiuroides, 263<br />
Echinothambematidae, 263<br />
edithae, Paracerceis, 218, 221<br />
Edo tea<br />
lyonsi, 269<br />
montosa, 269<br />
samariensis, 276<br />
edulis, Processa, 113<br />
edwardsi, Plesionika, 113<br />
Edwinjoycea horologium, 252, 269<br />
eglanteria, Raja, 122<br />
Eisothistos, 16, 38, 39<br />
petrensis, 39<br />
ten, 39<br />
mm, Plesionika, 113<br />
Entoniscidae, 107, 109<br />
Entophilinae, 107<br />
Eophrixus subcaudalis, 111, 267<br />
Epicaridea, 4, 14, 107, 267<br />
Epinephelus, 122, 172, 183<br />
cruentatus, 172, 179<br />
>/z^, 172, 179<br />
guttatus, 172, 179<br />
*7, 122, 172, 190<br />
morio, 122<br />
Erichsonella, 255, 256, 257<br />
attenuata, 256, 258, 269<br />
Jiliformis, 256, 258, 269<br />
tropicalis, 257<br />
floridana, 256, 258, 269<br />
isabelensis, 269<br />
Eriphia gonagra, 111<br />
Eubranchiatae, 203<br />
Eurycopidae, 263<br />
Eurydice, 143, 147<br />
con^xfl, 147, 148, 149, 268<br />
littoralis, 148, 149, 268<br />
personata, 147, 149, 270<br />
piperata, 147, 149, 268<br />
Eurydicinae, 123, 139, 143<br />
excavata, Chiridotea, 269<br />
Excirolana, 144, 149, 150<br />
braziliensis, 150, 266, 268<br />
mayana, 150, 153, 268<br />
Atrial, Cymothoa, 172, 173, 182, 268<br />
Excorallana, 157, 159, 161<br />
antillensis, 161, 162, 268<br />
berbicensis, 161, 162<br />
delaneyi, 160, 161<br />
fissicauda, 161, 162<br />
mexicana, 160, 161, 268<br />
oculata, 161, 163<br />
quadricornis, 161, 165, 270<br />
sexticornis, 161, 165<br />
subtilis, 160<br />
tricornis, 266, 268<br />
occidentalis, 167<br />
tricornis, 161, 165<br />
warmingii, 161, 167<br />
exilipes, Palaemonetes, 113<br />
Exocoettus, 172, 184<br />
Exosphaeroma, 226, 229, 231<br />
fl/Afl, 229<br />
antillense, 229, 230<br />
diminuta, 229, 231<br />
productatelson, 229, 231<br />
yucatanum, 229, 231<br />
exotica, Ligia, 247, 249<br />
faber, Chaetodipterus, 171, 190<br />
fasciata, Mesanthura, 47, 49<br />
faustinum, Macrobrachium, 112<br />
/flxwii, Harrieta, 232, 268<br />
felis,Arius, 171, 190<br />
filiforme, Syringodium, 253, 260<br />
Jiliformis, Erichsonella, 256, 258, 269<br />
fimbriata<br />
Pleurocryptella, 112<br />
Processa, 113<br />
fissicauda, Excorallana, 161, 162<br />
fistularis, Aphysina, 223<br />
Flabellifera, 2, 14, 114, 115, 268, 269<br />
Jlavolineatum, Haemulon, 122, 172, 178
Jlinti, Munida, 111<br />
jloridana<br />
Erichsonella, 256, 258, 269<br />
Pleurocrypta, 112<br />
florid anus<br />
Allodiplophryxus, 267<br />
Antarcturus, 269<br />
Thor, 110, 111<br />
jloridensis<br />
Carpias, 267<br />
Gnathic, 268<br />
Mesanthura, 53, 267<br />
Paranthura, 69, 71, 267<br />
Pleurocope, 98, 267<br />
joetens, Synodus, 173, 183<br />
foliatus, Parathelges, 112<br />
formosa, Kupellonura, 267<br />
formosus, Alpheus, 111, 112<br />
fritzmuelleri, Synalpheus, 110, 111<br />
jucorum, Latreutes, 112<br />
Jucosus, Dardanus, 112<br />
Julvus, Epinephelus, 172, 179<br />
Jurcifer, Paranthias, 173, 179<br />
Galathea rostrata, 112<br />
galathinus, Petrolisthes, 110<br />
Galeocerdo cuvieri, 122<br />
geminsula, Amakusanthura, 18<br />
Geocerceis, 210, 215<br />
barbarae, 215<br />
geracei, Bahalana, 128<br />
Genes rhombeus, 172, 187<br />
giardi, Synalpheion, 113<br />
giganteus, Bathynomus, 131, 268<br />
Gigantione<br />
mortenseni, 111, 267<br />
uberlackerae, 267<br />
Ginglymostoma cirratum, 122, 158<br />
glabrothorax, var., Dynamenella acutitelson, 214<br />
Glossobius, 170, 172, 183, 184<br />
hemiramphi, 172, 184<br />
impressus, 172, 184<br />
Paracerceis, 218, 221<br />
Paraleptosphaeroma, 210, 266<br />
Cwato, 238<br />
beethoveni, 238, 239<br />
Jloridensis, 268<br />
gonzalezi, 238, 239<br />
Johanna, 238, 239, 276<br />
magdalenensis, 238, 239<br />
puertoricensis, 238, 239<br />
ra^z, 238, 241<br />
samariensis, 238, 241<br />
triospathiona, 238, 241<br />
ye/
300 INDEX<br />
hemiramphi3 Glossobius, 172, 184<br />
Hemiramphidae, 187<br />
Hemiramphus<br />
bermudensis, 188<br />
brasiliensis, 172, 184<br />
hemphilli, Synalpheus, 110, 111<br />
hendleri, Pendanthura, 56<br />
herbstii, Panopeus, 111<br />
herrerai, Microcharon, 93<br />
heterocarpus, Plesionika, 113<br />
heterochaelis, A Ipheus, 112<br />
Heteromesus bijurcatus, 263<br />
Hexapanopeus angustifrons, 111<br />
Hippolyte<br />
curacao ensis 3 110<br />
pleutacanthus, 110, 111<br />
Zostericola, 110<br />
hippos, Caranx, 171, 183<br />
Hirundichthys speculifer, 172, 184<br />
hispida, Domecia, 111<br />
holacanthi, Anilocra, 172, 175, 179<br />
Holacanthus tricolor, 172, 179<br />
holocentri, Anilocra, 175, 179<br />
Holocentrus ascensionis, 172, 179<br />
hopkinsi, Mesanthura, 47, 51, 267<br />
Horoloanthura irpex, 267<br />
horologium, Edwinjoycea, 252, 269<br />
hummelincki, Cyathura (Stygocyathura), 35<br />
Hydroniscus quadrifrons, 263<br />
Hyperphrixus castrensis, 267<br />
Hypoconcha<br />
sabulosa, 111<br />
spinosissima, 111<br />
Hyporhamphus unifasciatus, 172, 188<br />
hyptius, Stegophryxus, 113, 268<br />
Hyssuridae, 16, 58, 60<br />
fanirella<br />
caribbica3 263<br />
vemae, 263<br />
Afotai, 255, 259<br />
balthica, 259<br />
metallicay 259, 269<br />
Idoteidae, 251, 252, 255<br />
Idoteinae, 255, 256<br />
Iliacantha<br />
liodactyla, 111<br />
subglobosa3 111<br />
imbricata, Parapagurion, 112<br />
impressa, Politolana, 140<br />
impressus, Glossobius, 172, 184<br />
imswe, Kupellonura, 60<br />
imw/a, Arubolana, 144, 145<br />
/nriftZj 4*£A, 268<br />
indica, Limnoria, 194, 195<br />
infundibulata, Paranthura, 69, 71, 270<br />
insulae, Limnoria, 195<br />
insular is<br />
Alcirona, 158<br />
A'ttrtfl, 170<br />
Rocinela, 119, 120, 268<br />
integra, Synsynella, 268<br />
interger, Cyclograpsus, 111<br />
intermedius, Palaemonetes, 113<br />
loninae, 107<br />
Iridopagurus, 112, 113<br />
zm, 113<br />
z'm, Iridopagurus, 113<br />
irmae, Haliophasma, 43<br />
fr/fcx, Horoloanthura, 267<br />
irrasa, Munida, 111<br />
irritans, Munidion, 111<br />
isabelensis, Erichsonella, 269<br />
Ischnomesidae, 263<br />
Ischnomesus<br />
armatus, 263<br />
caribbicus, 263<br />
multispinis, 263<br />
Ischyromene, 210, 214, 217<br />
bamardi, 218<br />
iVfljflrfl, Epinephelus, 122, 172, 190<br />
jacobus, Myripristis, 172, 180<br />
jacqueti, Sclerocrangon, 110<br />
Janiridae, 80, 81, 263<br />
Janiroidea, 75, 79, 80, 81<br />
Joeropsidae, 80, 87<br />
Joeropsis, 87, 88<br />
bifasciatus, 88<br />
coralicola, 88, 267<br />
personatus, 88, 90<br />
rathbunae, 88, 90, 267, 270<br />
JOAHHM, G/ifltfifl, 238, 239, 292<br />
jonesi, Anopsilana, 124, 127<br />
kadiakensis, Palaemonetes, 113<br />
kensleyi, Mexicope, 81, 267<br />
kohleri, Mesosignum, 264<br />
AWAJI, Alcirona, 158, 268, 270<br />
tfiwifl, 170, 184<br />
insularis, 171, 186<br />
Kupellonura, 60<br />
formosa, 267<br />
imswe, 60<br />
lachneri, Apogon, 171, 188<br />
Lachnolaimus maximus, 122<br />
Laminaria, 201<br />
lamprotaenia, Anchoa, 171, 187<br />
laodicense, Gnathostenetroides, 78
lasallae, <strong>As</strong>tacilla, 252, 253<br />
lata, Parabopyrella, 112<br />
lathridia, Amakusanthura, 18, 20<br />
laticauda, Anilocra, 268<br />
laticeps, Skuphonura, 58<br />
latreillei, Tylos, 247, 250<br />
Latreutes Jucorum, 112<br />
latreuticola, Probopyrinella, 112, 268, 270<br />
latus, Caranx, 171, 183<br />
/flw#?, <strong>As</strong>tacilla, 269<br />
Laurencia, 219<br />
Aimini, 111, 270<br />
distorta, 111<br />
Leiostomus xanthurus, 172, 183, 187,<br />
190<br />
Lepisosteus spatula, 172, 190<br />
leptorhynchus, Pandalus, 113<br />
lewisi, Chalixanthura, 27, 29<br />
Licranthura, 16, 43<br />
amyle, 43<br />
Lfcifl, 247, 249<br />
baudiniana, 247, 249<br />
wrrtiai, 247, 249<br />
302 INDEX<br />
mayana<br />
Bahalana, 128<br />
Excirolana, 150, 153, 268<br />
mcclendoni, Synalpheus, 110, 111<br />
Megalops atlantica, 172, 183<br />
menziesi, Metacirolana, 153, 154<br />
Mesantkura, 17, 45<br />
bivittata, 47<br />
decorata, 53<br />
fasciata, 47, 49<br />
JloridensiSy 53, 267<br />
hopkinsit479 51, 267<br />
looensis, 47, 51<br />
paucidens, 47, 51<br />
pulchra, 47, 52, 267<br />
punctillata, 47, 53<br />
reticulata, 47, 53<br />
Mesosignidae, 264<br />
Mesosignum kohleri, 264<br />
Metacirolana, 144, 153<br />
agaricicola, 153, 154<br />
Afl/ifl, 153, 154<br />
menziesi, 153, 154<br />
sphaeromiformis, 153, 154<br />
metallica, Idotea, 259, 269<br />
Metaphrixus carolii, 111<br />
mexicana, Excorallana, 160, 161, 268<br />
mexicanus, Microcerberus t 269<br />
Mexicope, 80, 81<br />
kensleyiy 81, 267<br />
Mexilana, 273<br />
mgrayiy Serolis, 202, 268<br />
Microcerberidae, 243, 244<br />
Microcerbendea, 14, 243, 269, 273<br />
Microcerberus, 244, 273<br />
littoralis, 244<br />
mexicanuSy 269<br />
minutuSy 244<br />
mirabiliSy 244<br />
nunezi, 244<br />
renaudiy 244<br />
simplex, 244<br />
syrtiaiSy 244<br />
Microcharon, 90, 91<br />
herrerai, 93<br />
phreaticus, 93<br />
sabulurrty 91<br />
Micropanope barbadensis, 111<br />
Microparasellidae, 80, 90, 273<br />
Microphrys bicornutus, 110<br />
//nV^r, Munida} 112<br />
millae, Apanthuroides, 27<br />
milleri, Santiay 99, 267<br />
minocule, Stenetrium, 100, 102<br />
minusy Synalpheus, 110, 113<br />
minuta, Cirolana, 132, 135<br />
Cfl#wj, 82, 84, 270<br />
MacrostyliSy 263<br />
Microcerberusy 244<br />
Minyanthura, 16, 53<br />
corallicola, 53<br />
mirabilis<br />
MadraciSy 29, 41<br />
Microcerberusy 244<br />
Miratidoteay 255, 259<br />
bruscai> 260<br />
Monacanthus ciliatuSy 172, 190<br />
montaguiy Pandalus, 113<br />
montosa, Edoteat 269<br />
morioy Epinephelus, 122<br />
Gigantione, 111, 267<br />
Parabopyrellay 112, 267<br />
mosaica, Cassidinidea, 208<br />
motasiy Cyathura (Stygocyathura), 35, 36<br />
Mothocya, 170, 187<br />
bohlkeorum, 171, 173, 187, 188<br />
wflwfl, 172, 187, 188<br />
Mugil cephalus, 172, 190<br />
multilineatus, Chromis, 172, 177<br />
multipunctata, Limnoria, 195, 196<br />
multispinis, Ischnomesus, 263<br />
constricta, 112<br />
TKnrt, 111<br />
irrasas 111<br />
longipeSy 112<br />
772 £/w, 112<br />
schroederiy 112<br />
simplex, 110<br />
stimpsoni, 111<br />
valida, 110<br />
Munidion<br />
cubensCy 111<br />
irritans, 111<br />
longipedisy 112, 267<br />
Munna, 93<br />
petronastes, 94<br />
Munnidae, 80, 93<br />
Munnogoniumy 96<br />
wilsoni, 96<br />
Mycteroperca<br />
bonaci, 122<br />
venenosa, 122<br />
Myripristis jacobus, 172, 180
myripristis, Anilocra, 172, 175, 180<br />
Nalicora, 157, 168<br />
rapax, 169, 268<br />
nana, Mothocya, 172, 187, 188<br />
Nannoniscidae, 264<br />
Nannoniscus camayae, 264<br />
narinari, Aetobatus, 166<br />
Natatolana, 139<br />
gracilis, 140<br />
nebulosus, Cynoscion, 172, 183<br />
Nemanthura, 43<br />
Neoanthura coeca, 263<br />
Neopanope<br />
packardii, 111<br />
ttxana sayi, 111<br />
Neostenetroides, 77, 78<br />
stocki, 78<br />
Nerocila, 170, 172, 188<br />
acuminata, 171, 172, 173, 190, 266, 268<br />
forma acuminata, 190<br />
forma aj/er, 190<br />
ninae, Armidilloniscus, 246, 247<br />
nfzwtf, T^/ar, 247, 250<br />
normanni, Alpheus, 112<br />
northropi, Palaemon, 113<br />
nunezi, Microcerberus, 244<br />
nuttingi, Paracerceis, 218, 223<br />
obtruncata, Cirolana, 132, 135, 268<br />
occiden talis<br />
Achelion, 110<br />
Cleantioides, 256<br />
Excorallana tricornis, 167<br />
Parathelges, 112<br />
ocellatus, Chaetodon, 171, 177 I<br />
Excorallana, 161, 163<br />
Rocinela, 119, 120, 266, 268<br />
Oculina arbuscula, 88<br />
Ocyurus chrysurus, 172, 183<br />
owfram, Cymothoa, 171, 172, 173, 182, 183,<br />
268<br />
ohione, Macrobrachium, 112<br />
Olencira praegustator, 268<br />
Zigia, 247, 249<br />
Macrobrachium, 112<br />
Ontilorpheus, 124, 139<br />
stebbingi, 139<br />
Oniscidea, 1, 4, 14, 15, 246, 247, 262<br />
ophiuroides, Echinothambema, 263<br />
Orbioninae, 107<br />
orghidani, Cyathura (Stygocyathura), 35,<br />
36<br />
Orthopristis<br />
chrysoptera, 172, 183<br />
raA^r, 122, 173, 179<br />
ovalis<br />
Cassidinidea, 207, 208, 268<br />
Lironeca, 268<br />
Ovobopyrus alphezemiotes, 267<br />
oxyophthalmus, Paguristes, 112<br />
Pachygrapsus transversus, 111<br />
packardii, Neopanope, 111<br />
Paguristes<br />
oxyophthalmus, 112<br />
tortugae, 112<br />
Pagurus<br />
annulipes, 113<br />
bonairensis, 110, 113<br />
brevidactylus, 112, 113<br />
longicarpus, 110, 113<br />
provenzanoi, 110, 112, 113<br />
Palaemon<br />
northropi, 113<br />
pandaliformis, 113<br />
Palaemonetes<br />
exilipes, 113<br />
intermedius, 113<br />
kadiakensis, 113<br />
paludosus, 113<br />
pugio, 113<br />
vulgaris, 113<br />
paludosus, Palaemonetes, 113<br />
pandalicola, Probopyrus, 112, 266<br />
pandaliformis, Palaemon, 113<br />
Pandalus<br />
annul ico rni s, 113<br />
Ari, 113<br />
leptorhynchus, 113<br />
montagui, 113<br />
pandionis, Synalpheus, 111, 113<br />
Panopeus herbstii, 111<br />
Panoplax depressa, 111<br />
Parabopyrella<br />
lata, 112<br />
mortenseni, 112, 267<br />
richardsonae, 112, 267<br />
thomasi, 112<br />
Parabopyriscus stellatus, 267<br />
Paracerceis, 210, 218, 223<br />
cflurfate, 219, 268, 270<br />
var. brevipes, 219<br />
roA«ifl*, 219, 220<br />
wfifAae, 218, 221<br />
£/>wii, 218, 221<br />
Hatting, 218, 223
304 INDEX<br />
Paradella, 210, 214, 223<br />
dianae, 224, 266, 268<br />
plicatura, 223, 224<br />
quadripunctata, 223, 224<br />
tumidicauda, 223, 226<br />
Paradynamene benjamensis, 268<br />
Paragnathia, 237<br />
Paraleptosphaeroma, 207, 208<br />
^iww, 210, 266<br />
Paralimnoria, 193, 199<br />
andrewsi, 199<br />
forma A, 200<br />
forma B, 201<br />
forma typica, 200<br />
Paraliomera dispar, 111<br />
Paramunnidae, 80, 96<br />
Paranthias farcifer, 173, 179<br />
Paranthura, 64, 69<br />
antillensis, 69<br />
barnardiy 69, 71<br />
floridensis, 69, 71, 267<br />
infundibulatay 69, 71, 270<br />
Paranthuridae, 16, 64, 263, 270<br />
Parapagurion imbricata, 112<br />
Parapagurus, 112<br />
parapotamica, Cyathura (Stygocyathura), 35,<br />
Para theIges<br />
foliatus, 112<br />
occidentalism 112<br />
piriformis, 112, 270<br />
tumidipes, 112, 270<br />
partiti, Anilocra, 173, 175, 180<br />
partitus, Pomacentrus3 173, 180<br />
parva, Cirolana, 132, 135, 268<br />
parvioculata, Arubolana, 144, 145<br />
parviramus, Pseudione, 276<br />
parvus, Colopisthus, 147, 270<br />
patulipalma, Stenetrium, 100, 102<br />
paucidens, Mesantkura, 47, 51<br />
pectiniger, Synalpheus, 110, 111, 113<br />
penascoensis; Cortezura, 31<br />
Pendanthura, 17, 56<br />
hendleri, 56<br />
tanaiformis, 56, 270<br />
penna, Calamus, 122<br />
perforata, Dynamenella, 213, 215, 270<br />
Periclimenes<br />
americanus, 111, 113<br />
longicaudatus, 113<br />
periclimenis, Dicropleon, 111<br />
personata, Eurydice, 147, 149, 270<br />
personatus, Joeropsis, 88, 90<br />
petrensis, Eisotkistos, 39<br />
Petrochirus diogenes, 110<br />
Petrolisthes<br />
armatus, 110<br />
galathinus, 110<br />
marginatus, 110<br />
petronastesy Munna, 94<br />
pfefferi, Limnoria, 195, 198<br />
Phaeoptyx<br />
conklini, 173, 188<br />
pigmentaria, 173, 188<br />
phreaticuSy Microcharoriy 93<br />
Phreatocoidea, 14<br />
Phycolimnoria, 193, 201<br />
bacescuiy 276<br />
clarkae, 201<br />
Phyllodurinae, 107<br />
pigmentariay Phaeoptyx, 173, 188<br />
piperata, Eurydice, 147, 149, 268<br />
piriformis, Parathelges, 112, 270<br />
planicauda, Cleantioides, 256, 266, 268<br />
Platybranchiatae, 203<br />
platycauda, Limnoria, 195, 198<br />
Plesionika<br />
antiguai, 113<br />
edwardsiy 113<br />
mw, 113<br />
heterocarpus, 113<br />
martia, 113<br />
p leuracanthus, Hip poly te, 110, 111<br />
Pleurocope, 97<br />
floridensisy 98, 267<br />
Pleurocopidae, 80, 81, 96<br />
Pleurocryptella fimbriata, 112<br />
plicatura, Paradella, 223, 224<br />
plicifera, Callispongia, 221<br />
plumieri, Haemulon, 172, 179<br />
Pogonias cromis, 173, 190<br />
polita<br />
Cyathura, 267<br />
Politolana, 140, 143, 268<br />
Politolana, 139, 140<br />
impressa, 140<br />
^o/ito, 140, 143, 268<br />
Pomacentrus partitus, 173, 180<br />
Pontonia margarita, 113<br />
Porce liana say ana, 110<br />
A>n7^, 88, 90<br />
poteriophora, Akidognathia, 264<br />
praegustator, Olencira, 268<br />
Priacanthus arenatus, 173, 183<br />
probatocephalus, Archosargus, 122<br />
Probopyria alphei, 112, 267
Probopyrinella<br />
heardiy 267<br />
latreuticola, 112, 268, 270<br />
Probopyrus pandalicola, 112, 266<br />
Processa<br />
acutirostris y 113<br />
canaliculata y 113<br />
eduliSy 113<br />
Jimbriatay 113<br />
tenuipes, 113<br />
processaey Urobopyrusy 113, 268<br />
Prodajus cf. bigelowiensis, 268<br />
productatelson, Exosphaeroma3 229, 231<br />
provenzanoiy Pagurus> 110, 112, 113<br />
psamathus, Anglieray 91<br />
Pseudasymmetrione, 113<br />
Pseudione<br />
qffinis, 113, 270<br />
cognata, 268<br />
parviramuSy 276<br />
upogebiae, 268<br />
Pseudioninae, 107<br />
Ptilanthura tricarina, 267<br />
puertoricensis, Gnathia} 238, 241<br />
pugilatoty Uca, 111<br />
GnathostenetroideSy 77', 267<br />
PalaemoneteSy 113<br />
pulchra<br />
caribbedy Storthyngura, 263<br />
Mesanthura, 47, 52, 267<br />
punctatuSy Carpias, 82, 85<br />
punctillatdy Mesanthura, 47, 53<br />
purpureus, Arcturus3 264<br />
PylopaguruSy 110<br />
corallinus, 112<br />
quadricornisy Excorallana, 161, 165, 270<br />
quadridentata, Sphaeroma, 234, 268<br />
quadrifrons, Hydroniscus, 263<br />
quadriliratdy Dynamenella, 213, 215<br />
quadripunctata, Paradella, 223, 223<br />
racovitzai, Angliera, 91<br />
radicicola<br />
Anopsilana, 124, 127<br />
Xylolana, 157<br />
i?a/a eglanteridy 122<br />
rapaXy Nalicora, 169, 268<br />
rathbunae<br />
JoeropsiSy 88, 90, 267, 270<br />
Lysmata, 112<br />
rafAi, Gnathic, 238, 241<br />
redmanni, Lironecay 172, 173, 186, 268<br />
regalis, Scomberomorus, 173, 187<br />
regina, <strong>As</strong>tacilla, 252, 253<br />
renaudiy Microcerberus, 244<br />
Renocila, 170, 191<br />
bowmani, 173, 191<br />
«/!/!!, 171, 191<br />
waldneriy 173, 191, 193<br />
reticulatas Mesanthura, 47, 53<br />
INDEX 305<br />
reynoldsiy Uromunnay 94, 95, 266, 267<br />
RhamphioUy see i4«ga (Ramphion)<br />
Rhizophora mangle, 235<br />
rhombeus, GerreSy 172, 187<br />
Rhyscotus, 247, 249<br />
fexwuij, 247, 249<br />
richardsonae, Parabopyrella, 112, 267<br />
ricordiy Sesarma, 111<br />
nVrf/z, Vandeloscia, 247, 251<br />
Ritkropanopeus karrisiiy 111<br />
Rocinela, 116, 119<br />
cubensisy 119, 120<br />
insularis, 119, 120, 268<br />
oca/ate, 119, 120, 266, 268<br />
jignafa, 119, 120, 266, 268<br />
rostratay Galathea, 112<br />
Caranx, 171, 183<br />
Orthopristisy 122, 173, 179<br />
ruetzleri, Cymodoce, 227<br />
sabulosa, Hypoconcha, 111<br />
sabulum, Microcharony 91<br />
salpiscinalis, Cyathura (Stygocyathura), 35, 38<br />
samariensis<br />
Edotea, 276<br />
GnafAia, 238, 251<br />
Santiay 98<br />
ffiiV/m, 99, 267<br />
Santiidae, 80, 98<br />
Sargassum, 84, 201<br />
saseboensiSy Limnoria, 195, 198<br />
saxatilis, Abudefdufy 171, 175, 186<br />
sayana, Porcellana, 110<br />
ja>^", Neopanope texana, 111<br />
sbordoniiy Cyathura (Stygocyathura), 35,<br />
38<br />
Schizobopyrina urocaridis, 113, 268<br />
schmittiy Azygopleon, 110, 267<br />
schoepfi<br />
Alutera, 171, 190<br />
Chilomycterusy 172, 190<br />
schroederi, Munida, 112<br />
sciuruSy Haemulon, 172, 179<br />
Sclerocrangon jacqueti, 110<br />
Scomberomorus<br />
cavalla, 173, 187
306 INDEX<br />
Scomberomorus (cont.)<br />
maculatus, 173, 187<br />
regain, 173, 187<br />
scopulosa, Chalixanthura, 27, 29<br />
sedentarius, Chaetodon, 171, 177<br />
Selar crumenophthalmuSy 173, 183<br />
Serolidae, 114, 115, 201<br />
Serolis, 202<br />
mgrayi, 202, 268<br />
Serranus tigrinus, 173, 191, 193<br />
serrata, Spinianirella, 263<br />
serratum, Stenetrium, 100, 102<br />
serricaudus, Carpias, 82, 87<br />
Sesarma ricordi, 111<br />
seticornis, Stenorhynchus, 110<br />
setifer, Macrostylis, 264<br />
setosa, Accalathura, 64, 65<br />
sexticorniSy Excorallana, 161, 165<br />
Amakusanthura, 18, 21<br />
Rocinela, 119, 120, 266, 268<br />
signijica, Amakusanthura, 18, 23<br />
Microcerberus, 244<br />
Munida, 110<br />
simulata, Limnoria, 195, 198<br />
Skuphonura, 17, 58<br />
laticeps, 58<br />
lindae, 267<br />
snanoi, Storthyngura, 263<br />
somala, Haptolana, 138<br />
Sparisoma viride, 122<br />
spathulicarpusy Stenetrium, 100, 104<br />
spatula, Lepisosteus, 172, 190<br />
speculifer, Hirundichthys, 172, 184<br />
specus, Cyathura (Stygocyathura), 35, 38<br />
Speocirolana, 273<br />
Sphaerolana> 273<br />
Sphaeroma, 226, 232, 234<br />
annandalei, 234<br />
destructor, 235<br />
quadridentata, 234, 268<br />
terebrans, 234, 235, 268<br />
ttWfen, 234, 235<br />
<strong>Sphaeromatidae</strong>, 114, 115, 202, 204<br />
Sphaeromatinae, 204, 226<br />
sphaeromiformis, Metacirolana, 153,<br />
154<br />
Sphoeroides maculatus, 173, 190<br />
Sphyraena barracuda, 122<br />
j/>i»ata, Arcturella, 252, 269<br />
Spinianirella serrata, 263<br />
spinosissima<br />
Acanthocope, 263<br />
Hypoconcha, 111<br />
Oncilorpheus, 139<br />
Stenetrium, 100, 104, 267, 270<br />
Stegias clibanarii, 113, 270<br />
Stegophryxus hyptius, 113, 268<br />
steindachneri, Haemulon, 122<br />
<strong>As</strong>trapogon, 171, 188<br />
Parabopyriscus, 267<br />
Stenetriidae, 99<br />
Stenetrioidea, 99<br />
Stenetrium, 99, 100<br />
bowmani, 100<br />
minocule, 100, 102<br />
patulipalma, 100, 102<br />
serratum, 100, 102<br />
spathulicarpus, 100, 104<br />
stebbingi, 100, 104, 267, 270<br />
Stenobermuda, 99, 106<br />
acutirostrata, 106, 270<br />
Stenorhynchus seticornis, 110<br />
stenotelson, Anthomuda, 23, 270<br />
stimpsoni, Munida, 111<br />
stocki, Neostenetroides, 78<br />
pulchra caribbea, 263<br />
snanoi, 263<br />
striata, Yvesia, 246<br />
striatus, Chaetodon, 171, 177<br />
Stygocyathura, see Cyathura (Stygocyathura)<br />
subcaudalis, Eophrixus, 111, 267<br />
subglobosa, Iliacantha, 111<br />
subtilis, Excorallana, 160<br />
sulcipes, Dactylokepon, 267<br />
surinamensis, Batrachoides, 171, 190<br />
surinamicum, Macrobrachium, 112<br />
symmetricus, Ambidexter, 113<br />
synagris, Lutjanus, 172, 183<br />
synalphei<br />
Bopyrione, 110, 267<br />
Hemiarthrus, 111, 267<br />
Synalpheion giardi, 113<br />
Synalpheus<br />
bousfieldi, 110<br />
brevicarpus, I ] 0<br />
AiwwW, 110, 111, 113<br />
fritzmuelleri, 110, 111<br />
goodei, 110, 111<br />
hemphilli, 110, 111
longicarpus, 110, 111, 113<br />
mcclendoni, 110, 111<br />
minus, 110, 113<br />
pandionis, 111, 113<br />
pectiniger, 110, 111, 113<br />
Synodus foe tens, 173, 183<br />
Synsynella, 110, 113<br />
choprae, 113, 268, 270<br />
deformans, 110, 113, 268, 270<br />
Integra, 268<br />
Syringodium, 166, 232<br />
filiforme, 253, 260<br />
syrticus, Microcerberus, 244<br />
tanaiformis, Pendanthura, 56, 270<br />
Tecticeps, 204<br />
Tecticipitinae, 204<br />
/l^, 117, 119<br />
Processa, 113<br />
tenuis, Colanthura, 65, 270<br />
tenuistylis, Lironeca, 171, 186, 187<br />
terebrans, Sphaeroma, 234, 235, 268<br />
ten, Eisothistos, 39<br />
testudinea, Thalassia, 251<br />
texana, Lironeca, 268<br />
texensis, Rhyscotus, 247, 249<br />
Thalassia, 166, 232<br />
testudinea, 251<br />
Thermarcturus, 252, 255<br />
venezuelensis, 255<br />
thomasi, Parabopyrella, 112<br />
floridanus, 110, 111<br />
manningi, 111<br />
f/Wn, Bopyrinella, 110<br />
tigrinus, Serranus, 173, 191, 193<br />
tortugae, Paguristes, 112<br />
tortuganus, Balanopleon, 110<br />
Tozeuma carolinense, 112<br />
transversa, Ceratothoa, 268<br />
transversus, Pachygrapsus, 111<br />
tricarina, Ptilanthura, 267<br />
tricarinata, Booralana, 275<br />
trichostoma, Haptolana, 138<br />
Clibanarius, 110, 112, 113<br />
Holacanthus, 172, 179<br />
tricornis, Excorallana, 161, 165, 266, 268<br />
occidentalis, 167<br />
tricornis, 161, 165<br />
Tridentella, 236<br />
virginiana, 236<br />
Tridentellidae, 115, 235, 236<br />
triospathiona, Gnathia, 238, 241<br />
tripunctata, Limnoria, 199<br />
/n/ow, Carpias, 82, 87<br />
Troglocirolana, 273<br />
tropicalis<br />
Erichsonella filiformis, 2bl<br />
Haplomesus, 263<br />
Lironeca, 268<br />
INDEX 307<br />
tuberculata, Limnoria, 194, 195, 199, 268<br />
tuberculatus, Chiton, 229<br />
tumidicauda, Paradella, 223, 226<br />
tumidipes, Parathelges, 112, 270<br />
Turbinaria, 166, 219<br />
7>/Wj 247, 250<br />
/
308 INDEX<br />
Virganthura, 64, 73<br />
crassa, 73<br />
virginalis, Gnathia, 238, 243<br />
virginiana, Tridentella, 236<br />
viridari, Alpheus, 112<br />
viride, Sparisoma, 122<br />
vittatus, Clibanarius, 110, 112<br />
vulgaris, Palaemonetes, 113<br />
waldneri, Renocila, 173, 191, 193<br />
walkeri, Sphaeroma, 234, 235<br />
warmingii, Excorallana, 161, 167<br />
wegeneri, Tylos, 247, 250<br />
wilsoni, Munnogonium, 96<br />
wolffi, Bopyrissa, 110<br />
wurdemanni, Lysmata, 112<br />
xanthurus, Leiostomus, 172, 183, 187, 190<br />
Xenanthura, 60<br />
brevitelson, 62, 267<br />
A>/