Lecture 23
The Homeotic genes
Introduction:
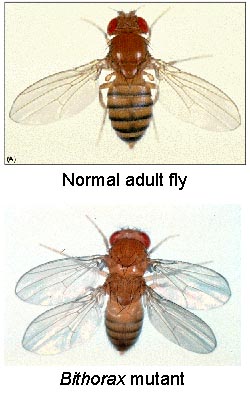
Some of the more fascinating mutant phenotypes in animals are those in which one body part is replaced by another. In 1894, William Bateson described these phenotypes as homeotic transformations. In 1915, Calvin Bridges isolated a spontaneous Drosophila mutant in which the haltere took on the identity of wing. This mutant was called bithorax as it is a duplication of a thoracic segment (see figure below). Subsequent genetic analyses in Drosophila determined that the genes controlling segmental identity along the anterior-posterior body axis were transcription factors. These genes were named the homeotic genes and it was determined that the fate of a given segment was selected by the expression of specific homeotic genes. The homeotic genes can be thought of as genetic switches that turn different programs of cellular differentiation on or off. The activities of a number of homeotic genes are required to establish the identity of parasegments in the embryo.

Photos of Ed Lewis
Homeotic genes have been found in all major animal phyla, and share three key characteristics:
1. They are organized in gene complexes. This means that related genes are found in close proximity on the chromosome, and suggests that the original genes were elaborated by duplication events.
2. There is a correlation between the 3' - 5' order of genes along the chromosome and the anterior-posterior location of gene products in the embryo. This phenomenon is known as temporal and spatial colinearity and is unique to homeotic genes. It is unknown why this occurs.
3. Homeotic genes contain a highly conserved 180bp sequence called the homeobox. The homeobox encodes for the homeodomain, a DNA-binding domain, confirming that the homeotic genes are regulatory proteins.
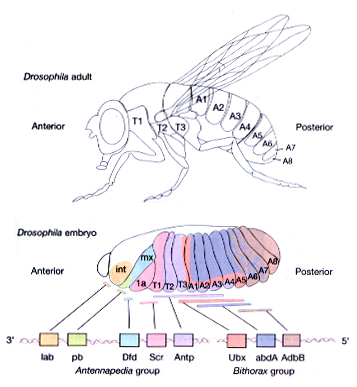
The Drosophila homeotic genes are clustered in two major groups called the Antennapedia complex (ANT-C) and the bithorax complex (BX-C). The genes of the ANT-C complex are expressed in the head and anterior thoracic regions and are thus responsible for the formation of head and thoracic structures. The genes of the BX-C are mainly expressed in the posterior thorax and abdominal regions and are thus responsible for the formation of posterior thoracic and abdominal segments.
One of the best studied examples of the homeotic genes is Antennapedia (Antp), which as the name suggests causes the transformation of antennal structures to the corresponding (homologous) leg structures.

The dominant phenotype of Antp mutants is a result of inappropriate expression of the normal Antp gene product. Antp is normally required in PS4 which corresponds to the posterior part of the first thoracic segment (T1p) and the anterior part of the second thoracic segment (T2a). If ANTP is expressed in the antenna (far anterior to PS4) transformation of antennal structures to leg structures occurs. This can result from a mutation that brings normal coding sequences under inappropriate control or by regulated mis-expression of a cDNA encoding the protein under heat shock control.
The Bithorax Complex:
Initial genetic experiments on the Bithorax complex (BX-C) suggested that there was one homeotic gene specifying the identity of each segment. However, molecular analysis of the BX-C indicated that it is composed of three genes, Ultrabithorax (Ubx), abdominal-A (abdA), and Abdominal-B (AbdB). The genes are large (they span approx. 300 kb) and have very complex regulatory regions. In fact, most of the initial putative "genes" are actually cis-acting regulatory elements that control the pattern of expression of these three genes.
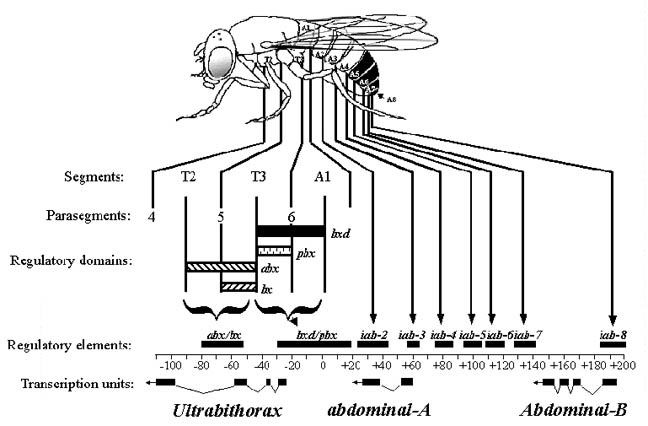
The regulatory regions of the BX-C are defined by a large number of mutations
affecting the expression of one or more of the genes in different regions of the
body. For example: anterobithorax (abx), bithorax (bx),
bithoraxoid (bxd) and postbithorax (pbx) are 4
classes of recessive mutations that all act on the Ubx transcription
unit. abx and bx are required for normal development of PS5.
bxd and pbx are required for the correct development of PS6.
As an example, in bx mutations there is a transformation of the anterior
part of the haltere to the anterior part of the wing. There is also a
transformation of the anterior part of the third leg such that it develops as
the anterior part of the second leg (T3a develops as T2a). pbx
mutations for example affect another set of structures and result in the
transformation of the posterior part of the haltere into the posterior part of
the wing and the posterior part of the third leg into the posterior part of the
second leg (T3p -> T2p). The same situation exists for abdA and AbdB.
Regulatory elements that affect the expression of these genes are spaced along
the DNA in the same order as the segments of the abdomen that they affect. These
elements are called infra-abdominal (iab) and are designated as
follows: iab-2 controls abdA in abdominal segment 2, iab-3
controls expression in abdominal segment 3 etc. The regulatory elements
found in the BX-C are complex, modular and will be discussed in the next
lecture.
Effects of Mutations in the Bithorax Complex:
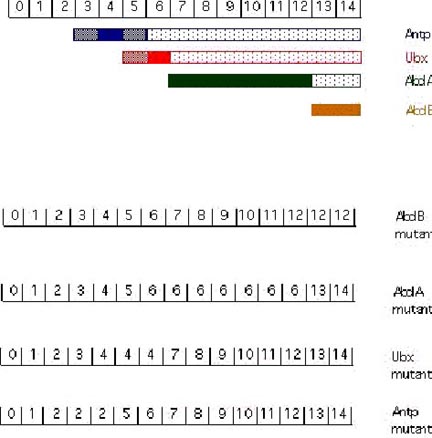
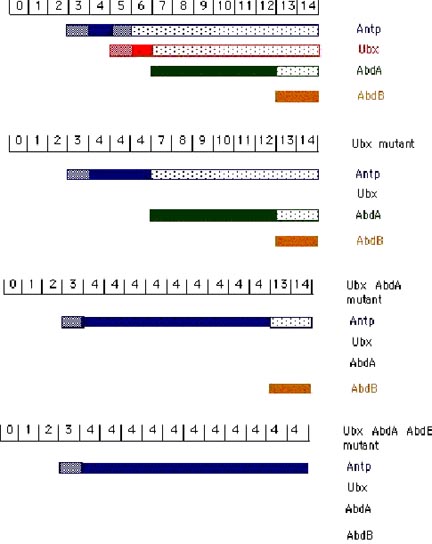
The cuticular pattern of larva is used to assign an identity to each parasegment in the developing fly larva. The numbers in the above exampled represent parasegments (PS). [From Akam, 1987]. In AbdB mutant embryos: PS 13 and PS 14 resemble PS7-12 (the abdA domain). In abdA mutant embryos: PS 7-12 resemble PS 6 (the Ubx domain). In Ubx mutant embryos PS 5 and 6 resemble PS 4 (the Antp domain). In Antp mutant embryos PS 3 and 4 resemble PS 2 (the Scr domain; Scr is an additional gene of the ANT-C).
Cross-regulatory interactions between the homeotic genes are important in defining the domains of expression. As shown above, the more posterior acting genes function as negative regulators of their more anterior neighbors. AbdB represses abdA, both repress Ubx, all three repress Antp. Removal of an anteriorly-acting homeotic gene has no effect on expression or phenotype in the domain of a more posteriorly acting homeotic gene.
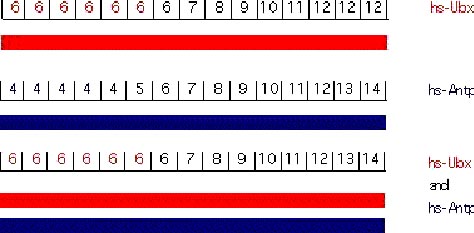
The function of a given homeotic gene is epistatic to its anterior neighbors (a similar phenomenon in vertebrates is called "posterior prevalence"). Under normal circumstances, the dominance of a posterior gene in repressing its anterior is obvious in molecular terms as the more posterior genes will repress the transcription of the more anterior genes. However, it is possible to force the expression of homeotic genes outside their normal domain of expression either using transgenes or mutations. The results from these experiments are quite surprising. Using a heat shock promoter it is possible to drive high levels of Ubx expression, hence UBX protein, throughout the embryo. In the abdA and AbdB domains there is no phenotypic consequence of Ubx overexpression. In more anterior regions, ectopic UBX has the expected consequence of transforming segments to PS6 identity. Similarly, ectopic Antp has no effect in the more posterior domains but can transform more anterior domains. The epistasis of Ubx over Antp can be strikingly demonstrated by experiments where both are ectopically expressed. Each alone transforms the anterior segments to its own character. When co-expressed, Ubx dominates and all structures anterior to the Ubx domain are transformed to PS6, despite the presence of high levels of Antp protein (Gonzalez-Reyes et al., 1990; Mann and Hogness, 1990). These results indicate that more posteriorly-acting genes are able to block not only the transcription of more anteriorly-acting genes but can also block their activity if both are present.
Hox
Gene Expression and Colinearity
Mann, R.S. 1997. Why are Hox
genes clustered? BioEssays
Whereas the colinearity that occurs between the homeotic complex and
anterior-posterior expression was first recognized by Ed Lewis in his genetic
experiments, it was formally demonstrated in mouse using in situ
hybridization.
Here,
members of the HoxB
cluster have different anterior boundaries of expression in the CNS and
prevertebra, which reflected their relative chromosomal organization. In
1989, Hox genes were given names according to the order in which they
were discovered. In 1997, we know that the anteriorly expressed genes
reside at the 3' end of the complex and that unlike in Drosophila (whose
homeotic genes are all expressed at the same time), the mouse homeotic complex
is also temporally colinear, meaning that as the body plan develops in an
anterior-posterior direction during gastrulation, there is a sequential
expression of the homeotic complex. We also know that the paralogue groups
are expressed with roughly the same anterior boundaries in segmented tissues and
that the next more posterior group is expressed one or two segments more
posterior.
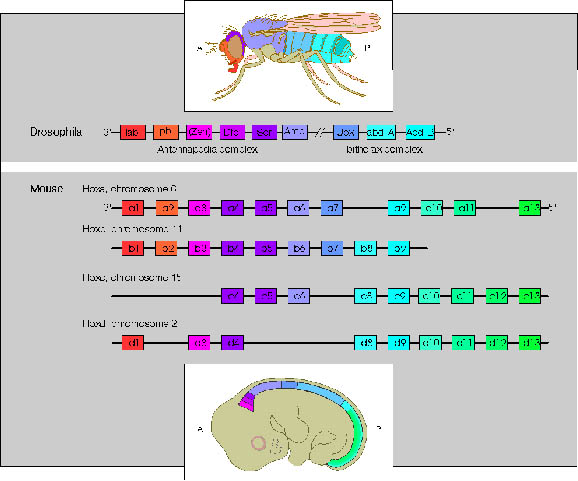
Comparison of Drosophila ANT-C and BX-C genes and the four human Hox complexes. Genes in different Hox complexes with the same number designation and colour are paralogues.
There are some interesting differences in the regulation between
vertebrate and Drosophila homeotic genes.
In
vertebrates, the main evidence of segmentation is in the vertebral column.
The head, the fore- and hindlimbs are later adaptations onto the early
vertebrate skeleton. The earliest expression studies focused on the
expression of Hox genes in the prevertebra of mid-gestation embryos.
In these experiments, it was realized that specific paralogue groups had
anterior boundaries within prevertebra and that each vertebra had its own Hox
address or code. Whereas the anterior boundaries are well defined, the posterior
boundaries of hox genes are not; suffice it to say that many more homeotic genes
are expressed within posterior segments than in anterior segments.