Monograph |
|
Corresponding author: Marek Borowiec ( mlborowiec@ucdavis.edu ) Academic editor: Brian Lee Fisher
© 2016 Marek Borowiec.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Borowiec ML (2016) Generic revision of the ant subfamily Dorylinae (Hymenoptera, Formicidae). ZooKeys 608: 1-280. https://doi.org/10.3897/zookeys.608.9427
|
Abstract
The generic classification of the ant subfamily Dorylinae is revised, with the aim of facilitating identification of easily-diagnosable monophyletic genera. The new classification is based on recent molecular phylogenetic evidence and a critical reappraisal of doryline morphology. New keys and diagnoses based on workers and males are provided, along with reviews of natural history and phylogenetic relationships, distribution maps, and a list of valid species for each lineage. Twenty-eight genera (27 extant and 1 extinct) are recognized within the subfamily, an increase from 20 in the previous classification scheme. Species classified in the polyphyletic Cerapachys and Sphinctomyrmex prior to this publication are here distributed among 9 and 3 different genera, respectively. Amyrmex and Asphinctanilloides are synonymized under Leptanilloides and the currently recognized subgenera are synonymized for Dorylus. No tribal classification is proposed for the subfamily, but several apparently monophyletic genus-groups are discussed. Valid generic names recognized here include: Acanthostichus (= Ctenopyga), Aenictogiton, Aenictus (= Paraenictus, Typhlatta), Cerapachys (= Ceratopachys), Cheliomyrmex, Chrysapace gen. rev., Cylindromyrmex (= Holcoponera, Hypocylindromyrmex, Metacylindromyrmex), Dorylus (= Alaopone syn. n., Anomma syn. n., Cosmaecetes, Dichthadia syn. n., Rhogmus syn. n., Shuckardia, Sphecomyrmex, Sphegomyrmex, Typhlopone syn. n.), Eburopone gen. n., Eciton (= Camptognatha, Holopone, Mayromyrmex), Eusphinctus gen. rev., Labidus (= Nycteresia, Pseudodichthadia), Leptanilloides (= Amyrmex syn. n., Asphinctanilloides syn. n.), Lioponera gen. rev. (= Neophyracaces syn. n., Phyracaces syn. n.), Lividopone, Neivamyrmex (= Acamatus, Woitkowskia), Neocerapachys gen. n., Nomamyrmex, Ooceraea gen. rev. (= Cysias syn. n.), Parasyscia gen. rev., †Procerapachys, Simopone, Sphinctomyrmex, Syscia gen. rev., Tanipone, Vicinopone, Yunodorylus gen. rev., Zasphinctus gen. rev. (= Aethiopopone syn. n., Nothosphinctus syn. n.).
Keywords
Taxonomy, systematics, morphology, dorylomorphs, doryline section, army ants
Preface
The ant subfamily Dorylinae is a monophyletic group of predatory ants, occurring throughout most of the tropical and subtropical regions of the world, with an appreciable number of species in warm temperate environments. The relatively few dorylines for which foraging biology is known usually prey on other ants or social insects, although notable exceptions occur and several of the charismatic ‘army ants’ evolved more generalized predatory habits. There are about 680 described species with an estimate of the total diversity being at least 1,000. The diversity of both habits and morphology within the subfamily is high and nesting can be subterranean or arboreal, with colony sizes ranging from a few dozen to millions of workers. These workers vary from having well-developed compound eyes to being entirely blind, having very short to very long slender appendages, and with the cuticle varying from coarsely sculptured to polished and shiny, with dull or conspicuous coloration.
Although numerous studies focusing on the biology of the few conspicuous species have been published, our overall knowledge of this clade is poor. A likely contributing factor is that many species are subterranean or occur at low abundances. It is also likely that comparative studies of doryline biology have been thwarted by poor taxonomic knowledge, lack of identification resources, and a classification that does not reflect evolutionary relationships.
The taxonomic limits of the Dorylinae have been in considerable flux since its establishment and, as currently circumscribed, the group has never received a focused treatment at the genus level. Until recently, our understanding of doryline morphology and phylogeny was insufficient to provide a stable classification based on easilydiagnosed monophyletic groupings. The aim of this study is to highlight the diversity of dorylines and provide a more natural genus-level classification, along with new identification resources. It is my hope that this effort will foster renewed interest in this highly diverse but neglected group of ants.
Material and methods
The taxonomic decisions of the present work reflect the evidence from examination of morphological characters in most doryline species and from recently published molecular phylogenetic research (
Specimens used in the course of this study come from the following institutions and individuals:
American Museum of Natural History, New York, USA.
Andreas Schulz personal collection, Leverkusen, Germany.
Bohart Museum of Entomology, Davis, California, USA.
California Academy of Sciences, San Francisco, California, USA.
John T. Longino personal collection, Salt Lake City, Utah, USA.
Los Angeles County Museum of Natural History, Los Angeles, California, USA.
Lund Zoological Museum, University of Lund, Lund, Sweden.
Marek L. Borowiec personal collection, Davis, California, USA.
Muséum d’Histoire Naturelle, Geneva, Switzerland.
Muséum National d’Histoire Naturelle, Paris, France.
Museum of Comparative Zoology, Cambridge, Massachusetts, USA.
Phil S. Ward personal collection, Davis, California, USA.
Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt am Main, Germany.
Smithsonian National Museum of Natural History, Washington, D.C., USA.
The Natural History Museum, London, UK.
Color photographs were prepared using a Leica MZ 16 stereomicroscope with a JVC digital video camera. All images were processed using Syncroscopy Automontage and Zerene Systems Zerene Stacker software and cleaned and adjusted using Adobe Photoshop. Wing venation images were prepared in Adobe Illustrator, based on wing automontage photographs.
Representative specimens imaged for each genus were assigned unique CASENT identifiers and their collection data is available on AntWeb (www.antweb.org). Along with the name and the original description reference, species lists give the country of type locality or verbatim type locality if the country could not be determined with confidence.
Distribution maps were collated from the published records and material examined by the author. For each genus every valid species and subspecies is listed, along with the country of its type locality; where the country could not be confidently identified, the type locality is listed verbatim from the original description.
Brief introduction to doryline ants
Below I provide an outline of doryline biology and diversity. More information on the natural history of each lineage can be found under individual genus accounts.
The 28 genera of the Dorylinae recognized here form a well-supported monophyletic group that is in turn a part of a more inclusive formicoid clade (
Dorylines occur on all continents except Antarctica but are the most prominent in tropical regions of the world. A few species range into the warm temperate zone, as far as the state of New Jersey in northeastern United States and western Turkey and the Dodecanese in the Mediterranean. In the southern hemisphere, they reach southern Australia and Tasmania, South Africa, and at least as far as Chubut province in southern Argentina.
Within the subfamily, a group of genera sometimes termed ‘the true army ants’ (or ‘AenEcDo’ army ants;
The true army ants currently account for the majority of doryline diversity. More than 45% of the described species of Dorylinae are classified in just two true army ant genera: Aenictus (Figure
The sexual dimorphism of the true army ants is remarkable, even compared to other ants, and has contributed to a complex early taxonomic history and the establishment of a ‘dual taxonomy’ in which descriptions of new species were often based on males unassociated with any females (see History of taxonomic and phylogenetic research below).
Aenictogiton, an African lineage now recognized as the sister group to Dorylus and thus in phylogenetic terms nested within the true army ant clade (
In addition to the true army ants, the subfamily Dorylinae comprises a variety of forms that prior to the study of
History of taxonomic and phylogenetic research
In much of the myrmecological literature, the true army ants and other ants classified here as dorylines (sometimes referred to as ‘non-army ant dorylines’; all other genera of the former subfamilies Cerapachyinae and Leptanilloidinae) have not been universally recognized as close relatives. As a result of this, the research on the taxonomy and phylogeny of these assemblages has followed largely separate paths. Because of this I keep these histories separate in the account that follows, concluding with a review of how phylogenetic considerations brought these groups together. The summary presented here focuses on the classification at the genus level and above. Remarks on species-level taxonomy can be found under the individual genus accounts.
The first taxon that would eventually be included in the Dorylinae is Dorylus helvolus, which was described from a male by Linnaeus in 1764 as Vespa helvola. The convoluted taxonomic history of this species and the true army ants in general has been vividly described by
The descriptive work on the true army ants continued with unfortunate proliferation of taxa described based on workers, males, or even gynes unassociated with other sexes or castes. This practice has been especially common in Aenictus and Dorylus but has impacted most true army ant lineages. The result of this approach is a ‘dual taxonomy’ with many forms known from only the worker caste or from males, but not both. In the genus Aenictus, for example, about 50 out of the 180 currently recognized species are known only from the male and no species is known from worker, gyne, and male.
Morphologically distinct lineages of the true army ants exist in the Old and New World and the two regions have not shared a genus-level taxon since at least 1840 (
At the genus level, the current classification of the New World army ants was firmly established by Borgmeier in his monographic studies on these taxa (
The taxonomic history of non-army ant dorylines began with Alfred Russell Wallace collecting a worker specimen later described by Fredrick Smith of the British Museum (
The general trend in genus-level taxonomy can be summarized as a progressive addition of new names coupled with increasing number of Cerapachys synonyms. This lumping of names with Cerapachys began with Emery (
In 1923 a genus of distinctive, minute and blind ants, Leptanilloides, was first described (
The study of doryline phylogeny and evolution is intertwined with taxonomic considerations, although a summary somewhat independent from the above account, which focuses primarily on the nomenclature, is possible. The work on relationships within the true army ants centers on the controversy of whether they evolved once (the monophyly hypothesis) or more times independently (the polyphyly hypotheses;
Early views on the relationship of the ‘cerapachyines’ to the true army ants were conflicting; these opposing perspectives were succinctly summarized by William Morton
Modern research on the phylogeny of the Dorylinae began with Bolton’s two influential works, as already signaled above (
Current views on doryline classification and evolution
The current limits of the subfamily were not established until the molecular study of Brady et al. published in 2014. As explained above, prior to that, the genera of this group had been classified in as many as six subfamilies. The affinities of those subfamilies had not always been recognized, but a close relationship has since been convincingly demonstrated through a series of independent morphological (
The above mentioned study of Brady and colleagues (
Recent advances in DNA sequencing techniques provide orders of magnitude more data than has been used in phylogeny reconstruction in previous studies (
Phylogenetic relationships among the genera of Dorylinae based on
Morphological characters uniting morphologically disparate genera in clade (1) are not obvious, although all these ants currently occur in the Indomalayan region (at least one Chrysapace is known from Eocene Baltic amber). The members of the three genera in clade (2) are mostly soil- and leaf litter-dwelling species and are characterized by small or entirely absent eyes in the worker and reduced antennal segment count in both females (from 12 to 11 or 9) and males (from 13 to 12 or 11). This antennal count reduction is universal across species of this group, although independent reductions occurred elsewhere in the Dorylinae. Clade (3) is well-defined and phylogenetic relationships within it are also very well-supported. The members of this group share the loss of the forewing costal vein, a trait which is present in many other dorylines. Genomic data also strongly suggest a ‘New World Clade’ (4). Within that large clade, the termite hunters Acanthostichus and Cylindromyrmex form are sister genera, and New World army ants (equivalent of the former Ecitoninae) form another well-supported monophyletic group. It is unclear which genus is sister to New World army ants but the data suggest either Leptanilloides or Sphinctomyrmex. Morphological synapomorphies for both the large New World clade and the clade uniting New World army ants with Leptanilloides and Sphinctomyrmex remain elusive. The New World army ants, or Eciton genus-group, which by themselves undoubtedly form a clade, however, possess a highly derived morphology and are well-differentiated from other dorylines. Many of the characters found in New World army ants appear to be independently derived in the Old World army ants clade (clade 5), which comprises Aenictogiton, Aenictus, and Dorylus. Within that group Aenictogiton is sister to Dorylus, forming a clade from which Aenictus apparently diverged a long time ago (Borowiec, in prep.).
A pattern emerges when combining morphological scrutiny of doryline ants with the molecular phylogenetic results: most groupings for which a strong signal of monophyly was recovered in the molecular phylogenies are also easily diagnosed using morphology. This is especially true for all the genera recognized here, but less so for most above-genus groupings. The present revision builds upon this fact and resurrects a number of names hitherto treated as synonyms of Cerapachys or Sphinctomyrmex to better reflect evolutionary relationships. It also introduces two new generic names for taxa previously placed in Cerapachys, and it synonymizes others. The total number of recognized doryline genera is raised from 19 (
It is worth stating here that the division of these taxa into multiple genera is not motivated solely by the need for monophyletic groupings in modern classifications, but also by the fact that the species hitherto classified under Cerapachys exhibit unusual and confusing variation in morphological characters that have traditionally been used to delimit genera in other ant groups. These characters include the number of palpal segments, number and development of tibial spurs, the development of abdominal segment III (postpetiole), as well as other features. I believe that the revised classification not only better reflects the phylogeny, but also targets for assignment of names those clades that are most morphologically distinct. Reorganization of the ‘Cerapachys’ diversity into more manageable taxa will hopefully stimulate future species-level revisions and permit easy integration of newly discovered forms into this new framework.
Classification of the Dorylinae proposed here
The classification proposed here is outlined below. The general distribution and the number of valid described species (excluding subspecies) are given in parentheses. Biogeographic divisions follow those outlined by
Dorylinae Leach, 1815 (worldwide, 27 extant genera and 1 extinct genus, 685 described extant and 8 extinct species)
= Acanthostichini Emery, 1901a
= Aenictinae Emery, 1901a
= Aenictogitoninae Ashmead, 1905
= Cerapachyinae Forel, 1893a
= Cheliomyrmecini Wheeler, W. M., 1921
= Cylindromyrmecini Emery, 1901a
= Ecitoninae Forel, 1893a
= Eusphinctinae Clark, 1951
= Leptanilloidinae Baroni Urbani, Bolton & Ward, 1992
= Lioponerini Ashmead, 1905
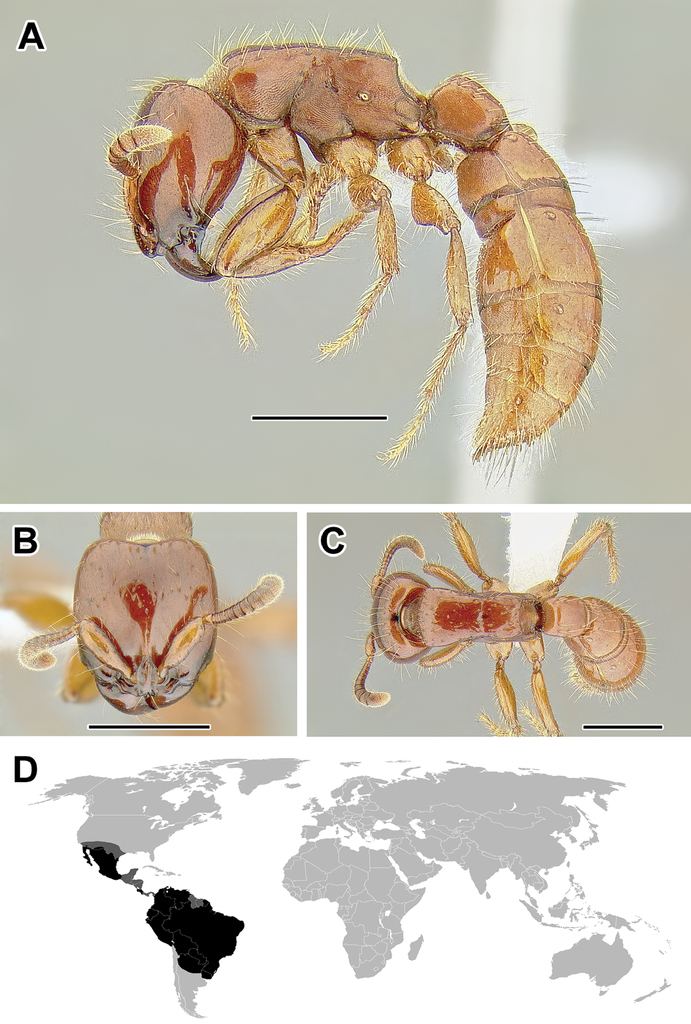
Acanthostichus Mayr, 1887 (Nearctic, Neotropical, and Dominican amber, 23 extant and1 fossil species)
= Ctenopyga Ashmead, 1906
Aenictogiton Emery, 1901b (Afrotropical, 7 extant species)
Aenictus Shuckard, 1840b (Palearctic, Afrotropical, Indomalayan, and Australasian, 184 extant species)
= Paraenictus Wheeler, W. M., 1929
= Typhlatta Smith, 1857
Cerapachys Smith, F., 1857 (Indomalayan, 5 extant species)
= Ceratopachys Schulz, 1906
Cheliomyrmex Mayr, 1870 (Neotropical, 4 extant species)
Chrysapace Crawley, 1924a, gen. rev. (Malagasy, Indomalayan, and Baltic amber, 3 extant and 1 undescribed fossil species)
Cylindromyrmex Mayr, 1870 (Neotropical and Dominican amber, 10 extant and 3 fossil species)
= Holcoponera Cameron, 1891
= Hypocylindromyrmex Wheeler, W. M., 1924a
= Metacylindromyrmex Wheeler, W. M., 1924a
Dorylus Fabricius, 1793 (Palearctic, Afrotropical, and Indomalayan, 60 extant species)
= Alaopone Emery, 1881, syn. n.
= Anomma Shuckard, 1840c, syn. n.
= Cosmaecetes Spinola, 1851
= Dichthadia Gerstäcker, 1863, syn. n.
= Rhogmus Shuckard, 1840c, syn. n.
= Shuckardia Emery, 1895b
= Sphecomyrmex Schulz, 1906
= Sphegomyrmex Imhoff, 1852
= Typhlopone Westwood, 1839, syn. n.
Eburopone Borowiec, gen. n. (Afrotropical and Malagasy, 1 extant species)
Eciton Latreille, 1804 (Neotropical, 12 extant species)
= Camptognatha Grey, 1832
= Holopone Santschi, 1925
= Mayromyrmex Ashmead, 1905
Eusphinctus Emery, 1893a, gen. rev. (Indomalayan, 2 extant species)
Labidus Jurine, 1807 (Nearctic and Neotropical, 7 extant species)
= Nycteresia Roger, 1861
= Pseudodichthadia André, 1885
Leptanilloides Mann, 1923 (Nearctic and Neotropical, 19 extant species)
= Amyrmex Kusnezov, 1953, syn. n.
= Asphinctanilloides Brandão, Diniz, Agosti & Delabie, 1999, syn. n.
Lioponera Mayr, 1879, gen. rev. (Palearctic, Afrotropical, Malagasy, Indomalayan, and Australasian, 73 extant species)
= Neophyracaces Clark, 1941, syn. n.
= Phyracaces Emery, 1902, syn. n.
Lividopone Fisher and Bolton, 2016 (Malagasy, 1 extant species)
Neivamyrmex Borgmeier, 1940 (Nearctic, Neotropical, and Dominican amber, 127 extant and 1 fossil species)
= Acamatus Emery, 1894
= Woitkowskia Enzmann, 1952
Neocerapachys Borowiec, gen. n. (Neotropical, 2 extant species)
Nomamyrmex Borgmeier, 1936 (Nearctic and Neotropical, 2 extant species)
Ooceraea Roger, 1862, gen. rev. (Pantropical; native in Indomalayan and Australasian, 11 extant species)
= Cysias Emery, 1902, syn. n.
Parasyscia Emery, 1882, gen. rev. (Palearctic, Afrotropical, Malagasy, Indomalayan, and Australasian, 50 extant species)
†Procerapachys Wheeler, W. M., 1915b (Baltic amber, 3 fossil species)
Simopone Forel, 1891 (Afrotropical, Malagasy, Indomalayan, and Australasian, 39 extant species)
Sphinctomyrmex Mayr, 1866b (Neotropical, 3 extant species)
Syscia Roger, 1861, gen. rev. (Nearctic, Neotropical, and Indomalayan, 5 extant species)
Tanipone Bolton & Fisher, 2012 (Malagasy, 10 extant species)
Vicinopone Bolton and Fisher, 2012 (Afrotropical, 1 extant species)
Yunodorylus Xu, 2000b, gen. rev. (Indomalayan, 4 extant species)
Zasphinctus Wheeler, W. M., 1918, gen. rev. (Afrotropical and Australasian, 20 extant species)
= Aethiopopone Santschi, 1930, syn. n.
= Nothosphinctus Wheeler, W. M., 1918, syn. n.
Morphology
The Dorylinae possess a number of characteristic morphological traits, which are discussed in detail below. A
An illustration of morphological characters used in this revision is presented in Figures
Doryline ants are characterized by their predation on other social insects, a condition that appears to be apomorphic for the group, but there is also a plethora of morphological characters that distinguish them from other ant lineages. Extensive work grounded in examination of morphology has been done to infer ant phylogeny (
Diagnostic characters of the worker
1. Lateral area of clypeus very narrow in full face view; distance from anterior clypeal margin to paraoculoclypeal sulcus greater than distance from anterior clypeal margin to frontoclypeal sulcus where antennae insert.
The clypeal area of the head capsule (Figure
A relatively narrow clypeus is present in several ant subfamilies, but the condition described above appears to be restricted to the Dorylinae, Martialis, Leptanillinae, Amblyoponinae, and Proceratiinae. I suspect that a thorough study of the clypeal area in the dorylines may reveal new characters of diagnostic or phylogenetic utility within the subfamily.
2. Parafrontal ridges present, i.e. genae carinate laterally of antennal sockets.
Another feature characteristic and likely synapomorphic for the Dorylinae is the presence of often prominent ridges extending some distance back from the paraoculoclypeal sulcus (when visible), laterally to the antennal socket (Figure
A–C Worker of Acanthostichus cf. serratulus (CASENT0732109) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Acanthostichus (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
3. Torulo-posttorular complex present, often vertical, occasionally horizontal; antennal sockets exposed or (more rarely) partially concealed by torulo-posttorular complex in full-face view.
The vertical configuration of the torulo-posttorular complex is characteristic and probably synapomorphic to the Dorylinae. A similar morphology is present in some proceratiines, in particular in Probolomyrmex. Similar configuration exists in the Leptanillinae and in Apomyrma (Amblyoponinae). A horizontally expanded torulo-posttorular complex is also found in the Amblyoponinae (Myopopone).
4. Stipes of maxilla sharply divided into proximal and distal faces, with proximal face extending beyond inner margin of stipes; prementum concealed when mouthparts closed.
The major sclerite of the maxilla, the stipes, is in dorylines sharply divided into a raised proximal face and sunken distal face on its outer surface (
A similar condition has apparently independently evolved in some Amblyoponinae where the prementum is also concealed (
5. Eyes frequently reduced or absent.
Eyes are poorly developed in most species of the doryline workers, although large and multifaceted eyes are present in a number of lineages. A few speciose lineages lack the eyes completely and without exception in any of the species (e.g. Aenictus, Dorylus), while many other genera are either blind in most species or with very small eyes present (e.g. Acanthostichus, Eciton, Neivamyrmex, Syscia, Ooceraea). Large worker eyes can be present in some or all species of Cerapachys, Chrysapace, Cylindromyrmex, Lioponera, Lividopone, Simopone, Tanipone, and Vicinopone. Many of the species with large eyes are arboreal, but others are surface-foragers or their natural history is unknown.
6. Mesosoma internally with fused meso- and metafurcal arms, externally corresponding to an endophragmal pit.
The mesosoma of the Dorylinae worker ants possess a pit in the cuticle, located anteriorly to the propodeal spiracle and near mesometapleural Pronotomesopleural suture (where present; Figures
7. Metapleural gland orifice concealed beneath a ventrally directed cuticular flap or flange.
The metapleural gland is a feature found exclusively in ants and is believed to aid in colony sanitation (
I suspect that a careful study focused on the structures surrounding metapleural gland orifice would reveal additional genus-level diagnostic characters in the Dorylinae.
8. Helcium sternite bulging ventrally and articulated on the inner wall of the tergite.
The helcium is a term used for the presclerites of abdominal segment III. The relative development and place of articulation of the helcial sternite varies in ants. The dorylines exhibit a rare condition where the sternite is well developed and bulging ventrally, not obscured by the tergite in lateral view. The sternite is also articulated to the tergite some distance up on the inner wall of the latter, so that the tergite overlaps the sternite. A ventrally bulging helcial sternite appears to occur only in some Proceratiinae, Tatuidris, and in the Myrmicinae. In the myrmicine ants, however, the articulation of the sternite and the tergite is along the lateral margins, and thus the sclerites are not overlapping. In all other ants the helcial sternite is flat or only slightly convex, not readily visible in lateral view.
9. Abdominal segment III with complete tergosternal fusion.
The degree of fusion of sternites to the tergites of abdominal segments varies in ants (
10. Abdominal segment IV without tergosternal fusion.
Complete fusion of tergites and sternites of abdominal segment IV is rare in ants and apparently restricted to Agroecomyrmecinae, Ponerinae, and Proceratiinae. In other subfamilies the sclerites are unfused or only presclerites exhibit fusion (
11. Spiracles of abdominal segments V–VII shifted posteriorly on each segment, not concealed by the posterior margin of the preceding tergite and visible without distension or dissection.
This is a character that is a likely synapomorphy of the subfamily and does not appear to occur in any other ants. In most ants the spiracles of abdominal segments I (the propodeum) through IV are visible, but those of abdominal segments V, VI, and VII are ordinarily concealed by the posttergites of their respective preceding segments. These spiracles cannot be thus seen without distension or dissection of the gaster. In the dorylines, however, the spiracles are shifted posteriorly on the posttergites and visible in specimens without any manipulation (Figures
12. Pygidium modified: either large and with dorsum flattened and armed with teeth or spines, or reduced to a narrow V-shaped sclerite.
In general, the pygidium (last visible abdominal tergite) is derived in the dorylines, departing from a condition of a large, evenly rounded sclerite that is presumed to be plesiomorphic for ants. The degree and nature of the modification varies, however. In many genera previously classified under the Cerapachyinae the pygidium has a flattened medial area and is armed with thick, specialized setae that are thought to have sensory function (
13. Sting apparatus with furcula fused to base of sting or absent in most species.
The furcula is a Y- or wishbone-shaped sclerite flexibly attached at the base of the sting (
14. Metacoxal cavities fully closed, without a Pronotomesopleural suture in the broad annulus.
The morphology of the cuticle surrounding the sockets where hind coxae articulate (the coxal cavities) varies among ants. The primitive condition is presumably one where the cavities are not fully surrounded by the cuticle (the annulus) and the cavities are connected to the petiolar foramen. This condition is present in Myrmeciinae, Aneuretinae, Platythyrea in the Ponerinae, and Ectatomminae. A modification of this state occurs where the annulus surrounds the cavities, so that the cuticle is continuous around the openings, although a Pronotomesopleural suture can be discerned where the outgrowths of the cuticle closing the foramen meet. This state is present in some Amblyoponinae, most Ponerinae, some Heteroponerinae, Paraponera, and some Proceratiinae. Finally, the cuticle can be entirely fused and no Pronotomesopleural suture is visible in the cuticle surrounding coxal cavities. This is the condition observed in the Dorylinae. This character state is common among the subfamilies of the formicoid clade, including Dolichoderinae, Formicinae, Myrmicinae, Pseudomyrmecinae, and some Heteroponerinae (
15. Metatibial gland present, located distally on the ventral surface of hind tibia.
Multiple ant lineages have been found to possess a glandular structure on the ventral (flexor) surface of their hind tibiae (
Glands on the hind tibiae also occur in the Ponerinae (
As mentioned above, several Dorylinae genera are lacking externally visible metatibial glands and there has been no comprehensive histological study of all the lineages. Given this, coupled with unresolved relationships among most lineages of the subfamily, it is somewhat uncertain whether this character is a synapomorphy of the whole clade or if it is primitively absent from early-branching lineages.
Despite its presence in some of the better-studied species (true army ants, O. biroi), the function of the gland in the Dorylinae is unknown (
The worker Dorylinae can be thus easily recognized through a combination of metapleural gland orifice concealed by a dorsal cuticular flap, large and convex sternite of the helcium, and exposed abdominal spiracles of segments V–VII.
Diagnostic characters of the male
1. Abdominal sternite IX (hypopygium) modified, bidentate to biaculeate.
The appearance of the abdominal sternite IX of the male is distinctive in the Dorylinae. A simple sclerite with convex or medially tapered posterior margin appears to be the plesiomorphic condition, present in most ants. In the dorylines the hypopygium often has a convex posterior margin and is laterally drawn into two processes, ranging from blunt triangular denticles to long, parallel prongs. Occasionally further modifications, including folds, excisions, and additional teeth are also present on the hypopygium. In some lineages the male hypopygium is useful for species identification. Leptanilloides is again the exception, and the sclerite in this lineage is relatively simple, sometimes medially convex or concave. Outside of the Dorylinae, a biaculeate male hypopygium is present in at least two genera, Paraponera and Nothomyrmecia.
2. Cerci absent from male genitalia.
The cerci (also called pygostyles) are paired sensory structures articulating with the last abdominal tergite. Most male ants have cerci but their loss has occurred several times independently, in Leptanillinae, Martialis, some Amblyoponinae, and some Proceratiinae (
3. Genitalia completely retractile.
The doryline males are able to retract their genital capsule into the abdomen. In other ant taxa, even those that also lack cerci, the genitalia cannot be completely retracted. The genitalia of true army ants are always well-concealed in dead specimens and not visible without dissection. The genitalia of other, particularly smaller dorylines, however, can be partially visible in dead males. The small-sized males of Leptanilloides may be an exception among the dorylines, as the known specimens have exerted genitalia, with most of the genital capsule visible without artificial distension or dissection of the abdomen. The abdomen of some Leptanilloides species also appears too small to allow for full retraction of the genital capsule.
4. Jugal lobe absent from hindwing.
The jugal lobe is a basally located projection of the wing membrane.
The male Dorylinae can be thus recognized by the lack cerci, almost universally bispinose hypopygium, and retractable genital capsule. The last two characters do not apply to Leptanilloides, but the cerci are lacking in this genus, too. Leptanilloides also has extremely reduced tegulae or is lacking them entirely, a loss perhaps unique among male ants.
Diagnostic characters of the gyne
Doryline gynes share many worker characteristics and can be recognized by the same putative synapomorphies as the worker, except perhaps for the highly specialized ‘dichthadiigyne’ queens of the true army ants. The latter may be difficult to distinguish from convergently evolved specialized queens of Leptanilla (Leptanillinae;
Characters used to describe worker morphology
Number of antennal segments. The ant antenna includes only three ‘true’ segments (scape, pedicel, and funiculus), that is metameric structures connected to other segments via muscles, with funiculus further subdivided into secondary structures. Together, the scape, pedicel, and funicular segments are thus sometimes referred to as antennomeres. In the taxonomic literature concerning ants, however, use of ‘antennal segments’ instead of ‘antennomeres’ is widespread and I follow this convention here. In the Dorylinae, the plesiomorphic condition is 12-segmented antennae (Figure
Relative size of the apical antennal segment. The size of the last (apical or terminal) antennal segment varies widely within the Dorylinae, both in length and width relative to other segments. The apical segment ranges from small in Simopone (Figure
Cuticular apron of the clypeus. Many species in various genera possess a semi-translucent to opaque cuticular projection, or lamella, that arises from the clypeus and closes the gap between mandibles and the head capsule. This trait varies among and within genera.
Lateroclypeal teeth. Many dorylines possess cuticular projections that are arising from lateral portions of the clypeus and overhang mandibles (Figure
Parafrontal ridges. As discussed above under worker diagnostic character 2, this is one of distinguishing characters of the Dorylinae worker. A few lineages such as Acanthostichus and Dorylus seem to lack this trait completely, although reductions of various degree occurred in several genera (see also above under Diagnostic characters of the worker).
Torulo-posttorular complex. Another defining feature of the Dorylinae, this character is discussed above under worker diagnostic character 3.
Antennal scrobes. Depressions of the cuticle that receive retracted antennal scapes are uncommon in the Dorylinae, well developed only in Cylindromyrmex and some species of Simopone. Although not considered scrobes here, feebly marked depressions that apparently receive antennal scape can be seen in certain species of Parasyscia and Lividopone.
Labrum shape. Most dorylines have a labrum that is notched medially on its distal (non-articulated) margin, although in a few (Aenictogiton, some Dorylus, Leptanilloides) the margin is evenly rounded across.
Proximal face of stipes. The stipites concealing the prementum are another trait of the subfamily that is discussed above under worker diagnostic character 4.
Number of maxillary palp segments. This character varies from the plesiomorphic number of six segments in Tanipone and most Simopone to only one segment in Aenictogiton. Although the palpal segment count is apparently constant throughout some genera, its reduction appears to often correlate with small size. As such, and because this character is often impossible to see without dissection, it is of limited utility for genus-level identification.
Number of labial palp segments. The discussion regarding maxillary palp segmentation applies to labial palps as well. The labial palps are never composed of more than four segments and as a rule have fewer segments than maxillary palps of the same individual. Exceptions to this rule are the New World army ants and Acanthostichus, where the labial palps are longer than maxillary palps, with three and two segments, respectively. In most Dorylus both maxillary palps are 2- or 1-segmented and labial palps are 2-segmented but the labial palps are more slender and longer than the maxillary palps. Taken together, the number of maxillary and labial palp segments is sometimes expressed as ‘palp formula’ which simply gives the two numbers separated by a comma. For example, palp formula 4,3 means the maxillary palps are 4-segmented and labial palps are 3-segmented.
Mandible shape and dentition. Shape of the mandibles varies across the Dorylinae, with many species retaining plesiomorphic triangular mandibles with well-differentiated basal and masticatory margins and numerous denticles of uniform shape on the latter (Figure
Eye size. In general, eyes are small or absent in dorylines, although exceptions do occur, as discussed under worker diagnostic character 5 above.
Presence of ocelli. The ocelli are rare in worker dorylines, although Chrysapace, Simopone (Figure
Head capsule above occipital foramen. Dorylines vary in the degree of differentiation between dorsal (or frontal) and posterior (or occipital) faces of the head. Most species have a distinct posterior surface of the head just anterior to the attachment with the mesosoma. In Simopone and Vicinopone (Figure
Carina on ventrolateral surface of head. Ventrally on the head of most dorylines, a carina surrounding occipital foramen can be found (see below). Additionally, in some species there may be additional ridges that originate at the lateral corners of that ventral occipital carina and run partways or down the length of ventral head surface towards mandibular insertions (Figure
Carina surrounding occipital foramen. Many dorylines possess a carina around the occipital foramen. In some species, this carina joins ventrally to separate the area immediately anterior to the occipital foramen from the rest of the head capsule. This character is variable within and among genera.
Pronotal flange delimited by a ridge. The sloping surface of the mesosoma immediately behind the occipital articulation is known as the pronotal flange. This area can be evenly rounding into the pronotal dorsum, also called the pronotal neck, or separated from it by a variously developed cuticular margin. This feature can be consistently present within certain genera like in Cerapachys or Eburopone (Figure
Promesonotal connection. The connection between the first mesosomal notum, the pronotum, and the rest of the mesosoma is variously developed in ants. The pronotum is dorsally adjacent to the mesonotum and laterally to the mesopleuron. The connection can be fully articulated as in most Formicinae where a well-developed Pronotomesopleural suture is present, or rigidly fused as in the Myrmicinae where the entire mesosoma forms a single rigid block and usually there is no trace of Pronotomesopleural suture dorsally. Among the dorylines both of the above mentioned conditions can be found, along with intergradations. A completely unfused and mobile connection is present only in certain Leptanilloides, while in Dorylus, for example, a conspicuous Pronotomesopleural suture is present but the connection is not mobile. In others, only lateral portions of the Pronotomesopleural suture are unfused (see next character below) or, as is the case in Parasyscia, the connection is fully fused with no trace of Pronotomesopleural suture.
Pronotomesopleural suture. In the Dorylinae, the promesonotum and mesopleuron are often linked by a Pronotomesopleural suture (sometimes termed the 'promesopleural' Pronotomesopleural suture). This character is linked to the preceding one but it is treated separately because in many genera the Pronotomesopleural suture may be completely fused dorsally, at the same time being unfused laterally on the mesosoma (Figure
Mesometapleural groove. Directly posterior to the pronotomesopleural Pronotomesopleural suture (or the area where the Pronotomesopleural suture would be found) is the mesopleuron. This area can be delimited posteriorly from the succeeding sclerite, the metapleuron, by a groove (Figure
Transverse groove dividing mesopleuron. The mesopleuron can be undivided or separated into two parts by a Pronotomesopleural suture or a cuticular ridge (Figure
Concavity surrounding pleural endophragmal pit. As explained in the discussion of the internal mesosomal structure above, an endophragmal pit is an impression in the cuticle that corresponds to invaginations of the cuticle. An examination of the pit itself often requires scanning electron microscope but the pit can be surrounded by a variously pronounced concavity of the cuticle, making it more easily discernable. When visible, the concavity is usually placed some distance anterior to and/or below the propodeal spiracle.
Margination of various body segments. This category encompasses characters that include dorsolateral margination of the mesosoma, petiole, or other segments. Several genera have some form of margination at the junction of the lateral and dorsal faces of the mesosoma or above the petiolar spiracle. The most characteristic margination of dorsolateral corners of the body is present in Lioponera, where it can range from being confined to the anterior half of abdominal segment II (petiole) to well-defined margins present across the posterior half of the head, most of mesosoma, posteriorly to abdominal segment IV (Figure
Metanotal depression or groove on mesosoma. Dorsally the mesosoma may possess a groove or depression marking the division between the thorax and the propodeum (which is anatomically the first abdominal segment). Most dorylines have no such groove, but in Aenictus and New World army ants this distinction is usually pronounced (Figures
Propodeal spiracle position. The position of propodeal spiracle on the lateral wall of the mesosoma can serve as a good character distinguishing army ants from other dorylines. When inspected in lateral view, the spiracle opening is almost always at or below the midheight of the mesosoma in the genera that are not considered army ants (Figure
Shape and margination of propodeal declivity. The propodeum has a sloping or vertical posterior face that can be variously shaped and dorsally immarginate or with a distinct margin, bound by a carina. The most common shape of the doryline propodeum when viewed from behind is approximately rectangular. However, in Aenictus a more triangular shape is common. The dorsal margination appears to be more common in certain genera then in others, although there is often variability within genus.
Metapleural gland bulla. The metapleural gland is positioned at the posterior end of the metapleuron, on the lateral mesosoma directly above where the hind coxa articulates. The gland opens through the cuticle and has a chamber, or bulla, situated below the opening. The metapleural gland orifice is further discussed above under diagnostic characteristics. In the descriptions I indicate whether the gland bulla is visible through (Figure
Propodeal lobes. Propodeal lobes are projections of the cuticle on the propodeum, arising immediately lateral to the propodeal foramen, the opening in the mesosoma where abdominal segment II (petiole) articulates. These lobes are variously developed in the Dorylinae. They are completely absent in Aenictogiton, Dorylus, and Leptanilloides and absent or short in the Eciton genus-group. In other genera the lobes are well-developed and visible laterally as semicircular extensions of the propodeum projecting past the metapleural gland bulla (Figure
Position of helcium. Two major portions can be distinguished in each abdominal segment starting with segment II (the petiole; the propodeum corresponds to segment I): presclerites and postsclerites. Presclerites form the portion that articulates with the preceding segment. Postsclerites constitute the part of the segment that is always exposed without dissection or extension of gastral sclerites. The helcium comprises the presclerites of abdominal segment III, that is its anterior portion that articulates with the petiole. The helcium can be positioned at or above the tergosternal Pronotomesopleural suture of segment III. The position of helcium can also be described relative to the midheight of postsclerites of abdominal segment III in lateral view. Axial helcium means positioned at the midheight, infraaxial below, and supraaxial above. The helcium can thus be positioned at the tergosternal Pronotomesopleural suture and at the same time be considered infraaxial if the Pronotomesopleural suture occurs below the midheight of the segment. In most dorylines the helcium is placed at the Pronotomesopleural suture and more or less axially. The most obvious departure from this state is when the helcium is in a supraaxial position and above the Pronotomesopleural suture (Figure
Prora. Prora is used to describe a protrusion on the anterior face of abdominal poststernite III, below the helcium. In the dorylines this feature is variously developed, as a simple angle not delimited by carinae (Figure
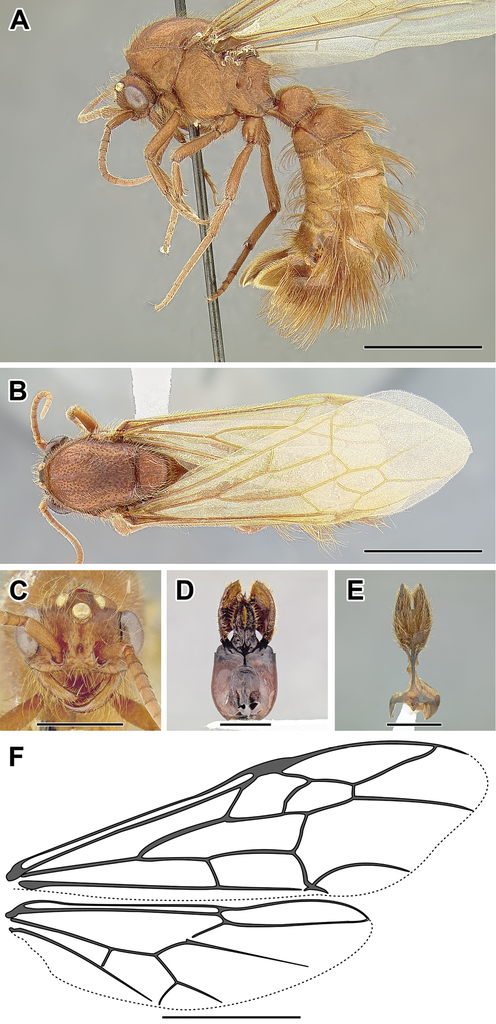
A–F Male of Acanthostichus sp. (CASENT0731087) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm.
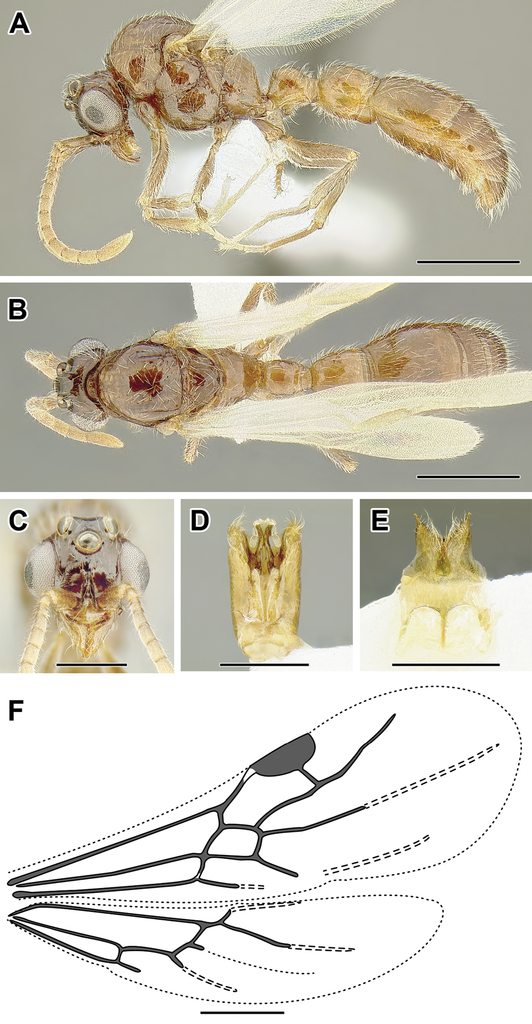
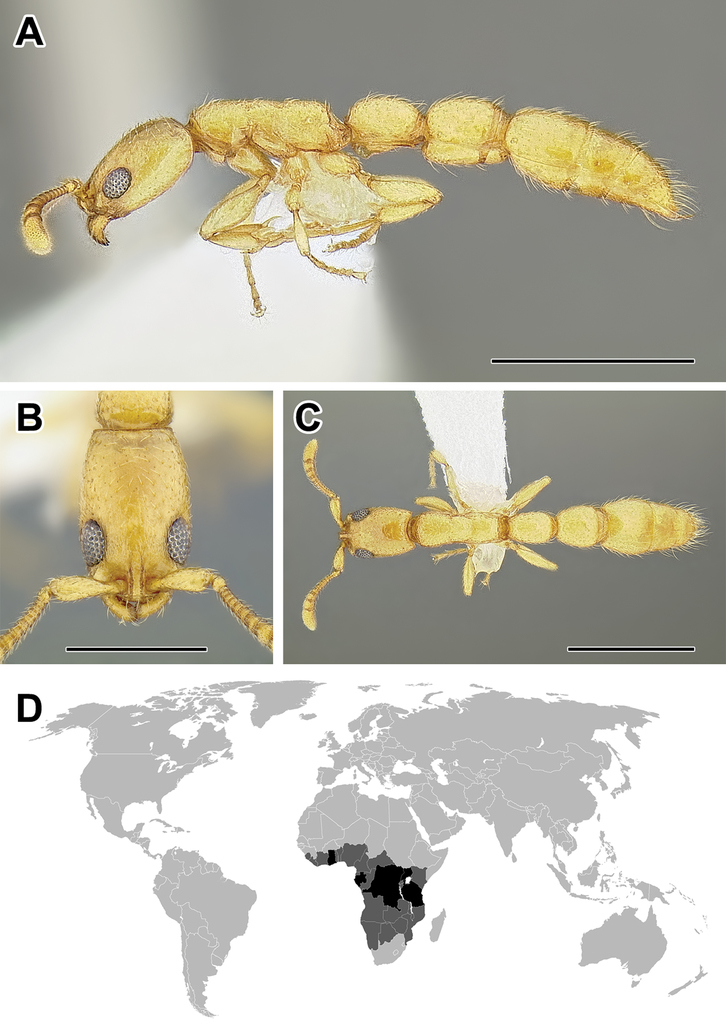
A–C Worker of Aenictogiton sp. (CASENT0317577) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Aenictogiton (black: present, dark grey: likely present). Scale bar equals 0.5 mm
Spiracle openings of abdominal segments IV–VI. Spiracles are visible on the gaster in the Dorylinae without dissection and their orifices can be variously shaped, from round to narrow and slit-shaped. Slit-shaped openings are rare and are found only in Eciton, Nomamyrmex, and some Neivamyrmex. Sometimes the spiracle opening on segment IV is more round than those of succeeding segments.
Girdling constriction of segment IV. The boundary between pre- and postsclerites of segment IV can be inconspicuous (Figure
Sculpturing of cinctus of abdominal segment IV. When the girdling constriction between pre- and postsclerites is present, it can take different forms. It can be a simple dip or a defined trench or gutter-like concavity. The constriction can also be smooth or sculptured, most often cross-ribbed with short lines (Figure
Relative size of abdominal segment IV. This abdominal segment can form the bulk of the metasoma in certain species (Figure
A–F Male of Aenictogiton sp. (CASENT0731199) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm.
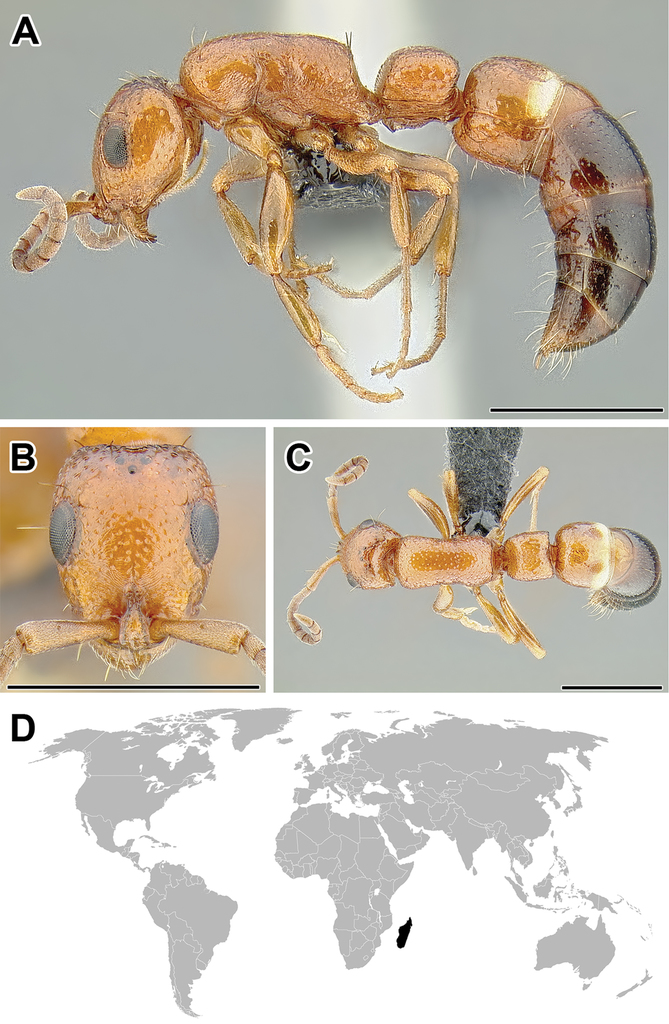
A–C Worker of Aenictus sp. (CASENT0249272) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Aenictus (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
Anterior folding of abdominal tergite IV. In most dorylines the tergosternal Pronotomesopleural suture runs across the midheight of the segment in lateral view such that both poststernite and posttergite are visible across the entire length of the segment. In Syscia, however, the anterior portion of the Pronotomesopleural suture drops down in lateral view resulting in only the tergite being visible posterior to the cinctus (Figure
Girdling constrictions on tergites or sternites V and VI. Although most dorylines possess some form of constriction on abdominal segment IV, such constrictions can be also present posterior to that segment (Figure
Size and shape of pygidium. A modified pygidium, or tergite of abdominal segment VII, is likely a synapomorphy of the Dorylinae. See diagnostic characters above for a discussion.
Hypopygium. The hypopygium is the sternal portion of abdominal segment VII of workers. Occasionally, as in some species of Syscia and Ooceraea, the hypopygium can be lined with specialized thick setae similar to those on the pygidium.
Number and shape of tibial spurs. One or two multicellular articulated projections, known as spurs, may occur at the apex of tibiae. The configuration of spurs on the middle and hind tibiae can be useful in identification of doryline genera. There can be two spurs on both middle and hind tibiae, which is the condition seen in Aenictus, Chrysapace, Cylindromyrmex, and Yunodorylus, as well as at least one Leptanilloides species. More commonly, there is one spur on both middle and hind tibiae. In a few genera there are no spurs on middle tibiae but one spur is present on the apex of hind tibia. These include Simopone, Tanipone, and Vicinopone. The shape of the spurs may also vary, from spurs that have a well-defined comb-like or pectinate margin, through barbulate surface, to simple seta- or spike-like spurs.
Form of hind basitarsus. In most dorylines the first segment of hind tarsus, the basitarsus, is circular in cross-section and as wide basally as distally. Syscia is an exception where the basitarsus is oval in cross-section, gradually widening towards the apex (Figure
Posterior flange of hind coxa. The joint between the coxa and femur is marked by a pronounced concavity in the former. Just posterior of that concavity, a thin lamella can be found in Lioponera (Figure
Metatibial gland. See the discussion under diagnostic character 15 above.
Metabasitarsal gland. See discussion under diagnostic character 15 above.
Hind pretarsal claws. The pretarsal claws can be simple or armed with a tooth in certain lineages. This feature is generally consistent within a genus and thus a reliable character for identification. Pretarsal claws are armed with a tooth at least on the hind leg in some Cerapachys, all Chrysapace, Simopone, Tanipone, Vicinopone, and the Eciton genus-group species except Neivamyrmex.
Characters used to describe male morphology
Because several features of male morphology are similar to those of the worker, I focus on the characters and character systems unique to the male, including flight sclerites, genitalia, and wing venation.
Number of antennal segments. This character varies as in the worker caste, but the plesiomorphic condition is 13 segments (Figure
Notauli. The notauli are grooves on the mesoscutum, or the anterior plate of the male mesonotum. When present, they are usually well-developed as V- or Y-shaped grooves converging towards the posterior (Figures
Metapleural gland opening. Unlike doryline workers, where the orifice of the metapleural gland is always present, many males have lost this feature. Even in the case of males possessing a concavity or orifice in the cuticle where the gland would be located, it is not clear whether this structure is connected to functioning glandular tissue. Because this character appears to be of some diagnostic value, however, I coded its presence and absence in the doryline males without any assumptions on gland activity.
Propodeal lobes. See discussion of this character under worker morphology above. The lobes are well-developed in males of non-army ant dorylines, where they seem to nearly always project beyond the dorsal margin of propodeal foramen. They are somewhat better developed in many Eciton genus-group males relative to the worker but in these genera the dorsal margin of propodeal foramen projects about as far posteriorly as the propodeal lobes.
Shape of abdominal sternite VII. The abdominal sternite VII is often a simple sclerite with a flat surface and no protrusions. In most Ooceraea, however, this sternite is modified to be notched, often with extensions on either side of the sclerite supporting thick setation, sometimes forming a brush.
Shape of abdominal sternite IX. See discussion under male diagnostic character 1.
Male genitalia. In the descriptions I provide a very general account of the genital morphology. The terminology I use here follows
Cupula. The basalmost sclerites form the cupula, also known as the basal ring. In most general terms, the doryline cupula can be short or long relative to the length of the genital capsule. The cupula is best developed in the Eciton genus-group, where it is characteristically nearing or exceeding the length of the rest of the genital capsule. In most non-army ant dorylines the cupula is shorter than half the length of the rest of genital capsule but nevertheless conspicuous. A few genera have a cupula that is very short, a narrow ring of cuticle at the base of genital capsule. These include Aenictus, Dorylus, Leptanilloides, and Yunodorylus. Apparent cupula length can also vary depending on whether viewed from above or ventrally.
Basimere and telomere. The outermost valve of the genital capsule is the paramere. The paramere can be divided into the basal portion called the basimere and the distal portion the telomere. In most dorylines these two portions are broadly connected but the New World army ants are an exception where the telomeres are very narrowly connected to dome-like basimeres (Figure
A–F Male of Aenictus sp. (CASENT0731090) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 2.0 mm.
Volsella. The second outermost valve is the volsella. Similarly to the telomere, volsella can be of variable shape.
Penisvalvae. The innermost valves, the penisvalvae, aedeagal valves, or collectively the aedeagus, are similarly variously shaped and can be apically rounded, grossly expanded, and straight or hooked. The setation of the apex of penisvalvae is a reliable character distinguishing otherwise similar males of New World army ant genera Cheliomyrmex, Labidus, and Nomamyrmex, which possess hairs, from Eciton and Neivamyrmex with hairless penisvalvae. Given much intrageneric variability of the genital capsule, and especially its inner valves, it is likely that this revision does not provide a complete description of its morphological diversity. Statements about male genitalia will certainly be revised and refined when additional males are examined.
Tegula. The tegula is a small, dome- or strap-shaped sclerite that covers the base of the fore wing. In the Dorylinae the tegula varies in shape from broadly oval to thin and strap-shaped but it is generally conspicuous (Figure
Fore wing venation. Wing venation often varies considerably within a genus. However, because it is obvious and because a certain pattern can be characteristic for the vast majority of species within a given genus despite occasional reductions, this is a character system valuable for identification (Figure
Costal vein (C). The vein on the leading margin of the fore wing anterior to the pterostigma is called the costal vein. This vein is an important character for genus identification although its presence may be challenging to ascertain when the leading wing margin is folded onto itself. The costal vein is often present (Figure
Radial vein (R). In ants, this vein is considered to be fused with subcostal vein and radial sector (Sc+R+Rs) proximally, bifurcating into Sc+R and radial sector (R) before reaching pterostigma. The free abscissae R·f1–2 are fused with the pterostigma and R·f3 projects distally of the pterostigma on the leading edge of the fore wing. In the Dorylinae, R·f3 is generally associated with overall well-developed venation and is found in all New World army ant genera. Among non-army dorylines, it can be found in Acanthostichus, Cerapachys, Chrysapace, Cylindromyrmex, most Eburopone, Neocerapachys, Procerapachys, and Yunodorylus.
Radial sector (Rs). Past the separation from Sc+R, the radial sector continues posterior to the pterostigma, first as a usually short free abscissa Rs·f1, then merging with median vein (M) and continuing fused (Rs+M) for some time, followed by the free abscissae Rs·f2–5 that, when present, constitute the next longitudinal vein system posterior to the pterostigma. Rs·f5 may distally connect with R·f3 to close a marginal cell. When Rs is present distally to Rs+M, it connects to the pterostigma via the second radial-radial sector cross-vein (2r-rs). Various levels of reduction of the radial sector are found within the Dorylinae. The most complete development is found in Eciton genus-group, Cerapachys, Chrysapace, Cylindromyrmex, Procerapachys, Sphinctomyrmex, and some Neocerapachys and Yunodorylus. In these ants the free abscissae of the radial sector continue to close the marginal vein by joining R·f3 at the wing margin, in some species interrupted only at the connection with Rs+M. In other genera such as Parasyscia, Lividopone, or Zasphinctus radial sector abscissae Rs·f2–3 connect to Rs+M but radial abscissa R·f3 is absent and Rs·f4–5 do not close the marginal cell. In Aenictogiton and Simopone the radial sector is further reduced, interrupted near Rs+M junction and not reaching wing margin. In Lioponera, Eburopone, and some Ooceraea and Syscia the abscissae Rs·f2–3 are absent and Rs·f4–5 together with 2r-rs form a ‘free stigmal vein’ that does not reach wing margin. More reduction is found in various genera where smaller species sometime lost all radial sector veins past Rs+M.
Median vein (M). Further away from the leading wing margin is the median vein, proximally fused with cubital vein (M+Cu), following separation continuing as a free abscissa M·f1 before joining with radial sector to form Rs+M. In the Dorylinae the next free abscissa (M·f2) may be separated from Rs+M if Rs·f2–3 or continuous with Rs+M in the absence of radial sector. If median vein is present past the junction with the radial sector, further free abscissae M·f3 and M·f4 can be differentiated in the presence of second radial sector-median cross-vein (2rs-m) and M·f4 may extend all the way to the distal wing margin. Various reductions are possible from this basic pattern. The median vein is highly variable, from the best developed in the Eciton genus-group, where it almost always reaches wing margin as a tubular vein, through a state where nebulous or spectral free abscissae M·f3–4 are disconnected from other veins, to entirely absent past Rs+M as in certain Leptanilloides.
Cubital vein (Cu). Proximally the cubital vein is fused with median vein (M+Cu) and can have up to three free abscissae, Cu·f1 through Cu·f3. The first median-cubital cross-vein (1m-cu) may connect the cubital vein to the median vein between Cu·f2 and Cu·f3. Cu·f3 can further distally branch into as many as three branches, Cu1–3. Cu1 is often present, even in species with venation otherwise reduced in the radial sector and the median vein. Cu1 is the long branch running towards the distal wing margin. Cu2 is short and sometimes connects to the anal vein (A). When present, Cu3 is always a short stub directed towards the posterior wing margin. In dorylines it is found only in the largest of males, in the New World army ants and some Dorylus.
Anal vein (A). The anal vein is the longitudinal vein running near the posterior wing margin. In dorylines it consists of at least one free abscissa fused to or terminating near cubital-anal cross-vein (cu-a), a connection to the cubital vein, and often two abscissae are present (A·f1–2) if continuing past cu-a. The position of cu-a relative to the branching of M·f1 can help distinguish a male of the Eciton genus-group from an Old World army ant: in the former M·f1 arises much closer to the base of the wing than cu-a while in Aenictogiton, Aenictus, or Dorylus M·f1 arises either distally to cu-a, directly below it, or only slightly proximally.
Hind wing venation. Veins in the hind wing follow a similar pattern to that found in the fore wing but there is no pterostigma and the venation is simplified (Figure
Characters used to describe gyne morphology
Because the morphology of the majority of doryline gynes is much like the worker, this revision does not describe gynes in detail. Instead, a general morphology is indicated, including how gynes differ from the worker, whether the known forms are alate, ergatoid (wingless and worker-like), or dichthadiigyne (see below), and references to more detailed descriptions are provided where available. The gyne morphology can be variable within a genus or even within species where intercastes, or individuals with morphology intermediate between workers and gynes, are known in addition to fully-developed gynes.
As mentioned above, the doryline gynes can be classified as alate, worker-like, or ‘dichthadiigyne’ or ‘subdichthadiigyne’, although a gradation of intermediates between all these morphologies is also observed among the doryline species. Fully alate gynes possess the usual complement of flight-associated sclerites, relatively large eyes and ocelli, and are apparently capable of flight. Alate gyne material available is often scarce and inference about the presence of wings from dealated gynes is difficult because obvious mesosomal sutures and wing scar-like structures are not always indicative of presence of fully developed wings in the virgin gynes. At least one species is known to have brachypterous (short-winged) gynes, bridging the gap between fully alate and worker-like morphologies. Wingless, ergatoid gynes are very common in dorylines. They exhibit variation in how different they are from the workers, ranging from gynes essentially indistinguishable from the worker to ones that have enlarged gasters, large eyes and ocelli, and wing scar-like structures on the mesosoma. The term ‘dichthadiigyne’ (
Alate or apparently alate (known only from dealated specimens) gynes are so far known in Acanthostichus, Cerapachys, Chrysapace, Cylindromyrmex, Eburopone, Lioponera, Lividopone, Neocerapachys, Parasyscia, Simopone, Syscia, Vicinopone, and Zasphinctus. Ergatoid gynes are found in Cerapachys, Eburopone, Eusphinctus, Lioponera, Ooceraea, Parasyscia, Simopone, Sphinctomyrmex, Tanipone, and Zasphinctus. Subdichthadiigynes or dichthadiigynes are found in Acanthostichus, Leptanilloides, Ooceraea, Zasphinctus, and ‘true army ants’ in the genera Aenictus, Dorylus, Eciton, Labidus, Neivamyrmex, and Nomamyrmex. A description of a Yunodorylus subdichthadiigyne is currently awaiting publication (
A–F Morphological diversity of Aenictus. A A. latifemoratus (CASENT0249279) B A. inflatus (CASENT0732111) C A. laeviceps (CASENT0732112) D A. hottai (CASENT0249278) E A. cornutus (CASENT0249267) FA. cf. eugenii (CASENT0249274). Scale bar equals 1.0 mm.
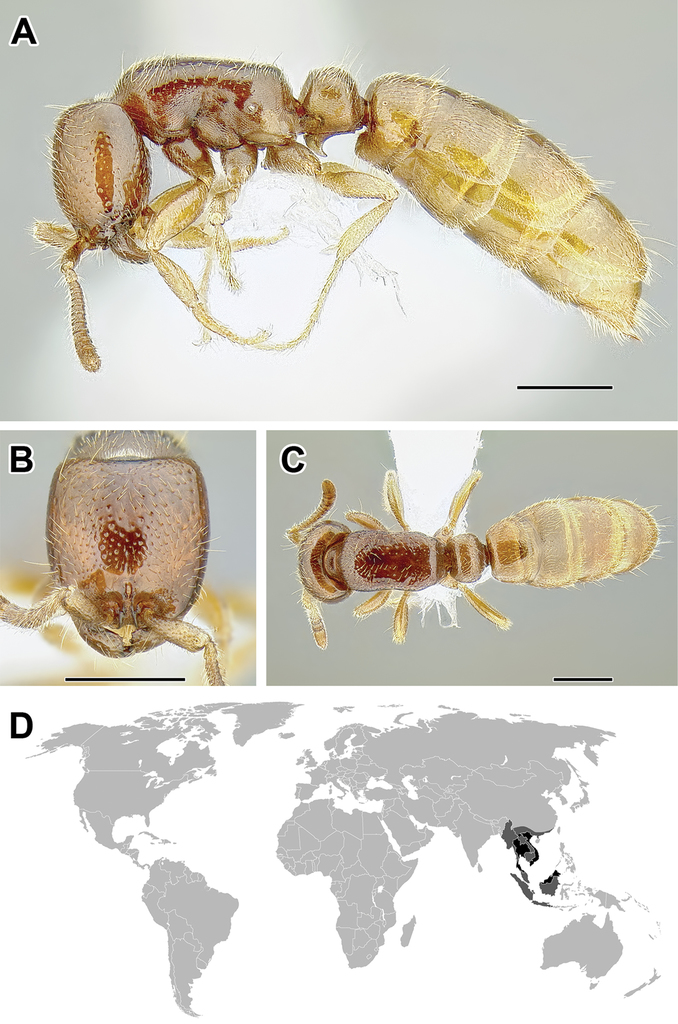
A–C Worker of Cerapachys sp. (CASENT0162338) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Cerapachys (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Cerapachys antennatus (CASENT0731091) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 2.0 mm in A–C and F, 0.5 mm in D and E.
A–C Worker of Cheliomyrmex morosus (CASENT0731129) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Cheliomyrmex (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Cheliomyrmex morosus (CASENT0731092) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 5.0 mm in A and B, D–F, 2.0 mm in C.
A–C Worker of Chrysapace sp. (CASENT0731133) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Chrysapace (black: present, dark grey: likely present). Scale bar equals 2.0 mm in A and C, 1.0 mm in B.
A–F Male of Chrysapace sp. (CASENT0731113) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A–C and F, 0.5 mm in D and E.
A–C Worker of Cylindromyrmex brasiliensis (CASENT0731132) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Cylindromyrmex (black: present, dark grey: likely present). Scale bar equals 2.0 mm.
A–F Male of Cylindromyrmex brevitarsus (CASENT0731094) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A–C and F, 0.5 mm in D and E.
A–C Worker of Dorylus nigricans terrificus (CASENT0731192) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Dorylus (black: present, dark grey: likely present). Scale bar equals 2.0 mm.
A–F Male of Dorylus nigricans terrificus (CASENT0731198). A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 5.0 mm.
A–C Worker of Eburopone sp. (CASENT073120) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Eburopone (black: present, dark grey: likely present). Scale bar equals 0.5 mm.
A–F Male of Eburopone sp. (A–CCASENT0731095D–F CASEN0113882) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A, B, and F, 0.5 mm in C–E.
A–C Worker of Eciton hamatum (CASENT0731194) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Eciton (black: present, dark grey: likely present). Scale bar equals 2.0 mm.
A–F Male of Eciton burchelli (CASENT0731197) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 2.0 mm.
A–C Worker of Eusphinctus furcatus (CASENT0173056) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Eusphinctus (black: present, dark grey: likely present). Scale bar equals 1.0 mm. Photographs courtesy of www.antweb.org (April Nobile).
A–F Male of Eusphinctus sp. (A–C: CASENT0278069, D–F: CASENT0131978) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A–C and F, 0.5 mm in D and E.
A–C Worker of Labidus coecus (CASENT0731195) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Labidus (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Labidus coecus (A–C: CASENT0731124, D–F: CASENT0731218) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 5.0 mm in A, B, and F, 2.0 mm in C–E.
A–C Worker of Leptanilloides gracilis (CASENT0234574) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Leptanilloides (black: present, dark grey: likely present). Scale bar equals 0.5 mm in A and C, 0.25 mm in B.
A–F Male of Leptanilloides sp. (A–C, F: CASENT0234556, D and E: CASENT0731110) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 0.5 mm in A–C and F, 0.25 mm in D and E.
A–C Worker of Lioponera clarus (CASENT0731128) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Lioponera (black: present, dark grey: likely present). Scale bar equals 2.0 mm.
A–F Male of Lioponera cf. mayri (A–CCASENT0731200D–FCASENT0234856) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm.
A–F Morphological diversity of Lioponera. A L. longitarsus (CASENT0731207) B L. sp. (CASENT0215877) C L. foreli (CASENT0731206) D L. elegans (CASENT0249293) EL. cf. kraepelini (CASENT0731204) FL. cf. suscitata (CASENT0731205). Scale bar equals 1.0 mm.
A–C Worker of Lividopone livida (CASENT0731209) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Lividopone (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Lividopone sp. (CASENT0234857) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A and B, 0.5 mm in C–F.
A–C Worker of Neivamyrmex nigrescens (CASENT0249493) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Neivamyrmex (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Neivamyrmex nigrescens (CASENT0732110) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 2.0 mm.
A–F Morphological diversity of Neivamyrmex. A N. melanocephalus (CASENT0731183) B N. adnepos (CASENT0249470) C N. iridescens (CASENT0249488) D N. gibbatus (CASENT0731189) E N. diversinodis (CASENT0249480) F N. cornutus (CASENT0249478). Scale bar equals 1.0 mm.
A–F Male of Neocerapachys sp. (A–C: CASENT0731109, D–F: CASENT0731210) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A, B, and F, 0.5 mm in C–E.
A–C Worker of Nomamyrmex esenbeckii (CASENT0731191) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Nomamyrmex (black: present, dark grey: likely present). Scale bar equals 2.0 mm.
A–F Male of Nomamyrmex esenbeckii (CASENT0731217) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 5.0 mm in A, B, and F, 2.0 mm in C–E.
A–C Worker of Ooceraea biroi (CASENT0731215) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Ooceraea (black: present, dark grey: likely present, asterisk: introduced). Scale bar equals 0.5 mm.
A–F Male of Ooceraea sp. (A–C, FCASENT0731100D and ECASENT0731098) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 0.5 mm.
A–C Worker of Parasyscia kodecorum (CASENT0731152) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Parasyscia (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Parasyscia sp. (A–CCASENT0731116D–FCASENT0731101) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 in A and B, 0.5 mm in C–F.
A–C Morphological diversity of Parasyscia. A P. sp. A (CASENT0731212) B P. sp. B (CASENT0216859) C P. imerinensis (CASENT0731170). Scale bar equals 1.0 mm.
A–C Worker of Simopone conradti (CASENT0731157) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Simopone (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Simopone grandidieri (A–CCASENT0148973), S. marleyi (D–FCASENT0731102). A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A, B, and F, 0.5 mm in C–E. Photographs A–C courtesy of www.antweb.org (Michele Esposito).
A–C Worker of Sphinctomyrmex cf. marcoyi (CASENT0731146) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Sphinctomyrmex (black: present, dark grey: likely present). Scale bar equals 1.0 mm A and C, 0.5 mm in B.
A–F Male of Sphinctomyrmex sp. (CASENT0731118) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A and B, 0.5 mm in C–F.
A–C Worker of Syscia augustae (CASENT0731214) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Syscia (black: present, dark grey: likely present). Scale bar equals 1.0 mm in A and C, 0.5 mm in B.
A–F Male of Syscia humicola (A–CCASENT0731213), Syscia sp. (D–FCASENT0731104) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 0.5 mm.
A–C Worker of Tanipone aversa (CASENT0207895) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Tanipone (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Tanipone sp. (A–CCASENT0154714D and ECASENT0731105FCASENT0217353) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A, B, and F, 0.5 mm in C–E.
A–C Worker of Vicinopone conciliatrix (CASENT0731137) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Vicinopone (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–C Worker of Yunodorylus eguchii (CASENT0731166) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Yunodorylus (black: present, dark grey: likely present). Scale bar equals 0.5 mm.
A–F Male of Yunodorylus sp. (CASENT0278751) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm.
A–C Worker of Zasphinctus trux (CASENT0731216) A Body in lateral view B Head in full-face view C Body in dorsal view D World distribution of Zasphinctus (black: present, dark grey: likely present). Scale bar equals 1.0 mm.
A–F Male of Zasphinctus sp. (A–C: CASENT0731115, D–F: CASENT0731106) A Body in lateral view B Body in dorsal view C Head in full-face view D Genital capsule in ventral view E Abdominal segment IX (subgenital plate) F Wing venation. Scale bar equals 1.0 mm in A, B, and F, 0.5 mm in C–E.
Key to the genera of doryline ants based on workers
Certain couplets build upon keys in
| 1 | Last visible abdominal tergite, the pygidium, not armed with numerous modified setae, at most with only one or two pairs of thick setae or cuticular projections (Figures A, B). Propodeal lobes short or absent | 2 |
| – | Pygidium armed with numerous specialized, peg-like or spiniform setae much thicker than surrounding fine hairs (Figure C); setae more than four in number, often more numerous. If pygidium small or with few specialized setae, then propodeal lobes conspicuous | 11 |
|
|
||
| 2 (1) | Propodeal spiracles positioned low on propodeum, at or below mid-height of the sclerite (Figure A) | 3 |
| – | Propodeal spiracles positioned high on propodeum, above mid-height of the sclerite (Figures B, C) | 4 |
|
|
||
| 3 (2) | Pygidium large. Propodeal lobes present (Baltic amber) | Procerapachys |
| – | Pygidium small. Propodeal lobes absent (Nearctic, Neotropical) | Leptanilloides |
| 4 (2) | Abdominal segment II (petiole) and segment III differentiated and both segments much smaller than the succeeding segment IV. Abdominal segment IV always conspicuously the largest segment (Figure A). | 5 |
| – | Only abdominal segment II (petiole) differentiated and smaller than succeeding segments III and IV (Figure B). If abdominal segment III attached to segment IV through a strong constriction and somewhat differentiated, then abdominal segment IV not conspicuously the largest segment. | 9 |
|
|
||
| 5 (4) | Antennae with 8–10 segments. Old World species (Figure A) (Palearctic, Afrotropical, Indomalayan, Australasian) | Aenictus |
| – | Antennae with 12 segments (Figure B). New World species | 6 |
|
|
||
| 6 (5) | Tarsal claws simple, without teeth (Figure A) (Nearctic, Neotropical, Dominican amber) | Neivamyrmex |
| – | Tarsal claws armed with teeth (Figure B) | 7 |
|
|
||
| 7 (6) | Inner (flexor) surface of hind tibiae without any sign of differentiated pale cuticle (Figure A) (Nearctic, Neotropical) | Nomamyrmex |
| – | Inner surface of hind tibiae with differentiated surface of pale cuticle (metatibial gland), from elongately oval patch near tibial spur to a narrow stripe spanning much of the length of tibia (Figure B) | 8 |
|
|
||
| 8 (7) | Propodeum armed with cuticular lamellae or spines (Figure A) (Neotropical) | Eciton |
| – | Propodeum unarmed, dorsal propodeal surface rounding into propodeal declivity (Figure B) (Nearctic, Neotropical) | Labidus |
|
|
||
| 9 (4) | Constrictions present at anterior end of abdominal segments V and VI (Figure A) (Afrotropical) | Aenictogiton |
| – | Constrictions absent from anterior end of abdominal segments V and VI (Figure B) | 10 |
|
|
||
| 10 (9) | Promesonotal Pronotomesopleural suture conspicuous (Figure A). Pygidium large and impressed at apex, armed with one or two cuticular teeth or spines on each side (Figure C). Pretarsal claws unarmed (Palearctic, Afrotropical, Indomalayan) | Dorylus |
| – | Promesonotal Pronotomesopleural suture absent (Figure B). Pygidium small and convex at apex, unarmed or with one or two peg-like setae on each side (Figure D). Pretarsal claws armed with teeth (Neotropical) | Cheliomyrmex |
|
|
||
| 11 (1) | Waist consisting only of abdominal segment II (petiole) and abdominal segment III broadly attached to segment IV, without conspicuous constrictions between pre- and postsclerites of abdominal segment IV (Figure A) (Indomalayan) | Yunodorylus |
| – | Waist with abdominal segment III at least weakly differentiated from segment IV; the latter with a constriction between its pre- and postsclerites (Figure B) | 12 |
|
|
||
| 12 (11) | Mid and hind tibiae each with two spurs (Figure A) | 13 |
| – | Middle tibiae with a single spur (Figure B) or without spurs (Figure C) and hind tibiae always with a single spur | 14 |
|
|
||
| 13 (12) | Antennal sockets at least partly concealed in full face view (Figure A). Pretarsal claws simple (Figure C). Maxillary palps 2-segmented, labial palps 3-segmented (Neotropical, Dominican amber) | Cylindromyrmex |
| – | Antennal sockets exposed in full face view (Figure B). Pretarsal claws armed with a tooth (Figure D). Maxillary palps 5-segmented, labial palps 3-segmented (Malagasy, Indomalayan, Baltic amber) | Chrysapace |
|
|
||
| 14 (12) | Pretarsal claws of hind leg armed ventrally with a tooth or at least a small denticle (Figure A); teeth can be difficult to discern below 50× magnification | 15 |
| – | Pretarsal claws of hind leg simple (Figure B) | 18 |
|
|
||
| 15 (14) | Middle tibiae always with a pectinate spur. Hind tibiae with a patch light cuticle near distal end (Figure A; metatibial gland) (Indomalayan) | Cerapachys (part) |
| – | Middle tibiae without spurs. Hind tibiae without a patch differentiated cuticle, but a conspicuous sulcus or groove on hind basitarsus may be present (Figure B; metabasitarsal gland) | 16 |
|
|
||
| 16 (15) | Antennae with 11 segments (Figure A). Longitudinal glandular groove (metabasitarsal gland) present on basal half of ventral surface of hind basitarsi (Afrotropical, Malagasy, Indomalayan, Australasian) | Simopone |
| – | Antennae with 12 segments (Figures B, C). Longitudinal glandular groove (metabasitarsal gland) absent from basal half of ventral surface of hind basitarsi. | 17 |
|
|
||
| 17 (16) | Ocelli absent. Posterior margin of eyes anterior to midlength of head capsule (Figure A). Maxillary palps 3-segmented and labial palps 2-segments. Maxillary palps short and often not exposed in pinned specimens. When palps extended, the maxillary palp terminates well before occipital foramen (Figure C) (Afrotropical) | Vicinopone |
| – | Ocelli present. Posterior margin of eyes behind midlength of head capsule (Figure B). Maxillary palps 6-segmented and labial palps 4-segmented. Maxillary palps very long, when extended almost reaching occipital foramen (Figure D) (Malagasy) | Tanipone |
|
|
||
| 18 (14) | At least anterior dorsolateral portions of abdominal segment II (petiole) marginate (Figure B) and often entire length of petiolar tergite with pronounced margins (Figure A). Hind coxa usually with posterior flange drawn into a vertical, opaque or semi-translucent lamella (Figure D). Metatibial gland pore plate usually in a depression or invagination of the cuticle, appearing as a slit (Figure F) or, more rarely, a circular opening (Figure G) or inconspicuous (Palearctic, Afrotropical, Malagasy, Indomalayan, Australasian) | Lioponera |
| – | No segment of body conspicuously dorsolaterally marginate although lateral crest immediately above abdominal segment II (petiolar) spiracle may be present (Figure C). If abdominal segment II appearing marginate, hind coxae without posterior vertical lamella (Figure E). Metatibial gland pore plate not in a depression, either an oval whitish patch (Figure H), or not discernable (Figure I) | 19 |
|
|
||
| 19 (18) | In lateral view pronotomesopleural Pronotomesopleural suture either completely or partially fused, never a curved cut in cuticular surface approaching dorsolateral margins of promesonotum (Figures A, B). Sometimes in place of Pronotomesopleural suture a groove (especially Neocerapachys and Sphinctomyrmex) or a row of punctures present, or the Pronotomesopleural suture short; there is never a lining of short pubescence | 20 |
| – | In lateral view pronotomesopleural Pronotomesopleural suture present as a deep cut in the cuticle, often curved below dorsolateral margins of mesosoma and with inside lined with short pubescence (Figures C, D) | 24 |
|
|
||
| 20 (19) | Helcium circumference large relative to abdominal segment II (petiole) and placed above midheight of the segment, resulting in low, undifferentiated posterior face of abdominal segment II and low anterior face of abdominal segment III (Figure A) (Malagasy) | Lividopone |
| – | Helcium circumference small relative to abdominal segment II (petiole) and placed at about midheight of segment, resulting in pronounced posterior face to abdominal segment II and conspicuous anterior face of abdominal segment III (Figure B) | 21 |
|
|
||
| 21 (20) | Metapleural gland trench an inconspicuous narrow slit, with posterior opening smaller than the diameter of propodeal spiracle (Figure A) (Neotropical) | 22 |
| – | Metapleural gland trench conspicuous, throughout its length broader than the diameter of propodeal spiracle opening (Figure B). If the posterior opening of the trench narrow (rarely), it is through a constriction made by an elevated ventral flange of the trench, the latter being broad and deep anteriorly to the constriction (Old World) | 23 |
|
|
||
| 22 (21) | Constrictions present at anterior end of abdominal segments V and VI (Figure A). No patches of differentiated cuticle on abdominal tergite IV (Figure C) (Neotropical) | Sphinctomyrmex |
| – | Constrictions absent from anterior end of abdominal segments V and VI (Figure B). Circular porous and pubescent patches differentiated from surrounding cuticle (occasionally indistinct) present laterally on abdominal tergite IV, just medial to the spiracles (Figure D) (Neotropical) | Neocerapachys |
|
|
||
| 23 (21) | Constrictions present at anterior end of abdominal segments V and VI (Figure A) (Afrotropical, Australasian) | Zasphinctus |
| – | Constrictions absent from anterior end of abdominal segments V and VI (Figure B) (Palearctic, Afrotropical, Malagasy, Indomalayan, Australasian) | Parasyscia |
|
|
||
| 24 (19) | Helcium circumference large relative to abdominal segment II (petiole) and placed above midheight of the segment, resulting in very low, undifferentiated posterior face of petiole and low anterior face of abdominal segment III (Figure A) | 25 |
| – | Helcium circumference small relative to abdominal segment II (petiole) placed at about midheight of segment, usually resulting in differentiated posterior face to abdominal segment II and conspicuous anterior face of abdominal segment III (Figure B) | 26 |
|
|
||
| 25 (24) | Pronotal flange not separated from collar by distinct ridge (Figure A). Eyes small, composed of few weakly differentiated ommatidia (Nearctic, Neotropical, Dominican amber) | Acanthostichus |
| – | Pronotal flange separated from collar by distinct ridge (Figure B). Eyes composed of more than 20 well-defined ommatidia (Indomalayan) | Cerapachys (part) |
|
|
||
| 26 (24) | Constrictions present at anterior end of abdominal segment V and abdominal segment VI (similar to couplet 23, Figure A) (Indomalayan) | Eusphinctus |
| – | Constrictions absent from anterior end of abdominal segment V and abdominal segment VI (similar to couplet 23, Figure B) | 27 |
| 27 (26) | Antennae with 12 segments (Figure A). A pale oval or finger-like patch of cuticle often conspicuous in the middle at posterior margin of abdominal sternite IV (Figure D) (Afrotropical, Malagasy) | Eburopone |
| – | Antennae with 9 to 11 segments (Figures B, C). No visible glandular patch in the middle at posterior margin of abdominal sternite IV (Figure E) | 28 |
|
|
||
| 28 (27) | Abdominal segment III relatively narrow in dorsal view and similar in size to the preceding abdominal segment II segment (petiole). In lateral view, abdominal tergite IV not folding over sternite and the anterior portion of the sternite visible (Figure A). Hind basitarsi not dilating distally, circular in cross-section (Figure C). Metabasitarsal glands absent (Indomalayan, Australasian, O. biroi is a pantropical tramp species) | Ooceraea |
| – | Abdominal segment III relatively wide in dorsal view and larger than the preceding abdominal segment II segment (petiole). In lateral view, abdominal tergite IV folding over sternite and the anterior portion of sternite at least partly obscured (Figure B). Hind basitarsi swollen at about two thirds of their length, oval in cross-section (Figure D). Metabasitarsal glands present in addition to metatibial glands, although difficult to discern under magnification lower than 100× (Nearctic, Neotropical, Palearctic, Indomalayan) | Syscia |
|
|
Provisional key to the genera of doryline ants based on males
Keys to the true army ants are modified from
| 1 | Tegula inconspicuous or absent, not covering the base of the wing (Figure A). Discal cell (DC) open (Nearctic, Neotropical) | Leptanilloides |
| – | Tegula present, broad or narrow but always covering the base of the wing and easily discernible (Figure B). Discal cell (DC) open or closed | 2 |
|
|
||
| 2 (1) | Propodeal lobes inconspicuous or absent. If present, then not projecting beyond dorsal margin of propodeal foramen (Figure A). Pronotum usually with dorsal and postero-ventral margins meeting at a sharp angle anterior of tegula (Figure C). Head relatively small compared to the mesosoma. Notauli always absent (‘the true army ants’) | 3 |
| – | Propodeal lobes present, occasionally inconspicuous, projecting beyond dorsal margin of propodeal foramen (Figure B). Pronotum usually with a defined posterior margin in front of tegula, meeting the dorsal margin at approximately right angle (Figure D). Head relatively large compared to the mesosoma. Notauli present or absent (non-army ant dorylines) | 10 |
|
|
||
| 3 (2) | M·f1 vein of fore wing arising from M+Cu at angle lower than 45° and conspicuously proximal relative to cu-a. Two submarginal cells present (SMC), Rs·f2–3 connecting to M·f1 and marginal cell closed (MC; Figure A) | 4 |
| – | M·f1 vein of fore wing arises from M+Cu at angle close to or higher than 45° and near cu-a, distal or, less commonly, slightly proximal. Usually one submarginal cell present (SMC; Figures B, C). If Rs·f2–3 dividing the submarginal cell, the marginal cell open (Figure D) | 8 |
|
|
||
| 4 (3) | Abdominal segments III–VII with dense tufts of long setae, distributed throughout the center of tergites; longest setae as long or longer than fore femur (Figure A). Apex of penisvalvae with setae (Nearctic, Neotropical) | Nomamyrmex |
| – | Abdominal segments III–VII without dense tufts of setae. If long setae present, then either confined to posterior half of dorsum of abdominal terga IV–VII or conspicuously shorter than fore femur (Figures B, C). Apex of penisvalvae with or without setae | 5 |
|
|
||
| 5 (4) | Apex of penisvalvae without setae (Figure A) | 6 |
| – | Apex of penisvalvae with setae (Figure B) | 7 |
|
|
||
| 6 (5) | Abdominal segment II (petiole) dorsum convex, flat or slightly depressed but not deeply excavated (Figure A). Volsella sharply pointed apically, often forked or curving downwards (Figure C). Legs relatively short, in mounted specimens hind femur not reaching past posterior margin of abdominal sternite IV (Nearctic, Neotropical, Dominican amber) | Neivamyrmex |
| – | Abdominal segment II (petiole) dorsum strongly concave (Figure B). Volsella gradually tapering to a blunt apex (Figure D). Legs longer, in mounted specimens hind femur reaching past posterior margin of abdominal sternite IV (Neotropical) | Eciton |
|
|
||
| 7 (5) | Abdominal sternite IX (subgenital plate) with four teeth (Figure A). Basal tarsal segment of hind leg flattened, without grooves (Figure C) (Neotropical) | Cheliomyrmex |
| – | Abdominal sternite IX with two teeth (Figure B). Basal tarsal segment of hind leg complex, with oblique groove accommodating tibial spur (Figure D) (Nearctic, Neotropical) | Labidus |
|
|
||
| 8 (3) | Submarginal cell (SMC) in fore wing partly or entirely divided by Rs·f2–3 vein (Figure A). In full face view head capsule excluding eyes and mandibles longer than wide (Figure C) (Afrotropical) | Aenictogiton |
| – | Submarginal cell (SMC) in fore wing not divided (Figure B). In full face view head capsule excluding eyes and mandibles wider than long (Figure D) | 9 |
|
|
||
| 9 (8) | Pterostigma narrow or inconspicuous and anterior wing margin pigmented (Figure A) Trochanters and femora compressed, broad relative to cylindrical tibiae (Figure C) (Palearctic, Afrotropical, Indomalayan) | Dorylus |
| – | Pterostigma broad, often with convex posterior edge, wing margin not pigmented past pterostigma (Figure B). Trochanters and femora never compressed. If femora flattened and broad, trochanter cylindrical and tibia not conspicuously more narrow than femora (Figure D) (Afrotropical, Palearctic, Indomalayan, Australasian) | Aenictus |
|
|
||
| 10 (2) | Maxillary palps very long and reaching occipital foramen, 6-segmented and visible in mounted specimens (Figure A) (Malagasy) | Tanipone |
| – | Maxillary palps never reaching occipital foramen, usually not visible without dissection and often with fewer than six segments (Figure B) | 11 |
|
|
||
| 11 (10) | Constriction present between pre- and postsclerites of abdominal segment V, both dorsally and ventrally and helcium circumference small with helcium positioned at about the midheight of segment III (Figures A, B) | 12 |
| – | No constriction between pre- and postsclerites of abdominal segment V (Figure C) or helcium circumference large and helcium positioned above midheight of segment III. Rarely, pre- and posttergites may be separated by a gutter-like cinctus but in lateral view there is no constriction, the surface of pre- and postsclerites is contiguous and there is no cinctus on the sternite | 13 |
|
|
||
| 12 (11) | Antennae with 12 segments (Indomalayan) | Eusphinctus |
| – | Antennae with 13 segments (Neotropical) | Sphinctomyrmex |
| 13 (11) | Veins C and R·f3 absent from the fore wing (Figure A). Sternite of abdominal segment IX (subgenital plate) usually visible without dissection as two thin spines, in lateral view curved upwards. Posttergite of abdominal segment VIII (pygidium) often flat or impressed and delimited by a carina (Afrotropical, Indomalayan, Australasian) | Zasphinctus |
| – | Veins C and R·f3 present in the fore wing (Figure B). Sternite of abdominal segment IX (subgenital plate) visible without dissection as two thin spines, in lateral view more or less straight or slightly upcurved. Posttergite of abdominal segment VIII (pygidium) not delimited by a carina | 14 |
|
|
||
| 14 (13) | Submarginal cell (SMC) in fore wing closed by Rs·f2–3 (a fenestra may be present at junction of Rs+M and Rs·f2–3) (Figure A) or SMC open but Rs·f2–3 present and 2rs-m also developed, closing SMC or not (Figure B) | 15 |
| – | SMC not closed by Rs·f2–3, either open (Figure C) or Rs·f2–3 completely absent and SMC closed by vein 2rs-m (Figure D) | 23 |
|
|
||
| 15 (14) | Vein 2rs-m present, partial or complete in fore wing (Figure A) | 16 |
| – | Vein 2rs-m absent or at most stub-like in fore wing (Figure B) | 19 |
|
|
||
| 16 (15) | Hind tibiae with one spur (Figure A) | 17 |
| – | Hind tibiae with two spurs (Figure B) | 18 |
|
|
||
| 17 (16) | Marginal cell closed (Baltic amber) | Procerapachys |
| – | Marginal cell open (Nearctic, Neotropical, Dominican amber) | Acanthostichus (part) |
| 18 (16) | Mesopleuron divided by oblique groove, irregularly sculptured (Figure A) (Malagasy, Indomalayan, Baltic amber) | Chrysapace |
| – | Mesopleuron not divided by a groove, mostly smooth with longitudinal rugae (Figure B) (Neotropical, Dominican amber) | Cylindromyrmex |
|
|
||
| 19 (15) | Costal vein (C) absent in fore wing, R·f3 absent or at most a stub past pterostigma (Figure A) | 20 |
| – | Costal vein (C) present in fore wing, R·f3 present past pterostigma (Figure B) | 21 |
|
|
||
| 20 (19) | Helcium circumference large and helcium positioned supraaxially; posterior face of abdominal tergite II (petiolar node) and anterior face of abdominal tergite III poorly developed (Figure A) (Malagasy) | Lividopone (part) |
| – | Helcium circumference small and helcium positioned axially; posterior face of abdominal tergite II (petiolar node) and anterior face of abdominal tergite III well developed (Figure B) (Palearctic, Afrotropical, Malagasy, Indomalayan, Australasian) | Parasyscia (part) |
|
|
||
| 21 (19) | Abdominal segment III very broadly attached to segment IV such that waist appears composed of one segment. Gaster usually widest posterior to abdominal segment IV (Figure A) (Indomalayan) | Yunodorylus (part) |
| – | Abdominal segment III narrowly attached to segment IV such that second segment of the waist (postpetiole) somewhat differentiated from rest of gaster. Gaster widest at abdominal segment IV (Figure B) | 22 |
|
|
||
| 22 (21) | Antennal segment III the shortest segment (Figure A). In lateral view, anterior margin of eye situated very close to mandibular insertion, separated by less than maximum scape diameter. Maxillary palps with 4 segments, labial palps with 3 segments (Neotropical) | Neocerapachys (part) |
| – | Antennal segment II is the shortest segment (Figure B). In lateral view, anterior margin of eye is situated relatively far from mandibular insertion, separated by more than maximum scape diameter. Maxillary palp with 5 segments, labial palps with 3 segments (Indomalayan) | Cerapachys |
|
|
||
| 23 (14) | Notauli absent (Figure A) | 24 |
| – | Notauli present, at least anteriorly (Figure B) | 27 |
|
|
||
| 24 (23) | Helcium circumference large and helcium positioned supraaxially; posterior face of abdominal tergite III and anterior face of abdominal tergite IV poorly developed (Figure A). Maxillary palps 2-segmented, labial palps 3-segmented (Nearctic, Neotropical, Dominican amber) | Acanthostichus (part) |
| – | Helcium circumference small and helcium positioned axially; posterior face of abdominal tergite III and anterior face of abdominal tergite IV developed (Figure B). Maxillary palps not 2-segmented in combination with 3-segmented labial palps | 25 |
|
|
||
| 25 (24) | R·f3 vein present in fore wing (Figure A), long and conspicuous, sometimes joining Rs·f4–5 to form a closed marginal vein (Indomalayan) | Yunodorylus (part) |
| – | R·f3 vein absent in fore wing, at most a stub (Figure B) | 26 |
|
|
||
| 26 (25) | Rs·f2–3 vein absent in fore wing. Pterostigma gives origin to a ‘free stigmal vein’ composed of 2r-rs&Rs·f4–5 (Figure A) or Rs connected to M through 2rs-m or, in smaller species, the free stigmal vein entirely absent or only a stub of 2r-rs present (Palearctic, Afrotropical, Malagasy, Indomalayan, Australasian) | Lioponera (part) |
| – | Rs·f2–3 vein present in fore wing, long or a stub (Figure B) (Palearctic, Afrotropical, Malagasy, Indomalayan, Australasian) | Parasyscia (part) |
|
|
||
| 27 (23) | Inner margins of antennal sockets concealed by ‘frontal carinae’ (torulo-posttorular complex) in full-face view (Figure A). Middle tibiae without spurs (Figure C) (Afrotropical, Malagasy, Indomalayan, Australasian) | Simopone |
| – | Antennal sockets completely exposed in full-face view (Figure B). Middle tibiae with a single spur, which may be simple and inconspicuous (Figure D) | 28 |
|
|
||
| 28 (27) | Helcium circumference large and helcium positioned supraaxially; posterior face of abdominal tergite II (petiolar node) and anterior face of abdominal tergite III poorly developed (similar to couplet 12 Figure A) (Malagasy) | Lividopone (part) |
| – | Helcium circumference small and helcium positioned axially; posterior face of abdominal tergite II (petiolar node) and anterior face of abdominal tergite III well developed (similar to couplet 12 Figure B) | 29 |
| 29 (28) | Antennae with 11 or 12 segments | 30 |
| – | Antennae with 13 segments | 31 |
| 30 (29) | Discal cell (DC) often closed (Figure A). Abdominal segment III distinctly smaller than the succeeding segment IV, i.e. postpetiole well differentiated and often similar in size to abdominal segment II (petiole). Abdominal sternite VII almost always modified, notched, equipped with tufts of setae, with palpiform or flat projections, or otherwise hypertrophied (Figure C). Most species with 11-segmented antennae, some with 12-segmented (Indomalayan, Australasian, O. biroi is a pantropical tramp species) | Ooceraea |
| – | Discal cell (DC) open (Figure B). Abdominal segment III may be smaller than the succeeding segment IV but usually larger than abdominal segment II (petiole). Abdominal sternite VII simple, never modified (Figure D). Antennae 12-segmented (Palearctic, Nearctic, Neotropical, Indomalayan) | Syscia |
|
|
||
| 31 (29) | Costal (C) and R·f3 veins absent from fore wing (Figure A) (Palearctic, Afrotropical, Malagasy, Indomalayan, Australasian) | Lioponera (part) |
| – | Costal (C) vein always present in fore wing, R·f3 often present (Figure B) | 32 |
|
|
||
| 32 (31) | Rs·f2–3 vein present in fore wing (Figure A). Propodeal declivity and anterior face of abdominal segment II (petiole) surrounded by a conspicuous carina (Neotropical) | Neocerapachys (part) |
| – | R·f2–3 vein absent in fore wing (Figure B). Propodeal declivity and anterior face of abdominal segment II usually not surrounded by a carina (Afrotropical, Malagasy) | Eburopone |
|
|
Taxonomic treatment of the genera of Dorylinae
Acanthostichus
= Ctenopyga Ashmead, 1906
Type-species
Typhlopone serratula, by monotypy.
Acanthostichus is a New World genus of termite-hunting dorylines most closely related to Cylindromyrmex.
Diagnosis
Worker. The workers of this distinctive lineage can be recognized by a combination of 12-segmented antennae, absence of ridge on pronotal collar, unfused pronotomesopleural Pronotomesopleural suture, highly positioned helcium, a single pectinate spur on mid and hind tibiae, propodeal spiracle usually positioned below the midheight of the sclerite, and large pygidium armed with modified finger-like setae. Restricted to the New World, the species of Acanthostichus are medium-sized ants that are often brown or yellowish in coloration and lack distinctive sulcate or striate sculpturing characteristic of its close relative Cylindromyrmex or very conspicuous constrictions between gastral segments of Sphinctomyrmex. Other New World dorylines (army ants related to Eciton, species of Leptanilloides) all have simple, small pygidium, at most armed with several thick setae. Workers of Syscia and the introduced Ooceraea biroi, also found in the New World, can be distinguished by antennal segment count reduced to 11 or 9, respectively.
Male. The male of Acanthostichus can be separated from all other dorylines by a combination of propodeal lobes conspicuous, supraaxial helcium, single spur on each mid and hind tibiae, costal vein (C) present in fore wing, and R·f3 present past pterostigma but not enclosing a cell with Rs·f5. Most species appear to have 12-segmented antennae but at least A. texanus and A. davisi are known to have 13 antennal segments. Among New World dorylines the habitus of males is similar to that of Cylindromyrmex, Neocerapachys, Syscia, and Sphinctomyrmex. Sphinctomyrmex has conspicuous constrictions between abdominal segments IV, V, and VI in combination with narrow axial helcium, Cylindromyrmex has two tibial spurs, and Syscia lacks the costal vein in the fore wing. Neocerapachys has either a closed marginal cell or lacks cross-vein 2rs-m. Furthermore, Acanthostichus males that lack 2rs-m have a broader helcium and more poorly developed posterior face of the petiole than is characteristic of Neocerapachys. Other dorylines found in the New World include the army ants, and males in these genera always have marginal cell closed by R·f3 and Rs·f5, only one well-differentiated waist segment, and no constriction between abdominal segments III and IV. The remaining neotropical genus, Leptanilloides, has no conspicuous tegula and venation reduced, without R·f3 or discal cell.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges absent. Torulo-posttorular complex horizontal. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 3-segmented. Mandibles triangular, with median tooth or triangular, edentate. Eyes present, composed of 1–20 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen absent or present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove replaced by cuticular ridge. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally weakly to conspicuously marginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low or high on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate, dorsolaterally marginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a simple U-shaped margin or reduced to small longitudinal ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured or weakly cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent or present. Pygidium large, with impressed medial field, and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent or oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 12 or 13 segments. Antennal scapes dorsoventrally flattened. Clypeus with cuticular apron, not translucent. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 3-segmented. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent or present. Transverse groove dividing mesopleuron absent or present. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening present. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a simple U-shaped margin or V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent or present. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms abutting or separated. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally flattened, at apex hooked ventrally. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate or narrow, demilanceolate in shape. Abscissa R·f3 present and running toward distal wing margin but not enclosing cell with Rs·f5. Abscissae Rs·f2–3 absent or present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, connected to Rs·f2–3&Rs·f4, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m or differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing absent or present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent or with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein Sc+R+Rs present. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m, sometimes a stub. Abscissa Rs·f1 in hind wing present, longer than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing absent or present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing absent or present. Abscissa M·f2 in hind wing absent or present. Cross-vein cu-a in hind wing absent or present. Vein Cu in hind wing absent or present. Vein A in hind wing absent or with abscissa A·f1 present.
Gyne. Acanthostichus gynes are known either as alates or subdichthadiiform, i.e. ergatoid without wing sclerites but possessing hypertrophied gasters. The former are currently known for A. emmae and A. texanus, the latter in A. brevicornis, A. quadratus, and A. laticornis. In the fully alate gynes the eyes are large and three ocelli are present and abdominal segment III is differentiated from succeeding segments. In the subdichthadiigynes the eyes are present but small, three small ocelli are present, the head is more round than in workers, and mandibles are falcate; there are no flight-associated sclerites, abdominal segment II (petiole) is broadly attached posteriorly to segment III, which is also enlarged, not separated from the rest of the gaster by a constriction (Emery 1895,
Larva. Described in
Distribution
Acanthostichus is a genus of 24 described species, occurring in southern United States, Mexico, and most of South America. The genus has long been thought absent from Central America, but at least one specimen is known from Costa Rica. This is unlikely due to undersampling, as Central American countries have been the subject to some of the most intensive surveys of ant faunas (
Taxonomy and phylogeny
Acanthostichus was erected by
Males described by Marion
It is now established that Acanthostichus is most closely related to Cylindromyrmex (
Biology
Along with their close relatives in Cylindromyrmex, Acanthostichus species are predators of termites, unlike most other doryline species which prey on ants (
Species of Acanthostichus
A. arizonensis Mackay, W.P., 1996: United States
A. bentoni Mackay, W.P., 1996: Brazil
A. brevicornis Emery, 1894: French Guiana
A. brevinodis Mackay, W.P., 1996: Brazil
A. concavinodis Mackay, W.P., 1996: Bolivia
A. davisi (Smith, M. R., 1942a): United States
A. emmae Mackay, W.P., 1996: Mexico
A. femoralis Kusnezov, 1962: Argentina
A. flexuosus Mackay, W.P., 1996: Brazil
A. fuscipennis Emery, 1895b: Brazil
†A. hispaniolicus De Andrade, 1998b: Dominican amber
A. kirbyi Emery, 1895b: Paraguay
A. laevigatus Mackay, W.P., 1996: Venezuela
A. laticornis Forel, 1908: Paraguay
A. lattkei Mackay, W.P., 1996: Venezuela
A. longinodis Mackay, W.P., 2004: Paraguay
A. punctiscapus Mackay, W.P., 1996: United States
A. quadratus Emery, 1895: Bolivia
A. quirozi Mackay, W.P., 1996: Mexico
A. sanchezorum Mackay, W.P., 1985: Colombia
A. serratulus (Smith, F., 1858): Brazil
A. skwarrae Wheeler, W. M., 1934: Mexico
A. texanus Forel, 1904: United States
A. truncatus Mackay, W.P., 1996: Colombia
Aenictogiton
Type-species
Aenictogiton fossiceps, by monotypy.
This Afrotropical genus was until recently known only from male specimens and little is known about its biology except that it is likely a subterranean nester and forager.
Diagnosis
Worker. The workers of the one Aenictogiton species for which this caste is known so far are unique in having propodeal spiracles situated high on the sclerite and propodeal lobes reduced, pygidium large but not armed with modified setae, and possessing marked constrictions between abdominal segments IV, V, and VI. Small body size is also characteristic, with mesosoma length under 0.65 mm in the only species known from workers. The same characters will serve to distinguish Aenictogiton from other Afrotropical dorylines that either have spiracles situated low on the propodeum, propodeal lobes well-developed and pygidium armed (Eburopone, Lioponera, Ooceraea, Parasyscia, Zasphinctus) or are markedly larger and have at most weakly impressed abdominal sternites at junction of segments IV, V, and VI, never conspicuous constrictions on both tergites and sternites (Aenictus, Dorylus).
Male. Aenictogiton males have distinctive wing venation where cross-vein cu-a in the fore wing arises proximal to M·f1, R·f3 is absent and is Rs·f3 ‘hanging’ free in the submarginal cell in the absence of Rs·f2. This, combined with the ‘army ant-like’ habitus that includes the lack of constriction between abdominal segments III and IV (no postpetiole), will serve to distinguish it from all other dorylines. The two other army ant genera that occur in the Afrotropics, Aenictus and Dorylus, do not have free Rs·f3 in fore wings.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum without median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 1-segmented. Labial palps 1-segmented. Mandibles falcate, with teeth on elongated masticatory margin. Eyes absent. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture conspicuous and complete, but immobile. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity absent, but a minute pit present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated high on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and sculptured but not cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI present. Girdling constriction between pre- and poststernites of abdominal segments V and VI present. Pygidium large, without impressed medial field, and simple, not armed with cuticular spines or modified setae. Hypopygium unarmed. Legs: Mid tibia with single simple/barbulate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Apparently monomorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical, reduced small, single vertical carina. Maxillary palps 1-segmented. Labial palps 1-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent but horizontal depression may be present. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head); all apodemes very short. Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere expanded at apex. Volsella narrow, hook-shaped, occasionally forming two hooks at apex. Penisvalva laterally compressed, narrow and lance-shaped at apex. Legs: Mid tibia with pectinate and simple spur. Hind tibia with pectinate and simple spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, disconnected from Rs+M. Cross-vein 2r-rs present, connected to Rs·f2–3&Rs·f4. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein Sc+R+Rs present. Vein R in hind wing present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, contiguous with Rs·f2. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing absent. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Not described.
Larva. Not described.
Distribution
This is an exclusively Afrotropical lineage and most species have been described from the Congo Basin but records extend to southern and eastern Africa.
Taxonomy and phylogeny
The taxonomic history of Aenictogiton begins with Emery’s description of A. fossiceps, a male-based taxon that he placed in the Dorylinae (
Aenictogiton is the sister taxon to Dorylus (
Biology
Virtually nothing is known about the biology of Aenictogiton. Most records of males coming to light are associated with forest habitats (
Species of Aenictogiton
A. attenuatus Santschi, 1919b: Democratic Republic of the Congo
A. bequaerti Forel, 1913a: Democratic Republic of the Congo
A. elongatus Santschi, 1919b: Democratic Republic of the Congo
A. emeryi Forel, 1913a: Democratic Republic of the Congo
A. fossiceps Emery, 1901b: Democratic Republic of the Congo
A. schoutedeni Santschi, 1924: Democratic Republic of the Congo
A. sulcatus Santschi, 1919b: Democratic Republic of the Congo
Aenictus
= Paraenictus Wheeler, W. M., 1929
= Typhlatta Smith, 1857
Type-species
Aenictus ambiguus, by original designation.
This Old World lineage contains some of the more conspicuous army ants and is the largest doryline genus with 183 described species.
Diagnosis
Worker. The workers of Aenictus be recognized by a combination of 8 to 10-segmented antennae, propodeal spiracle positioned high on the propodeum, and conspicuously binodal waist (abdominal segment IV is conspicuously the largest abdominal segment). Aenictus is most similar to the New World genus Neivamyrmex, which can be distinguished by 12-segmented antennae. Two other army ant genera co-occur with Aenictus: Aenictogiton and Dorylus. In Aenictogiton there are also constrictions between abdominal segments IV–VI, absent from Aenictus. Dorylus has a uninodal waist with no tapering towards the anterior of abdominal segment IV.
Male. The males of Aenictus are of decidedly army ant-like habitus and distinguishable from other dorylines by a combination of single segment in the waist, femora never extremely flattened relative to tibia, M·f1 vein of fore wing situated distal or near to cu-a, Rs·f2–3 absent, pterostigma broad and conspicuous. All New World army ant genera with similar habitus can be distinguished by fore wing venation, in particular presence of Rs·f2–3 and marginal cell closed along the leading edge by R·f3 connected to Rs·f5. In the Old World, Aenictogiton males can be easily told apart by their ‘hanging’ Rs·f2–3 vein in the fore wing, while Dorylus have a narrow pterostigma and dramatically flattened femora that contrast with tibiae that are more circular in cross-section.
Description
Worker.Head: Antennae with 8, 9, or 10 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined to moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges absent or reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes not projecting beyond inner margin of sclerite, prementum exposed when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth or with one median tooth, or falcate. Eyes absent. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen; in some species differentiation weak. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture completely fused; A. philippinensis group species with grooved cuticular lip anteriorly. Mesometapleural groove not impressed to deeply impressed, conspicuous. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity absent. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent or present. Propodeal spiracle situated high on sclerite. Propodeal declivity with or without distinct dorsal edge or margin and triangular or broadly oval in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, short. Metasoma: Petiole anterodorsally marginate with carina low on anterior face, dorsolaterally immarginate, and laterally above spiracle immarginate or marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and infraaxial. Prora forming a V-shaped protrusion or narrowed into anteriorly directed spine. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate, dorsolaterally immarginate. Abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV a gradual concavity, not gutter-like. Abdominal segment IV conspicuously the largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium small, reduced to narrow strip, without impressed medial field and simple, not armed with cuticular spines or modified setae. Hypopygium unarmed. Legs: Mid tibia with two spurs, one barbulate and one simple, or with two simple spurs. Hind tibia with two barbulate/simple spurs or with one barbulate and one pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle to patch occupying at least half of tibia length. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic to moderately polymorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical, reduced to vertical carina or entirely absent. Maxillary palps 2-segmented. Labial palps 1-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI circular, oval, or slit-shaped. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula strap-like, very short relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, basimere with no sulcus trace at junction, and ventrally with left and right arms separated. Telomere expanded at apex. Volsella variable. Penisvalva not flattened at apex, expanded. Legs: Mid tibia without spurs or with two simple spurs. Hind tibia without spurs or with two simple spurs. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, narrow, demilanceolate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 absent. Cross-vein 2r-rs present, connected to Rs·f2–3&Rs·f4. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, reaching or not reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent or present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, contiguous with Rs·f2. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Dichthadiiform, blind and with one or none ocelli, so far known in 13 species (
Larva. Larvae of several Indomalayan and Australasian Aenictus species have been described (
Distribution
Aenictus is widely distributed in the Old World. The vast majority of species is found in Southeast Asia, with the Afrotropics being the other center of diversity. A few species range into the southern parts of the Palearctic region, and there is a number of species known from Australia.
Taxonomy and phylogeny
The phylogenetic position of Aenictus has been difficult to infer. Phylogenomic data suggests that it is sister to the Aenictogiton plus Dorylus clade but they also show that these two lineages diverged very long ago, most likely in the Cretaceous (Borowiec, in prep.). The comprehensive morphology-based study of
Aenictus was first described based on a male from India, named for its ‘aenigmatical structure’ by
The trend of describing unassociated males unfortunately continued and Aenictus is an example of ‘dual taxonomy’. Many names are either worker- or male-based, and there is no single species known from workers, queens and males (
Biology
Given the number of described species and their abundance and importance as insect predators in the Old World tropics, the biology of Aenictus is poorly studied. The impressive species and morphological diversity is likely reflected in the diversity of habits, although all thus far observed species seem to be specialized predators of other ants (but see
Species of Aenictus
A. abeillei (André, 1886): Algeria
A. acerbus Shattuck, 2008: Australia
A. aitkenii Forel, 1901a: India
A. alluaudi Santschi, 1910c: Kenya
A. alluaudi falcifer Santschi, 1924: Democratic Republic of the Congo
A. alticola Wheeler, W. M. and Chapman, 1930a: Philippines
A. ambiguus Shuckard, 1840b: India
A. anceps Forel, 1910b: Eritrea
A. annae Forel, 1911a: Indonesia (Java)
A. appressipilosus Jaitrong andYamane, 2013: Malaysia (Sabah)
A. arabicus Sharaf and Aldawood, 2012: Saudi Arabia
A. aratus Forel, 1900a: Australia
A. artipus Wilson, 1964: Thailand
A. arya Forel, 1901a: India
A. asantei Campione, Novak and Gotwald, 1983: Ghana
A. asperivalvus Santschi, 1919a: Ivory Coast
A. bakeri Menozzi, 1925: Philippines
A. baliensis Jaitrong and Yamane, 2013: Indonesia (Bali)
A. bayoni Menozzi, 1932: Uganda
A. binghami Forel, 1900a: Myanmar
A. biroi Forel, 1907a: Sri Lanka
A. bobaiensis Zhou and Chen, 1999: China
A. bodongjaya Jaitrong and Yamane, 2011a: Indonesia (Sumatra)
A. bottegoi Emery, 1899a: Ethiopia
A. bottegoi noctivagus Santschi, 1913: Ethiopia
A. brazzai Santschi, 1910: Republic of the Congo
A. breviceps Forel, 1912b: Indonesia (Java)
A. brevicornis (Mayr, 1879): India
A. brevinodus Jaitrong and Yamane, 2011a: Indonesia (Sulawesi)
A. brevipodus Jaitrong and Yamane, 2013: Vietnam
A. buttelreepeni Forel, 1913c: Indonesia (Sumatra)
A. buttgenbachi Forel, 1913a: Democratic Republic of the Congo
A. camposi Wheeler, W. M. and Chapman, 1925: Philippines
A. carolianus Zettel and Sorger, 2010: Philippines
A. certus Westwood, 1842: India
A. ceylonicus (Mayr, 1866a): Sri Lanka
A. changmaianus Terayama and Kubota, 1993: Thailand
A. chapmani Wilson, 1964: Papua New Guinea
A. clavatus Forel, 1901a: India
A. clavatus atripennis Forel, 1913c: Indonesia (Sumatra)
A. clavatus kanariensis Forel, 1901a: India
A. clavatus sundaicus Forel, 1909c: Indonesia (Java)
A. clavitibia Forel, 1901a: India
A. clavitibia facetus Forel, 1911a: Indonesia (Java)
A. concavus Jaitrong and Yamane, 2013: Thailand
A. congolensis Santschi, 1911a: ‘Congo français’
A. cornutus Forel, 1900a: Malaysia (Sarawak)
A. crucifer Santschi, 1914a: Kenya
A. crucifer tuberculatus Arnold, 1915: Zimbabwe
A. currax Emery, 1900a: Papua New Guinea
A. cylindripetiolus Jaitrong and Yamane, 2013: Thailand
A. decolor (Mayr, 1879): ‘Ost-Afrika’
A. dentatus Forel, 1911c: Malaysia (Negeri Sembilan)
A. diclops Shattuck, 2008: Australia
A. dlusskyi Arnol’di, 1968: Armenia
A. doryloides Wilson, 1964: India
A. doydeei Jaitrong and Yamane, 2011b: Laos,
A. duengkaei Jaitrong and Yamane, 2012: Thailand
A. eguchii Jaitrong and Yamane, 2013: Vietnam
A. eugenii Emery, 1895a: South Africa
A. eugenii caroli Forel, 1910b: Eritrea
A. eugenii henrii Santschi, 1924: Democratic Republic of the Congo
A. exilis Wilson, 1964: Papua New Guinea
A. feae Emery, 1889: Myanmar
A. fergusoni Forel, 1901a: India
A. foreli Santschi, 1919a: Ivory Coast
A. formosensis Forel, 1913b: Taiwan
A. fuchuanensis Zhou, 2001: China
A. fulvus Jaitrong and Yamane, 2011a: Thailand
A. furculatus Santschi, 1919a: Senegal
A. furculatus andrieui Santschi, 1930: Sudan
A. furibundus Arnold, 1959: Zimbabwe
A. fuscipennis Forel, 1913c: Indonesia (Sumatra)
A. fuscovarius Gerstäcker, 1859: Mozambique
A. fuscovarius laetior Forel, 1910b: Eritrea
A. fuscovarius magrettii Emery, 1892: Sudan
A. fuscovarius sagittarius Santschi, 1938: Egypt
A. gibbosus Dalla Torre, 1893: Indonesia (Sumatra)
A. gibbosus ashaverus Forel, 1913c: Indonesia
A. glabratus Jaitrong and Nur-Zati, 2010: Malaysia (Selangor)
A. glabrinotum Jaitrong and Yamane, 2011: Malaysia (Sabah)
A. gleadowii Forel, 1901a: India
A. gonioccipus Jaitrong and Yamane, 2013: Indonesia (Sulawesi)
A. gracilis Emery, 1893b: Malaysia (Sarawak)
A. grandis Bingham, 1903: Myanmar
A. gutianshanensis
A. hamifer Emery, 1896d: Ethiopia/Somalia
A. hamifer spinosior Stitz, 1917: Algeria
A. henanensis Li and Wang, 2005: China
A. hilli Clark, 1928: Australia
A. hodgsoni Forel, 1901a: Myanmar
A. hoelldobleri Staab, 2015: China
A. hottai Terayama and Yamane, 1989: Indonesia (Sumatra)
A. humeralis Santschi, 1910c: Mali
A. humeralis chevalieri Santschi, 1910c: Senegal
A. humeralis viridans Santschi, 1915: Benin
A. huonicus Wilson, 1964: Papua New Guinea
A. icarus Forel, 1911a: Indonesia (Java)
A. icarus incautus Forel, 1911a: Indonesia (Java)
A. idoneus Menozzi, 1928: Indonesia (Java)
A. inconspicuus Westwood, 1845: South Africa
A. indicus Bharti, Wachkoo and Kumar, 2012: India
A. inflatus Yamane and Hashimoto, 1999: Malaysia (Sarawak)
A. itoi Jaitrong and Yamane, 2013: Indonesia (Sumatra)
A. jacobsoni Forel, 1909c: Indonesia (Java)
A. jarujini Jaitrong and Yamane, 2010a: Thailand
A. javanus Emery, 1896a: Indonesia (Java)
A. jawadwipa Jaitrong and Yamane, 2013: Indonesia (Java)
A. khaoyaiensis Jaitrong and Yamane, 2013: Thailand
A. kutai Jaitrong and Yamane, 2013: Indonesia
A. laeviceps (Smith, F., 1857): Malaysia (Sarawak)
A. latifemoratus Terayama and Yamane, 1989: Indonesia (Sumatra)
A. latiscapus Forel, 1901a: India
A. latiscapus fumatus Wheeler, W. M., 1927: China
A. latiscapus sauteri Forel, 1913b: Taiwan
A. leliepvrei Bernard, 1953a: Algeria
A. leptotyphlatta Jaitrong and Eguchi, 2010: Thailand
A. levior (Karavaiev, 1926): Indonesia (Buru Is.)
A. lifuiae Terayama, 1984: Taiwan
A. longi Forel, 1901a: India
A. longi taivanae Forel, 1913b: Taiwan
A. longicephalus Jaitrong and Yamane, 2013: Indonesia (Lombok)
A. longinodus Jaitrong and Yamane, 2012: Thailand
A. luteus Emery, 1892: Sierra Leone
A. luteus moestus Santschi, 1930: Mali
A. luzoni Wheeler, W. M. and Chapman, 1925: Philippines
A. maneerati Jaitrong and Yamane, 2013: Thailand
A. mariae Emery, 1895a: South Africa
A. mariae natalensis Forel, 1901c: South Africa
A. mauritanicus Santschi, 1910c: probably Morocco
A. mentu Weber, 1942: South Sudan
A. minimus Jaitrong and Hashimoto, 2012: Vietnam
A. minipetiolus Jaitrong and Yamane, 2013: Indonesia (Lombok)
A. minutulus Terayama and Yamane, 1989: Indonesia (Sumatra)
A. mocsaryi Emery, 1901c: Papua New Guinea
A. moebii Emery, 1895b: Togo
A. moebii sankisianus Forel, 1913a: Democratic Republic of the Congo
A. montivagus Jaitrong and Yamane, 2011a: Malaysia (Sabah)
A. mutatus Santschi, 1913: Ivory Coast
A. mutatus pudicus Santschi, 1919a: Ivory Coast
A. nesiotis Wheeler, W. M. and Chapman, 1930b: Philippines
A. nganduensis Wilson, 1964: Papua New Guinea
A. nishimurai Terayama and Kubota, 1993: Thailand
A. obscurus Smith, F., 1865: ‘New Guinea’
A. orientalis (Karavaiev, 1926): Indonesia (Aru Is.)
A. pachycerus (Smith, F., 1858): India
A. pangantihoni Zettel and Sorger, 2010: Philippines
A. paradentatus Jaitrong and Yamane, 2012: Thailand
A. parahuonicus Jaitrong and Yamane, 2011a: Thailand
A. peguensis Emery, 1895c: Myanmar
A. pfeifferi Zettel and Sorger, 2010: Malaysia (Sarawak)
A. pharao Santschi, 1924: Sudan
A. philiporum Wilson, 1964: Australia
A. philippinensis Chapman, 1963: Philippines
A. piercei Wheeler, W. M. and Chapman, 1930d: Philippines
A. pilosus Jaitrong and Yamane, 2013: Philippines
A. pinkaewi Jaitrong and Yamane, 2013: Thailand
A. porizonoides Walker, 1860: Sri Lanka
A. powersi Wheeler, W. M. and Chapman, 1930e: Philippines
A. prolixus Shattuck, 2008: Australia
A. pubescens Smith, F., 1859: India
A. punctatus Jaitrong and Yamane, 2012: Brunei
A. punctiventris Emery, 1901b: Indonesia (Laut Island)
A. punctiventris scutellaris Forel, 1912d: Indonesia (Sumatra)
A. punensis Forel, 1901a: India
A. rabori Chapman, 1963: Philippines
A. raptor Forel, 1913a: Democratic Republic of the Congo
A. reyesi Chapman, 1963: Philippines
A. rhodiensis Menozzi, 1936: Greece
A. rixator Forel, 1901: South Africa
A. rotundatus Mayr, 1901: South Africa
A. rotundatus guineensis Santschi, 1924: Guinea
A. rotundatus merwei Santschi, 1932: South Africa
A. rotundicollis Jaitrong and Yamane, 2011a: Malaysia (Sarawak)
A. rougieri André, 1893: Tunisia
A. sagei Forel, 1901a: India
A. schneirlai Wilson, 1964: Papua New Guinea
A. seletarius Wong and Guénard, 2016: Singapore
A. shillongensis Mathew and Tiwari, 2000: India
A. shuckardi Forel, 1901a: India
A. siamensis Jaitrong and Yamane, 2011a: Thailand
A. silvestrii Wheeler, W. M., 1929: West Malaysia
A. sirenicus Yamane and Wang, 2015: Malaysia (Sabah)
A. sonchaengi Jaitrong and Yamane, 2011a: Thailand
A. soudanicus Santschi, 1910c: Senegal?
A. soudanicus brunneus Forel, 1913a: Democratic Republic of the Congo
A. spathifer Santschi, 1928: Indonesia (Sumatra)
A. steindachneri Mayr, 1901: South Africa
A. stenocephalus Jaitrong, Yamane and Wiwatwitaya, 2010: Thailand
A. subterraneus Jaitrong and Hashimoto, 2012: Malaysia (Sabah)
A. sulawesiensis Jaitrong and Yamane, 2013: Indonesia (Sulawesi)
A. sumatrensis Forel, 1913c: Indonesia (Sumatra)
A. sumatrensis maxillosus Forel, 1913c: Indonesia (Sumatra)
A. sundalandensis Jaitrong and Yamane, 2013: Indonesia (Java)
A. thailandianus Terayama and Kubota, 1993: Thailand
A. togoensis Santschi, 1915: Togo
A. trigonus Forel, 1911a: Indonesia (Java)
A. turneri Forel, 1900a: Australia
A. vagans Santschi, 1924: Niger
A. vaucheri Emery, 1915c: Morocco
A. vieti Jaitrong and Yamane, 2010a: Vietnam
A. villiersi Bernard, 1953b: Guinea
A. watanasiti Jaitrong and Yamane, 2013: Thailand
A. wayani Jaitrong and Yamane, 2011a: Indonesia (Sulawesi)
A. weissi Santschi, 1910: Democratic Republic of the Congo
A. westwoodi Forel, 1901a: India
A. wilaiae Jaitrong and Yamane, 2013: Thailand
A. wilsoni Bharti, Wachkoo and Kumar, 2012: India
A. wiwatwitayai Jaitrong and Yamane, 2013: Thailand
A. wroughtonii Forel, 1890: India
A. wudangshanensis Wang, W., 2006: China
A. yamanei Wiwatwitaya and Jaitrong, 2011: Malaysia (Sarawak)
A. yangi Liu, Hita Garcia, Peng and Economo, 2015: China
A. zhengi Zhang, 1995: China
Cerapachys
= Ceratopachys Schulz, 1906
Type-species
Cerapachys antennatus, by subsequent designation of Bingham, 1903
This relatively species-poor lineage is apparently restricted to forest habitats of Southeast Asia.
Diagnosis
Worker. Cerapachys belongs to non-army ant dorylines with spiracle positioned below midheight of the propodeum and pygidium well-developed, armed with modified setae. It has a well-developed carina on the pronotal collar and a distinct pronotomesopleural Pronotomesopleural suture, a single pectinate spur on each mid and hind tibia, and helcium positioned supraaxially, above midheight of abdominal segment III. Some species have pretarsal claws armed with a tooth. Cerapachys is a genus of medium-sized, universally dark-colored ants that could be confused Lividopone. Distributions of the two genera do not overlap, however, with Lividopone being so far known only from Madagascar. Lividopone is further distinguished by almost complete fusion of pronotomesopleural Pronotomesopleural suture, which is unfused in Cerapachys. Lioponera overlaps in range with Cerapachys and certain species can be superficially similar but a more narrow and axially positioned helcium, dorsolaterally carinate petiole, and a flange on the posterior face of the coxae will distinguish Lioponera.
Male. The male of Cerapachys has 12-segmented antennae, a transverse groove running diagonally across the mesopleuron, vein C in fore wing present, one submarginal cell closed by Rs·f2–3, 2rs-m absent, and marginal cell closed by R·f3 and Rs·f4–5. Neocerapachys males have similar wing venation but in Cerapachys the antennal segment III is similar in length to segment IV, while in Neocerapachys the segment III is conspicuously the shortest antennal segment. Furthermore, Neocerapachys is only found in the New World.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus with or without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 3-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth. Eyes present, composed of more than 20 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron absent. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally marginate, dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field, and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple or each claw armed with a tooth. Polymorphism: Monomorphic to moderately polymorphic.
Male.Head: Antennae with 13 segments. Clypeus with cuticular apron. Parafrontal ridges present. Torulo-posttorular complex vertical. Maxillary palps 5-segmented. Labial palps likely 3-segmented (uncertain in-situ count). Mandibles triangular, edentate or crenulate. Ventrolateral margins of head with cuticular ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge or not separated. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity reduced, with or without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate or marginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV, occasionally slightly smaller; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured or cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines. with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal glands absent. Hind pretarsal claws each armed with a tooth; simple claws not observed but presumably absent from certain species as in worker. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M or separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, not reaching wing margin to almost reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing fused with M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing absent. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Alate, fully winged with large eyes and three ocelli, or also possibly ergatoid (
Larva. Not described. Cocoons absent.
Distribution
Cerapachys is distributed from northwestern India and Tibet, through southern China to Java, Borneo and the Philippines.
Taxonomy and phylogeny
This is the lineage where the type species of Cerapachys, C. antennatus, belongs. The type series was collected by A. R. Wallace in Sarawak and described by F. Smith in 1857.
Cerapachys is a member of a predominantly South East Asian clade that also includes Chrysapace and Yunodorylus (Borowiec, in prep.). All three lineages diverged long ago and although it seems that Cerapachys is the sister genus to Chrysapace, this relationship is not certain.
Biology
Almost nothing is known about the biology of this group. Most records seem to come from forest habitats. Brood development may be synchronized, based on the author’s observation of brood of uniform size in the single examined nest collection of C. antennatus.
Species of Cerapachys
C. antennatus Smith, F., 1857: Malaysia (Sarawak)
C. jacobsoni Forel, 1912b: Indonesia (Java)
C. manni Crawley, 1926: Indonesia (Sumatra)
C. sulcinodis Emery, 1889c: Myanmar
C. xizangensis Tang and Li, 1982: Tibet
Cheliomyrmex
Type-species
Cheliomyrmex nortoni (junior synonym of Labidus morosus), by monotypy.
Cheliomyrmex is a rarely encountered genus of New World army ants that is a mostly subterranean predator with likely a specialized diet.
Diagnosis
Worker. Workers of Cheliomyrmex can be recognized by a combination of propodeal spiracle positioned high on the propodeum, propodeal declivity simple and not armed with cuticular ridges or denticles, abdominal segment III small but broad posteriorly and thus waist appearing one-segmented, pygidium small and armed with at most a pair of modified setae, and pretarsal claws armed with a tooth. Cheliomyrmex is perhaps most similar to Labidus and certain Neivamyrmex but it is unique among New World army ants in having abdominal segment III broadly attached to segment IV (i.e. it has a uninodal waist) and thus easily told apart from all other army ant genera in this region.
Male. The males of Cheliomyrmex share the following wing venation characters with other New World army ants (Eciton, Labidus, Neivamyrmex and Nomamyrmex): costal (C) vein present in the fore wing, relatively narrow pterostigma, presence of vein 2rs-m and two closed submarginal cells, marginal cell closed by R·f3 and Rs·f4–5, 2rs-m present, and M·f1 vein arising from M+Cu at an angle lower than 45° and conspicuously proximal to cu-a. This characteristic venation pattern serves to distinguish New World army ants from the Old World army ants (Aenictogiton, Aenictus, Dorylus) that have no vein R·f3 and where M·f1 arises near cu-a and at an angle close to or higher than 45°. Aenictus and Dorylus additionally have no vein Rs·f2–3 and so only one submarginal cell that is closed distally by 2rs-m. In other dorylines with well-developed wing venation (e.g. Chrysapace, Cylindromyrmex) the vein M·f1 arises distal to cu-a and pterostigma is very broad and conspicuous. Within the New World army ants, wing venation is relatively conserved and thus of little use in discrimination of genera. Genitalic characters have been found to be the most reliable (
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes not projecting beyond inner margin of sclerite, prementum exposed when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 3-segmented. Mandibles polymorphic, from triangular with teeth through triangular with median tooth to falcate, with teeth on elongated masticatory margin. Eyes present, composed of 1–5 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated high on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and infraaxial. Prora narrowed into anteriorly directed spine. Spiracle openings of abdominal segments IV–VI oval. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium small, reduced to narrow strip, without impressed medial field, and simple, not armed with cuticular spines or modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws each armed with a tooth. Polymorphism: Polymorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 3-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI slit-shaped. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two outer spines and additional two inner denticles, with lateral apodemes longer than much reduced medial apodeme, directed anteriorly (towards head). Genitalia: Cupula very long, nearing or surpassing length of rest of genital capsule and shorter ventrally than dorsally. Basimere narrowly fused to telomere, with sulcus visible at least partway through junction, and ventrally with left and right arms abutting. Telomere expanded at apex. Volsella narrow, hook-shaped. Penisvalva not flattened at apex, expanded. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma narrow. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing present. Vein R in hind wing present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, reaching wing margin. Cross-vein 1rs-m in hind wing present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Not described.
Larva. Larvae of Cheliomyrmex megalonyx have been described (
Distribution
Cheliomyrmex is present in most of Central America, including southern Mexico, Belize, Guatemala, Honduras, and Panama, but so far it has not been collected in Nicaragua or Costa Rica. It is also known from northern and northwestern South America south to Peru and Bolivia.
Taxonomy and phylogeny
Cheliomyrmex was introduced by Mayr in 1870 who described C. nortoni, now a junior synonym of C. morosus (
Biology
Ants in this lineage have been rarely observed or collected. The raids and emigrations of these ants are mostly subterranean, only occasionally seen above ground. Raids have been observed mostly under stones or rotting wood (
Species of Cheliomyrmex
C. andicola Emery, 1894: Peru
C. audax Santschi, 1921b: Ecuador
C. megalonyx Wheeler, W. M., 1921: Guyana
C. morosus (Smith, 1859): Mexico
Chrysapace , gen. rev.
Type-species
Chrysapace jacobsoni, by monotypy.
Chrysapace is the only extant doryline genus also known from Baltic amber (late Eocene). These ants are extremely rarely collected and no observations of their biology have ever been published.
Diagnosis
Worker. The workers of this lineage are recognizable by a combination of prominent costate sculpture present on most of body surface, large eyes, exposed antennal sockets, two spurs on mid and hind tibiae, and pretarsal claws with a tooth. The New World Cylindromyrmex are the only other dorylines that have two pectinate tibial spurs and strongly costate or rugose sculpture but they are recognized by at least moderately developed antennal scrobes and horizontal torulo-posttorular complex that partly conceals antennal sockets. In Chrysapace there are no scrobes and antennal sockets are fully exposed.
Male. The males share the characteristic spur formula with the workers, have a well-defined groove on the mesopleuron, two submarginal cells, the marginal cell enclosed by R·f1 and Rs·f4–5, and pretarsal claws armed with a tooth. Acanthostichus and Cylindromyrmex can have similar wing venation. The former has only one pectinate tibial spur on each mid and hind tibiae and the latter has no transverse groove on the mesopleuron and simple pretarsal claws. Males attributed to Procerapachys also have similar wing venation but only a single tibial spur and no transverse groove on the mesopleuron.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 5-segmented. Labial palps 3-segmented. Mandibles triangular, with teeth. Eyes present, composed of more than 20 ommatidia. Ocelli present. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate or marginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove replaced by cuticular ridge. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity absent. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with or without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally marginate, dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and sculptured, cross-ribbed or not. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field and armed with modified setae and sometimes emarginate to deeply notched. Hypopygium unarmed. Legs: Mid tibia with two pectinate spurs. Hind tibia with two pectinate spurs or with one barbulate and one pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal gland absent. Hind pretarsal claws each armed with a tooth, sometimes very small. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges present. Torulo-posttorular complex vertical. Maxillary palps unknown. Labial palps unknown. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen unknown. Mesosoma: Pronotal flange separated from collar by distinct ridge. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening present. Propodeal lobes present. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms separated. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with two pectinate spurs. Hind tibia with two pectinate spurs. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing fused with M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Alate, similar to worker except for flight-adapted mesosoma. See
Larva. Not described. Presence of cocoons unknown.
Distribution
This rarely collected lineage is represented by at least four extant species of unusual geographic distribution. Chrysapace costatus, C. crawleyi and C. sauteri occur in Asia, while an additional, undescribed species has been recently found in northern Madagascar (Brian Fisher pers. comm.). One species is known from Baltic amber.
Taxonomy and phylogeny
The genus Chrysapace was proposed by Crawley in 1924 for C. jacobsoni from Sumatra as distinct from the then recognized Cerapachys and Phyracaces. The same year W. M.
Chrysapace is a member of a well-supported clade that also includes Cerapachys and Yunodorylus, and is possibly the sister genus of Cerapachys although this relationship received less support (Borowiec, in prep.).
Biology
To the best of my knowledge, nothing on the foraging, nesting, or other aspects of Chrysapace biology has ever been published.
Species of Chrysapace
C. costatus (Bharti and Wachkoo, 2013): India, comb. n.
C. jacobsoni Crawley, 1924: Indonesia (Sumatra), nom. rev.
C. sauteri (Forel, 1913b): Taiwan, comb. n.
Cylindromyrmex
= Holcoponera Cameron, 1891
= Hypocylindromyrmex Wheeler, W. M., 1924a
= Metacylindromyrmex Wheeler, W. M., 1924a
Type-species
Cylindromyrmex striatus, by monotypy.
Cylindromyrmex is a genus of mostly arboreal-nesting termite hunters, rarely encountered but distributed throughout New World tropics, including the Antilles.
Diagnosis
Worker. With a combination of large eyes, conspicuously costate or striate sculpture, torulo-posttorular complex horizontal and concealing antennal sockets, two pectinate spurs on mid and hind tibiae, and simple pretarsal claws, the workers of Cylindromyrmex can be readily distinguished from all other dorylines. The only other genus with large eyes, conspicuously sulcate sculpture, and two tibial spurs is Chrysapace, but it has fully exposed antennal sockets, possesses toothed pretarsal claws, and occurs only in the Old World. The extinct Procerapachys, which can also have sulcate sculpturing, has a single pectinate spur on each mid and hind tibiae.
Male. The males of Cylindromyrmex are also easily differentiated from all other genera by two tibial spurs, simple pretarsal claws, no transverse groove on the mesopleuron, and well-developed wing venation with costal (C) vein present in fore wing, two submarginal cells and marginal cell closed. The only other genus with two tibial spurs and similar venation is the Old World genus Chrysapace, but it has a transverse groove on the mesopleuron and pretarsal claws armed with a tooth. Putative males of the extinct Procerapachys have only one spur on each mid and hind tibiae.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges absent. Torulo-posttorular complex horizontal. Antennal scrobes present. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 3- or 2-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth or edentate. Eyes present, always composed of more than 5 ommatidia and usually more than 20 ommatidia. Ocelli present or absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge or not. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland bulla visible or not visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, dorsolaterally marginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI present or absent. Pygidium large, with impressed medial field and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with two pectinate spurs. Hind tibia with two pectinate spurs. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 3- or 2-segmented. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge or not separated. Notauli absent or present. Transverse groove dividing mesopleuron absent. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening present. Propodeal lobes present. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured or cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella laterally flattened, at apex with dorsal lobe and hooked ventrally. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with two pectinate spurs. Hind tibia with two pectinate spurs. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing fused with M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Alate, similar to worker except for the mesosoma; known for several species. See descriptions in
Larva. Not described. Cocoons absent.
Distribution
Cylindromyrmex is an exclusively Neotropical lineage with ten extant species and three extinct species known from Dominican amber (
Taxonomy and phylogeny
Cylindromyrmex has three generic synonyms: Holcoponera Cameron, Hypocylindromyrmex Wheeler, and Metacylindromyrmex Wheeler. Cameron’s Holcoponera has been considered a synonym since the end of 19th century (
Cylindromyrmex is the sister genus to Acanthostichus (
Biology
Members of this lineage have been reported to be termite predators (
A colony of C. whymperi has been recently found in Peru and studied in captivity by Josh Richards, an ant keeper from Lima, Peru. He has observed that these ants readily pursue and sting termites, which are brought to the nest paralyzed but apparently not dead. When outnumbered in a confrontation, Cylindromyrmex workers first sting as many termites as possible before attempting to carry some of them back to the nest (Josh Richards pers. comm.).
All known queens of Cylindromyrmex are winged and brood production is apparently synchronized (
Species of Cylindromyrmex
†C. antillanus De Andrade, 1998a: Dominican amber
C. boliviae Wheeler, W. M., 1924a: Bolivia
C. brasiliensis Emery, 1901a: Brazil
C. brevitarsus Santschi, 1925: Brazil
C. darlingtoni Wheeler, W. M., 1937: Cuba
†C. electrinus De Andrade, 1998a: Dominican amber
C. escobari De Andrade, 1998a: Colombia
C. godmani Forel, 1899: Panama
†C. inopinatus De Andrade, 2001: Dominican amber
C. longiceps André, 1892: Brazil
C. meinerti Forel, 1905: Venezuela
C. striatus Mayr, 1870: Suriname
C. whymperi (Cameron, 1891): Ecuador
Dorylus
= Alaopone Emery, 1881, syn. n.
= Anomma Shuckard, 1840c, syn. n.
= Cosmaecetes Spinola, 1851
= Dichthadia Gerstäcker, 1863, syn. n.
= Rhogmus Shuckard, 1840c, syn. n.
= Shuckardia Emery, 1895b
= Sphecomyrmex Schulz, 1906
= Sphegomyrmex Imhoff, 1852
= Typhlopone Westwood, 1839, syn. n.
Type-species
Vespa helvola, by monotypy.
The Afrotropical ‘driver ants’ of this genus epitomize the army ant lifestyle, but they represent only a fraction of the diversity of Dorylus. Most species are much less commonly observed, and forage underground or in leaf litter.
Diagnosis
Worker. The workers of Dorylus are readily recognized by a combination of well-developed promesonotal Pronotomesopleural suture, propodeal spiracle positioned high on the propodeum and lack of propodeal lobes, single waist segment, pygidium large and with a flattened surface and armed with two cuticular projections, and pretarsal claws simple. Other army ants of the Old World, Aenictus and Aenictogiton, are not easily confused with Dorylus as the former always has a well-differentiated second waist segment (postpetiole) and in Aenictogiton the gaster has more developed constrictions between gastral pre- and post-sclerites, resulting in apparent constriction between abdominal segments IV, V, and VI. Yunodorylus is superficially similar but is easily distinguished from all army ants by the propodeal spiracle situated low and presence of propodeal lobes. Among the New World army ants only Cheliomyrmex has one-segmented waist but Cheliomyrmex does not have a promesonotal Pronotomesopleural suture, its pygidium is reduced and never armed with cuticular projections, and its pretarsal claws are armed with a tooth.
Male. In general appearance Dorylus males are similar to other army ant genera but possess flattened femora that are much broader and more compressed than the tibiae and tarsi. This trait alone is sufficient to separate them from all other male dorylines, but a combination of single-segmented waist, M·f1 vein of fore wing arising from M+Cu at about 45° and situated near to cu-a, Rs·f2–3 lost, pterostigma narrow and inconspicuous can also be used to recognize Dorylus. The Old World army ant genera Aenictus and Aenictogiton have similar fore wing venation but both have a well-developed and broad pterostigma and the latter has a ‘free-hanging’ Rs·f3 vein. In the New World army ants M·f1 arises at a lower angle and is conspicuously proximal to cu-a, and Rs·f2–3 are present, forming two submarginal cells. Dorylus males also possess unique genital capsule morphology, where a tiny diamond-shaped structure is formed from a fragment of the basimeres and visible dorsally over the aedeagus (‘patella’ of
Description
Worker.Head: Antennae with 8, 9, 11, or 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges absent. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum without median notch or concavity. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2- or 1-segmented. Labial palps 2-segmented. Mandibles elongately triangular to falcate, with teeth on elongated masticatory margin. Eyes absent. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen entirely absent, including ventrally. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture conspicuous and complete, but immobile. Pronotomesopleural suture complete, continuous with promesonotal Pronotomesopleural suture. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated high on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle in smaller workers, mostly obscured in large workers. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and sculptured but not cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI present. Girdling constriction between pre- and poststernites of abdominal segments V and VI present. Pygidium large, with impressed medial field, and armed with cuticular spines. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Highly polymorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical, carinae separated by broad flat or convex area between exposed antennal sockets. Maxillary palps 2- or 1-segmented. Labial palps 1-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI slit-shaped. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes short, directed sideways. Genitalia: Cupula short relative to rest of genital capsule and shorter ventrally than dorsally. Basimere fused basally, with a fragment reduced to tiny, plate-like sclerite. Telomere folding backwards and then over rest of genital capsule, concealing it dorsally. Volsella gradually tapering toward apex. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma narrow. Abscissa R·f3 absent. Abscissae Rs·f2–3 absent. Cross-vein 2r-rs present, connected to Rs·f2–3&Rs·f4. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing unknown. Vein C in hind wing present. Vein R in hind wing present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing present, shorter than M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Dichthadiiform, blind, with median ocellus (see e.g.
Larva. Larvae of Dorylus have been described in
Distribution
Dorylus ranges from Sub-Saharan Africa throughout North Africa and Asia Minor to Borneo in Southeast Asia. The Afrotropics harbor the highest number of species and are the home of the surface- and leaf litter-foraging species.
Taxonomy and phylogeny
The long and confusing taxonomic history of the genus begins with a male ant from South Africa, described as Vespa helvola by Linnaeus in 1764. Later
Dorylus orientalis-group (equivalent of Alaopone), comprising species acutus, aethiopicus, atriceps, attenuatus, brevis, buyssoni, conradti, diadema, distinctus, ductor, katanensis, montanus, orientalis, vishnui.
Dorylus nigricans-group (equivalent of Anomma excluding emeryi and kohli (
Dorylus laevigatus-group (equivalent of Dichthadia), comprising species laevigatus.
Dorylus politus-group (species excluded from Doryluss. str. based on phylogeny in
Dorylus helvolus-group (equivalent of Doryluss. str. but excluding species of politus-group and including two species previously assigned to Anomma), comprising species affinis, agressor, alluaudi, bequaerti, bishyiganus, braunsi, brevipennis, congolensis, depilis, emeryi, faurei, furcatus, gaudens, ghanensis, gribodoi, helvolus, kohli, mandibularis, moestus, schoutedeni, stadelmani, staudingeri, striatidens, titan.
Dorylus fimbriatus-group (equivalent of Rhogmus), comprising species fimbriatus, fuscipennis, leo, ocellatus, savagei, termitarius.
Dorylus fulvus-group (equivalent of Typhlopone), comprising species fulvus, labiatus.
Species unassigned to species-groups: atratus, westwoodii.
Dorylus is the sister taxon to Aenictogiton (
Biology
Because some species of this lineage are so conspicuous and are the most important arthropod predators of the Afrotropics, this group has attracted considerable attention.
The best studied species include the Afrotropical species that forage above ground (
The life cycle of Dorylus colony is similar to that of Eciton and many other army ants but there are no pronounced nomadic and statary phases. The brood production is not synchronized (
Copulation in Dorylus has been observed only once (
The reproductive potential of Dorylus queens is impressive, at least in the surface-foraging species studied thus far. The queen mates between 15–20 times (
These army ants always occupy subterranean nests, either constructed by excavating large amounts of soil and/or taking advantage of a preexisting cavity (
Dorylus emigrate irregularly and the colony often returns to the same nesting spot.
Dorylus gynes may or may not be able to move on their own during nest emigration. All queen specimens known so far are missing tarsal segments (
A diversity of foraging habits and prey preferences has been documented for Dorylus (
Similarly to New World army ants, Dorylus colonies have numerous invertebrate and vertebrate associates, although these companion faunas are not as well described (
A variety of other research has been carried out on Dorylus, but most of these studies are isolated in nature.
Species of Dorylus
D. acutus Santschi, 1937a: Democratic Republic of the Congo
D. aethiopicus Emery, 1895b: ‘Sudan, Abessinien, Tunis’
D. affinis Shuckard, 1840c: Gambia
D. affinis aegyptiacus Mayr, 1865: Egypt
D. affinis denudatus Santschi, 1910c: Niger
D. affinis exilis Santschi, 1914a: Tanzania
D. affinis hirsutus Wheeler, W. M., 1922a: Egypt, Ethiopia
D. affinis loewyi Forel, 1907b: Tanzania
D. affinis parapsidalis Santschi, 1917: Malawi
D. affinis pulliceps Santschi, 1917: Ivory Coast
D. affinis sudanicus Santschi, 1917: Chad
D. affinis ugandensis Santschi, 1914a: Uganda
D. agressor Santschi, 1923b: Democratic Republic of the Congo
D. alluaudi Santschi, 1914a: Uganda
D. alluaudi lobatus Santschi, 1919b: Democratic Republic of the Congo
D. atratus Smith, F., 1859: Nigeria
D. atriceps Shuckard, 1840c: Gambia
D. attenuatus Shuckard, 1840c: Gambia
D. attenuatus acuminatus Emery, 1899b: South Africa
D. attenuatus australis Santschi, 1919a: South Africa
D. attenuatus bondroiti Santschi, 1912: South Africa
D. attenuatus latinodis Forel, 1920: Democratic Republic of the Congo
D. bequaerti Forel, 1913a: Democratic Republic of the Congo
D. bishyiganus (Boven, 1972): Rwanda
D. braunsi Emery, 1895b: Liberia
D. braunsi anceps Forel, 1914: Zimbabwe
D. brevipennis Emery, 1895b: Tanzania
D. brevipennis marshalli Emery, 1901d: Zimbabwe
D. brevipennis zimmermanni Santschi, 1910c: Republic of the Congo
D. brevis Santschi, 1919b: Democratic Republic of the Congo
D. buyssoni Santschi, 1910c: Kenya
D. buyssoni conjugens Santschi, 1910c: Kenya
D. congolensis Santschi, 1910: Republic of the Congo
D. conradti Emery, 1895b: Togo
D. conradti berlandi Santschi, 1926a: Ivory Coast
D. depilis Emery, 1895b: Cameroon
D. depilis clarior Santschi, 1917: Democratic Republic of the Congo
D. diadema Gerstäcker, 1859: Mozambique
D. diadema arnoldi Forel, 1914: Zimbabwe
D. diadema fusciceps Emery, 1899b: Malawi
D. distinctus Santschi, 1910c: Guinea
D. ductor Santschi, 1939: ‘Congo’
D. emeryi Mayr, 1896: Cameroon
D. emeryi opacus Forel, 1909b: Democratic Republic of the Congo
D. emeryi pulsi (Forel, 1904): ‘Afrique occidentale’
D. erraticus (Smith, F., 1865): ‘New Guinea’ (labeling error:
D. faurei Arnold, 1946: South Africa
D. fimbriatus (Shuckard, 1840c): Gambia
D. fimbriatus crampeli Santschi, 1919a: Central African Republic
D. fimbriatus laevipodex Santschi, 1919a: Kenya
D. fimbriatus poweri Forel, 1914: South Africa
D. fulvus (Westwood, 1839): ‘North Africa’
D. fulvus badius Gerstäcker, 1859: Mozambique
D. fulvus crosi Santschi, 1926b: Algeria
D. fulvus dentifrons Wasmann, 1904: Democratic Republic of the Congo
D. fulvus eurous Emery, 1915b: Ethiopia
D. fulvus glabratus Shuckard, 1840c: Gambia
D. fulvus juvenculus Shuckard, 1840c: Morocco
D. fulvus mordax Santschi, 1931: Ivory Coast
D. fulvus obscurior Wheeler, W. M., 1925a: Guinea
D. fulvus punicus Santschi, 1926b: Tunisia
D. fulvus ruficeps Santschi, 1926b: Lebanon
D. fulvus saharensis Santschi, 1926b: ‘Sahara’
D. funereus Emery, 1895b: Ghana
D. funereus acherontus Santschi, 1937b: Cameroon
D. funereus pardus Santschi, 1937b: Democratic Republic of the Congo
D. funereus stygis Santschi, 1937b: Democratic Republic of the Congo
D. funereus zumpti Santschi, 1937b: Cameroon
D. furcatus (Gerstäcker, 1872): South Africa
D. fuscipennis (Emery, 1892): Ghana
D. fuscipennis lugubris Santschi, 1919a: Ivory Coast
D. fuscipennis marginiventris Santschi, 1919a: Ivory Coast
D. gaudens Santschi, 1919b: Democratic Republic of the Congo
D. ghanensis Boven, 1975: Ghana
D. gribodoi Emery, 1892: Togo
D. helvolus (Linnaeus, 1764): South Africa
D. helvolus pretoriae Arnold, 1946: South Africa
D. katanensis Stitz, 1911: Democratic Republic of the Congo
D. kohli Wasmann, 1904: Democratic Republic of the Congo
D. kohli chapini Wheeler, W. M., 1922a: Democratic Republic of the Congo
D. kohli frenisyi Forel, 1916: Democratic Republic of the Congo
D. kohli indocilis Santschi, 1933: Democratic Republic of the Congo
D. kohli langi Wheeler, W. M., 1922a: Democratic Republic of the Congo
D. kohli militaris Santschi, 1923b: Democratic Republic of the Congo
D. kohli minor Santschi, 1911a: Angola
D. kohli victoriae Santschi, 1921a: Uganda
D. labiatus Shuckard, 1840c: India
D. laevigatus (Smith, F., 1857): Malaysia (Sarawak)
D. leo Santschi, 1919a: Ivory Coast
D. mandibularis Mayr, 1896: Cameroon
D. mandibularis pulchellus Santschi, 1920a: Ivory Coast
D. mayri Santschi, 1912: Cameroon
D. moestus Emery, 1895b: Democratic Republic of the Congo
D. moestus claripennis Santschi, 1919b: Democratic Republic of the Congo
D. moestus morio Santschi, 1919b: Republic of the Congo
D. moestus schereri Forel, 1911d: Liberia
D. montanus Santschi, 1910c: Tanzania
D. niarembensis (Boven, 1972): Democratic Republic of the Congo
D. nigricans Illiger, 1802: Sierra Leone
D. nigricans arcens (Westwood, 1847): Liberia
D. nigricans burmeisteri (Shuckard, 1840c): Sierra Leone
D. nigricans molestus (Gerstäcker, 1859): Mozambique
D. nigricans pallidus Santschi, 1921a: Cameroon
D. nigricans rubellus (Savage, 1849): Gabon
D. nigricans sjoestedti Emery, 1899b: Cameroon
D. nigricans sjostedtiwilverthi (Wasmann, 1917): Cameroon
D. nigricans terrificus Santschi, 1923b: Democratic Republic of the Congo
D. ocellatus (Stitz, 1910): Cameroon
D. orientalis Westwood, 1835: India
D. orientalis obscuriceps Santschi, 1920b: India
D. politus Emery, 1901d: Cameroon
D. rufescens Santschi, 1915: Cameroon
D. savagei Emery, 1895b: ‘Gabon und Congo’
D. savagei mucronatus Emery, 1899b: Nigeria
D. schoutedeni Santschi, 1923b: Democratic Republic of the Congo
D. spininodis Emery, 1901d: Cameroon
D. spininodis longiceps Viehmeyer, 1914: Tanzania
D. stadelmanni Emery, 1895b: Democratic Republic of the Congo
D. stanleyi Forel, 1909b: Democratic Republic of the Congo
D. staudingeri Emery, 1895b: Democratic Republic of the Congo
D. striatidens Santschi, 1910c: Senegal
D. termitarius Wasmann, 1911: Democratic Republic of the Congo
D. titan Santschi, 1923b: Democratic Republic of the Congo
D. titan vinalli Santschi, 1933: Democratic Republic of the Congo
D. vishnui Wheeler, W. M., 1913: Myanmar
D. westwoodii (Shuckard, 1840b): ‘South America’ (locality incorrect)
D. wilverthi Emery, 1899b: Democratic Republic of the Congo
Eburopone gen. n.
Type-species
Cerapachys wroughtoni, by present designation.
Only one species of this group has been described from Afrotropics, but Madagascar harbors a considerable undescribed diversity.
Diagnosis
Worker. Workers of Eburopone are most easily recognized from other dorylines by a unique whitish patch of cuticle of presumably glandular function present on the posterior edge of abdominal sternite IV, although the patch may be faint in small or pale-colored specimens. A combination of 12-segmented antennae, propodeal spiracle placed low on the sclerite and propodeal lobes present, petiole dorsolaterally immarginate, lack of conspicuous constrictions posterior to abdominal segment IV, helcium narrow and placed at about mid-height of the segment, pronotomesopleural Pronotomesopleural suture present, and mid and hind tibiae each with a single pectinate spur will serve to distinguish Eburopone workers from other dorylines. In the Afrotropics and in Madagascar other non-army dorylines include Ooceraea, Parasyscia, Lividopone, Lioponera, and Zasphinctus. None of these genera possesses the characteristic, apparently glandular, patch on the underside of gaster, but if that character is not obvious or obscured, it is still relatively easy to distinguish Eburopone: Ooceraea found in this region (O. biroi) have 9-segmented antennae, Parasyscia and Lividopone have pronotomesopleural sutures fused, and Lioponera has a dorsolaterally marginate petiole and a raised flange on hind coxa. Zasphinctus belongs to the genera with pronounced constrictions between abdominal segments IV, V, and VI.
Male. The male morphology of Eburopone is very variable, including wing venation, but the following combination of characters usually allows separation from other genera: Antennae with 13 segments, at least weak constriction present anterior to abdominal segment IV, costal vein (C) present in the fore wing, submarginal cell open, presence of R·f3 and a free ‘stigmal vein’ formed by 2r-rs and Rs·f4–5 in the absence of Rs·f2–3 or 2rs-m, not running to the wing margin. Among non-army ant dorylines that overlap in range with Eburopone, Lioponera and Ooceraea can have a free stigmal vein but these genera never have costal vein running along the anterior margin of the fore wing in combination with R·f3 present past pterostigma.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment moderately enlarged, broader than and about equal in length to two preceding segments combined to conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth present. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 2-segmented. Mandibles triangular, edentate. Eyes absent or present, composed of at most several weakly differentiated ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange often separated from collar by ridge, usually distinct but rarely poorly developed or absent. Promesonotal connection with Pronotomesopleural suture present, weakly differentiated or with Pronotomesopleural suture conspicuous and complete but immobile. Pronotomesopleural suture visible as groove but not unfused. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland usually with bulla visible through cuticle, sometimes obscured. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed; sculpturing may be weak. Abdominal segment IV not conspicuously largest segment. Abdominal segment IV conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field, and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus with or without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 4- or 3-segmented. Labial palps 3- or 2-segmented. Mandibles triangular with teeth or falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent or present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli present at least anteriorly, very rarely absent. Transverse groove dividing mesopleuron absent or present. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere not apically expanded, very reduced relative to basimere. Volsella variable. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present and running toward distal wing margin but not enclosing cell with Rs·f5 or rarely absent. Abscissae Rs·f2–3 absent. Cross-vein 2r-rs present, forming base of ‘free stigmal vein’ (2r-rs&Rs·f4–5) in absence of Rs·f3 and 2rs-m or rarely absent. Abscissae Rs·f4–5 fused in absence of 2rs-m or rarely absent. Abscissa M·f2 in fore wing contiguous with Rs+M or rarely absent. Abscissa M·f4 in fore wing present, reaching wing margin or not, rarely entirely absent. Cross-vein 1m-cu in fore wing present or rarely absent. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent or absent past M+Cu. Vein A in fore wing with abscissae A·f1 and A·f2 or only A·f1 present. Vein C in hind wing absent. Vein R in hind wing present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing absent or present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing absent or present, not reaching wing margin. Cross-vein 1rs-m in hind wing fused with M·f1 or absent. Vein M+Cu in hind wing absent or present. Abscissa M·f1 in hind wing absent or present. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent or present. Vein Cu in hind wing absent or present. Vein A in hind wing present with abscissa A·f1 present or absent.
Gyne. At least one dealate gyne specimen with fully developed wing sclerites is known, but ergatoid queens have also been collected (Peter Hawkes pers. comm.).
Larva. Larvae have not been described. Cocoons present.
Distribution
One species of Eburopone, E. wroughtoni, has been described so far from South Africa and Zimbabwe, but more species are evidently to be found throughout Sub-Saharan Africa, as evidenced by unassociated males and gynes present in collections. Specimens belonging to this group have also been collected in Cameroon and Mozambique, suggesting that Eburopone is widely distributed in Africa. This lineage is also represented by a major radiation in Madagascar with dozens of species, none of which has been described.
Taxonomy and phylogeny
Cerapachys wroughtoni was originally described by Forel from South Africa and the same author subsequently described C. wroughtoni var. rhodesiana and C. roberti, both considered junior synonyms of wroughtoni by
The position of Eburopone on the doryline tree is uncertain (
Biology
There are no published reports on the biology of this lineage, although field observations suggest that most species are subterranean, have relatively populous colonies, and forage on brood of other ants. Based on several nest samples of undescribed Malagasy species where only larvae or pupae were collected, brood production appears to be synchronized (Brian Fisher pers. comm., author’s observations).
Species of Eburopone
E. wroughtoni (Forel, 1910c): South Africa, comb. n.
Eciton
= Camptognatha Grey, 1832
= Holopone Santschi, 1925
= Mayromyrmex Ashmead, 1905
Type-species
Formica hamata, by subsequent designation of Shuckard, in Swainson and Shuckard, 1840.
Eciton comprises the most conspicuous army ants in the New World. The huge colony size combined with epigaeic nesting and foraging habits makes these ants major invertebrate predators and key species of the tropical ecosystems.
Diagnosis
Worker. Eciton is recognized by a combination of 12-segmented antennae, propodeal spiracle high on the propodeum, propodeal declivity armed with cuticular tubercles or lamellae, binodal waist, pretarsal claws armed with a tooth and presence of a prominent metatibial gland visible as an elongate patch of whitish or yellowish cuticle on the flexor (inner) surface of tibia. Among New World army ants, Eciton is similar to its closest relative Nomamyrmex, with which it shares propodeal armament, but workers of all sizes are easily separated by a conspicuous white stripe on inner hind tibiae that is absent in Nomamyrmex. Labidus species can be distinguished from Eciton by their smooth, unarmed propodeum.
Male. The males of Eciton possess wing venation characteristic of all the New World army ants (also see under Cheliomyrmex male diagnosis). A combination of absence of very long setae approaching femur length on abdomen, apices of penisvalvae without setae, gradually tapering volsellae, and deeply concave dorsal surface of the petiole will distinguish Eciton males from all other army ant genera in the New World. The dense tufts of long setae on abdomen are characteristic of Nomamyrmex, although Eciton setigaster also has long setae abdominal setae; those are not quite as long and abundant as in Nomamyrmex, however, not approaching fore femur length. The penisvalvae without setae are also found in Neivamyrmex but in that genus the volsellae taper to a sharp point and often turn downwards towards the apex or are forked, not simply gradually narrowing to a blunt apex as in Eciton. In addition, Eciton males have a very conspicuously excavated dorsal surface of the petiole, which is usually more flattened in Neivamyrmex.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 3-segmented. Mandibles polymorphic, from triangular with teeth through falcate with teeth on masticatory margin, to falcate without teeth on elongated masticatory margin. Eyes present, appearing as single large and convex ommatidium, in reality composed from fused ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture completely fused. Mesometapleural groove not impressed. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated high on sclerite. Propodeal declivity with distinct dorsal edge or margin and in form of narrow strip. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, short. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora narrowed into anteriorly directed spine. Spiracle openings of abdominal segments IV–VI slit-shaped or oval in small workers. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV a gradual concavity, not gutter-like. Abdominal segment IV conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium small, reduced to narrow strip, without impressed medial field and simple, not armed with cuticular spines or modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as patch of whitish cuticle occupying at least half of tibia length. Metabasitarsal gland absent. Hind pretarsal claws each armed with a tooth. Polymorphism: Highly polymorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 2-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora forming a simple, wide U-shaped margin not delimited by ridge. Spiracle openings of abdominal segments IV–VI slit-shaped. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes longer than much reduced medial apodeme, directed anteriorly (towards head). Genitalia: Cupula very long, nearing or surpassing length of rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere narrowly fused to telomere, with Pronotomesopleural suture modified into membrane at junction, and ventrally with left and right arms abutting. Telomere expanded at apex. Volsella laterally flattened, narrow and tapered towards tip. Penisvalva hook-like, strongly curved ventrally. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma narrow. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing present, reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, reaching wing margin. Cross-vein 1rs-m in hind wing fused with M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Dichthadiiform, with eyes but no ocelli (see e.g.
Larva. Larvae of several Eciton species have been described by
Distribution
From northern Mexico to northern Argentina.
Taxonomy and phylogeny
Eciton is the sister lineage to Nomamyrmex (
Biology
Eciton is the best studied lineage of the dorylines, owing to the lifetime efforts by pioneers of army ant biology, including Thomas Schneirla, Thomas Borgmeier and Carl Rettenmeyer.
Among the twelve described species, Eciton burchellii has attracted the most attention, followed by E. hamatum, although most species have been at least briefly observed in the field. Most accounts of Eciton biology are based on the two well-known species.
The literature on Eciton is vast, and it is impossible to cite all of the even more significant original contributions. Good overviews of Eciton biology can be found in
The life of an Eciton colony can be summarized as follows. The colony alternates between the so-called statary and nomadic phases. The cycles are understood to be regulated by brood development rather than an endogenous rhythm in adult ants. During the statary phase a single queen is laying eggs and the brood inside the nest consists of pupae and eggs; foraging does not happen every day and raids are relatively much less intensive. There are no emigrations to new nesting sites. In the nomadic phase, the queen stops producing new eggs and her abdomen contracts; the colony contains many developing larvae that need nutrition. Raids and emigrations usually occur every day. In Eciton burchellii, the statary phase lasts on average 20 days and the nomadic phase is 14 days long.
A mature colony containing a single mated queen will eventually produce up to six virgin queens and hundreds to thousands of males, depending on the species. Usually the queen that emerges first leaves the colony with workers clustered around her. She has the best chance to survive and lead the fissioning part of the nest. About half of the workers eventually leave with the virgin queen. Because the colony is divided into approximately equal halves, the workers represent a substantial part of the reproductive investment. This explains the highly male-biased sex ratio, also typical of other social insects with colony fission (
Although mature colonies have been observed to occasionally admit new males, there is strong evidence that all of the mating occurs when the queen is young (
Colony structure and nesting behavior has been studied in some detail in several species. Temporary nests are made up of bodies of workers, hanging together by their legs from a supporting structure. These bivouacs can be found in a variety of microhabitats, but common nesting sites include hollow logs, spaces between buttresses of large trees, and empty soil cavities such as abandoned mammal burrows. Eciton species vary in their preferences for bivouac sites, with E. burchellii and E. hamatum nesting in exposed sites, the former often hanging above ground without touching the surface. Eciton dulcius and E. mexicanum are known to nest only in underground cavities, and E. vagans is intermediate, sometimes found in relatively exposed sites, but often nesting under logs and in rock crevices.
Colony size estimates vary widely and reliable data exists only for E. burchellii and E. hamatum. Rettenmeyer estimated that mature colonies of E. burchellii contain from 300,000 to 700,000 worker ants before fission and 100,000 to 500,000 for E. hamatum. Colony densities have been estimated in several localities for Eciton burchellii, ranging from 3.5 colonies per 100 ha on Barro Colorado Island, Panama, to 11 colonies in Corcovado, Costa Rica (
Foraging behavior in Eciton has been studied extensively. Workers forage either mostly above ground (E. burchellii, E. hamatum, E. rapax) or with some part of the raid unfolding underground. The latter mode has been reported for most other species, but the paucity of data precludes comparisons. The surface foragers also ascend vegetation and are capable of foraging arboreally. Ant brood constitutes a major portion of Eciton prey, although other arthropods, especially other social insects, are often targeted. Eciton burchellii is the most generalist predator, still hunting ants, but also actively preying on a variety of other arthropods and even opportunistically killing small vertebrates.
At the beginning of a raid, foragers emerge from the nest and gradually assemble into narrow trails that often branch and extend for up to 100 m (200 m in Eciton rapax) from the bivouac. These columns are typical of most species except for Eciton burchellii where the front of each raid progresses as a ‘swarm’, a continuous front up to 10 m wide. Group foraging is a self-organizing process with no scouts to guide the ants to a particular source of food, but the workers do follow trail pheromones produced by sternal glands (
During the nomadic phase, Eciton conducts raids every day and at some point these raids transition into an exodus of workers and finally an emigration of the entire colony. The emigration doesn not always follow the same route as the day’s raid and can be sustained by agitated returning foragers carrying booty past the bivouac. Other workers follow these foragers and eventually start to carry brood away from the bivouac. When the transport of brood is well advanced, myrmecophiles appear in the emigration column and the queen passes, surrounded by an entourage of workers. The duration of emigration is dependent on the colony size and species, and distances covered vary greatly as well;
Eciton colonies have an extraordinarily rich associate fauna and over 300 species, from mites to birds, have been recorded to depend on E. burchellii (
Eciton species are important predators of ants and other social insects and elicit a wide range of responses from its prey.
Species of Eciton
E. burchellii (Westwood, 1842): Brazil
E. burchellii cupiens Santschi, 1923a: French Guiana
E. burchellii foreli Mayr, 1886b: Panama
E. burchellii parvispinum Forel, 1899: Guatemala
E. burchellii urichi Forel, 1899: Trinidad and Tobago
E. drepanophorum Smith, F., 1858: Brazil
E. dulcium Forel, 1912a: Brazil
E. dulcium crassinode Borgmeier, 1955: Panama
E. hamatum (Fabricius, 1782): French Guiana
E. jansoni Forel, 1912a: Nicaragua
E. lucanoides Emery, 1894: Peru
E. lucanoides conquistador Weber, 1949b: Panama
E. mexicanum Roger, 1863: Mexico
E. mexicanum argentinum Borgmeier, 1955: Argentina
E. mexicanum goianum Borgmeier, 1955: Brazil
E. mexicanum latidens Santschi, 1911b: French Guiana
E. mexicanum moralum Santschi, 1923c: French Guiana
E. mexicanum panamense Borgmeier, 1955: Panama
E. quadriglume (Haliday, 1836): Brazil
E. rapax Smith, F., 1855: Brazil
E. setigaster Borgmeier, 1953: Brazil
E. uncinatum Borgmeier, 1953: Ecuador
E. vagans (Olivier, 1792): French Guiana
E. vagans allognathum Borgmeier, 1955: Venezuela
E. vagans angustatum Roger, 1863: Mexico
E. vagans dispar Borgmeier, 1955: Brazil
E. vagans dubitatum Emery, 1896b: Paraguay
E. vagans fur Borgmeier, 1955: Brazil
E. vagans mutatum Borgmeier, 1955: Costa Rica
Eusphinctus , gen. rev.
Type-species
Eusphinctus furcatus, by monotypy.
Eusphinctus is a species-poor South East Asian genus with apparently small colonies.
Diagnosis
Worker. Eusphinctus workers belong to dorylines with conspicuous gastral constrictions visible between abdominal segments IV, V, and VI. This morphology is also seen in Aenictogiton, certain species of Leptanilloides, Sphinctomyrmex, and Zasphinctus. Eusphinctus is unique in the combination of propodeal spiracle situated low on the sclerite and propodeal lobes present, a large pygidium armed with modified setae, pronotomesopleural Pronotomesopleural suture present, and cinctus of abdominal segment IV simple and not cross-ribbed. This genus is thus far known only from India, Bangladesh, Myanmar, and Thailand and the only lineage with gastral constriction that is currently known to overlap with it is Zasphinctus. In Zasphinctus the pronotomesopleural Pronotomesopleural suture is fused, in Aenictogiton the propodeal spiracle is positioned high and there are no propodeal lobes, Leptanilloides has a reduced and unarmed pygidium, and the neotropical Sphinctomyrmex has 12-segmented antennae and the cinctus on abdominal segment IV smooth. The relative proportions of abdominal segments are also different, with segments IV, V, and VI being about equal in size in Sphinctomyrmex and Zasphinctus, while in Eusphinctus segment IV is the largest of the three.
Male. The male of Eusphinctus can be recognized by a combination of 12-segmented antennae, pronounced propodeal lobes, narrow axial helcium, conspicuous constrictions present between abdominal segments IV, V, and VI, costal (C) cell present in the fore wing, submarginal cell closed by Rs·f2–3, R·f3 present past pterostigma, and marginal cell open. Abdominal sternite IX (subgenital plate) in Eusphinctus gradually tapers caudad and has simple, straight spines directed posteriorly. Sphinctomyrmex and Zasphinctus also have constrictions between abdominal segments IV, V, and VI but the former always has 13-segmented antennae and the latter lacks veins C and R·f3 in the fore wing.
Description
Worker.Head: Antennae with 11 segments. Apical antennal segment moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth present. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum unknown. Proximal face of stipes unknown. Maxillary palps unknown. Labial palps unknown. Mandibles triangular, with teeth. Eyes present, composed of fewer than five ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI present. Girdling constriction between pre- and poststernites of abdominal segments V and VI present. Pygidium large, with impressed medial field, armed with modified setae, and deeply notched at apex. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 12 segments. Clypeus with cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps unknown. Labial palps unknown. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Girdling constriction between pre- and postsclerites of abdominal segments V and VI present. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella narrow, hook-shaped. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, disconnected from Rs+M. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing absent. Cross-vein 1rs-m in hind wing fused with M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Ergatoid, ‘scarcely different in size from the workers’ (
Larva. Larva not known. Presence of cocoons unknown.
Distribution
Indomalayan, known from India, Bangladesh, Myanmar, and Thailand.
Taxonomy and phylogeny
Based on morphological and molecular evidence (Borowiec in prep.), I revive Eusphinctus from synonymy with Sphinctomyrmex. The taxonomic history of taxa classified under Sphinctomyrmex is somewhat complicated. Detailed discussions can be found in
Here I propose a new classification where all the New World species are retained in Sphinctomyrmex, while most of the described Old World forms are relegated to Zasphinctus. The two remaining above mentioned Old World species are separated from Zasphinctus as Eusphinctus. Molecular data shows that all three genera arose independently on the dorylomorph tree (see Figure
Eusphinctus belongs to a clade that also includes Ooceraea and Syscia (Borowiec, in prep.). The members of this clade share the universal reduction in the number of antennal segments, from 12 to 11 or fewer in the worker caste and from 13 to 12 or fewer in males.
There are only two species of Eusphinctus, both quite similar, and
Biology
A. B. Soans and W. L. Brown collected two colonies of E. furcatus in Kottiyoor, Kerala, India. One was located in leaf litter near a rotting log and the other one was found under a stone in a shaded creek bottom. There were about 50 workers in each of the observed nests, and one colony contained two ergatoid gynes (
Species of Eusphinctus
E. furcatus Emery, 1893a: Myanmar, comb. rev.
E. taylori (Forel, 1900b): Bangladesh, India, comb. n.
Labidus
= Nycteresia Roger, 1861
= Pseudodichthadia André, 1885
Type-species
Labidus latreillii (junior synonym of Formica coeca), by monotypy.
With seven described species, Labidus is a relatively small but widely distributed genus. Its members are more generalized predators than most other New World army ants and may have the greatest overall ecological impact due to high densities.
Diagnosis
Worker. Labidus workers are easily recognized by a combination of spiracle positioned high on the propodeum, 12-segmented antennae, propodeum not armed with spines or cuticular lamellae, short propodeal lobes, two-segmented waist, metatibial gland present, and pretarsal claws with a tooth. Labidus belongs to New World army ants with an unarmed propodeum and it could only be confused with Cheliomyrmex and certain larger species of Neivamyrmex. The former have one-segmented waist, and the latter always lack teeth on pretarsal claws.
Male. Labidus males have the army ant habitus with abdominal segment III much larger than the preceding segment II (petiole), and head small relative to mesosoma. See discussion under Cheliomyrmex for characters differentiating New World army ant males from those of Old World Aenictus, Aenictogiton, and Dorylus. Among New World army ants, Labidus possesses the following unique character combination: no conspicuous tufts of long setae on gaster, apices of penisvalvae with setae, abdominal sternite IX (subgenital plate) with two spines, and hind basitarsus with a groove that accommodates the tibial spur. The lack of long gastral setae differentiates Labidus from Nomamyrmex, the apices of penisvalvae are hairy in Eciton and Neivamyrmex, and in Cheliomyrmex there are four spines on the abdominal sternite IX and hind basitarsus has no oblique grooves.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus with or without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 3-segmented. Mandibles polymorphic, from triangular with teeth to falcate with teeth on elongated masticatory margin. Eyes present, composed of seemingly single large ommatidium, in reality composed from multiple fused ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture completely fused. Mesometapleural groove not impressed. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated high on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes absent or very short. Metasoma: Petiole anterodorsally marginate with carina low on anterior face, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI oval. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and sculptured but not cross-ribbed. Abdominal segment IV conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium small, reduced to narrow strip, without impressed medial field and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as patch of whitish cuticle occupying at least half of tibia length. Metabasitarsal gland absent. Hind pretarsal claws each armed with a tooth. Polymorphism: Highly polymorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 2-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora forming a simple U-shaped margin or a broad cuticular lip, not delimited by carina; central protuberance may be present. Spiracle openings of abdominal segments IV–VI slit-shaped. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes longer than much reduced medial apodeme, directed anteriorly (towards head). Genitalia: Cupula very long, nearing or surpassing length of rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere narrowly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms abutting. Telomere expanded at apex. Volsella laterally flattened, narrow and tapered towards tip. Penisvalva not flattened at apex, expanded. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma narrow. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing present. Vein R in hind wing present, reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, reaching wing margin. Cross-vein 1rs-m in hind wing fused with M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Dichthadiiform, with minute eyes and no ocelli. The queen is known for L. coecus and L. praedator. For more details and a description of the former see
Larva. Larvae of Labidus have been described in
Distribution
Sout Central United States to northern Argentina.
Taxonomy and phylogeny
The species-level taxonomy of Labidus requires revision. There are currently seven valid species names and three of those are based only on males. In addition, morphology and preliminary molecular analyses suggest that the widely distributed L. praedator may be in fact a complex of reproductively isolated species (
Biology
Labidus are often the most common army ants throughout their range, with up to three species occurring in a given area (do Nascimiento et al. 2004, author’s personal observations). They nest mostly underground (
Labidus forages in swarm raids similar to those of Eciton burchellii (
The reproductive biology of Labidus is poorly known. There is conflicting evidence as to whether brood production is synchronized or not, with available brood samples consisting of immatures at one or multiple stages of development and queen specimens with either extended or contracted gasters (
Species of Labidus
L. auropubens (Santschi, 1920a): French Guiana
L. coecus (Latreille, 1802): ‘Amérique méridionale’
L. curvipes (Emery, 1900b): Costa Rica
L. mars (Forel, 1912a): Brazil
L. mars denticulatus Borgmeier, 1955: Brazil
L. praedator (Smith, F., 1858): Brazil
L. praedator sedulus (Menozzi, 1926): Colombia
L. spininodis (Emery, 1890): Costa Rica
L. truncatidens (Santschi, 1920a): French Guiana
Leptanilloides
= Amyrmex Kusnezov, 1953, syn. n.
= Asphinctanilloides Brandão, Diniz, Agosti and Delabie, 1999, syn. n.
Type-species
Leptanilloides biconstricta, by original designation.
This is a lineage of subterranean ants from Central and South America. Its members had been extremely rarely encountered before collecting methods targeting soil-dwelling ants were popularized: 17 out of the 19 currently known species were described after 1998.
Diagnosis
Worker. The workers of Leptanilloides are rather variable but can be distinguished from all other dorylines by a combination of promesonotal Pronotomesopleural suture variously developed but often conspicuous, propodeal spiracles positioned low, lack of metanotal groove, absence of propodeal lobes, and small and unarmed pygidium. The positioning of the propodeal spiracle may be rather high on the sclerite in some species and so these could conceivably be mistaken for small Neivamyrmex. The latter, however, can be distinguished by a complete lack of a promesonotal Pronotomesopleural suture in dorsal view and abdominal segment IV much larger than the succeeding segment V, with no apparent constrictions between abdominal segments IV, V, and VI. Although some larger Leptanilloides species can have an inconspicuous promesonotal Pronotomesopleural suture, those will have visible constrictions on the gaster and abdominal segment IV and that segment is never much larger than succeeding gastral segments.
Male. The males of Leptanilloides are distinct in their extreme reduction of tegulae. The wing venation is reduced and unusual among dorylines because of the combination of costal cell (C) present in fore wing and discal cell always being open. The males of Leptanilloides also lack conspicuously bispinose abdominal sternite IX (subgenital plate) characteristic of almost all other male dorylines.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent or present. Parafrontal ridges absent or reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum without median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2- or, rarely, 1-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth or triangular with median tooth. Eyes absent. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent or present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection variable, with Pronotomesopleural suture present, weakly differentiated, immobile or with Pronotomesopleural suture conspicuous and complete, but immobile, or with Pronotomesopleural suture complete and mobile. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural grooveweakly impressed to deeply impressed and conspicuous. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity absent. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated low on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial or slightly supraaxial. Prora simple, not delimited by carina, a simple U-shaped margin, or a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III variable, more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist) or abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured or a gradual concavity, not gutter-like. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent or present. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent or present. Pygidium small, reduced to narrow strip, without impressed medial field and simple, not armed with cuticular spines or modified setae. Hypopygium unarmed. Legs: Mid tibia with single simple/barbulate spur, with single pectinate spur, or rarely with two simple spurs. Hind tibia with pectinate spur or rarely with one barbulate and one pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus with or without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps unknown. Labial palps unknown. Mandibles falcate or, more rarely, elongately triangular, edentate, or intermediate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes absent. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite or at Pronotomesopleural suture and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX simple or cleft, with lateral apodemes reduced, only medial apodeme conspicuous, short. Genitalia: Cupula short relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, with sulcus discernable at junction or no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single simple/barbulate spur or with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula absent or extremely small. Vein C in fore wing present. Pterostigma narrow or broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M or absent. Abscissa M·f4 in fore wing absent or present, not reaching wing margin. Cross-vein 1m-cu in fore wing absent. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissa A·f1 present. Vein C in hind wing absent or present. Vein R in hind wing absent. Vein Sc+R in hind wing absent or present. Abscissa Rs·f1 in hind wing absent or present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing absent or present, not reaching wing margin. Cross-vein 1rs-m in hind wing absent. Vein M+Cu in hind wing absent. Abscissa M·f1 in hind wing absent. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent. Vein Cu in hind wing absent. Vein A in hind wing absent.
Gyne. The queens of Leptanilloides collected so far have been ‘subdichthadiiform’, or wingless ergatoids with eyes and hypertrophied gaster, including abdominal segment III. The gynes possess eyes but no ocelli. See description of L. erinys gyne in
Larva. Larva was described by
Distribution
Leptanilloides is a genus found throughout Central America, from Chiapas, Mexico to Panama, and from scattered, mostly high-elevation records from Bolivia, Colombia, Ecuador, and Venezuela. It is also present in the Amazon Basin and one species has been described from the Atlantic forest habitat of São Paulo, Brazil. A recent collection from western Texas (
Taxonomy and phylogeny
Leptanilloides was described with one species, L. biconstricta, in 1923 (
The phylogenetic position of Leptanilloides within Dorylinae was difficult to ascertain with Sanger sequencing data (
Biology
L. nomada was observed foraging at night in partly subterranean, partly exposed, columns but the ants did not carry any prey (
Species of Leptanilloides
L. amazona Brandão, Diniz, Agosti and Delabie, 1999: Brazil, comb. n.
L. anae Brandão, Diniz, Agosti and Delabie, 1999: Brazil, comb. n.
L. atlantica Silva, Brandão, Feitosa and Freitas, 2013: Brazil
L. biconstricta Mann, 1923: Bolivia
L. caracola Donoso, Vieira and Wild, 2006: Ecuador
L. chihuahuaensis MacGown, Schiefer and Branstetter 2015: United States
L. copalinga
L. erinys Borowiec and Longino, 2011: Ecuador
L. femoralis Borowiec and Longino, 2011: Venezuela
L. golbachi Kusnezov, 1953: Argentina, comb. n.
L. gracilis Borowiec and Longino, 2011: Mexico
L. improvisa Brandão, Diniz, Agosti and Delabie, 1999: Ecuador
L. legionaria Brandão, Diniz, Agosti and Delabie, 1999: Colombia
L. manaura Brandão, Diniz, Agosti and Delabie, 1999: Brazil, comb. n.
L. mckennae Longino, 2003: Costa Rica
L. nomada Donoso, Vieira and Wild, 2006: Ecuador
L. nubecula Donoso, Vieira and Wild, 2006: Ecuador
L. prometea Delsinne and Donoso, 2015: Ecuador
L. sculpturata Brandão, Diniz, Agosti and Delabie, 1999: Colombia
Lioponera , gen. rev.
= Neophyracaces Clark, 1941, syn. n.
= Phyracaces Emery, 1902, syn. n.
Type-species
Lioponera longitarsus, by monotypy.
This is the most species-rich genus that is revived here from synonymy under Cerapachys. Lioponera occurs only in the Old World and all species observed thus far prey on other ants.
Diagnosis
Worker. The workers of Lioponera are distinguishable using the combination of a unique cuticular flange present on the posterior edge of the hind coxa, just posterior to the femur attachment, at least the anterior half of the petiole being dorsolaterally marginate, and a peculiar development of the metatibial gland that forms an open slit in the cuticle. The coxal flange should not to be confused with the elevated faces of coxa that can be conspicuous when there is a deep trench leading to the articulation with the femur, as can be sometimes seen in e.g. Simopone. The coxal flange and metatibial gland have been reduced in a handful of Australasian species, but in these the dorsolateral carinae of petiole and (usually) also postpetiole and mesosoma, are prominent. The dorsolateral margination of the body is characteristic and in most species very conspicuous on the abdominal segments II (petiole) and III. In a few species the margination extends from the head to the abdominal segment IV. The only other genera that can possess somewhat similar dorsolateral carinae on the petiole are Acanthostichus, Cerapachys, and Cylindromyrmex. The workers of those groups, however, do not possess a coxal flange.
Male. The males of the many species of Lioponera are variable. Several characteristics can point to the affinity with this genus: antennae are 13-segmented, costal vein (C) is always absent from the fore wing, a ‘free stigmal vein’ (2r-rs&Rs·f4–5) is present and R·f3 and Rs·f2–3 are always absent. Cross-vein 2rs-m is usually absent but its traces can be rarely seen as a weak spectral vein arising close to 2r-rs. There is a constriction between abdominal segments III and IV but no succeeding segments and the middle tibiae are armed with a single spur. The notauli are present or absent, pretarsal claws are unarmed, and the palp formula is either 4,3 or 3,2. Compare diagnoses of the genera where a free stigmal vein is also found (Eburopone, Ooceraea, Syscia, Tanipone). Eburopone can be distinguished by costal (C) vein always present in the fore wing, Ooceraea and Syscia have 12- or 11-segmented antennae, and Tanipone has very long, 6-segmented maxillary palps that are extruded in mounted specimens and reach the occipital foramen.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined to moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 4- or 3-segmented. Labial palps 3- or 2-segmented. Mandibles triangular, with teeth. Eyes present, composed of more than 20 ommatidia. Ocelli absent or more rarely present. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head with or without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate or marginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate, weakly marginate, or conspicuously marginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with or without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate, dorsolaterally marginate, and laterally above spiracle immarginate or marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora simple, not delimited by carina or a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate or marginate and dorsolaterally immarginate or marginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured or cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with pectinate spur or, more rarely, with barbulate spur and simple spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa produced as raised lamella or rarely without lamella. Metatibial gland present in form of slit or orifice in cuticle or rarely absent. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus with cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 3-segmented. Labial palps 2-segmented. Mandibles triangular, edentate. Ventrolateral margins of head with or without cuticular ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent or present. Transverse groove dividing mesopleuron present. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening present. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally marginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora forming a simple U-shaped margin or U-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX cleft or modified into two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva constricted basally, distally a widening triangle, serrated ventrally. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa produced as raised lamella or not. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 absent. Cross-vein 2r-rs most often present and forming base of ‘free stigmal vein’ (2r-rs&Rs·f4–5) in absence of Rs·f3 and 2rs-m or present and connected to Rs·f2–3&Rs·f4 or, rarely, absent. Abscissae Rs·f4–5 absent or present, fused in absence of 2rs-m or, more rarely, differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing absent or contiguous with Rs+M. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing present or more rarely absent. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1 or more rarely absent. Vein Cu in fore wing present, with only Cu1 branch prominent or absent past M+Cu. Vein A in fore wing with abscissa A·f1 present with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing absent or present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing absent or present, not reaching wing margin. Cross-vein 1rs-m in hind wing absent or present, about as long as M·f1. Vein M+Cu in hind wing absent or present. Abscissa M·f1 in hind wing absent or present. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent or present. Vein Cu in hind wing absent or present. Vein A in hind wing absent or with abscissa A·f1 present.
Gyne. Alate or ergatoid; apparent intercastes have also been reported. Alate or apparently alate (known from dealated specimens) gynes are known in a number of species, for example in L. clarki, L. daikoku, L. fervida, L. huode, L. pubescens, L. turneri. Ergatoid gynes are known to vary in morphology, from scarcely different from the worker, through possessing relatively larger eyes and ocelli, to having the mesosomal morphology identical to that of winged queens but possessing no wings. Ergatoids have been reported in, for example, L. angustata, L. bicolor, L. constricta, L. elegans, L. gilesi, L. nigriventris, L. punctatissima, L. simmonsae. Malagasy species related to L. kraepelini and L. mayri also possess ergatoid gynes (author’s observations). Intercastes with morphologies intermediate between workers and alate gynes have been reported to occur along with fully developed gynes in L. clarki (
Larva. Larvae of Lioponera have been described by
Distribution
This is the most species-rich lineage outside the true army ants, distributed throughout the Old World, from Africa to Oceania, with a major radiation in Australia.
Taxonomy and phylogeny
Originally Lioponera was described for L. longitarsus, a species from India (
Lioponera is part of a well-supported Old World clade where the intergeneric relationships are known (
Biology
Members of this lineage have been observed both in the field and in the laboratory (
Lioponera nests are found in a variety of microhabitats, including soil, under stones, in rotting logs or arboreally in hollow twigs (
Species of Lioponera
L. aberrans (Clark, 1934): Australia, comb. n.
L. adama (Forel, 1910a): Australia, comb. n.
L. angustata (Clark, 1924b): Australia, comb. n.
L. anokha (Bharti and Akbar, 2013): India, comb. n.
L. bakeri Wheeler, W. M. and Chapman, 1925: Philippines, comb. rev.
L. bicolor (Clark, 1924b): Australia, comb. n.
L. binodis (Forel, 1910a): Australia, comb. n.
L. braunsi (Emery, 1902): South Africa, comb. n.
L. braytoni (Weber, 1949a): Kenya, comb. n.
L. brevicollis (Clark, 1924a): Australia, comb. n.
L. brevis (Clark, 1924b): Australia, comb. n.
L. clarki (Crawley, 1922): Australia, comb. n.
L. clarus (Clark, 1930): Australia, nom. rev.
L. cohici (Wilson, 1957): New Caledonia, comb. n.
L. collingwoodi (Sharaf, 2007): Egypt, comb. n.
L. constricta (Clark, 1924a): Australia, comb. n.
L. coxalis (Arnold, 1926): Zimbabwe, comb. n.
L. crassa (Clark, 1941): Australia, comb. n.
L. daikoku (Terayama, 1996): Japan, comb. n.
L. decorsei Santschi, 1912: Chad, comb. rev.
L. desertorum (Dlussky, 1990): Uzbekistan, comb. n.
L. dumbletoni (Wilson, 1957): New Caledonia, comb. n.
L. elegans (Wheeler, W. M., 1918): Australia, comb. n.
L. emeryi (Viehmeyer, 1914): Australia, nom. rev.
L. fervida (Wheeler, W. M., 1918): Australia, comb. n.
L. ficosa (Wheeler, W. M., 1918): Australia, comb. n.
L. flammea (Clark, 1930): Australia, comb. n.
L. foreli (Santschi, 1914b): Ghana, comb. n.
L. gilesi (Clark, 1924a): Australia, comb. n.
L. grandis (Clark, 1934): Australia, comb. n.
L. greavesi (Clark, 1934): Australia, comb. n.
L. gwynethae (Clark, 1941): Australia, comb. n.
L. heros (Wheeler, W. M., 1918): Australia, comb. n.
L. hewitti (Wheeler, W. M., 1919): Malaysia (Sarawak), comb. n.
L. huode (Terayama, 2009): Taiwan, comb. n.
L. inconspicua (Clark, 1924b): Australia, nom. rev.
L. jovis (Forel, 1915): Australia, comb. n.
L. kraepelinii (Forel, 1895d): Madagascar, comb. n.
L. krombeini (Donisthorpe, 1947): Papua New Guinea, comb. n.
L. larvata (Wheeler, W. M., 1918): Australia, comb. n.
L. longitarsus Mayr, 1879: India, comb. rev.
L. luzuriagae Wheeler, W. M. and Chapman, 1925: Philippines, comb. rev.
L. macrops (Clark, 1941): Australia, comb. n.
L. marginata (Emery, 1897): Papua New Guinea, comb. n.
L. mayri (Forel, 1892b): Madagascar, comb. n.
L. mjoebergi (Forel, 1915): Australia, comb. n.
L. mullewana (Wheeler, W. M., 1918): Australia, comb. n.
L. nayana (Bharti and Akbar, 2013): India, comb. n.
L. nigra Santschi, 1914a: Kenya, comb. rev.
L. nigriventris (Clark, 1924b): Australia, comb. n.
L. nkomoensis (Forel, 1916): Democratic Republic of the Congo, comb. n.
L. noctambula Santschi, 1910a: Tunisia, comb. rev.
L. picipes (Clark, 1924b): Australia, comb. n.
L. picta (Clark, 1934): Australia, comb. n.
L. piliventris (Clark, 1941): Australia, comb. n.
L. potteri (Clark, 1941): Australia, comb. n.
L. pruinosa (Brown, 1975): Philippines, comb. n.
L. pubescens (Emery, 1902): Indonesia (Laut Island), comb. n.
L. punctatissima (Clark, 1924a): Australia, comb. n.
L. reticulata (Clark, 1926): Australia, nom. rev.
L. ruficornis (Clark, 1924a): Australia, comb. n.
L. rugulinodis (Wheeler, W. M., 1918): Australia, comb. n.
L. senescens (Wheeler, W. M., 1918): Australia, comb. n.
L. similis Santschi, 1930: Ivory Coast, comb. rev.
L. simmonsae (Clark, 1924a): Australia, comb. n.
L. singaporensis (Viehmeyer, 1916): Singapore, comb. n.
L. singularis (Forel, 1900a): Australia, comb. n.
L. sjoestedti (Forel, 1915): Australia, comb. n.
L. suscitata (Viehmeyer, 1913): Indonesia (Sulawesi, in copal), comb. n.
L. turneri (Forel, 1902): Australia, comb. n.
L. varians (Clark, 1924b): Australia, comb. n.
L. versicolor Donisthorpe, 1948: Indonesia (Papua), comb. rev.
L. vespula (Weber, 1949a): Kenya, comb. n.
Lividopone
Type-species
Cerapachys lividus, by subsequent designation.
This relatively speciose lineage includes species nesting in a variety of substrates, from soil to twigs. It is known that they prey on brood of other ants.
Diagnosis
Worker. The workers of Lividopone are recognized by a combination of 12-segmented antennae, pronotomesopleural Pronotomesopleural suture fused, propodeal spiracle positioned low and presence of propodeal lobes, pygidium large and armed with modified setae, helcium broad and positioned supraaxially on the sclerite, middle tibiae with a single pectinate spur, and pretarsal claws simple. Cerapachys is similar in general habitus but it is easily differentiated because of its unfused pronotomesopleural Pronotomesopleural suture. Parasyscia, certain Lioponera and Simopone may have a similar habitus but those genera never have a broad, highly positioned helcium.
Male. The male of Lividopone shares the broad supraaxial helcium with the worker caste, which makes it easy to distinguish from any other doryline when combined with well-developed propodeal lobes, antennal toruli exposed in full-face view, one spur on each mid and hind tibia, and no C or R·f3 veins in the fore wing. Males of Lividopone can potentially be confused with Lioponera and Parasyscia which also lack veins C and R·f3 and with which it co-occurs, but the highly broad positioned helcium alone can distinguish it from those two lineages.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus with or without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 3-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth or edentate. Eyes present, composed of more than 20 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Ventrolateral margins of head with cuticular ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent or present. Mesosoma: Pronotal flange separated or not from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture completely fused. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with or without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and supraaxial. Prora forming a simple U-shaped margin or V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field, and armed with modified setae, and in some species deeply notched at apex. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus with cuticular apron. Parafrontal ridges present. Torulo-posttorular complex vertical. Maxillary palps unknown. Labial palps unknown. Mandibles triangular, edentate. Ventrolateral margins of head with cuticular ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen unknown. Mesosoma: Pronotal flange separated from collar by distinct ridge. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and supraaxial. Prora forming a U-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva constricted basally, distally a widening to narrow triangle, serrated ventrally. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2 or more rarely absent. Cross-vein 2r-rs absent or present, forming base of ‘free stigmal vein’ (2r-rs&Rs·f4–5) in absence of Rs·f3 and 2rs-m, or (most commonly) present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 absent or more often present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing absent or present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing absent or a stub. Cross-vein 1m-cu in fore wing absent or present. Cross-vein cu-a in fore wing absent or fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing absent or with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing absent. Abscissa Rs·f1 in hind wing absent or present, longer than 1rs-m. Abscissa Rs·f2 in hind wing absent or stub present. Cross-vein 1rs-m in hind wing absent or present, about as long as M·f1. Vein M+Cu in hind wing absent or present. Abscissa M·f1 in hind wing absent or present. Abscissa M·f2 in hind wing absent or present. Cross-vein cu-a in hind wing absent. Vein Cu in hind wing absent or present. Vein A in hind wing absent.
Gyne. Alate with large eyes and ocelli or ergatoid. The latter variously developed, from forms possessing flight-associated mesosomal sutures but no wings to extremely worker-like, without ocelli and of similar size, with only erect pubescence distinguishing the gyne from worker. In some species intercastes occur in addition to alate gynes (Barry Bolton pers. comm.).
Larva. Not described. Cocoons are present.
Distribution
This group is relatively species-rich with only one named species but some 30 more species awaiting description. The genus appears to be restricted to Madagascar.
Taxonomy and phylogeny
Lividopone is sister to the Zasphinctus plus Parasyscia clade (
Biology
Species of Lividopone
L. livida (Brown, 1975): Madagascar
Neivamyrmex
= Acamatus Emery, 1894
= Woitkowskia Enzmann, 1952
Type-species
Eciton (Acamatus) schmitti (junior synonym of Labidus nigrescens), by subsequent designation of Ashmead, 1906.
Neivamyrmex is the most species-rich and widely distributed genus of New World army ants. The biology of the vast majority of the 130 described species is unknown, but N. nigrescens has become one of the best studied dorylines.
Diagnosis
Worker. Neivamyrmex have 12-segmented antennae, propodeal spiracle high on the sclerite, lack conspicuous propodeal lobes, pygidium small and without a central impressed field, waist two-segmented, and pretarsal claws without a tooth. The simple claws distinguish Neivamyrmex workers from all other New World army ant genera (Cheliomyrmex, Eciton, Labidus, and Nomamyrmex). Aenictus in the Old World will also match some of these diagnostic characters but workers of this genus never have more than 10 antennal segments.
Male. Neivamyrmex males share the army ant-like habitus with other members of the Eciton genus-group. See discussion under Cheliomyrmex male diagnosis for characters differentiating New World army ant males from those of the Old World. Among the New World army ants, Neivamyrmex can most reliably distinguished by a combination of apex penisvalvae without setae, no dense setation on gaster, and abdominal segment II (petiole) without a deeply notched or concave surface. The bare penisvalvae are shared only with Eciton and Nomamyrmex but the former always has a deeply excavated petiole and the latter has conspicuous tufts of dense setae on the gaster.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 3- or 2-segmented. Mandibles triangular, with teeth. Eyes absent or present, composed of few poorly defined ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen; differentiation sometimes weak. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge or separated by ridge that is low on pronotum. Promesonotal connection with Pronotomesopleural suture completely fused or Pronotomesopleural suture weakly differentiated, immobile. Pronotomesopleural suture completely fused or unfused partway to notal surface. Mesometapleural groove not impressed to weakly impressed. Transverse groove dividing mesopleuron absent or present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated high on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, short. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin or V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular, oval, or slit-shaped. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and sculptured but not cross-ribbed. Abdominal segment IV conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium small, reduced to narrow strip, without impressed medial field and simple, usually not armed with cuticular spines or modified setae but occasionally with one or two pairs of thick modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent to conspicuous patch of whitish cuticle occupying at least half of tibia length. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic to polymorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 3- or 2-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes absent or present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI oval or slit-shaped. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV absent, not impressed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, often with additional projections such as medial spine or paired median denticles, with lateral apodemes longer than much reduced medial apodeme, directed anteriorly (towards head). Genitalia: Cupula very long, nearing or surpassing length of rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere narrowly fused to telomere, with sulcus visible at least partway through junction, and ventrally with left and right arms abutting. Telomere expanded at apex. Volsella narrow, hook-shaped or laterally flattened, triangular in lateral view, narrowing towards tip. Penisvalva not flattened at apex, expanded. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple or each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing present. Vein R in hind wing present, reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, reaching wing margin. Cross-vein 1rs-m in hind wing present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Dichthadiiform, blind or with very small eyes, without ocelli. Known for several species. See e.g.
Larva. Described in
Distribution
Central and southern United States, south to central Argentina.
Taxonomy and phylogeny
First species now classified in Neivamyrmex were described from males by William Shuckard in his 1840 series ‘Monograph of the Dorylidae’. Borgmeier later erected the genus (
Biology
The majority of species has never been studied in any detail, and much of what we know comes from the observations made on one relatively common species, Neivamyrmex nigrescens, studied extensively by Howard Topoff and his students (
If N. nigrescens is representative of this genus, the lineage’s habits are similar to those of other New World army ants. There are marked nomadic and statary phases, lasting about 16 and 20 days, respectively. The colonies are of moderate size, containing 80,000 to 140,000 workers (
Nesting sites of Neivamyrmex are rarely observed.
The foraging biology of N. nigrescens in Arizona was studied in detail by
Several Neivamyrmex species can occur sympatrically, and it is likely that a diversity of prey preferences exists in the genus.
Neivamyrmex nigrescens uses both tactile and chemical cues in orientation (
Many species of ants respond to a Neivamyrmex attack by nest evacuation and this behavior has been highlighted as a tool for collecting colonies of soil-nesting species that are normally difficult to excavate.
Species of Neivamyrmex
N. adnepos (Wheeler, W. M., 1922b): Trinidad and Tobago
N. agilis Borgmeier, 1953: Mexico
N. albacorpus Varela-Hernández and Castaño-Meneses, 2011: Mexico
N. alfaroi (Emery, 1890): Costa Rica
N. andrei (Emery, 1901b): Mexico
N. angulimandibulatus Watkins, 1974: Mexico
N. angustinodis (Emery, 1888): Argentina
N. antillanus (Forel, 1897): Grenada
N. asper Borgmeier, 1955: Costa Rica
N. balzani (Emery, 1894): Bolivia
N. baylori Watkins, 1973: United States
N. bohlsi (Emery, 1896c): Paraguay
N. bruchi (Forel, 1912c): Argentina
N. bureni (Enzmann, E.V., 1952): Peru
N. californicus (Mayr, 1870): United States
N. carettei (Forel, 1913d): Argentina
N. carinifrons Borgmeier, 1953: Brazil
N. carolinensis (Emery, 1894): United States
N. chamelensis Watkins, 1986: Mexico
N. clavifemur Borgmeier, 1953: Brazil
N. cloosae (Forel, 1912a): Mexico
N. coeca (Buckley, 1867): United States
N. compressinodis Borgmeier, 1953: Costa Rica
N. cornutus Watkins, 1975a: Mexico
N. crassiscapus Watkins, 1990: Mexico
N. cratensis Borgmeier, 1953: Brazil
N. cristatus (André, 1889): ‘Amérique du Sud’
N. curvinotus Watkins, 1994: Peru
N. densepunctatus (Borgmeier, 1933): Brazil
N. detectus Borgmeier, 1953: Brazil
N. diabolus (Forel, 1912a): Mexico
N. diana (Forel, 1912c): Brazil
N. digitistipus Watkins, 1975b: Costa Rica
N. diversinodis (Borgmeier, 1933): Bolivia
N. dorbignii (Shuckard, 1840b): No locality given
†N. ectopus Wilson, 1985: Dominican amber
N. emersoni (Wheeler, W. M., 1921): Guyana
N. emeryi (Santschi, 1921b): Bolivia, Peru
N. erichsonii (Westwood, 1842): Brazil
N. falcifer (Emery, 1900b): Bolivia
N. foveolatus Borgmeier, 1953: Panama
N. fumosus (Forel, 1913d): Guatemala
N. fuscipennis (Smith, M.R., 1942b): United States
N. genalis Borgmeier, 1953: Bolivia
N. gibbatus Borgmeier, 1953: Costa Rica
N. goeldii (Forel, 1901d): Brazil
N. graciellae (Mann, 1926): Mexico
N. gracilis Borgmeier, 1955: Brazil
N. gradualis Borgmeier, 1953: Brazil
N. guerinii (Shuckard, 1840d): Brazil
N. guyanensis (Santschi, 1916): French Guiana
N. halidaii (Shuckard, 1840a): Brazil
N. harrisii (Haldeman, 1852): United States
N. hetschkoi (Mayr, 1886a): Brazil
N. hopei (Shuckard, 1840b): Brazil
N. humilis (Borgmeier, 1939): Costa Rica
N. iheringi (Forel, 1908): Brazil
N. imbellis (Emery, 1900b): Peru, Venezuela
N. impudens (Mann, 1922): Honduras
N. inca (Santschi, 1921b): Peru
N. inflatus Borgmeier, 1958: Mexico
N. iridescens Borgmeier, 1950: Guyana
N. jerrmanni (Forel, 1901e): Paraguay
N. kiowapache Snelling, G.C. and Snelling, R.R., 2007: United States
N. klugii (Shuckard, 1840b): Saint Vincent and the Grenadines
N. klugii distans Borgmeier, 1953: Costa Rica
N. kuertii (Enzmann, E.V., 1952): Peru
N. laevigatus (Borgmeier, 1948): Argentina
N. latiscapus (Emery, 1901b): Brazil
N. legionis (Smith, F., 1855): Argentina
N. leonardi (Wheeler, W. M., 1915a): United States
N. leptognathus (Emery, 1900b): Bolivia
N. lieselae (Forel, 1913d): Argentina
N. longiscapus Borgmeier, 1953: Costa Rica
N. macrodentatus (Menozzi, 1931): Costa Rica
N. mandibularis (Smith, M.R., 1942b): United States
N. manni (Wheeler, W. M., 1914): Mexico
N. maroccanus (Santschi, 1926b): Morocco (labeling error)
N. maxillosus (Emery, 1900b): Brazil
N. megathrix Kempf, 1961: Suriname
N. melanocephalus (Emery, 1895d): Mexico
N. melshaemeri (Haldeman, 1852): United States
N. micans Borgmeier, 1953: Brazil
N. microps Borgmeier, 1955: United States
N. minensis (Borgmeier, 1928): Brazil
N. minor (Cresson, 1872): United States
N. modestus (Borgmeier, 1933): Brazil
N. mojave (Smith, M.R., 1943): United States
N. moseri Watkins, 1969: United States
N. ndeh Snelling, G.C. and Snelling, R.R., 2007: United States
N. nigrescens (Cresson, 1872): United States
N. nordenskioldii (Holmgren, 1908): Peru
N. nyensis Watkins, 1977: United States
N. opacithorax (Emery, 1894): United States
N. orthonotus (Borgmeier, 1933): Brazil
N. pacificus Borgmeier, 1955: Peru
N. pauxillus (Wheeler, W. M., 1903a): United States
N. perplexus Borgmeier, 1953: Brazil
N. pertii (Shuckard, 1840b) : Brazil
N. physognathus (Emery, 1900b): Bolivia
N. pilosus (Smith, F., 1858): Brazil
N. piraticus Borgmeier, 1953: Brazil
N. planidens Borgmeier, 1953: Ecuador
N. planidorsus (Emery, 1906): Paraguay
N. postangustatus (Borgmeier, 1934): Suriname
N. postcarinatus Borgmeier, 1953: Panama
N. pseudops (Forel, 1909a): Paraguay
N. puerulus Borgmeier, 1955: Panama
N. pulchellus Borgmeier, 1955: Panama
N. pullus Borgmeier, 1953: Panama
N. punctaticeps (Emery, 1894): Brazil
N. quadratoocciputus Watkins, 1975c: El Salvador
N. radoszkowskii (Emery, 1900b): Peru
N. raptor (Forel, 1911b): Brazil
N. romandii (Shuckard, 1840b): Brazil
N. rosenbergi (Forel, 1911d): Ecuador
N. rugulosus Borgmeier, 1953: Mexico
N. scutellaris Borgmeier, 1953: Panama
N. shuckardi (Emery, 1900b): Paraguay
N. spatulatus (Borgmeier, 1939): Costa Rica
N. spoliator (Forel, 1899): Costa Rica
N. sulcatus (Mayr, 1868): Argentina
N. sumichrasti (Norton, 1868): Mexico
N. swainsonii (Shuckard, 1840a): Brazil
N. tenuis Borgmeier, 1953: Brazil
N. texanus Watkins, 1972: United States
N. tristis (Forel, 1901e): Mexico
N. vicinus Borgmeier, 1953: Brazil
N. walkerii (Westwood, 1842): Brazil
N. wilsoni Snelling, G.C. and Snelling, R.R., 2007: United States
Neocerapachys gen. n.
Type-species
Cerapachys (Cerapachys) neotropicus, by present designation.
Neocerapachys is a rarely encountered Neotropical lineage with unknown habits.
Diagnosis
Worker. Neocerapachys can be recognized by a combination of relatively low-positioned propodeal spiracle, propodeal lobes present, constriction present between abdominal segments III and IV, middle tibiae with a single spur, pretarsal claws unarmed, petiole dorsolaterally rounded (not marginate), constriction absent from between abdominal segments IV, V, and VI, pronotomesopleural Pronotomesopleural suture fused, helcium axial, abdominal segment III anterodorsally often marginate, and two spots where pilosity is denser than the surrounding hairs present laterally on abdominal tergite IV. Neocerapachys is superficially very similar to certain species of Parasyscia of the Old World but the latter never has lateral clumps of hair on abdominal tergite IV and its metapleural gland trench is broader than in Neocerapachys. Palp formulae also differ in these two lineages with 3,3 in Neocerapachys and 3,2 or 2,2 in Parasyscia. The neotropical Sphinctomyrmex shares several characters with Neocerapachys but is distinguished by constrictions between abdominal segments IV, V, and VI.
Male. Males of Neocerapachys possess well-developed propodeal lobes, mid and hind tibiae each with one spur, C and R·f3 veins in the fore wing, Rs·f2–3 abscissae present, cross-vein 2rs-m absent, third antennal segment conspicuously the shortest segment, and conspicuously marginate propodeal declivity. This combination will serve to distinguish it from all other lineages. Indomalayan Cerapachys is a relatively similar genus but it differs in longer, normally developed third antennal segment and eyes situated further away from mandibular insertions. In the Neotropics, Sphinctomyrmex males have similar wing venation but are easily told apart by constrictions visible between abdominal segments IV, V, and VI.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 3-segmented. Labial palps 3-segmented. Mandibles triangular, with teeth. Eyes present, composed of 1–5 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head with cuticular ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla partially obscured but often discernable through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally marginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field, and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus with cuticular apron. Parafrontal ridges present. Torulo-posttorular complex vertical. Maxillary palps 4-segmented. Labial palps 3-segmented. Mandibles triangular, edentate. Ventrolateral margins of head with cuticular ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and supraaxial. Prora forming a simple U-shaped margin. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV or about half size; latter weakly or strongly constricted at presegmental portion (transitional between uninodal waist and binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines curving dorsally at apices, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere broadly fused to telomere, basimere with no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella laterally flattened, at apex with dorsal lobe and hooked ventrally. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present and running toward distal wing margin and enclosing marginal cell with Rs·f5 or not. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2 or disconnected from Rs+M. Cross-vein 2r-rs absent. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing absent or present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Apparently alate or ergatoid with well-developed mesosomal sutures; with large eyes and three ocelli. This interpretation is based on one gyne specimen from Venezuela (John T. Longino personal collection, LACMENT 142669).
Larva. Not described. Presence of cocoons unknown.
Distribution
This lineage ranges from Costa Rica south to southern Brazil and apparently is not very species-rich with only two species described.
Taxonomy and phylogeny
Both currently named species have been described under Cerapachys and later discussed by
The exact phylogenetic position of Neocerapachys is not known with certainty, but in molecular analyses based on genomic data it is a part of a large New World clade that includes Acanthostichus, Cylindromyrmex, Leptanilloides, Sphinctomyrmex, and the Eciton genus-group (Borowiec, in prep.).
Biology
I am not aware of any nest collections or observations of behavior of Neocerapachys.
Species of Neocerapachys
N. neotropicus (Weber, 1939): Trinidad and Tobago, comb. n.
N. splendens (Borgmeier, 1957): Brazil, comb. n.
Nomamyrmex
Type-species
Eciton crassicornis (junior synonym of Labidus esenbeckii), by original designation.
Nomamyrmex is a relatively commonly observed genus with only two species and two additional subspecies recognized. It is the only army ant genus that has been reported to successfully attack well-defended and often enormous colonies of Atta leaf cutter ants.
Diagnosis
Worker. The workers of Nomamyrmex are easily recognized by a combination of highly positioned spiracle and lack of pronounced propodeal lobes, propodeum armed with cuticular projections, two-segmented waist, armed pretarsal claws, and absence of metatibial gland. The lack of conspicuous lighter area of cuticle on the inner side of hind tibia (the metatibial gland) distinguishes this genus from all other Eciton genus-group ants except for some Neivamyrmex, but those always have simple pretarsal claws.
Male. Nomamyrmex males possess traits characteristic of New World army ants; see discussion under Cheliomyrmex for characters distinguishing New World army ant males from those of the Old World. Nomamyrmex is also easily told apart from other New World army ant males by its dense tufts of very long hairs present on the gaster. Eciton setigaster is one species that could be mistaken for a Nomamyrmex, but the setae on its gaster are not as dense or as long, not approaching front femur length.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 3-segmented. Mandibles triangular, with teeth. Eyes present, appearing as single large and convex ommatidium, in reality composed from fused ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange separated from collar by distinct ridge or not. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove not impressed. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma present. Propodeal spiracle situated high on sclerite. Propodeal declivity with or without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, short. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora forming a simple U-shaped margin or V-shaped protrusion. Spiracle openings of abdominal segments IV–VI oval to slit-shaped. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and sculptured but not cross-ribbed. Abdominal segment IV conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium small, reduced to narrow strip, without impressed medial field and simple, not armed with cuticular spines or modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal gland absent. Hind pretarsal claws each armed with a tooth. Polymorphism: Polymorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 3- or 2-segmented. Mandibles falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora forming a simple U-shaped margin. Spiracle openings of abdominal segments IV–VI slit-shaped. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula very long, nearing or surpassing length of rest of genital capsule and of approximately equal length on both dorsal and ventral surfaces. Basimere narrowly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms abutting. Telomere expanded at apex. Volsella laterally flattened, narrowly triangular in lateral view, narrowing towards tip. Penisvalva curved ventrally at apex, with short dorsal and longer ventral process. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma narrow. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing present. Vein R in hind wing present, reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, reaching wing margin. Cross-vein 1rs-m in hind wing present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Dichthadiiform, with falcate mandibles, small eyes, and no ocelli. Known for N. esenbeckii (
Larva. Not described. Cocoons present.
Distribution
Both Nomamyrmex species are widely distributed and the genus is found from Texas to northern Argentina.
Taxonomy and phylogeny
The two species of Nomamyrmex were known since Westwood described them in 1842, but he treated them under Labidus. Borgmeier introduced Nomamyrmex as a subgenus of Eciton (
Biology
Henry Walter Bates was perhaps the first to report on the habits of Nomamyrmex in his famous narrative (
A remarkable aspect of Nomamyrmex biology is the capability to successfully raid the huge colonies of leaf-cutting ants in the genus Atta, otherwise mostly ignored by army ants. Most published records of Nomamyrmex foraging contain observations of raids on leaf cutters (
Nomamyrmex is capable of inflicting significant damage on a raided Atta colony. A subterranean raid on a partially excavated Atta mexicana colony was observed where the army ants killed a large proportion of adult Atta, including the queen (
Species of Nomamyrmex
N. esenbeckii (Westwood, 1842): Brazil
N. esenbeckii mordax (Santschi, 1929): Mexico
N. esenbeckii wilsoni (Santschi, 1920a): United States
N. hartigii (Westwood, 1842): Brazil
Ooceraea , gen. rev.
= Cysias Emery, 1902, syn. n.
Type-species
Ooceraea fragosa, by monotypy.
Ooceraea is an Old World lineage that contains a species emerging as the only model organism among dorylines, the clonal O. biroi.
Diagnosis
Worker. The workers of Ooceraea can be distinguished by a combination of propodeal spiracle positioned low on the sclerite and pygidium armed with modified setae, antennae with 11 or fewer segments, pronotomesopleural Pronotomesopleural suture developed, two-segmented waist with abdominal segment III strongly tubulated, and no constrictions between abdominal segments IV, V, and VI. The abdominal segment IV is conspicuously the largest and its tergite does not fold over the sternite anteriorly. The habitus of Ooceraea is distinctive, with conspicuously differentiated abdominal segment III forming a postpetiole, eyes small or absent, and coarse cuticular sculpturing. Among the non-army ant dorylines that exhibit reduction in antennomere count Ooceraea can only be confused with Syscia and Parasyscia. The former exhibits a conspicuous folding of the anterior portion of abdominal tergite IV and possesses a unique mid-tibial gland (see below). The few Parasyscia species that have 11 antennal segments can be distinguished from Ooceraea by fused pronotomesopleural Pronotomesopleural suture and larger abdominal segment III.
Male. The males of Ooceraea commonly have only 11 antennal segments, which is unique among male dorylines, but a few have 12-segmented antennae, a state shared with most Acanthostichus and all Eusphinctus, Simopone, and Syscia. In Acanthostichus and Eusphinctus the costal vein of fore wing is always present, while missing from the majority of Ooceraea. Additionally, in Acanthostichus the vein R·f3 is visible beyond pterostigma and in Eusphinctus the submarginal cell is closed by Rs·f2–3. Both of these veins are always absent in Ooceraea. Distinguishing between males of Ooceraea and Syscia is difficult. As mentioned above, the majority of species in Ooceraea have 11-segmented antennae, while in Syscia these seem to be always 12-segmented. In Ooceraea the discal cell is closed by cross-vein 1m-cu in the majority of males examined, except for the smallest of specimens, while in the limited material of Syscia I have examined this vein appears to be universally absent. Additionally, most Ooceraea males have a unique specialization of abdominal sternite VII, ranging from a deep cleft in the middle of the posterior margin and denser pilosity on lateral sides, to conspicuous cuticular projections with a brush of hairs. No Syscia have such modifications but in certain Ooceraea this character is not obvious (e.g. O. biroi) or absent (a male tentatively associated with O. coeca).
Description
Worker.Head: Antennae with 9, 10 (rarely) or 11 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus with or without cuticular apron. Lateroclypeal teeth present. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 3-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth. Eyes absent or present but small, composed of 1–5 ommatidia, very rarely composed of 6–20 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge or not. Promesonotal connection with Pronotomesopleural suture completely fused or Pronotomesopleural suture present, weakly differentiated, immobile. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove not impressed to weakly impressed. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with or without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland bulla visible or not through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora simple, not delimited by carina or a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III about half size of succeeding segment IV, which is strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Abdominal segment IV conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium medium-sized, with impressed medial field, and armed with modified setae. Hypopygium unarmed or armed with modified setae. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 11 segments or more rarely with 12 segments. Clypeus with cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 5-segmented. Labial palps 3-segmented. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge, occasionally ridge marked on sides. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity reduced, with or without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate, inconspicuously in small species. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin or a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III about half size of succeeding segment IV or less; latter strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV conspicuously largest segment. Abdominal sternite VII modified, rarely simple. Abdominal sternite IX cleft to modified into two spines, sometimes with additional medial projection or spine, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present or absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 absent. Cross-vein 2r-rs present, forming base of ‘free stigmal vein’ (2r-rs&Rs·f4–5) in absence of Rs·f3 and 2rs-m, although 2rs-m may be present as stub. Abscissae Rs·f4–5 present, fused in absence of 2rs-m or absent. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing absent. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing absent or present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissa A·f1 or with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent or present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing absent. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing absent or present, not reaching wing margin. Cross-vein 1rs-m in hind wing absent. Vein M+Cu in hind wing absent or present. Abscissa M·f1 in hind wing absent. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent or present. Vein Cu in hind wing absent. Vein A in hind wing absent or with abscissa A·f1 present.
Gyne. Ergatoid or replaced by fertile workers (
Larva. Larva has been described for Ooceraea australis (
Distribution
Ooceraea is a lineage confined to the Indomalayan and Australasian regions, including the Fijian archipelago. O. biroi is a tramp species that has been more widely introduced across tropical regions of the world.
Taxonomy and phylogeny
The taxonomic history of Ooceraea is complicated. The genus was originally described by
Cysias is a name introduced by
Genomic data show that Ooceraea is most closely related to Eusphinctus and Syscia (Borowiec, in prep.). No attempts to investigate the internal phylogeny have been made.
Biology
The members of this lineage are found primarily in leaf litter and soil core samples. Worker morphology (eyes often very small or absent) is also suggestive of subterranean habits.
Ooceraea biroi is perhaps the best studied doryline species. The army ants Eciton and Dorylus have been extensively researched in the field, but their huge colonies are exceptionally difficult to manipulate in laboratory conditions. In contrast, O. biroi is a species much more amenable to experimental manipulation and has been the focal organism for multiple published laboratory-based studies.
O. biroi is a clonal species where all workers in a colony have reproductive potential and multiple individuals are active egg layers (
In addition to offering a rare opportunity for studying the habits of a non-army ant doryline, O. biroi has also provided some important insights into social insect biology in general. A study by
It is unknown whether the clonal reproduction and brood production synchronicity in O. biroi is representative of other Ooceraea and if the species is a part of an older clade of parthenogenetic lineages or an exception. Subdichthadiigyne queens of Ooceraea crypta suggest more traditional reproduction in at least one other species. Many males of Ooceraea have a highly modified abdominal sternite VII, suggesting its involvement in copulation (see discussion of male characters above). An Australian species O. australis is relatively common throughout the continent and has been reported to form colonies with thousands of individuals (
Species of Ooceraea
O. alii (Bharti and Akbar, 2013): India, comb. n.
O. australis (Forel, 1900a): Australia, nom. rev.
O. biroi (Forel, 1907a): Singapore, comb. n.
O. besucheti (Brown, 1975): India, comb. n.
O. coeca Mayr, 1897: Sri Lanka, comb. rev.
O. crypta (Mann, 1921): Fiji, comb. n.
O. fuscior (Mann, 1921): Fiji, comb. n.
O. fragosa Roger, 1862: Sri Lanka, comb. rev.
O. papuana Emery, 1897: Papua New Guinea, comb. rev.
O. pawa (Mann, 1919): Solomon Islands, comb. n.
O. pusilla Emery, 1897: Papua New Guinea, comb. n.
Parasyscia , gen. rev.
Type-species
Parasyscia piochardi, by monotypy.
After Lioponera this is the most species-rich lineage formerly included in Cerapachys.
Diagnosis
Worker. Parasyscia workers are distinguished by a combination of propodeal spiracle positioned low on the sclerite and propodeal lobes present, constriction between abdominal segments III and IV, petiole dorsolaterally not marginate, no constriction between abdominal segments IV, V, and VI, pronotomesopleural Pronotomesopleural suture fused, helcium axial, middle tibiae with a single pectinate spur, pretarsal claws unarmed, and abdominal segment III anterodorsally often marginate. Parasyscia is an exclusively Old World group and is most similar to Neocerapachys of the New World, which can be differentiated by narrower trench leading to the metapleural gland orifice, the presence of two patches of denser pilosity on abdominal tergite IV, and different palp formula (3,3 in Neocerapachys versus 3,2 or 2,2 in Parasyscia).
Male. The males of Parasyscia have variably developed wing venation and are generally diverse, making them somewhat challenging to identify. Most species share characteristic venation: C and R·f3 are absent, Rs·f2–3 is present and runs all the way from Rs+M to 2r-rs, closing a submarginal cell. In addition, Parasyscia males have 13-segmented antennae, antennal toruli fully exposed, no constrictions between abdominal segments IV, V, and VI, narrow and axial helcium, and a single spur on each middle and hind tibia. Lividopone and Zasphinctus may have similar wing venation but the former has a broad supraaxial helcium and the latter has pronounced constrictions between abdominal segments IV, V, and VI. Along with Lioponera, Parasyscia males are among the most common of non-army ant doryline males in collections from the Old World. Except for specimens with much reduced wing venation, Lioponera can be distinguished by a ‘free stigmal vein’ formed in complete absence of Rs·f2–3.
Description
Worker.Head: Antennae with 11 or 12 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 3- or 2-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth. Mandibles triangular, edentate. Eyes present, composed of 1 to more than 20 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head with or without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated or not from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture completely fused. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron absent or present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with or without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus with cuticular apron. Parafrontal ridges present. Torulo-posttorular complex vertical. Maxillary palps 2-segmented. Labial palps 2-segmented. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange separated from collar by distinct ridge mostly on sides or not separated. Notauli absent or present. Transverse groove dividing mesopleuron present. Propodeal declivity reduced, with or without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms separated. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally flattened, at apex hooked ventrally. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, disconnected from Rs+M or connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2 or contiguous with Rs+M. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing absent or present. Cross-vein cu-a in fore wing present, arising from M+Cu at variable distance, proximal, distal to, at or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissa A·f1 present or with abscissae A·f1 and A·f2 present; A·f2 short. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing absent. Abscissa Rs·f1 in hind wing absent. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing absent or present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissa A·f1 or with abscissae A·f1 and A·f2 present.
Gyne. Alate gynes are known in a number of species, e.g. P. afer, P. imerinensis, P. reticulata. Ergatoid gynes are also known, for example in P. indica, P. nayana, P. schoedli, P. seema, and P. sudanensis. The ergatoid gyne of P. schoedli is extremely worker-like, does not possess ocelli, and differs form the worker mostly in relatively larger gaster and more erect pilosity. In P. seema intercastes or ergatoids with ocelli but no modifications to the mesosoma are known in addition to a gyne with well-developed mesosomal sutures. It is unclear, however, whether the latter is dealated or never possessed wings (
Larva. The larva of Parasyscia opaca has been described (
Distribution
50 species of Parasyscia are known, distributed throughout the warm temperate and tropical Old World, extending into New Guinea and many Pacific islands but absent from Australia.
Taxonomy and phylogeny
Parasyscia was described as a genus by Emery in 1882 and shortly afterwards treated as a subgenus by
Parasyscia has been identified as the sister group of Zasphinctus (
Biology
Two Parasyscia species, P. flavaclavata and P. opaca, were observed in the field in New Guinea (
Species of Parasyscia
P. afer (Forel, 1907a): Tanzania, comb. n.
P. aitkenii (Forel, 1900b): India, comb. n.
P. arnoldi (Forel, 1914): South Africa, comb. n.
P. browni (Bharti and Wachkoo, 2013): India, comb. n.
P. bryanti (Wheeler, W. M., 1919): Malaysia (Sarawak), comb. n.
P. centurio (Brown, 1975): Democratic Republic of the Congo, comb. n.
P. conservata (Viehmeyer, 1913): Indonesia (Sulawesi, in copal), comb. n.
P. cribrinodis Emery, 1899b: Cameroon, comb. rev.
P. desposyne (Wilson, 1959): Papua New Guinea, comb. n.
P. dohertyi (Emery, 1902): Indonesia (Laut Island), comb. n.
P. dominula (Wilson, 1959): Indonesia (Papua), comb. n.
P. faurei (Arnold, 1949): South Africa, comb. n.
P. flavaclavata (Donisthorpe, 1938): Indonesia (Papua), comb. n.
P. fossulata (Forel, 1895a): Sri Lanka, comb. n.
P. foveolata (Radchenko, 1993): Vietnam, comb. n.
P. hashimotoi (Terayama, 1996): Japan, comb. n.
P. imerinensis Forel, 1891: Madagascar, comb. rev.
P. inconspicua Emery, 1901c: Papua New Guinea, comb. n.
P. indica (Brown, 1975): India, comb. n.
P. kenyensis (Consani, 1951): Kenya, comb. n.
P. keralensis (Karmaly, 2012): India, comb. n.
P. kodecorum (Brown, 1975): Indonesia (South Kalimantan), comb. n.
P. lamborni (Crawley, 1923): Malawi, comb. n.
P. lindrothi (Wilson, 1959): Fiji, comb. n.
P. luteoviger (Brown, 1975): Sri Lanka, comb. n.
P. majuscula (Mann, 1921): Fiji, comb. n.
P. muiri (Wheeler, W. M. and Chapman, 1925): Philippines, comb. n.
P. natalensis (Forel, 1901d): South Africa, comb. n.
P. nitens (Donisthorpe, 1949): Indonesia (Papua), comb. n.
P. nitidula (Brown, 1975): Democratic Republic of the Congo, comb. n.
P. opaca (Emery, 1901c): Papua New Guinea, comb. n.
P. peringueyi Emery, 1886: South Africa, comb. rev.
P. piochardi Emery, 1882: Syrian Arab Republic, comb. rev.
P. polynikes (Wilson, 1959): Papua New Guinea, comb. n.
P. reticulata (Emery, 1923): Taiwan, comb. n.
P. rufithorax (Wheeler, W. M. and Chapman, 1925): Philippines, comb. n.
P. salimani (Karavaiev, 1925): Indonesia (Java), comb. n.
P. seema (Bharti and Akbar, 2013): India, comb. n.
P. schoedli (Bharti and Akbar, 2013): India, comb. n.
P. sculpturata (Mann, 1921): Fiji, comb. n.
P. sudanensis (Weber, 1942): South Sudan, comb. n.
P. sylvicola (Arnold, 1955): Zimbabwe, comb. n.
P. superata (Wilson, 1959): Papua New Guinea, comb. n.
P. terricola (Mann, 1919): Solomon Islands, comb. n.
P. valida (Arnold, 1960): South Africa, comb. n.
P. villiersi (Bernard, 1953b): Guinea, comb. n.
P. vitiensis (Mann, 1921): Fiji, comb. n.
P. wighti (Bharti and Akbar, 2013): India, comb. n.
P. wittmeri (Collingwood, 1985): Saudi Arabia, comb. n.
P. zimmermani (Wilson, 1959): Fiji, comb. n.
Procerapachys
Type-species
Procerapachys annosus, by original designation.
Procerapachys is an extinct genus known from Baltic amber.
Diagnosis
Worker. The extinct Procerapachys is apparently unique among non-army ant dorylines in having a large but unarmed pygidium. All other dorylines with unarmed pygidium have either highly positioned propodeal spiracles and no propodeal lobes (Aenictus, Aenictogiton, Dorylus) or a reduced pygidium (Leptanilloides). When pygidium is not clearly visible, these often heavily-sculptured ants can be confused with Chrysapace, which also occurs in Eocene ambers (see under that taxon above). Chrysapace and Procerapachys differ in spur formula, however. The former has two pectinate spurs on each mid and hind tibia, and the latter has only one pectinate spur. Procerapachys specimens were also reported to have palp formula 5,4, which is different from 5,3 counted in one of the extant Chrysapace.
Male. The status of the putative males of Procerapachys is uncertain, but the specimens originally attributed to this genus had well-developed wing venation with two submarginal cells and the marginal cell closed, similar to Chrysapace and Cylindromyrmex. The most reliable character that separates these males from these two genera is a single pectinate tibial spur in Procerapachys and two spurs present in both Chrysapace and Cylindromyrmex.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeal apron unknown. Lateroclypeal teeth unknown. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum shape unknown. Proximal face of stipes unknown. Maxillary palps 5-segmented. Labial palps 4-segmented. Mandibles triangular, edentate. Eyes present, composed of more than 20 ommatidia. Ocelli absent or present. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head unknown. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen unknown. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture conspicuous and complete, but immobile. Pronotomesopleural suture complete, continuous with promesonotal Pronotomesopleural suture. Mesometapleural groove not impressed. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity unknown. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland unknown. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally unknown, dorsolaterally immarginate, and laterally above spiracle marginate. Placement of helcium unknown. Prora unknown. Spiracle openings of abdominal segments IV–VI unknown. Abdominal segment III anterodorsally unknown and dorsolaterally unknown. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV unknown. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field and simple, not armed with cuticular spines or modified setae. Hypopygium unknown. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa unknown. Metatibial gland unknown. Metabasitarsal gland unknown. Hind pretarsal claws unknown. Hind pretarsal claws simple. Polymorphism: Unknown.
Male. (putative, see under Taxonomy and phylogeny below) Head: Antennae with 13 segments. Clypeal lamella unknown. Parafrontal ridges unknown. Torulo-posttorular complex vertical. Maxillary palps unknown. Labial palps unknown. Mandibles triangular, edentate. Ventrolateral margins of head unknown. Carina surrounding occipital foramen unknown. Mesosoma: Pronotal flange separated from collar by distinct ridge. Notauli unknown. Transverse groove dividing mesopleuron absent. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening unknown. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle unknown. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora unknown. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV unknown. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX unknown. Genitalia: Genital morphology unknown. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa unknown. Metatibial gland unknown. Metabasitarsal glands unknown. Hind pretarsal claws unknown. Wings: Tegula unknown. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from Cu and distal to, at or near M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Hind wing venation unknown.
Gyne. Not described. Wheeler (1915) mentioned that some of the specimens possessed ocelli while others did not and suggesting that these may represent ergatogynes.
Larva. Not described. Presence of cocoons unknown.
Taxonomy and phylogeny
Procerapachys was described based on several workers and two male specimens by W. M. Wheeler (1915) in his monograph on the Baltic amber collection of the Geological Institute of Königsberg (now Kaliningrad, Russia). Unfortunately, most of this collection was destroyed during WWII, including the Procerapachys material (Dlussky, 2009).
Distribution
Eocene Baltic and Bitterfeld ambers.
Species of Procerapachys
†P. annosus Wheeler, W. M. 1915b: Baltic amber
†P. favosus Wheeler, W. M. 1915b: Baltic amber
†P. sulcatus Dlussky, 2009: Baltic amber
Simopone
Type-species
Simopone grandidieri, by monotypy.
Simopone is a genus of arboreal predators of other ants, found in the Old World tropics.
Diagnosis
Worker. Workers of Simopone are unique among all dorylines in the combination of 11-segmented antennae, eyes and ocelli present, no spur on mid tibia, and the lack of metatibial gland. Simopone also possess a conspicuous groove on the interior surface of hind basitarsus. Other dorylines lacking mid tibial spur include certain species of Lioponera, all Tanipone, and Vicinopone. All these genera have 12-segmented antennae.
Male. The males are easily identified by a combination of antennal sockets partially concealed by the torulo-posttorular complex in full-face view, 12-segmented antennae, presence of notauli, and lack of spurs on middle tibiae. The only other non-army ant doryline genus that lacks spurs on middle tibiae is Tanipone, although it is possible that Vicinopone males will turn out to lack them also, when discovered. All Tanipone males known thus far have fully exposed antennal sockets, 13-segmented antennae, and no notauli, in addition to characteristically long maxillary palps that reach the occipital foramen.
Description
Worker.Head: Antennae with 11 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges absent or reduced. Torulo-posttorular complex horizontal. Antennal scrobes absent or present. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 6- or 5-segmented. Labial palps 4- or 3-segmented. Mandibles triangular, edentate. Eyes present, composed of more than 20 ommatidia. Ocelli present. Head capsule without differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange often separated from collar by distinct ridge, occasionally ridge absent. Promesonotal connection with Pronotomesopleural suture present, weakly differentiated, immobile. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove weakly impressed or not impressed. Transverse groove dividing mesopleuron absent or present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal groove on mesosoma absent or shallowly impressed but well-defined line. Propodeal spiracle situated low on sclerite. Propodeal declivity often without distinct dorsal edge or margin but occasionally marginate, rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate or marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora simple, not delimited by carina, a U-shaped margin, or U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field, armed with modified setae, and in some species deeply notched at apex. Hypopygium unarmed. Legs: Mid tibia without spurs. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal gland present. Hind pretarsal claws each armed with a tooth. Polymorphism: Monomorphic.
Male.Head: Antennae with 12 segments. Clypeus without cuticular apron. Parafrontal ridges present. Torulo-posttorular complex horizontal. Maxillary palps 6- or 5-segmented. Labial palps 4- or 3-segmented. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange separated from collar by distinct ridge. Notauli present. Transverse groove dividing mesopleuron absent. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms separated. Telomere expanded at apex. Volsella variable. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia without spurs. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3 or absent. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present or absent. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with both branches Cu1 and Cu2. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing unknown. Abscissa Rs·f2 in hind wing unknown. Cross-vein 1rs-m in hind wing present, about as long as M·f1, never tubular. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present.
Gyne. Alate or extremely ergatoid/ replaced by fertile workers. Alate and dealated queen specimens are known in all three Simopone species-groups recognized by
Larva. Not described. Cocoons absent.
Distribution
Simopone is limited to the Old World and currently contains 39 named species. Most occur in Madagascar and in the Afrotropics but five rare species have been recorded from the Indomalayan Region (China, Thailand, Vietnam, Singapore, Philippines) and New Guinea.
Taxonomy and phylogeny
Biology
Despite relatively high species diversity very little is known about the biology of Simopone, although several species have been recorded nesting arboreally (
Brood production appears not to be synchronized (author’s observations).
Species of Simopone
S. amana Bolton and Fisher, 2012: Gabon
S. annettae Kutter, 1976: Cameroon
S. bakeri Menozzi, 1926: Singapore
S. brunnea Bolton and Fisher, 2012: Gabon
S. chapmani Taylor, 1966: Philippines
S. conradti Emery, 1899b: Cameroon
S. consimilis Bolton and Fisher, 2012: Madagascar
S. dignita Bolton and Fisher, 2012: Madagascar
S. dryas Bolton and Fisher, 2012: Kenya
S. dux Bolton and Fisher, 2012: Madagascar
S. elegans Bolton and Fisher, 2012: Madagascar
S. emeryi Forel, 1892b: Madagascar
S. fera Bolton and Fisher, 2012: Madagascar
S. fulvinodis Santschi, 1923b: Democratic Republic of the Congo
S. grandidieri Forel, 1891: Madagascar
S. grandis Santschi, 1923b: Democratic Republic of the Congo
S. gressitti Taylor, 1965: Indonesia (Papua)
S. inculta Bolton and Fisher, 2012: Madagascar
S. laevissima Arnold, 1954: Uganda
S. latiscapa Bolton and Fisher, 2012: Ghana
S. marleyi Arnold, 1915: South Africa
S. matthiasi Kutter, 1977: Cameroon
S. mayri Emery, 1911: Madagascar
S. merita Bolton and Fisher, 2012: Madagascar
S. miniflava Bolton and Fisher, 2012: Gabon
S. nonnihil Bolton and Fisher, 2012: Madagascar
S. occulta Bolton and Fisher, 2012: Gabon
S. oculata Radchenko, 1993: Vietnam
S. persculpta Bolton and Fisher, 2012: Kenya
S. rabula Bolton and Fisher, 2012: Tanzania
S. rex Bolton and Fisher, 2012: Madagascar
S. schoutedeni Santschi, 1923b: Democratic Republic of the Congo
S. sicaria Bolton and Fisher, 2012: Madagascar
S. silens Bolton and Fisher, 2012: Madagascar
S. trita Bolton and Fisher, 2012: Madagascar
S. vepres Bolton and Fisher, 2012: Ghana
S. victrix Bolton and Fisher, 2012: Madagascar
S. wilburi Weber, 1949a: Democratic Republic of the Congo
S. yunnanensis Chen, Zhou and Liang, 2015: China
Sphinctomyrmex
Type-species
Sphinctomyrmex stali, by monotypy.
Sphinctomyrmex is a Neotropical lineage of extremely rarely encountered ants. Nothing is known about their biology.
Diagnosis
Worker. Workers of Sphinctomyrmex are among the dorylines with prominent girdling constrictions between abdominal segments IV, V, and VI. These include Aenictogiton, Eusphinctus, Leptanilloides, and Zasphinctus. Sphinctomyrmex can be differentiated from these genera by a combination of presence of propodeal lobes and propodeal spiracle positioned high (no lobes and spiracle low on propodeum in Aenictogiton), metapleural gland trench narrow (broad in Zasphinctus), large pygidium armed with modified setae (pygidium unarmed and reduced to a narrow strip in Leptanilloides), and girdling constriction between abdominal segments III and IV cross-ribbed and segment IV similar in size to segments V and VI (girdling constriction smooth and segment IV larger than V and VI in Eusphinctus). Among these genera, only Leptanilloides occurs in sympatry with Sphinctomyrmex.
Male. The males of Sphinctomyrmex also show girdling constrictions between abdominal segments III, IV, and V. Among male dorylines, this state is restricted to the Old World taxa Eusphinctus and Zasphinctus. They differ in wing venation, shape of abdominal sternite IX and genitalia and the venation characters are the easiest to assess for identification. The marginal cell is closed in Sphinctomyrmex (open in Eusphinctus) and the costal vein (C) is present in the fore wing (absent in Zasphinctus). Malagasy Tanipone may also have weak abdominal constrictions but are distinguished by very long, 6-segmented maxillary palps that are visible in mounted specimens and reach occipital foramen. Some Acanthostichus or Cylindromyrmex males may also have gastral constrictions but in the former helcium is broad and supraaxial and in the latter there are two spurs on hind tibiae.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 3-segmented. Labial palps 3-segmented. Mandibles triangular, edentate. Eyes present, composed of 1–5 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused or with Pronotomesopleural suture present, weakly differentiated, immobile. Pronotomesopleural suture completely fused but impressed line present. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI present. Girdling constriction between pre- and poststernites of abdominal segments V and VI present. Pygidium large, with impressed medial field, and armed with modified setae. Hypopygium unarmed. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus with cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps unknown. Labial palps unknown. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity with dorsal edge present, incomplete. Metapleural gland opening present. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI present. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella laterally flattened, at apex with dorsal lobe and hooked ventrally. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present, running toward distal wing margin and enclosing cell with Rs·f5. Abscissae Rs·f2–3 absent or present, very short and disconnected from Rs+M. Cross-vein 2r-rs present, forming base of 2r-rs&Rs·f4–5 in absence of 2rs-m or differentiated from Rs·f4 by presence of short Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing present, extending past Sc+R but not reaching distal wing margin. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, longer than 1rs-m. Abscissa Rs·f2 in hind wing present, short, not reaching wing margin. Cross-vein 1rs-m in hind wing absent. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing absent. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent or stub present. Vein Cu in hind wing present. Vein A in hind wing with abscissa A·f1 present.
Gyne. Gynes are so far only known for S. stali. One apparently dealated gyne has been collected in this species, in addition to ergatoid/intercaste specimens with relatively large eyes and ocelli. For a detailed discussion see
Larva. Not described. Cocoons unknown.
Distribution
So far only known from Amazonas, Santa Catarina, and São Paulo states in Brazil, and Jujuy province in Argentina but likely present throughout most of South America.
Taxonomy and phylogeny
For taxonomic history see under Eusphinctus.
The three known species of Sphinctomyrmex are reviewed and keyed in
The affinities of the genus are not known exactly but genomic data suggests that it forms a clade with Leptanilloides and the Eciton genus-group (Borowiec, in prep.).
Biology
Virtually nothing is known of this lineage’s biology, and no nest series have ever been collected (
Species of Sphinctomyrmex
S. marcoyi Feitosa, Brandão, Fernández and Delabie, 2011: Brazil
S. schoerederi Feitosa, Brandão, Fernández and Delabie, 2011: Brazil
S. stali Mayr, 1866b: Brazil
Syscia , gen. rev.
Type-species
Syscia typhla, by monotypy.
Syscia is the only doryline genus with a disjunct distribution between the Old and New World, and includes many cryptic, undescribed species.
Diagnosis
Worker. Syscia workers have 11- or 9-segmented antennae, eyes small to absent, and are usually heavily sculptured with abundant body pilosity. Body is usually uniformly colored and ranges from yellow through reddish to dark brown but never black. They possess apparently autapomorphic characters that serve to easily distinguish this lineage from all other dorylines: basal segment of hind tarsus widening distally with a light patch of cuticle on the inner (flexor) side, and abdominal tergite IV anteriorly folding over sternite. This combination is unique to Syscia and although species in other lineages may have similar habitus (Ooceraea, Parasyscia), none of these possess these characteristics.
Male. The males of Syscia have the number of antennal segments reduced to 12. They can be difficult to distinguish from Ooceraea (see under diagnosis for that genus), but a lack of constrictions between abdominal segments IV, V, and VI, presence of a spur on middle tibia, and no costal vein (C) in the fore wing will distinguish them from the other genera where a reduction in the number of antennal segments is currently known, which include Acanthostichus, Eusphinctus, and Simopone.
Description
Worker.Head: Antennae with 9 or 11 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth present. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 2-segmented. Mandibles triangular, edentate. Eyes absent or present, composed of 1–5 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove not impressed. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron present or absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured or cross-ribbed. Abdominal segment IV not conspicuously largest segment or conspicuously largest segment. Abdominal tergite IV folding over sternite, anterior portion of sternite concealing tergite in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium medium-sized, with impressed medial field, and armed with modified setae. Hypopygium unarmed or armed with modified setae. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus widening distally, oval in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland present. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 12 segments. Clypeus with cuticular apron. Parafrontal ridges present. Torulo-posttorular complex vertical. Maxillary palps 4-segmented. Labial palps 2-segmented. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli present. Transverse groove dividing mesopleuron present. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Abdominal segment III about half size of succeeding segment IV or less; latter strongly constricted at presegmental portion (binodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines. with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella not tapering much toward apex, relatively broad. Penisvalva laterally flattened, at apex hooked ventrally, in Neotropical forms also apparently curving outwards. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 absent. Cross-vein 2r-rs present, forming base of ‘free stigmal vein’ (2r-rs&Rs·f4–5) in absence of Rs·f3 and 2rs-m. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, short, not reaching wing margin. Cross-vein 1m-cu in fore wing absent. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing absent past M+Cu. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, longer than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing absent or present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing absent. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent. Vein Cu in hind wing absent. Vein A in hind wing with abscissa A·f1 present.
Gyne. Alate, brachypterous, or ergatoid with eyes of variable size and with or without ocelli. Gynes are known for S. augustae, S. honduriana, S. humicola, and S. typhla. In S. typhla the gynes have well-developed flight sclerites on the mesosoma but I have never examined a specimen with wings. Field observations of S. augustae or a closely related species suggest that virgin queens may be brachypterous (Michael Branstetter pers. comm.). Ergatoid queens have been reported in S. humicola (
Larva. Described for S. augustae (
Distribution
This is the only doryline genus with a disjunct distribution between the New and Old Worlds (except for tramp Ooceraea biroi), with one center of diversity in Central America, with records from the Antilles (Cuba and Dominican Republic) and as far north as Arkansas, United States, and the other center in Southeast Asia west of Wallace’s Line, especially Borneo, reaching Japan to the north and southern India to the west.
Taxonomy and phylogeny
Syscia was described by
Syscia is here recognized as a valid genus, following the molecular evidence that Neotropical and Indomalayan species form a clade (
This lineage belongs to a clade with Eusphinctus and Ooceraea (Borowiec, in prep.). No attempts of reconstructing the internal phylogeny have been made.
Biology
Syscia species are found in leaf litter samples and soil cores. The foraging habits are not known. Syscia augustae, a species present in southern United States, has been observed on diurnal emigrations and briefly studied under laboratory conditions (Clint Penick pers. comm.). The brood production in S. augustae is synchronized. Gyne morphology varies in this lineage, with ergatoid, brachypterous, and fully winged individuals known. There is an observation of a brachypterous gyne aggregation under a stone (Michael Branstetter pers. comm.). It is unknown whether this represents cooperative colony foundation.
Species of Syscia
S. augustae (Wheeler, W. M., 1902): United States, comb. n.
S. honduriana (Mann, 1922): Honduras, comb. n.
S. humicola (Ogata, 1983): Japan, comb. n.
S. tolteca (Forel, 1909a): Guatemala, comb. n.
S. typhla Roger, 1861: Sri Lanka, comb. rev.
Tanipone
Type-species
Tanipone hirsuta, by original designation.
Tanipone is a small genus with unknown biology, endemic to arid and semi-arid habitats of Madagascar.
Diagnosis
Worker. Tanipone workers are distinctive ants with large eyes and ocelli, very long palps and unique glandular patches on abdominal segment III. The latter are present in all species except one (T. aglandula). The body coloration is black or bicolored reddish and black, with a light band or two light spots present at the posterior edge of abdominal segment III. The workers of Tanipone lack a conspicuous mid tibial spur. Other genera without the spur include Simopone and Vicinopone. Additionally, in certain species of Lioponera the mid tibial spur may be reduced and not easily discernible. Tanipone workers can be easily distinguished from all three lineages by remarkably long palps that are always visible on preserved specimens, reaching the occipital foramen.
Male. The male of Tanipone is also easily distinguished from all other dorylines by the long maxillary palps, almost always extruded and reaching occipital foramen. The wing venation is variously developed but the submarginal cell (SMC) is open or closed by a faint 2rs-m cross-vein. There are no notauli and no spurs on middle tibiae.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 6-segmented. Labial palps 4-segmented. Mandibles triangular, edentate. Eyes present, composed of more than 20 ommatidia. Ocelli present. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused up to notal surface. Mesometapleural groove deeply impressed, conspicuous. Transverse groove dividing mesopleuron present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent or a weakly impressed line. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora simple, not delimited by carina. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Cinctus of abdominal segment IV gutter-like and smooth or cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI present or absent. Pygidium large, with weakly impressed medial field, and armed with few modified setae restricted to apex. Hypopygium unarmed. Legs: Mid tibia without spurs. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws each armed with a tooth. Polymorphism: Monomorphic.
Male.Head: Antennae with 13 segments. Clypeus without cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 6-segmented. Labial palps 4-segmented. Mandibles triangular, edentate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge or not. Notauli absent. Transverse groove dividing mesopleuron present. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin or U-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms separated. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia without spurs. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Hind pretarsal claws each armed with a tooth. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 absent. Cross-vein 2r-rs absent, present and forming base of ‘free stigmal vein’ (2r-rs&Rs·f4–5) in absence of Rs·f3 and 2rs-m, or present and connected to Rs·f2–3&Rs·f4. Abscissae Rs·f4–5 absent or differentiated into Rs·f4 and Rs·f5 by 2rs-m. Abscissa M·f2 in fore wing absent or contiguous with Rs+M. Abscissa M·f4 in fore wing absent or present, reaching wing margin. Cross-vein 1m-cu in fore wing absent or present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with only abscissa A·f1 present or with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, as long as 1rs-m or longer than 1rs-m. Abscissa Rs·f2 in hind wing absent or present, reaching wing margin. Cross-vein 1rs-m in hind wing absent or present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing absent or present. Vein Cu in hind wing present. Vein A in hind wing abscissa A·f1 or with abscissae A·f1 and A·f2 present.
Gyne. Presumably extremely ergatoid in all species; specimens identified as putative gynes differ from workers only in sculpturation. See
Larva. Not described. Cocoons absent.
Distribution
Endemic to Madagascar.
Taxonomy and phylogeny
There are ten Tanipone species currently known, all confined to Madagascar (
Biology
The known specimens have been collected mostly in a variety of drier habitats in Madagascar, including dry tropical forest, savannah, and spiny forest. Most workers were collected on low vegetation, on the ground or in rot holes on tree trunks. The sole nest sample of Tanipone (T. zona) was collected under a stone. Nothing is known about feeding habits of this lineage. The putative queen specimens are ergatoid. Based on nest collections where larvae of different sizes and pupae were collected together, known for Tanipone hirsuta, T. subpilosa, and T. zona, brood development appears not synchronized.
Species of Tanipone
T. aglandula Bolton and Fisher, 2012: Madagascar
T. aversa Bolton and Fisher, 2012: Madagascar
T. cognata Bolton and Fisher, 2012: Madagascar
T. hirsuta Bolton and Fisher, 2012: Madagascar
T. maculata Bolton and Fisher, 2012: Madagascar
T. pilosa Bolton and Fisher, 2012: Madagascar
T. scelesta Bolton and Fisher, 2012: Madagascar
T. subpilosa Bolton and Fisher, 2012: Madagascar
T. varia Bolton and Fisher, 2012: Madagascar
T. zona Bolton and Fisher, 2012: Madagascar
Vicinopone
Type-species
Simopone conciliatrix, by original designation.
Vicinopone is a monotypic lineage of arboreal ants.
Diagnosis
Worker. The workers of the sole species of Vicinopone are slender and small yellowish ants with large eyes and no ocelli. The cuticle is sculptured with weak punctation. Vicinopone is recognized by a combination of propodeal spiracle high on the sclerite and propodeal lobes present, no constrictions between abdominal segments IV, V, and VI, large pygidium armed with modified setae, no ocelli in the worker, 12-segmented antennae and no spur on the middle tibiae. This genus is most similar to Simopone with which it shares the lack of a mid tibial spur, but in Simopone the antennae are 11-segmented and workers have ocelli.
Male. The male of Vicinopone is unknown.
Description
Worker.Head: Antennae with 12 segments. Apical antennal segment moderately enlarged, broader than and about equal in length to two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth absent. Parafrontal ridges reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes not projecting beyond inner margin of sclerite, prementum exposed when mouthparts fully closed. Maxillary palps 3-segmented. Labial palps 2-segmented. Mandibles triangular, edentate. Eyes present, composed of more than 20 ommatidia. Ocelli absent. Head capsule without differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like and cross-ribbed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium large, with impressed medial field, armed with modified setae. Hypopygium unarmed. Legs: Mid tibia without spurs. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal gland absent. Hind pretarsal claws each armed with a tooth. Polymorphism: Monomorphic.
Male. Not described.
Gyne. Apparently alate, with ocelli and flight sclerites but only dealated specimens known (
Larva. Not described. Cocoons absent.
Distribution
So far Vicinopone has been collected in Ghana, Gabon, Democratic Republic of the Congo, Uganda, and Tanzania but it is likely that it is more widely distributed in sub-Saharan Africa.
Taxonomy and phylogeny
Vicinopone is a genus recently established by
The phylogenetic position of Vicinopone is uncertain (
Biology
Little is known of the species’ habits, but the two known nest samples have been taken from dead twigs on trees, suggesting that this is an obligatory arboreal nester. Two dealate gynes were collected with the type nest series (
Species of Vicinopone
V. conciliatrix (Brown, 1975): Ghana
Yunodorylus , gen. rev.
Type-species
Yunodorylus sexspinus, by original designation.
This is a poorly known genus with striking morphology somewhat reminiscent of Dorylus.
Diagnosis
Worker. Workers of Yunodorylus are stout ants with no eyes and body coloration ranging from yellow to reddish, with cuticle sculpture and pilosity moderate. Yunodorylus is the only non-army ant doryline with a single waist segment and no or very weak girdling constriction on abdominal segment IV. It can be distinguished from army ant dorylines by relatively high positioned propodeal spiracle and presence of propodeal lobes.
Male. The males of Yunodorylus have a distinctive habitus with abdominal segment III very broadly attached posteriorly to segment IV and gaster widest towards the apex, posterior of abdominal segment IV. A combination of propodeal lobes well-developed, 13-segmented antennae, helcium relatively narrow and axial, no constrictions between abdominal segments IV, V, and VI, and wings always with veins C, R·f3, and at least a stub of Rs·f2–3 will distinguish Yunodorylus males from other genera. Although there may be gutter-like constrictions on abdominal segment IV and beyond, these are restricted to the tergites and do not produce constricted appearance in lateral view as in, for example, Zasphinctus. Sharp and conspicuously ventrally hooked penisvalvae appear to be unique among dorylines.
Description
Worker.Head: Antennae with 11 or 12 segments. Apical antennal segment not enlarged, not broader and longer than two preceding segments combined. Clypeus without cuticular apron. Lateroclypeal teeth present. Parafrontal ridges absent or reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes projecting beyond inner margin of sclerite, concealing prementum when mouthparts fully closed. Maxillary palps 2-segmented. Labial palps 2-segmented. Mandibles triangular, with teeth or falcate, with teeth on elongated masticatory margin. Eyes absent. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture visible, unfused partway to notal surface. Mesometapleural groove weakly impressed. Transverse groove dividing mesopleuron absent. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity without distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland with bulla visible through cuticle. Propodeal lobes present, short. Metasoma: Petiole anterodorsally, dorsolaterally immarginate, and laterally above spiracle immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial or supraaxial. Prora forming a vertical carina. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV absent, i.e. pre- and postsclerites indistinct. Cinctus of abdominal segment IV not impressed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI absent. Girdling constriction between pre- and poststernites of abdominal segments V and VI absent. Pygidium medium-sized, with impressed medial field, and armed with cuticular spines. Hypopygium unarmed. Legs: Mid tibia with pectinate and simple spur. Hind tibia with pectinate and simple spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic to moderately polymorphic.
Male.Head: Antennae with 13 segments. Clypeus with cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 3-segmented. Labial palps 2-segmented. Mandibles triangular, edentate to falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally absent. Mesosoma: Pronotal flange not separated from collar by distinct ridge. Notauli absent. Transverse groove dividing mesopleuron absent or present. Propodeal declivity reduced, without distinct dorsal edge or margin. Metapleural gland opening absent or present. Propodeal lobes present. Metasoma: Petiole anterodorsally immarginate, dorsolaterally immarginate, and laterally above spiracle immarginate or marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and axial. Prora forming a simple U-shaped margin. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Girdling constriction between pre- and postsclerites of abdominal segments V and VI absent. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines, with lateral apodemes about as long as medial apodeme, directed anteriorly (towards head). Genitalia: Cupula short relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with no sulcus trace at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella gradually tapering toward apex. Penisvalva hook-like, strongly curved ventrally. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing present. Pterostigma broad. Abscissa R·f3 present and running toward distal wing margin and enclosing cell with Rs·f5 or not. Abscissae Rs·f2–3 present, disconnected from Rs+M, rarely closely approaching Rs+M. Cross-vein 2r-rs present, connected to Rs·f2–3&Rsf4. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing contiguous with Rs+M. Abscissa M·f4 in fore wing present, reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing present. Abscissa Rs·f1 in hind wing present, shorter than 1rs-m. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing present, about as long as M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing present. Cross-vein cu-a in hind wing absent. Vein Cu in hind wing present. Vein A in hind wing with abscissa A·f1 present.
Gyne. A detailed description of a gyne of Yunodorylus is currently in preparation (
Larva. Not described. Presence of cocoons unknown.
Distribution
This is a species-poor lineage, apparently restricted to mainland Southeast Asia and Borneo, although it is possible it will be eventually found on other islands.
Taxonomy and phylogeny
The genus was originally established for Y. sexspinus by
Based on genomic data, Yunodorylus is part of a well-supported clade also including Cerapachys and Chrysapace.
Biology
Very little is known about this lineage. Yunodorylus eguchii nests in soil in evergreen forests and dry dwarf forests in southern Vietnam. A colony sample (type series) of Y. eguchii contain larvae of various sizes. The field and laboratory observations of two queen-right colonies of Y. eguchii will be reported by
Species of Yunodorylus
Y. doryloides (Borowiec, 2009): Malaysia (Sarawak), comb. n.
Y. eguchii (Borowiec, 2009): Vietnam, comb. n.
Y. paradoxus (Borowiec, 2009): Malaysia (Sarawak), comb. n.
Y. sexspinus Xu, 2000: China, comb. rev.
Zasphinctus , gen. rev.
= Aethiopopone Santschi, 1930, syn. n.
= Nothosphinctus Wheeler, W. M. 1918, syn. n.
Type-species
Sphinctomyrmex turneri, by monotypy.
Zasphinctus is a moderately speciose lineage of specialized ant predators, most prominent in Australia.
Diagnosis
Worker. The workers of Zasphinctus are ants of variable size, color, and sculpturation, but always possessing conspicuous girdling constrictions between abdominal segments IV, V, and VI. The eyes absent in most species. Zasphinctus can be distinguished from other lineages with pronounced abdominal constrictions by highly-positioned propodeal spiracles, propodeal lobes present, pygidium large and armed with modified setae, and pronotomesopleural Pronotomesopleural suture fused. See also diagnoses of Eusphinctus and Sphinctomyrmex.
Male. The males of Zasphinctus also possess the characteristic abdominal constrictions between abdominal segments IV, V, and VI and can be recognized by a combination of costal vein (C) absent from the fore wing, submarginal cell (SMC) closed by Rs·f2-f3, vein 2rs-m absent, pronotum not marginate anterodorsally, and antennae 13-segmented. This venation is similar to Lividopone and Parasyscia but Zasphinctus can be recognized by the presence of abdominal constrictions and different appearance of abdominal sternite IX (subgenital plate). In Zasphinctus, the sternite is abruptly constricted proximal to where spines arise and is much wider at midlength. In Lividopone and Parasyscia in contrast, the sternite IX is usually gradually narrowing to the point of bifurcation. The males of Eusphinctus and Sphinctomyrmex have similar abdominal constrictions but the former has 12-segmented antennae and the latter has different wing venation with costal vein present.
Description
Worker.Head: Antennae with 11 or 12 segments. Apical antennal segment conspicuously enlarged, much broader than and longer than two preceding segments combined. Clypeus with cuticular apron. Lateroclypeal teeth absent or present. Parafrontal ridges absent or reduced. Torulo-posttorular complex vertical. Antennal scrobes absent. Labrum with median notch or concavity. Proximal face of stipes not projecting beyond inner margin of sclerite, prementum exposed when mouthparts fully closed. Maxillary palps 3-segmented. Labial palps 3-segmented. Mandibles triangular, with teeth or edentate. Eyes absent or present, composed of more than 20 ommatidia. Ocelli absent. Head capsule with differentiated vertical posterior surface above occipital foramen. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Posterior head corners dorsolaterally immarginate. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge or not. Promesonotal connection with Pronotomesopleural suture completely fused. Pronotomesopleural suture completely fused. Mesometapleural groove not impressed or weakly impressed. Transverse groove dividing mesopleuron absent or present. Pleural endophragmal pit concavity present. Mesosoma dorsolaterally immarginate. Metanotal depression or groove on mesosoma absent. Propodeal spiracle situated low on sclerite. Propodeal declivity with distinct dorsal edge or margin and rectangular in posterior view. Metapleural gland without bulla visible through cuticle. Propodeal lobes present, well developed. Metasoma: Petiole anterodorsally marginate or immarginate, dorsolaterally immarginate, and laterally above spiracle marginate or rarely immarginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at posttergite and helcium axial, occasionally slightly supraaxial. Prora simple, not delimited by carina. Prora forming a U-shaped margin with median ridge. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III anterodorsally immarginate and dorsolaterally immarginate. Abdominal segment III more than half size of succeeding segment IV, which is weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV not impressed. Abdominal segment IV not conspicuously largest segment. Abdominal tergite IV not folding over sternite, and anterior portions of sternite and tergite equally well visible in lateral view. Girdling constriction between pre- and posttergites of abdominal segments V and VI present. Girdling constriction between pre- and poststernites of abdominal segments V and VI present. Pygidium large, with impressed medial field, and armed with modified setae, sometimes notched. Hypopygium armed with modified setae. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Hind basitarsus not widening distally, circular in cross-section. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland absent or an oval patch of whitish cuticle. Metabasitarsal gland absent. Hind pretarsal claws simple. Polymorphism: Monomorphic.
Male.Head: Antennae with 12 or 13 segments. Clypeus with cuticular apron. Parafrontal ridges absent. Torulo-posttorular complex vertical. Maxillary palps 3-segmented. Labial palps 3-segmented. Mandibles triangular, edentate to falcate. Ventrolateral margins of head without lamella or ridge extending towards mandibles and beyond carina surrounding occipital foramen. Carina surrounding occipital foramen ventrally present. Mesosoma: Pronotal flange separated from collar by distinct ridge. Notauli present or, more rarely, absent. Transverse groove dividing mesopleuron present. Propodeal declivity with distinct dorsal edge or margin. Metapleural gland opening absent. Propodeal lobes present. Metasoma: Petiole anterodorsally marginate, dorsolaterally immarginate, and laterally above spiracle marginate. Helcium in relation to tergosternal Pronotomesopleural suture placed at Pronotomesopleural suture and axial. Prora forming a V-shaped protrusion. Spiracle openings of abdominal segments IV–VI circular. Abdominal segment III more than half size of succeeding segment IV; latter weakly constricted at presegmental portion (uninodal waist). Girdling constriction of segment IV present, i.e. pre- and postsclerites distinct. Cinctus of abdominal segment IV gutter-like, not sculptured. Girdling constriction between pre- and postsclerites of abdominal segments V and VI present. Abdominal segment IV not conspicuously largest segment. Abdominal sternite VII simple. Abdominal sternite IX distally armed with two spines curved dorsally at apices, with lateral apodemes shorter than or about as long as medial apodeme, directed anteriorly (towards head); all apodemes long. Genitalia: Cupula long relative to rest of genital capsule and shorter ventrally than dorsally. Basimere broadly fused to telomere, with sulcus discernable at junction, and ventrally with left and right arms abutting. Telomere gradually tapering toward apex. Volsella laterally flattened, at apex with dorsal lobe and hooked ventrally. Penisvalva laterally compressed, rounded at apex. Legs: Mid tibia with single pectinate spur. Hind tibia with single pectinate spur. Posterior flange of hind coxa not produced as raised lamella. Metatibial gland present as oval patch of whitish cuticle. Metabasitarsal glands absent. Hind pretarsal claws simple. Wings: Tegula present, broad, demiovate in shape. Vein C in fore wing absent. Pterostigma broad. Abscissa R·f3 absent. Abscissae Rs·f2–3 present, connecting with Rs+M&M·f2 or disconnected from Rs+M. Cross-vein 2r-rs present, differentiated from Rs·f4 by presence of Rs·f2–3. Abscissae Rs·f4–5 present, fused in absence of 2rs-m. Abscissa M·f2 in fore wing present, separated from Rs+M by Rs·f2. Abscissa M·f4 in fore wing present, not reaching wing margin. Cross-vein 1m-cu in fore wing present. Cross-vein cu-a in fore wing present, arising from M+Cu and proximal to M·f1 or near M·f1. Vein Cu in fore wing present, with only Cu1 branch prominent. Vein A in fore wing with abscissae A·f1 and A·f2 present. Vein C in hind wing absent. Vein R in hind wing absent. Vein Sc+R in hind wing absent. Abscissa Rs·f1 in hind wing not differentiated in absence of Sc+R. Abscissa Rs·f2 in hind wing present, not reaching wing margin. Cross-vein 1rs-m in hind wing fused with M·f1. Vein M+Cu in hind wing present. Abscissa M·f1 in hind wing present. Abscissa M·f2 in hind wing absent. Cross-vein cu-a in hind wing present. Vein Cu in hind wing present. Vein A in hind wing with abscissae A·f1 and A·f2 present; The latter a stub.
Gyne. Alate, ergatoid, or subdichthadiigyne. Alate gynes are known in an undescribed species from Africa (
Larva. Cocoons present.
Distribution
The twenty described species of Zasphinctus are distributed throughout Australasia, including New Caledonia and New Guinea, and the Afrotropics. Most species are known from Australia, with only three taxa described from Africa. Recently a species has been described from Thailand (Jaitrong et al. 2016), and unidentified Zasphinctus males are also known from Myanmar (author’s unpublished observations).
Taxonomy and phylogeny
This name is here revived from synonymy with Sphinctomyrmex. For a brief account of taxonomic history and justification see under Eusphinctus.
The position of Zasphinctus within dorylines appears to be well established as the sister group to Parasyscia, and it is reasonably certain that this lineage was derived independently from the Neotropical Sphinctomyrmex (Figure
Biology
Wilson provided notes on the biology of Zasphinctus caledonicus from New Caledonia and Z. steinheili from Australia. The former was observed raiding a nest of Stigmacros ants in the field, and the latter was feeding on ant brood of several species in the laboratory. Both were reported to have colonies containing multiple ergatoid gynes and synchronized brood.
Species of Zasphinctus
Z. asper (Brown, 1975): Australia, comb. n.
Z. caledonicus (Wilson, 1957): New Caledonia, comb. n.
Z. cedaris (Forel, 1915): Australia, comb. n.
Z. chariensis (Santschi, 1915): Chad, comb. n.
Z. clarus (Forel, 1893b): Australia, comb. n.
Z. cribratus (Emery, 1897): Papua New Guinea, comb. n.
Z. duchaussoyi (André, 1905): Australia, comb. n.
Z. emeryi (Forel, 1893b): Australia, comb. n.
Z. froggatti (Forel, 1900a): Australia, comb. n.
Z. imbecilis (Forel, 1907c): Australia, comb. n.
Z. mjobergi (Forel, 1915): Australia, comb. n.
Z. myops (Forel, 1895b): Australia, comb. n.
Z. nigricans (Clark, 1926): Australia, comb. n.
Z. occidentalis (Clark, 1924a): Australia, comb. n.
Z. rufiventris (Santschi, 1915): Benin, comb. n.
Z. septentrionalis (Crawley, 1925): Australia, comb. n.
Z. siamensis (Jaitrong, 2016): Thailand, comb. n.
Z. steinheili (Forel, 1900a): Australia, comb. n.
Z. trux (Brown, 1975): Australia, comb. n.
Z. turneri (Forel, 1900a): Australia, comb. n.
Acknowledgments
Special thanks to Phil Ward for his support, helpful discussions about this project, and much effort put into careful proof-reading of the manuscript. Many thanks to other people that helped to improve this revision: Gary Alpert, Bonnie Blaimer, Barry Bolton, Lech Borowiec, Brendon Boudinot, Stefan Cover, Katsuyuki Eguchi, Brian Fisher, Brian Heterick, Daniel Kronauer, Vincent Perrichot, Caspar Schöning, and Steve Shattuck. I would also like to thank all the curators and hosts at the institutions visited or loaned from during this work: Christine LeBeau (American Museum of Natural History), Andreas Schulz (personal collection), Flávia Esteves, Brian Fisher, and Michele Esposito (California Academy of Sciences), Jack Longino (personal collection), Weiping Xie (Los Angeles County Museum of Natural History), Roy Danielsson (Lund Zoological Museum), Bernhard Merz (Muséum d’Histoire Naturelle, Geneva), Virginie Beuetel and Claire Villemant (Muséum National d’Histoire Naturelle), Gary Alpert and Stefan Cover (Museum of Comparative Zoology, Cambridge), Mónica Solórzano Kraemer (Senckenberg Forschungsinstitut und Naturmuseum), Seán Brady, Eugenia Okonski, and Ted Schultz (Smithsonian National Museum of Natural History), and Gavin Broad and Suzanne Ryder (The Natural History Museum, London). Thank you to all who contributed crucial material used in this revision and in the molecular phylogeny of the Dorylinae: Brendon Boudinot, Júlio Chaul, Katsuyuki Eguchi, Flávia Esteves, Rodrigo Feitosa, Brian Fisher, Francisco Hita-Garcia, Milan Janda, Jack Longino, Andrea Lucky, Sean McKenzie, Matt Prebus, Caspar Schöning, Andy Suarez, and Phil Ward. This project was supported by SYNTHESYS grants FR-TAF-594 and GB-TAF-303, Ernst Mayr travel grants from the Museum of Comparative Zoology, and National Geographic Young Explorers Grant.
References
- Aktaç N, Radchenko AG, Kiran K (2004) On the taxonomy of the West Palaearctic Aenictinae ants (Hymenoptera: Formicidae). Annales Zoologici (Warsaw) 54: 361–364.
- André E (1885) Supplément aux fourmis. In: André, Edm. 1885. 1881–1886. Species des Hyménoptères d’Europe et d’Algérie. Tome Deuxième. Edmond André, Beaune, 833–854.
- André E (1886) Appendice aux fourmis. In: André, Edm. 1886. 1881–1886. Species des Hyménoptères d’Europe et d’Algérie. Tome Deuxième. Edmond André, Beaune, 854–859.
- André E (1889) Hyménoptères nouveaux appartenant au groupe des Formicides. Revue d’Entomologie (Caen) 8: 217–231.
- André E (1892) Matériaux myrmécologiques. Revue d’Entomologie (Caen) 11: 45–56.
- André E (1905) Description d’un genre nouveau et de deux espèces nouvelles de fourmis d’Australie. Revue d’Entomologie (Caen) 24: 205–208.
- Arnold G (1915) A monograph of the Formicidae of South Africa. Part I. Ponerinae, Dorylinae. Annals of the South African Museum 14: 1–159.
- Arnold G (1926) A monograph of the Formicidae of South Africa. Appendix. Annals of the South African Museum 23: 191–295.
- Arnold G (1946) New species of African Hymenoptera. No. 6. Occasional Papers of the National Museum of Southern Rhodesia 2: 49–97.
- Arnold G (1954) New Formicidae from Kenya and Uganda. Annales du Musée Royal du Congo Belge. Nouvelle Série in Quarto. Sciences Zoologiques 1: 291–295.
- Arnold G (1955) New species of African Hymenoptera. No. 11. Occasional Papers of the National Museum of Southern Rhodesia 2: 733–762.
- Arnold G (1959) New species of African Hymenoptera. No. 14. Occasional Papers of the National Museum of Southern Rhodesia. B. Natural Sciences 3: 316–339.
- Arnold G (1960) Aculeate Hymenoptera from the Drakensberg Mountains, Natal. Annals of the Natal Museum 15: 79–87.
- Arnol’di KV (1968) Important additions to the myrmecofauna (Hymenoptera, Formicidae) of the USSR, and descriptions of new forms. Zoologicheskii Zhurnal 47: 1800–1822. [In Russian]
- Ashmead WH (1905) A skeleton of a new arrangement of the families, subfamilies, tribes and genera of the ants, or the superfamily Formicoidea. Canadian Entomologist 37: 381–384. doi: 10.4039/Ent37381-11
- Ashmead WH (1906) Classification of the foraging and driver ants, or Family Dorylidae, with a description of the genus Ctenopyga Ashm. Proceedings of the Entomological Society of Washington 8: 21–31.
- Baroni Urbani C (1977) Materiali per una revisione della sottofamiglia Leptanillinae Emery (Hymenoptera: Formicidae). Entomologica Basiliensia 2: 427–488.
- Baroni Urbani C, Bolton B, Ward PS (1992) The internal phylogeny of ants (Hymenoptera: Formicidae). Systematic Entomology 17: 301–329. doi: 10.1111/j.1365-3113.1992.tb00553.x
- Barr D, Boven JKA, Gotwald WHJr (1985) Phenetic studies of African army ant queens of the genus Dorylus (Hymenoptera: Formicidae). Systematic Entomology 10: 1–10. doi: 10.1111/j.1365-3113.1985.tb00560.x
- Barth MB, Moritz RFA, Pirk CWW, Kraus FB (2013) Male-biased dispersal promotes large scale gene flow in a subterranean army ant, Dorylus (Typhlopone) fulvus. Population Ecology 55: 523–533. doi: 10.1007/s10144-013-0383-4
- Barth MB, Moritz RFA, Kraus FB (2015) Genetic differentiation at species level in the Neotropical army ant Labidus praedator. Insectes Sociaux 62: 299–306. doi: 10.1007/s00040-015-0410-x
- Bates HW (1863) The naturalist on the River Amazons, a record of adventures, habits of animals, sketches of Brazilian and Indian life, and aspects of nature under the Equator, during eleven years of travel. J. Murray, London, ix + 351 pp. doi: 10.5962/bhl.title.103298
- Beeren C, Maruyama M, Kronauer DJC (2016) Cryptic diversity, high host specificity and reproductive synchronization in army ant-associated Vatesus beetles. Molecular Ecology 25: 990–1005. doi: 10.1111/mec.13500
- Berghoff SM, Franks NR (2007) First record of the army ant Cheliomyrmex morosus in Panama and its high associate diversity. Biotropica 39: 771–773. doi: 10.1111/j.1744-7429.2007.00302.x
- Berghoff SM, Gadau J, Winter T, Linsenmair KE, Maschwitz U (2003a) Sociobiology of hypogaeic army ants: characterization of two sympatric Dorylus species on Borneo and their colony conflicts. Insectes Sociaux 50: 139–147. doi: 10.1007/s00040-003-0642-z
- Berghoff SM, Maschwitz U, Linsenmair KE (2003b) Influence of the hypogaeic army ant Dorylus (Dichthadia) laevigatus on tropical arthropod communities. Oecologia 135: 149–157. doi: 10.1007/s00442-002-1173-4
- Berghoff SM, Weissflog A, Linsenmair KE, Hashim R, Maschwitz U (2002a) Foraging of a hypogaeic army ant: a long neglected majority. Insectes Sociaux 49: 133–141. doi: 10.1007/s00040-002-8292-0
- Berghoff SM, Weissflog A, Linsenmair KE, Mohamed M, Maschwitz U (2002b) Nesting habits and colony com-position of the hypogaeic army ant Dorylus (Dichthadia) laevigatus Fr. Smith. Insectes Sociaux 49: 1–8. doi: 10.1007/PL00012662
- Bernard F (1953a) Les fourmis du Tassili des Ajjer. In: Bernard F (Ed.) Mission scientifique au Tassili des Ajjer (1949).Volume I. Recherches zoologiques et médicales. P. Lechevalier, Paris, 121–250.
- Bernard F (1953b) [‘1952’] La réserve naturelle intégrale du Mt Nimba. XI. Hyménoptères Formicidae. Mémoires de l’Institut Français d’Afrique Noire 19: 165–270.
- Bharti H (2003) Queen of the army ant Aenictus pachycerus (Hymenoptera, Formicidae, Aenictinae). Sociobiology 42: 715–718.
- Bharti H, Akbar SA (2013) Taxonomic studies on the ant genus Cerapachys Smith (Hymenoptera, Formicidae) from India. ZooKeys 336: 79–103. doi: 10.3897/zookeys.336.5719
- Bharti H, Wachkoo AA (2013) Cerapachys browni and Cerapachys costatus, two new rare ant species (Hymenoptera: Formicidae) from India. Biologia 68(6): 1189–1192. doi: 10.2478/s11756-013-0260-9
- Bharti H, Wachkoo AA, Kumar R (2012) Two remarkable new species of Aenictus (Hymenoptera: Formicidae) from India. Journal of Asia-Pacific Entomology 15: 291–294. doi: 10.1016/j.aspen.2012.02.002
- Billen J (2009) Occurrence and structural organization of the exocrine glands in the legs of ants. Arthropod Structure and Development 38: 2–15. doi: 10.1016/j.asd.2008.08.002
- Billen JPJ, Gotwald WHJr (1988) The crenellate lining of the Dufour gland in the genus Aenictus: a new character for interpreting the phylogeny of Old World army ants (Hymenoptera, Formicidae, Dorylinae). Zoologica Scripta 17: 293–295. doi: 10.1111/j.1463-6409.1988.tb00104.x
- Billen J, Gobin B (1996) Trail following in army ants (Hymenoptera, Formicidae). Netherlands Journal of Zoology 46: 272–280. doi: 10.1163/156854295X00221
- Billen J, Gobin B, Ito F (1999) Fine structure of the postpygidial gland in Aenictus army ants. Acta Zoologica (Stockholm) 80: 307–310. doi: 10.1046/j.1463-6395.1999.00026.x
- Bingham CT (1903) The fauna of British India, including Ceylon and Burma. Hymenoptera, Vol. II. Ants and Cuckoo-wasps. London: Taylor and Francis, 506 pp.
- Birket-Smith SJR (1981) The male genitalia of Hymenoptera - a review based on morphology in Dorylidae (Formicoidea). Entomologica Scandinavica. Supplementum 15: 377–397.
- Bolico CF, Oliveira EA, Gantes ML, Dumont LFC, Carrasco DS, D’Incao F (2012) Mirmecofauna (Hymenoptera, Formicidae) de duas marismas do Estuário da Lagoa dos Patos, RS: diversidade, flutuação de abundância e similaridade como indicadores de conservação. EntomoBrasilis 5: 11–20. doi: 10.12741/ebrasilis.v5i1.147
- Bolton B (1990a) Abdominal characters and status of the cerapachyine ants (Hymenoptera, Formicidae). Journal of Natural History 24: 53–68. doi: 10.1080/00222939000770051
- Bolton B (1990b) Army ants reassessed: the phylogeny and classification of the doryline section (Hymenoptera, Formicidae). Journal of Natural History 24: 1339–1364. doi: 10.1080/00222939000770811
- Bolton B (2003) Synopsis and classification of Formicidae. Memoirs of the American Entomological Institute 71: 1–370.
- Bolton B, Fisher BL (2012) Taxonomy of the cerapachyine ant genera Simopone Forel, Vicinopone gen. n. and Tanipone gen. n. (Hymenoptera: Formicidae). Zootaxa 3283: 1–101.
- Borgmeier T (1928) Algumas formigas do Museo Paulista. Boletim Biológico. Laboratório de Parasitologia. Faculdade de Medicina de São Paulo 12: 55–70.
- Borgmeier T (1933) Sobre algumas especies de formigas do genero Eciton Latreille. Archivos da Escola Superior da Agricultura e Medicina Veterinaria. Nictheroy 10: 161–168.
- Borgmeier T (1934) Contribuição para o conhecimento da fauna mirmecológica dos cafezais de Paramaribo, Guiana Holandesa (Hym. Formicidae). Archivos do Instituto de Biologia Vegetal (Rio de Janeiro) 1: 93–111.
- Borgmeier T (1936) Sobre algumas formigas dos generos Eciton e Cheliomyrmex (Hym. Formicidae). Archivos do Instituto de Biologia Vegetal (Rio de Janeiro) 3: 51–68.
- Borgmeier T (1939) Nova contribuição para o conhecimento das formigas neotropicas (Hym. Formicidae). Revista de Entomologia (Rio de Janeiro) 10: 403–428.
- Borgmeier T (1940) Duas notas myrmecologicas. Revista de Entomologia (Rio de Janeiro) 11: 606.
- Borgmeier T (1948) Einige Ameisen aus Argentinien (Hym. Formicidae). Revista de Entomologia (Rio de Janeiro) 19: 459–471.
- Borgmeier T (1950) Uma nova espécie do gênero Neivamyrmex Borgmeier (Hym. Formicidae). Revista de Entomologia (Rio de Janeiro) 21: 623–624.
- Borgmeier T (1953) Vorarbeiten zu einer Revision der neotropischen Wanderameisen. Studia Entomologica 2: 1–51.
- Borgmeier T (1955) Die Wanderameisen der neotropischen Region. Studia Entomologica 3: 1–720.
- Borgmeier T (1957) Myrmecologische Studien, I. Anais da Academia Brasileira de Ciencias 29: 103–128.
- Borgmeier T (1958) Nachtraege zu meiner Monographie der neotropischen Wanderameisen (Hym. Formicidae). Studia Entomologica (n.s.) 1: 197–208.
- Borowiec ML (2009) New ant species related to Cerapachys sexspinus and discussion of the status of Yunodorylus (Hymenoptera: Formicidae). Zootaxa 2069: 43–58.
- Borowiec ML, Longino JT (2011) Three new species and reassessment of the rare Neotropical ant genus Leptanilloides (Hymenoptera, Formicidae, Leptanilloidinae). ZooKeys 133: 19–48. doi: 10.3897/zookeys.133.1479
- Boudinot BE (2015) Contributions to the knowledge of Formicidae (Hymenoptera, Aculeata): a new diagnosis of the family, the first global male-based key to subfamilies, and a treatment of early branching lineages. European Journal of Taxonomy 120: 1–62. doi: 10.5852/ejt.2015.120
- Boven JKA (1972) Description de deux reines d’Anomma (Hymenoptera: Formicidae). Bulletin et Annales de la Société Royale Entomologique de Belgique 108: 133–146.
- Boven JKA (1975) Deux nouvelles reines du genre Dorylus Fabricius (Hymenoptera, Formicidae). Annales Zoologici (Warsaw) 33: 189–199.
- Boven JKA, Lévieux J (1968) Les Dorylinae de la savane de Lamto (Hymenoptera: Formicidae). Annales de l’Universite de l’Abidjan (Sciences), series E, 1: 351–358.
- Brady SG (2003) Evolution of the army ant syndrome: the origin and long-term evolutionary stasis of a complex of behavioral and reproductive adaptations. Proceedings of the National Academy of Sciences of the United States of America 100: 6575–6579. doi: 10.1073/pnas.1137809100
- Brady SG, Fisher BL, Schultz TR, Ward PS (2014) The rise of army ants and their relatives: diversification of specialized predatory doryline ants. BMC Evolutionary Biology 14: 93. doi: 10.1186/1471-2148-14-93
- Brady SG, Schultz TR, Fisher BL, Ward PS (2006) Evaluating alternative hypotheses for the early evolution and diversification of ants. Proceedings of the National Academy of Sciences of the United States of America 103: 18172–18177. doi: 10.1073/pnas.0605858103
- Brady SG, Ward PS (2005) Morphological phylogeny of army ants and other dorylomorphs (Hymenoptera: Formicidae). Systematic Entomology 30: 593–618. doi: 10.1111/j.1365-3113.2005.00290.x
- Brandão CRF, Diniz JLM, Agosti D, Delabie JH (1999a) Revision of the Neotropical ant subfamily Leptanilloidinae. Systematic Entomology 24: 17–36. doi: 10.1046/j.1365-3113.1999.00064.x
- Brandão CRF, da Silva RR, Diniz JLM, Yamamoto CI, Castro-Mello C (1999b) Biologia de Leptanilloidinae. Naturalia (São Paulo) 24: 45–47.
- Briese DT (1984) Interactions between a myrmecophagous ant and a prey species. Australian Journal of Entomology 23: 167–168. doi: 10.1111/j.1440-6055.1984.tb01936.x
- Brown WLJr (1973) A comparison of the Hylean and Congo-West African rain forest ant faunas. In: Meggers BJ, Ayensu ES, Duckworth WD (Eds) Tropical forest ecosystems in Africa and South America: a comparative review.Smithsonian Institution Press, Washington D.C., 161–185.
- Brown WLJr (1975) Contributions toward a reclassification of the Formicidae. V. Ponerinae, tribes Platythyreini, Cerapachyini, Cylindromyrmecini, Acanthostichini, and Aenictogitini. Search. Agriculture (Ithaca, New York) 5(1): 1–115.
- Bruch C (1925) Macho, larva y ninfa de Acanthostichus ramosmexiae Bruch. Physis (Buenos Aires) 8: 110–114.
- Buckley SB (1866) Descriptions of new species of North American Formicidae. Proceedings of the Entomological Society of Philadelphia 6: 152–172.
- Buschinger A, Peeters C, Crozier RH (1990) [‘1989’] Life-pattern studies of an Australian Sphinctomyrmex (Formicidae: Ponerinae; Cerapachyini): functional polygyny, brood periodicity and raiding behavior. Psyche (Cambridge) 96: 287–300. doi: 10.1155/1989/13614
- Cameron P (1891) Appendix. Hym. Formicidae. In: Whymper E (1891) Travels amongst the Great Andes. J. Murray, London, 89–95.
- Campione BM, Novak JA, Gotwald WHJr (1983) Taxonomy and morphology of the West African army ant, Aenictus asantei n. sp. (Hymenoptera: Formicidae). Annals of the Entomological Society of America 76: 873–883. doi: 10.1093/aesa/76.5.873
- Chadab-Crepet R, Rettenmeyer CW (1982) Comparative behavior of social wasps when attacked by army ants or other predators and parasites. In: Breed MD, Michener CD, Evans HE (Eds) The biology of social insects. Proceedings of the Ninth Congress of the IUSSI, Boulder, Colorado, August 1982. Westview Press, Boulder, 270–274.
- Chapman JW (1963) Some new and interesting Philippine ants (Hymenoptera: Formicidae). Philippine Journal of Science 92: 247–263.
- Chapman JW (1965) [‘1964’] Studies on the ecology of the army ants of the Philippines genus Aenictus Shuckard (Hymenoptera: Formicidae). Philippine Journal of Science 93: 551–595.
- Chen Z, Zhou S, Liang L (2015) Simopone yunnanensis sp. n. – the first record of Simopone Forel, 1891 from China (Hymenoptera, Formicidae, Cerapachyinae). Asian Myrmecology 7: 5–10.
- Clark J (1924) [‘1923’] Australian Formicidae. Journal and Proceedings of the Royal Society of Western Australia 9: 72–89.
- Clark J (1926) Australian Formicidae. Journal of the Royal Society of Western Australia 12: 43–51.
- Clark J (1928) Australian Formicidae. Journal of the Royal Society of Western Australia 14: 29–41.
- Clark J (1930) New Formicidae, with notes on some little-known species. Proceedings of the Royal Society of Victoria (n.s.) 43: 2–25.
- Clark J (1934) New Australian ants. Memoirs of the National Museum of Victoria 8: 21–47.
- Clark J (1941) Australian Formicidae. Notes and new species. Memoirs of the National Museum of Victoria 12: 71–94.
- Clark J (1951) The Formicidae of Australia. Vol. 1. Subfamily Myrmeciinae. CSIRO, Melbourne, 230 pp.
- Collingwood CA (1985) Hymenoptera: Fam. Formicidae of Saudi Arabia. Fauna of Saudi Arabia 7: 230–302.
- Consani M (1951) Formiche dell’Africa Orientale I. Bollettino dell’Istituto di Entomologia della Università degli Studi di Bologna 18: 167–172.
- Cox B (2001) The biogeographic regions reconsidered. Journal of Biogeography 28(4): 511–523. doi: 10.1046/j.1365-2699.2001.00566.x
- Crawley WC (1922) New ants from Australia. Annals and Magazine of Natural History (9)9: 427–448. doi: 10.1080/00222932208632695
- Crawley WC (1923) Myrmecological notes. Entomologist’s Record and Journal of Variation 35: 29–32.
- Crawley WC (1925) New ants from Australia. - II. Annals and Magazine of Natural History (9)16: 577–598. doi: 10.1080/00222932508633350
- Crawley WC (1926) A revision of some old types of Formicidae. Transactions of the Entomological Society of London 1925: 373–393.
- Cresson ET (1872) Hymenoptera Texana. Transactions of the American Entomological Society 4: 153–292. doi: 10.2307/25076272
- Czaczkes TJ, Ratnieks FL (2013) Cooperative transport in ants (Hymenoptera: Formicidae) and elsewhere. Myrmecological News 18: 1–11.
- Dalla Torre KW (1893) Catalogus Hymenopterorum hucusque descriptorum systematicus et synonymicus. Vol. 7. Formicidae (Heterogyna). W. Engelmann, Leipzig, 289 pp.
- Darlington JPEC (1985) Attacks by doryline ants and termite nest defences (Hymenoptera; Formicidae; Isoptera; Termitidae). Sociobiology 11: 189–200.
- De Andrade ML (1998a) Fossil and extant species of Cylindromyrmex (Hymenoptera: Formicidae). Revue Suisse de Zoologie 105: 581–664. doi: 10.5962/bhl.part.80052
- De Andrade ML (1998b) First description of fossil Acanthostichus from Dominican amber (Hymenoptera: Formicidae). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 71: 269–274.
- De Andrade ML (2001) A remarkable Dominican amber species of Cylindromyrmex with Brazilian affinities and additions to the generic revision (Hymenoptera: Formicidae). Beiträge zur Entomologie 51: 51–63.
- Dejean A, Corbara B, Roux O, Orivel J (2013) The antipredatory behaviours of Neotropical ants towards army ant raids (Hymenoptera: Formicidae). Myrmecological News 19: 17–24.
- Delabie JHC, Reis YT (2000) Sympatry and mating flight synchrony of three species of Cylindromyrmex (Hymenoptera, Formicidae) in southern Bahia, Brazil, and the importance of Malaise trap for rare ants inventory. Revista Brasileira de Entomologia 44: 109–110.
- Delsinne T, Donoso DA (2015) [New species of Leptanilloides attributed to Delsinne and Donoso]. Pp. 8–17 In: Delsinne T, Sonet G, Donoso DA (2015) Two new species of Leptanilloides Mann, 1823 (Formicidae: Dorylinae) from the Andes of southern Ecuador. European Journal of Taxonomy 143: 1–35.
- Delsinne T, Sonet G, Donoso DA (2015) Two new species of Leptanilloides Mann, 1823 (Formicidae: Dorylinae) from the Andes of southern Ecuador. European Journal of Taxonomy 143: 1–35. doi: 10.5852/ejt.2015.143
- Devries PJ, Austin GT, Martin NH (2009) Estimating species diversity in a guild of Neotropical skippers (Lepidoptera: Hesperiidae) with artificial lures is a sampling problem. Insect Conservation and Diversity 2: 125–134. doi: 10.1111/j.1752-4598.2009.00047.x
- Dlussky GM (1990) Cerapachys desertorum Dlussky, sp. n. In: Dlussky GM, Soyunov OS, Zabelin SI (1990) Ants of Turkmenistan. Ylym Press, Ashkabad, 177–179. [In Russian]
- Dlussky GM (2009) The ant subfamilies Ponerinae, Cerapachyinae, and Pseudomyrmecinae (Hymenoptera, Formicidae) in the Late Eocene ambers of Europe. Paleontological Journal 43: 1043–1086. doi: 10.1134/S0031030109090068
- Do Nascimento IC, Delabie JHC, Ferreira PSF, Del-La Lucia TMC (2004) Mating flight seasonality in the genus Labidus (Hymenoptera: Formicidae) at Minas Gerais, in the Brazilian Atlantic Forest Biome, and Labidus nero, junior synonym of Labidus mars. Sociobiology 44: 1–8.
- Donisthorpe H (1938) New species of ants and a new subgenus of Dolichoderus from various localities. Annals and Magazine of Natural History (11)2: 498–504.
- Donisthorpe H (1947) [‘1946’] Ants from New Guinea, including new species and a new genus. Annals and Magazine of Natural History (11)13: 577–595.
- Donisthorpe H (1948) A third instalment of the Ross Collection of ants from New Guinea. Annals and Magazine of Natural History (11)14: 589–604.
- Donisthorpe H (1949) [‘1948’] A fifth instalment of the Ross Collection of ants from New Guinea. Annals and Magazine of Natural History (12)1: 487–506.
- Donoso DA, Vieira JM, Wild AL (2006) Three new species of Leptanilloides Mann from Andean Ecuador (Formicidae: Leptanilloidinae). Zootaxa 1201: 47–62.
- Eguchi K, Bui TV, Oguri E, Maruyama M, Yamane S (2014) A new data of worker polymorphism in the ant genus Dorylus. Journal of Asia-Pacific Entomology 17: 31–36. doi: 10.1016/j.aspen.2013.09.004
- Eguchi K, Mizuno R, Ito F, Satria R, Dang VA, Bui TV, Phung THL (in press) First discovery of subdichthadiigyne in Yunodorylus Xu (Formicidae: Dorylinae). Revue Suisse de Zoologie 124(1).
- Emery C (1877) Saggio di un ordinamento naturale dei Mirmicidei, e considerazioni sulla filogenesi delle formiche. Bullettino della Società Entomologica Italiana 9: 67–83.
- Emery C (1881) Spedizione italiana nell’Africa equatoriale. Risultati zoologici. Formiche. Annali del Museo Civico di Storia Naturale 16: 270–276.
- Emery C (1882) [‘1881’] [Untitled. 3e Genre. - Parasyscia, Emery, n. gen.]. Pp. 235–236 In: André, Ern.1882. Les fourmis [part]. Pp. 233–280 In: André, Edm.1882. 1881–1886. Species des Hyménoptères d’Europe et d’Algérie. Tome Deuxième. Edmond André, Beaune, 919 + 48 pp.
- Emery C (1886) Alcune formiche africane. Bullettino della Società Entomologica Italiana 18: 355–366.
- Emery C (1888) Alcune formiche della Repubblica Argentina raccolte dal Dott. C. Spegazzini. Annali del Museo Civico di Storia Naturale 26[=(2)6]: 690–694.
- Emery C (1889) Formiche di Birmania e del Tenasserim raccolte da Leonardo Fea (1885–87). Annali del Museo Civico di Storia Naturale 27[=(2)7]: 485–520.
- Emery C (1890) Studii sulle formiche della fauna neotropica. Bullettino della Società Entomologica Italiana 22: 38–80.
- Emery C (1892) [‘1891’] Voyage de M. Ch. Alluaud dans le territoire d’Assinie (Afrique occidentale) en juillet et août 1886. Formicides. Annales de la Société Entomologique de France 60: 553–574.
- Emery C (1893a) [‘1892’] [Untitled. Introduced by: ‘M. C. Emery, de Bologne, envoie les diagnoses de cinq nouveaux genres de Formicides’.]. Bulletin Bimensuel de la Société Entomologique de France 1892: cclxxv-cclxxvii.
- Emery C (1893b) Formicides de l’Archipel Malais. Revue Suisse de Zoologie 1: 187–229. doi: 10.5962/bhl.part.3745
- Emery C (1894) Studi sulle formiche della fauna neotropica. VI-XVI. Bullettino della Società Entomologica Italiana 26: 137–241.
- Emery C (1895a) Voyage de M. E. Simon dans l’Afrique australe (janvier-avril 1893). 3e mémoire. Formicides. Annales de la Société Entomologique de France 64: 15–56.
- Emery C (1895b) Die Gattung Dorylus Fab. und die systematische Eintheilung der Formiciden. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 8: 685–778.
- Emery C (1895c) Viaggio di Leonardo Fea in Birmania e regioni vicine. LXIII. Formiche di Birmania del Tenasserim e dei Monti Carin raccolte da L. Fea. Parte II. Annali del Museo Civico di Storia Naturale 34[=(2)14]:450–483.
- Emery C (1896a) Formicides récoltés à Buitenzorg (Java), par M. Massart. Annales de la Société Entomologique de Belgique 40: 245–249.
- Emery C (1896b) Studi sulle formiche della fauna neotropica. XVII–XXV. Bullettino della Società Entomologica Italiana 28: 33–107.
- Emery C (1896c) Formiciden, gesammelt in Paraguay von Dr. J. Bohls. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 9: 625–638.
- Emery C (1896d) [‘1897’] Formiche raccolte dal Cap. V. Bottego nella regione dei Somali. Annali del Museo Civico di Storia Naturale 37[=(2)17]:153–160.
- Emery C (1897) Formicidarum species novae vel minus cognitae in collectione Musaei Nationalis Hungarici quas in Nova-Guinea, colonia germanica, collegit L. Biró. Természetrajzi Füzetek 20: 571–599.
- Emery C (1899a) Formiche dell’ultima spedizione Bottego. Annali del Museo Civico di Storia Naturale 39[=(2)19]: 499–501.
- Emery C (1899b) Fourmis d’Afrique. Annales de la Société Entomologique de Belgique 43: 459–504.
- Emery C (1899c) Intorno alle larve di alcune formiche. Memorie della Reale Accademia delle Scienze dell’Istituto di Bologna 5(8): 3–10.
- Emery C (1900a) Formicidarum species novae vel minus cognitae in collectione Musaei Nationalis Hungarici quas in Nova-Guinea, colonia germanica, collegit L. Biró. Publicatio secunda. Természetrajzi Füzetek 23: 310–338.
- Emery C (1900b) Nuovi studi sul genere Eciton. Memorie della Reale Accademia delle Scienze dell’Istituto di Bologna (5)8: 173–188[pagination of separate: 511–526].
- Emery C (1901a) Notes sur les sous-familles des Dorylines et Ponérines (Famille des Formicides). Annales de la Société Entomologique de Belgique 45: 32–54.
- Emery C (1901b) Note sulle Doriline. Bullettino della Società Entomologica Italiana 33: 43–56.
- Emery C (1901c) Formicidarum species novae vel minus cognitae in collectione Musaei Nationalis Hungarici, quas in Nova-Guinea, colonia germanica, collegit L. Biró. Publicatio tertia. Természetrajzi Füzetek 25: 152–160.
- Emery C (1901d) Studi sul polimorfismo e la metamorfosi nel genere Dorylus. Memorie della Reale Accademia delle Scienze dell’Istituto di Bologna (5)9: 183–201[pagination of separate: 415–433].
- Emery C (1902) Note mirmecologiche. Rendiconti delle Sessioni della Reale Accademia delle Scienze dell’Istituto di Bologna (n.s.) 6: 22–34.
- Emery C (1906) [‘1905’] Studi sulle formiche della fauna neotropica. XXVI. Bullettino della Società Entomologica Italiana 37: 107–194.
- Emery C (1910) Hymenoptera. Fam. Formicidae. Subfam. Dorylinae. Genera Insectorum 102: 1–35.
- Emery C (1911) Hymenoptera. Fam. Formicidae. Subfam. Ponerinae. Genera Insectorum 118: 1–125.
- Emery C (1915a) Contributo alla conoscenza delle formiche delle isole italiane. Descrizione di forme mediterrannee nuove o critiche. Annali del Museo Civico di Storia Naturale 46[=(3)6]: 244–270.
- Emery C (1915b) Formiche raccolte nell’Eritrea dal Prof. F. Silvestri. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d’Agricoltura. Portici 10: 3–26.
- Emery C (1923) Einige exotische Ameisen des Deutschen Entomologischen Institutes. Entomologische Mitteilungen. Berlin-Dahlem 12: 60–62.
- Enzmann EV (1952) Woitkowskia, a new genus of army ants. Proceedings of the Iowa Academy of Science 59: 442–447.
- Fabricius JC (1782) [‘1781’] Species insectorum exhibentes eorum differentias specificas, synonyma, auctorum loca natalia, metamorphosin adiectis observationibus, descriptionibus. Tome I. C. E. Bohn, Hamburgi et Kilonii [= Hamburg and Kiel], 552 pp.
- Fabricius JC (1793) Entomologia systematica emendata et aucta. Secundum classes, ordines, genera, species, adjectis synonimis, locis observationibus, descriptionibus. Tome 2. C. G. Proft, Hafniae [= Copenhagen], 519 pp.
- Feitosa RM, Brandão CRF, Fernández F, Delabie JHC (2011) The ant genus Sphinctomyrmex Mayr (Hymenoptera, Formicidae, Cerapachyinae) in the Neotropical region, with the description of two new species. Psyche 2012: Article ID 342623. doi: 10.1155/2012/342623
- Felsenstein J (1985) Phylogenies and the comparative method. American Naturalist1–15. doi: 10.1086/284325
- Fernández F, Escobar F (1997) Primer registro de Cylindromyrmex Mayr (Hymenoptera: Formicidae) para Colombia. Caldasia 19: 347.
- Fisher BL, Bolton B (2016) Ants of Africa and Madagascar. A guide to the genera. University of California Press, x + 497 pp.
- Forel A (1890) Aenictus-Typhlatta découverte de M. Wroughton. Nouveaux genres de Formicides. Annales de la Société Entomologique de Belgique 34: cii–cxiv.
- Forel A (1891) Les Formicides. [part]. In: Grandidier A (1891) Histoire physique, naturelle, et politique de Madagascar. Volume XX. Histoire naturelle des Hyménoptères. Deuxième partie (28e fascicule). Hachette et Cie, Paris, v + 237 pp.
- Forel A (1892a) Critique de: Peter Cameron. Hemenoptera [sic], Formicidae; Extracted from supplementary appendix to Travels amongst the Great Andes of the Equator by Edw. Whymper. London 1891. Annales de la Société Entomologique de Belgique 36: 255–256.
- Forel A (1892b) Les Formicides. [concl.]. In: Grandidier A (1892) Histoire physique, naturelle, et politique de Madagascar. Volume XX. Histoire naturelle des Hyménoptères. Deuxième partie. Supplèment au 28e fascicule. Hachette et Cie, Paris, 229–280.
- Forel A (1893a) Sur la classification de la famille des Formicides, avec remarques synonymiques. Annales de la Société Entomologique de Belgique 37: 161–167.
- Forel A (1893b) Nouvelles fourmis d’Australie et des Canaries. Annales de la Société Entomologique de Belgique 37: 454–466.
- Forel A (1895a) Nouvelles fourmis de diverses provenances, surtout d’Australie. Annales de la Société Entomologique de Belgique 39: 41–49.
- Forel A (1895b) Nouvelles fourmis d’Australie, récoltées à The Ridge, Mackay, Queensland, par M. Gilbert Turner. Annales de la Société Entomologique de Belgique 39: 417–428.
- Forel A (1897) Quelques Formicides de l’Antille de Grenada récoltés par M. H. H. Smith. Transactions of the Entomological Society of London 1897: 297–300. doi: 10.1111/j.1365-2311.1897.tb01683.x
- Forel A (1899) Formicidae. [part].Biologia Centrali-Americana. InsectaHymenoptera. 3: 1–24.
- Forel A (1900a) Ponerinae et Dorylinae d’Australie récoltés par MM. Turner, Froggatt, Nugent, Chase, Rothney J.-J. Walker, etc. Annales de la Société Entomologique de Belgique 44: 54–77.
- Forel A (1900b) Les Formicides de l’Empire des Indes et de Ceylan. Part VII. Journal of the Bombay Natural History Society 13: 303–332.
- Forel A (1901a) Les Formicides de l’Empire des Indes et de Ceylan. Part VIII. Journal of the Bombay Natural History Society 13: 462–477.
- Forel A (1901b) I. Fourmis mexicaines récoltées par M. le professeur W.-M. Wheeler. II. A propos de la classification des fourmis. Annales de la Société Entomologique de Belgique 45: 123–141.
- Forel A (1901c) [Untitled. Introduced by: ‘Faccio seguire le descrizioni mandatemi dal Prof. Forel di due specie africaine della sua collezione’.]. Pp. 48–49 In: Emery C (1901d) Note sulle Doriline. Bullettino della Società Entomologica Italiana 33: 43–56.
- Forel A (1901d) Nouvelles espèces de Ponerinae. (Avec un nouveau sous-genre et une espèce nouvelle d’Eciton). Revue Suisse de Zoologie 9: 325–353. doi: 10.5962/bhl.part.20305
- Forel A (1901e) Formiciden des Naturhistorischen Museums zu Hamburg. Neue Calyptomyrmex-, Dacryon-, Podomyrma- und Echinopla-Arten. Mitteilungen aus dem Naturhistorischen Museum in Hamburg 18: 43–82.
- Forel A (1902) Fourmis nouvelles d’Australie. Revue Suisse de Zoologie 10: 405–548. doi: 10.5962/bhl.part.13793
- Forel A (1904) Fourmis du Musée de Bruxelles. Annales de la Société Entomologique de Belgique 48: 168–177.
- Forel A (1905) Miscellanea myrmécologiques II (1905). Annales de la Société Entomologique de Belgique 49: 155–185.
- Forel A (1907a) Formicides du Musée National Hongrois. Annales Historico-Naturales Musei Nationalis Hungarici 5: 1–42.
- Forel A (1907b) Fourmis nouvelles de Kairouan et d’Orient. Annales de la Société Entomologique de Belgique 51: 201–208. doi: 10.5962/bhl.part.1582
- Forel A (1907c) Formicidae. In: Michaelsen W, Hartmeyer R (Eds) Die Fauna Südwest-Australiens. Band I, Lieferung 7. Gustav Fischer, Jena, 263–310.
- Forel A (1908) Ameisen aus Sao Paulo (Brasilien), Paraguay etc. gesammelt von Prof. Herm. v. Ihering, Dr. Lutz, Dr. Fiebrig, etc. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 58: 340–418.
- Forel A (1909a) Ameisen aus Guatemala usw., Paraguay und Argentinien (Hym.). Deutsche Entomologische Zeitschrift 1909: 239–269. doi: 10.1002/mmnd.48019090209
- Forel A (1909b) Fourmis du Musée de Bruxelles. Fourmis de Benguela récoltées par M. Creighton Wellman, et fourmis du Congo récoltées par MM. Luja, Kohl et Laurent. Annales de la Société Entomologique de Belgique 53: 51–73. doi: 10.5962/bhl.part.21869
- Forel A (1909c) Ameisen aus Java und Krakatau beobachtet und gesammelt von Herrn Edward Jacobson. Notes from the Leyden Museum 31: 221–232.
- Forel A (1910a) Formicides australiens reçus de MM. Froggatt et Rowland Turner. Revue Suisse de Zoologie 18: 1–94.
- Forel A (1910b) Ameisen aus der Kolonie Erythräa. Gesammelt von Prof. Dr. K. Escherich (nebst einigen in West-Abessinien von Herrn A. Ilg gesammelten Ameisen). Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 29: 243–274.
- Forel A (1910c) Note sur quelques fourmis d’Afrique. Annales de la Société Entomologique de Belgique 54: 421–458. doi: 10.5962/bhl.part.21462
- Forel A (1911a) Ameisen aus Java beobachtet und gesammelt von Herrn Edward Jacobson. II. Theil. Notes from the Leyden Museum 33: 193–218.
- Forel A (1911b) Ameisen des Herrn Prof. v. Ihering aus Brasilien (Sao Paulo usw.) nebst einigen anderen aus Südamerika und Afrika (Hym.). Deutsche Entomologische Zeitschrift 1911: 285–312. doi: 10.1002/mmnd.48019110305
- Forel A (1911c) Fourmis nouvelles ou intéressantes. Bulletin de la Société Vaudoise des Sciences Naturelles 47: 331–400.
- Forel A (1911d) Die Ameisen des K. Zoologischen Museums in München. Sitzungsberichte der Mathematischen-Physikalischen Klasse der Königlich Bayerischen Akademie der Wissenschaften zu München 11: 249–303.
- Forel A (1912a) Formicides néotropiques. Part I. Annales de la Société Entomologique de Belgique 56: 28–49.
- Forel A (1912b) Ameisen aus Java beobachtet und gesammelt von Edward Jacobson. III. Theil. Notes from the Leyden Museum 34: 97–112.
- Forel A (1912c) Formicides néotropiques. Part IV. 3me sous-famille Myrmicinae Lep. (suite). Mémoires de la Société Entomologique de Belgique 20: 1–32.
- Forel A (1912d) Einige neue und interessante Ameisenformen aus Sumatra etc. Zoologische Jahrbücher. Supplement 15(‘Erster Band’): 51–78.
- Forel A (1913a) Formicides du Congo Belge récoltés par MM. Bequaert, Luja, etc. Revue Zoologique Africaine (Brussels) 2: 306–351.
- Forel A (1913b) H. Sauter’s Formosa-Ausbeute: Formicidae II. Archiv für Naturgeschichte (A) 79(6): 183–202.
- Forel A (1913c) Wissenschaftliche Ergebnisse einer Forschungsreise nach Ostindien ausgeführt im Auftrage der Kgl. Preuss. Akademie der Wissenschaften zu Berlin von H. v. Buttel-Reepen. II. Ameisen aus Sumatra, Java, Malacca und Ceylon. Gesammelt von Herrn Prof. Dr. v. Buttel-Reepen in den Jahren 1911–1912. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 36: 1–148.
- Forel A (1913d) Fourmis d’Argentine, du Brésil, du Guatémala and de Cuba reçues de M. M. Bruch, Prof. v. Ihering, Mlle Baez M, Peper et M. Rovereto. Bulletin de la Société Vaudoise des Sciences Naturelles 49: 203–250.
- Forel A (1914) Formicides d’Afrique et d’Amérique nouveaux ou peu connus. Bulletin de la Société Vaudoise des Sciences Naturelles 50: 211–288.
- Forel A (1915) Results of Dr. E. Mjöbergs Swedish Scientific Expeditions to Australia 1910–13. 2. Ameisen. Arkiv för Zoologi 9(16): 1–119.
- Forel A (1916) Fourmis du Congo et d’autres provenances récoltées par MM. Hermann Kohl, Luja, Mayné, etc. Revue Suisse de Zoologie 24: 397–460. doi: 10.5962/bhl.part.4645
- Forel A (1920) [‘1919’] Deux fourmis nouvelles du Congo. Bulletin de la Société Vaudoise des Sciences Naturelles 52: 479–481.
- Fowler HG (1979) Notes on Labidus praedator (Fr. Smith) in Paraguay (Hymenoptera: Formicidae: Dorylinae: Ecitonini). Journal of Natural History 13: 3–10. doi: 10.1080/00222937900770021
- Franks NR (1982) A new method for censusing animal populations: the number of Eciton burchelli army ant colonies on Barro Colorado Island, Panama. Oecologia 52: 266–268. doi: 10.1007/BF00363847
- Gerstäcker A (1859) [Untitled. Introduced by: ‘Hr. Peters berichtete über sein Reisewerk, von dem die Insecten bis zum 64., die Botanik bis zum 34. Bogen gedruckt sind und theilte den Schluss der Diagnosen der von Hrn. Dr. Gerstäcker bearbeiteten Hymenopteren mit.’]. Monatsberichte der Königlichen Preussischen Akademie der Wissenschaften zu Berlin 1858: 261–264.
- Gerstäcker A (1863) Ueber ein merkwürdiges neues Hymenopteron aus der Abtheilung der Aculeata. Stettiner Entomologische Zeitung 24: 76–93.
- Gerstäcker A (1872) Hymenopterologische Beiträge. Stettiner Entomologische Zeitung 33: 250–308.
- Gobin B, Rüppell O, Hartmann A, Jungnickel H, Morgan ED, Billen J (2001) A new type of exocrine gland and its function in mass recruitment in the ant Cylindromyrmex whymperi (Formicidae, Cerapachyinae). Naturwissenschaften 88: 395–399. doi: 10.1007/s001140100251
- Gotwald WHJr (1969) Comparative morphological studies of the ants, with particular reference to the mouthparts (Hymenoptera: Formicidae). Memoirs of the Cornell University Agricultural Experiment Station 408: 1–150.
- Gotwald WHJr (1971) Phylogenetic affinities of the ant genus Cheliomyrmex (Hymenoptera: Formicidae). Journal of the New York Entomological Society 79: 161–173.
- Gotwald WHJr (1976) Behavioral observations on African army ants of the genus Aenictus (Hymenoptera: Formicidae). Biotropica 8: 59–65. doi: 10.2307/2387819
- Gotwald WHJr (1979) Phylogenetic implications of army ant zoogeography (Hymenoptera: Formicidae). Annals of the Entomological Society of America 72: 462–467. doi: 10.1093/aesa/72.4.462
- Gotwald WHJr (1982) Army ants. In: Hermann HR (Ed.) Social insects.Volume 4. Academic Press, New York, 157–254.
- Gotwald WHJr (1995) Army ants: the biology of social predation. Cornell University Press, Ithaca, New York, xviii + 302 pp.
- Gotwald WHJr, Barr D (1988) [‘1987’] Quantitative studies on workers of the Old World army ant genus Aenictus (Hymenoptera: Formicidae). Insectes Sociaux 34: 261–273. doi: 10.1007/BF02224358
- Gotwald WHJr, Brown WLJr (1967) [‘1966’] The ant genus Simopelta (Hymenoptera: Formicidae). Psyche (Cambridge) 73: 261–277. doi: 10.1155/1966/69869
- Gotwald WHJr, Cunningham-van Someren GR (1990) A year in the life of an Old World army ant colony: spatial patterns in raiding and emigration. In: Veeresh GK, Mallik B, Viraktamath CA. Social Insects and the Environment. Proceedings of the Eleventh IUSSI Congress. Oxford and IBH Publishing, New Delhi, 714–715.
- Gotwald WHJr, Cunningham-van Someren GR (1976) Taxonomic and behavioral notes on the African ant, Aenictus eugenii Emery, with a description of the queen (Hymenoptera: Formicidae). Journal of the New York Entomological Society 84: 182–188.
- Gotwald WHJr, Kupiec BM (1975) Taxonomic implications of doryline worker ant morphology: Cheliomyrmex morosus (Hymenoptera: Formicidae). Annals of the Entomological Society of America 68: 961–971. doi: 10.1093/aesa/68.6.961
- Gotwald WHJr, Leroux JM (1980) Taxonomy of the African army ant, Aenictus decolor (Mayr), with a description of the queen (Hymenoptera: Formicidae). Proceedings of the Entomological Society of Washington 82: 599–608.
- Gray GR (1832) [Untitled. Camptognatha testacea, attributed to Gray.]. In: Griffith E et al. (1832) The animal kingdom arranged in conformity with its organization, by the Baron Cuvier, with supplementary additions to each order by Edward Griffith F, L.S., A.S., and others. Volume the Fifteenth [= Class Insecta, Volume 2]. Whittaker, Treacher and Co., London, 516.
- Haldeman SS (1852) Appendix C. - Insects. In: Stansbury H (1852) An expedition to the Valley of the Great Salt Lake of Utah; including a description of its geography, natural history, and minerals, and an analysis of its waters. Sampson Low, Son and Co., London, 366–378.
- Haliday AH (1836) Descriptions, etc. of the Hymenoptera. Pp. 316–331 In: Curtis J, Haliday AH, Walker F (1836) Descriptions, etc. of the insects collected by Captain P.P. King R. N., F. R. S., in the survey of the Straits of Magellan.Transactions of the Linnean Society of London 17: 315–359. doi: 10.1111/j.1095-8339.1834.tb00026.x
- Hermann HR (1969) The hymenopterous poison apparatus: evolutionary trends in three closely related subfamilies of ants (Hymenoptera: Formicidae). Journal of the Georgia Entomological Society 4: 123–141.
- Hermann HR, Blum MS (1967) The morphology and histology of the hymenopterous poison apparatus. III. Eciton hamatum (Formicidae). Annals of the Entomological Society of America 60: 1282–1291. doi: 10.1093/aesa/60.3.661
- Hermann HR, Chao J-T (1983) Furcula, a major component of the hymenopterous venom apparatus. International Journal of Insect Morphology and Embryology 12: 321–337. doi: 10.1016/0020-7322(83)90027-2
- Heterick BE (2009) A guide to the ants of south-western Australia. Records of the Western Australian Museum Supplement 76: 1–206. doi: 10.18195/issn.0313-122x.76.2009.007-206
- Hirosawa H, Higashi S, Mohamed M (1998) Food habits of army ants Aenictus and their effects on ant community in a rainforest of Borneo. [Abstract.]. In: Schwarz MP, Hogendoorn K (Eds) Social insects at the turn of the millennium. Proceedings of the XIII International Congress of IUSSI. Adelaide, Australia. 29 December 1998 - 3 January 1999. XIII Congress of IUSSI, Adelaide, 210.
- Hita Garcia F, Fischer G, Peters MK, Snelling RR, Wägele JW (2009) A preliminary checklist of the ants (Hymenoptera: Formicidae) of Kakamega Forest (Kenya). Journal of East African Natural History 98: 147–165. doi: 10.2982/028.098.0201
- Hölldobler B (1982) Communication, raiding behavior and prey storage in Cerapachys (Hymenoptera: Formicidae). Psyche (Cambridge) 89: 3–23. doi: 10.1155/1982/28390
- Hölldobler B (2016) Queen specific exocrine glands in legionary ants and their possible function in sexual selection. PLoS ONE 11(3): e0151604. doi: 10.1371/journal.pone.0151604
- Hölldobler B, Obermayer M, Peeters C (1996) Comparative study of the metatibial gland in ants (Hymenoptera, Formicidae). Zoomorphology (Berlin) 116: 157–167. doi: 10.1007/BF02527156
- Holmgren N (1908) Über einige myrmecophile Insekten aus Bolivia und Peru. Zoologischer Anzeiger 33: 337–349.
- Humle T (2011) Ant-dipping: how ants have shed light on culture. In: Matsuzawa T, Humle T, Sugiyama Y (Eds) The chmipanzees of Bossou and Nimba.Primatology Monographs. Springer, Japan, 97–105. doi: 10.1007/978-4-431-53921-6_10
- Illiger K (1802) Neue Insekten. Magazin für Insektenkunde (Illiger) 1: 163–208.
- Imhoff L (1852) Ueber eine Art afrikanischer Ameisen. Bericht über die Verhandlungen der Naturforschenden Gesellschaft in Basel 10: 175–177.
- Jaitrong W (2016) [New species of Sphinctomyrmex attributed to W. Jaitrong]. Pp. 6–8 In: Jaitrong W, Wiwatwitaya D, Sakchoowong W (2016) Review of the Thai species of the genus Sphinctomyrmex Mayr, 1866 (Hymenoptera: Formicidae, Dorylinae), with description of a new species. Far Eastern Entomologist 305: 1–9.
- Jaitrong W, Eguchi K (2010) A new army ant of the genus Aenictus from Thailand (Hymenoptera: Formicidae). The Thailand Natural History Museum Journal 4(1): 13–17.
- Jaitrong W, Hashimoto Y (2012) Revision of the Aenictus minutulus species group (Hymenoptera: Formicidae: Aenictinae) from Southeast Asia. Zootaxa 3426: 29–44.
- Jaitrong W, Nur-Zati AM (2010) A new species of the ant genus Aenictus (Hymenoptera: Formicidae: Aenictinae) from the Malay Peninsula. Sociobiology 56: 449–454. doi: 10.1111/j.1479-8298.2010.00385.x
- Jaitrong W, Wiwatwitaya D (2013) Two new new species of the Aenictus pachycerus species group (Hymenoptera: Formicidae: Aenictinae) from Southeast Asia. Raffles Bulletin of Zoology 61: 97–102.
- Jaitrong W, Yamane S (2010) The army ant Aenictus silvestrii and its related species in Southeast Asia, with a description of a new species (Hymenoptera: Formicidae: Aenictinae). Entomological Science 13: 328–333.
- Jaitrong W, Yamane S (2011a) Synopsis of Aenictus species groups and revision of the A. currax and A. laeviceps groups in the eastern Oriental, Indo-Australian, and Australasian regions (Hymenoptera: Formicidae: Aenictinae). Zootaxa 3128: 1–46.
- Jaitrong W, Yamane S (2011b) Aenictus doydeei Jaitrong et Yamane, sp. n. Pp. 319–320 In: Jaitrong W, Yamane S, Chanthalangsy N (2011) The ant genus Aenictus from Laos, with description of a new species (Hymenoptera: Formicidae: Aenictinae). Journal of Asia-Pacific Entomology 14: 317–322. doi: 10.1016/j.aspen.2010.12.012
- Jaitrong W, Yamane S (2012a) Aenictus paradentatus Jaitrong and Yamane sp.n. Pp. 136–137 In: Jaitrong W, Yamane S, Tasen W (2012) A sibling species of Aenictus dentatus Forel, 1911 (Hymenoptera: Formicidae) from continental Southeast Asia. Myrmecological News 16: 133–138.
- Jaitrong W, Yamane S (2012b) Review of the Southeast Asian species of the Aenictus javanus and Aenictus philippinensis species groups (Hymenoptera, Formicidae, Aenictinae). ZooKeys 193: 49–78. doi: 10.3897/zookeys.193.2768
- Jaitrong W, Yamane S (2013) The Aenictus ceylonicus species group (Hymenoptera, Formicidae, Aenictinae) from Southeast Asia. Journal of Hymenoptera Research 31: 165–233. doi: 10.3897/jhr.31.4274
- Jaitrong W, Yamane S, Chanthalangsy N (2011) The ant genus Aenictus from Laos, with description of a new species (Hymenoptera: Formicidae: Aenictinae). Journal of Asia-Pacific Entomology 14: 317–322. doi: 10.1016/j.aspen.2010.12.012
- Jaitrong W, Yamane S, Tasen W (2012) A sibling species of Aenictus dentatus Forel, 1911 (Hymenoptera: Formicidae) from continental Southeast Asia. Myrmecological News 16: 133–138.
- Jaitrong W, Yamane S, Wiwatwitaya D (2010) The army ant Aenictus wroughtonii (Hymenoptera, Formicidae, Aenictinae) and related species in the Oriental region, with descriptions of two new species. Japanese Journal of Systematic Entomology 16: 33–46.
- Jurine L (1807) Nouvelle méthode de classer les Hyménoptères et les Diptères. Hyménoptères. Vol. 1. Paschoud, Genève, 319 pp.
- Karavaiev V (1925) Ponerinen (Fam. Formicidae) aus dem Indo-Australischen Gebiet. Konowia 4: 69–81.
- Karavaiev V (1926) Ameisen aus dem Indo-Australischen Gebiet. Treubia 8: 413–445.
- Karmaly KA (2012) Cerapachys keralensis, Karmaly sp.n. Pp. 157–158 In: Karmaly KA, Rabeesh TP, Sumesh S (2012) Description of new species of genus Cerapachys, Smith (Hymenoptera : Formicidae) from Kerala, India. Journal of Entomological Research (New Delhi) 36: 157–159.
- Keller RA (2011) A phylogenetic analysis of ant morphology (Hymenoptera: Formicidae) with special reference to the poneromorph subfamilies. Bulletin of the American Museum of Natural History 355: 1–90. doi: 10.1206/355.1
- Kempf WW (1961) A survey of the ants of the soil fauna in Surinam (Hymenoptera: Formicidae). Studia Entomologica 4: 481–524.
- Kempf WW (1972) Catálogo abreviado das formigas da região Neotropical. Studia Entomologica 15: 3–344.
- Kingdon J (1997) The Kingdon field guide to African mammals. Academic Press, San Diego, 464 pp.
- Kistner DH (1982) The social insects’ bestiary. In: Hermann HR (Ed.) Social insects.Volume 4. Academic Press, New York, 1–244.
- Kronauer DJC (2009) Recent advances in army ant biology (Hymenoptera: Formicidae). Myrmecological News 12: 51–65.
- Kronauer DJC, Berghoff SM, Powell S, Denny AJ, Edwards KJ, Franks NR, Boomsma JJ (2006) A reassessment of the mating system characteristics of the army ant Eciton burchellii. Naturwissenschaften 93: 402–406. doi: 10.1007/s00114-006-0121-2
- Kronauer DJC, Boomsma JJ (2007a) Do army ant queens re-mate later in life? Insectes Sociaux 54: 20–28. doi: 10.1007/s00040-007-0904-2
- Kronauer DJC, Boomsma JJ (2007b) Multiple queens means fewer mates. Current Biology 17:R753-R755. doi: 10.1016/j.cub.2007.06.057
- Kronauer DJC, Peters MK, Schöning C, Boomsma JJ (2011) Hybridization in East African swarm-raiding army ants. Frontiers in Zoology 8: 20, 13 pp.
- Kronauer DJC, Pierce NE, Keller L (2012) Asexual reproduction in introduced and native populations of the ant Cerapachys biroi. Molecular Ecology 21: 5221–5235. doi: 10.1111/mec.12041
- Kronauer DJC, Schöning C, Boomsma JJ (2006) Male parentage in army ants. Molecular Ecology 15: 1147–1151. doi: 10.1111/j.1365-294X.2005.02850.x
- Kronauer DJC, Schöning C, Pedersen JS, Boomsma JJ, Gadau J (2004) Extreme queen-mating frequency and colony fission in African army ants. Molecular Ecology 13: 2381–2388. doi: 10.1111/j.1365-294X.2004.02262.x
- Kronauer DJC, Schöning C, Vilhelmsen LB, Boomsma JJ (2007) A molecular phylogeny of Dorylus army ants provides evidence for multiple evolutionary transitions in foraging niche. BMC Evolutionary Biology 7: Article 56, 11 pp. doi: 10.1186/1471-2148-7-56
- Kusnezov N (1953) Lista de las hormigas de Tucumán con descripción de dos nuevos géneros (Hymenoptera, Formicidae). Acta Zoologica Lilloana 13: 327–339.
- Kusnezov N (1955) Un nuevo caracter de importancia filogenética en las hormigas (Hymenoptera, Formicidae). Dusenia 6: 183–186.
- Kusnezov N (1962) El género Acanthostichus Mayr (Hymenoptera, Formicidae). Acta Zoologica Lilloana 18: 121–138.
- Kutter H (1976) Beitrag zur Kenntnis der Gattung Simopone (Hym. Formicidae Subfam. Cerapachyinae resp. Ponerinae). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 49: 273–276.
- Kutter H (1977) Zweiter Beitrag zur Kenntnis der Gattung Simopone Forel (Hym. Formicidae, Subf. Cerapachyinae resp. Ponerinae). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 50: 173–176.
- Latreille PA (1802) Histoire naturelle des fourmis, et recueil de mémoires et d’observations sur les abeilles, les araignées, les faucheurs, et autres insectes. Impr. Crapelet (chez T. Barrois), Paris, xvi + 445 pp.
- Latreille PA (1804) Tableau méthodique des insectes. In: Société de Naturalistes et d’Agriculteurs 1804. Nouveau dictionnaire d’histoire naturelle. Tome 24. Déterville, Paris, 129–200.
- Leach WE (1815) Entomology. In: Brewster D (Ed.) The Edinburgh encyclopedia, Volume 9.William Blackwood, Edinburgh, 57–172.
- Leroux JM (1977) Densité des colonies et observations sur les nids de dorylines Anomma nigricans Illiger (Hym. Formicidae) dans la région de Lamto (Côte d’Ivoire). Bulletin de la Société Zoologique de France 102: 51–62.
- Leroux JM (1982) Ecologie des populations de dorylines Anomma nigricans dans la région de Lamto (Côte d’Ivoire). Pub-lications du Laboratoire de Zoologie, No. 22. – Ecole Nor-male Supérieure, Paris, 158 pp.
- Li S-P, Wang Y-L (2005) A new species of the ant genus Aenictus Shuckard (Hymenoptera: Formicidae) from Henan, China. Entomotaxonomia 27: 157–160.
- Liu C, Hita Garcia F, Peng Y-Q, Economo EP (2015) Aenictus yangi sp. n. – a new species of the A. ceylonicus species group (Hymenoptera, Formicidae, Dorylinae) from Yunnan, China. Journal of Hymenoptera Research 42: 33–45. doi: 10.3897/JHR.42.8859
- Linnaeus C (1764) Museum S:ae R:ae M:tis Ludovicae Ulricae Reginae Svecorum, Gothorum, Vandalorumque, &c. In quo animalia rariora, exotica, imprimis. Insecta and Conchilia describuntur and determinantur. Prodromi instar.Salvius, Holmiae [= Stockholm], 8 + 720 pp.
- Longino JT (2003) A new Costa Rican species of Leptanilloides (Hymenoptera: Formicidae: doryline section: Leptanilloidinae). Zootaxa 264: 1–6.
- Longino JT, Branstetter MG, Colwell RK (2014) How ants drop out: Ant abundance on tropical mountains. PLoS ONE 9(8): e104030. doi: 10.1371/journal.pone.0104030
- MacGown JA, Schiefer TL, Branstetter MG (2015) First record of the genus Leptanilloides (Hymenoptera: Formicidae: Dorylinae) from the United States. Zootaxa 4006(2): 392–400. doi: 10.11646/zootaxa.4006.2.10
- MacKay WP (1985) Acanthostichus sanchezorum (Hymenoptera: Formicidae), una nueva especie de Colombia. Sociobiology 11: 127–131.
- MacKay WP (1996) A revision of the ant genus Acanthostichus (Hymenoptera: Formicidae). Sociobiology 27: 129–179.
- Mackay WP (2004) A new species of the ant genus Acanthostichus Mayr (Hymenoptera: Formicidae) from Paraguay, and a description of the gyne of A. brevicornis Emery. Proceedings of the Entomological Society of Washington 106: 97–101.
- Mann WM (1919) The ants of the British Solomon Islands. Bulletin of the Museum of Comparative Zoology 63: 273–391.
- Mann WM (1921) The ants of the Fiji Islands. Bulletin of the Museum of Comparative Zoology 64: 401–499.
- Mann WM (1922) Ants from Honduras and Guatemala. Proceedings of the United States National Museum 61: 1–54. doi: 10.5479/si.00963801.61-2434.1
- Mann WM (1923) Two new ants from Bolivia. (Results of the Mulford Biological Exploration. - Entomology.). Psyche (Cambridge) 30: 13–18. doi: 10.1155/1923/58289
- Mann WM (1926) Some new neotropical ants. Psyche (Cambridge) 33: 97–107. doi: 10.1155/1926/45492
- Mariano CSF, Delabie JHC, Pompolo SG (2004) Nota sobre uma colônia e o cariótipo da formiga Neotropical Cylindromyrmex brasiliensis Emery (Hymenoptera: Formicidae: Cerapachyinae). Neotropical Entomology 33: 267–269. doi: 10.1590/S1519-566X2004000200020
- Maruyama M, Akino T, Hashim R, Komatsu T (2009) Behavior and cuticular hydrocarbons of myrmecophilous insects (Coleoptera: Staphylinidae; Diptera: Phoridae; Thysanura) associated with asian Aenictus army ants (Hymenoptera; Formicidae). Sociobiology 54: 19–35.
- Mason WRM (1986) Standard drawing conventions and definitions for venational and other features of wings of Hymenoptera. Proceedings of the Entomological Society of Washington 88: 1–7.
- Mathew R, Tiwari RN (2000) Insecta: Hymenoptera: Formicidae. In: Director; Zoological Survey of India (Ed.) Fauna of of Meghalaya. Part 7. [State Fauna Series 4.] Insecta 2000. Zoological Survey of India, Calcutta, 251–409.
- Mayr G (1865) Formicidae. In: Novara Expedition 1865. Reise der Österreichischen Fregatte ‘Novara’ um die Erde in den Jahren 1857, 1858, 1859. Zoologischer Theil. Bd. II.Abt. 1. K. Gerold’s Sohn, Wien, 119 pp.
- Mayr G (1866a) Myrmecologische Beiträge. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien. Mathematisch-Naturwissenschaftliche Classe. Abteilung I 53: 484–517. doi: 10.5962/bhl.part.20570
- Mayr G (1866b) Diagnosen neuer und wenig gekannter Formiciden. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 16: 885–908. doi: 10.5962/bhl.part.20570
- Mayr G (1868) Formicidae novae Americanae collectae a Prof. P. de Strobel. Annuario della Società dei Naturalisti e Matematici, Modena 3: 161–178.
- Mayr G (1870) Neue Formiciden. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 20: 939–996.
- Mayr G (1879) Beiträge zur Ameisen-Fauna Asiens. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 28: 645–686.
- Mayr G (1886a) Ueber Eciton-Labidus. Wiener Entomologische Zeitung 5: 33–36.
- Mayr G (1886b) Ueber Eciton-Labidus. (Schluss). Wiener Entomologische Zeitung 5: 115–122.
- Mayr G (1887) Südamerikanische Formiciden. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 37: 511–632.
- Mayr G (1896) Beiträge zur Kenntniss der Insektenfauna von Kamerun. 5. Formiciden gesammelt von Herrn Yngve Sjöstedt. Entomologisk Tidskrift 17: 225–252. doi: 10.5962/bhl.part.18240
- Mayr G (1897) Formiciden aus Ceylon und Singapur. Természetrajzi Füzetek 20: 420–436.
- Mayr G (1901) Südafrikanische Formiciden, gesammelt von Dr. Hans Brauns. Annalen des Kaiserlich-Königlichen Naturhistorischen Museums in Wien 16: 1–30. doi: 10.5962/bhl.part.29039
- McCreery HF, Breed MD (2014) Cooperative transport in ants: a review of proximate mechanisms. Insectes Sociaux 61: 99–110. doi: 10.1007/s00040-013-0333-3
- Menozzi C (1925) Nouvelles fourmis des Philippines. Philippine Journal of Science 28: 439–451.
- Menozzi C (1926) Due nuove specie di Eciton Latr. (Hymenoptera - Formicidae). Folia Myrmecologica et Termitologica 1: 29–32.
- Menozzi C (1926) [‘1925’] Nuove formiche delle isole Filippine e di Singapore. Atti della Società dei Naturalisti e Matematici di Modena 56[=(6)4]: 92–103.
- Menozzi C (1928) Tre nuove formiche della Sumatra orientale. Miscellanea Zoologica Sumatrana 30: 1–5.
- Menozzi C (1931) Contribuzione alla conoscenza del ‘microgenton’ di Costa Rica. III. Hymenoptera - Formicidae. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d’Agricoltura. Portici 25: 259–274.
- Menozzi C (1932) Raccolte mirmecologiche dell’Africa orientale conservate nel Museo Civico di Storia Naturale ‘Giacomo Doria’ di Genova. Parte II. Formiche dell’Uganda e delle isole Sesse raccolte dal Dr. E. Bayon. [part]. Annali del Museo Civico di Storia Naturale ‘Giacomo Doria’ 56: 93–112.
- Menozzi C (1936) Nuovi contributi alla conoscenza della fauna delle Isole italiane dell’Egeo. VI. Hymenoptera - Formicidae. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d’Agricoltura. Portici 29: 262–311.
- Mirenda JT, Topoff H (1980) Nomadic behavior of army ants in a desert-grassland habitat. Behavioral Ecology and Sociobiology 7: 129–135. doi: 10.1007/BF00299518
- Mirenda JT, Eakins DG, Gravelle K, Topoff H (1980) Predatory behavior and prey selection by army ants in a desert-grassland habitat. Behavioral Ecology and Sociobiology 7: 119–127. doi: 10.1007/BF00299517
- Monteiro AF, Sujii ER, Morais HC (2008) Chemically based interactions and nutritional ecology of Labidus praedator (Formicidae: Ecitoninae) in an agroecosystem adjacent to a gallery forest. Revista Brasileira de Zoologia 25: 674–681. doi: 10.1590/S0101-81752008000400012
- Moreau CS, Bell CD, Vila R, Archibald SB, Pierce NE (2006) Phylogeny of the ants: diversification in the age of angiosperms. Science (Washington DC) 312: 101–104. doi: 10.1126/science.1124891
- Morgan ED, Jungnickel H, Billen J, Ito F, Bergmann J, Gobin B (2008) Contents of the exocrine glands of the ant subfamily Cerapachyinae. Biochemical Systematics and Ecology 36: 260–265. doi: 10.1016/j.bse.2007.02.007
- Norton E (1868) Notes on Mexican ants. American Naturalist 2: 57–72. doi: 10.1086/270185
- O’Donnell S, Kaspari M, Lattke J (2005) Extraordinary predation by the neotropical army ant Cheliomyrmex andicola: implications for the evolution of the army ant syndrome. Biotropica 37: 706–709. doi: 10.1111/j.1744-7429.2005.00091.x
- O’Donnell S, Lattke J, Powell S, Kaspari M (2009) Species and site differences in Neotropical army ant emigration behaviour. Ecological Entomology 34: 476–482. doi: 10.1111/j.1365-2311.2008.01074.x
- Ogata K (1983) The ant genus Cerapachys F. Smith of Japan, with description of a new species (Hymenoptera, Formicidae). Esakia 20: 131–137.
- Oldham NJ, Morgan ED, Gobin B, Billen JPJ (1994) First identification of a trail pheromone of an army ant (Aenictus species). Experientia (Basel) 50: 763–765. doi: 10.1007/BF01919378
- Oldroyd BP (2013) Social evolution: policing without genetic conflict. Current Biology 23: R208–R210. doi: 10.1016/j.cub.2013.01.051
- Olivier AG (1792) Encyclopédie méthodique. Histoire naturelle. Insectes. Tome 6. (pt. 2). Panckoucke, Paris, 369–704.
- Overal WL, Bandeira AG (1985) Nota sobre hábitos de Cylindromyrmex striatus Mayr, 1870, na Amazônia (Formicidae, Ponerinae). Revista Brasileira de Entomologia 29: 521–522.
- Oxley PR, Ji L, Fetter-Pruneda I, McKenzie SK, Li C, Hu H, Zhang G, Kronauer DJ (2014) The genome of the clonal raider ant Cerapachys biroi. Current Biology 24: 451–458. doi: 10.1016/j.cub.2014.01.018
- Pamilo P (1991) Evolution of colony characteristics in social insects. II. Number of reproductive individuals. American Naturalist 138: 412–433. doi: 10.1086/285224
- Parr CL, Robertson HG, Chown SL (2003) Apomyrminae and Aenictogitoninae: two new subfamilies of ant (Hymenoptera: Formicidae) for southern Africa. African Entomology 11: 128–129.
- Perfecto I (1992) Observations of a Labidus coecus (Latreille) underground raid in the central highlands of Costa Rica. Psyche (Cambridge) 99: 214–220. doi: 10.1155/1992/47525
- Peters MK, Likare S, Kraemer M (2008) Effects of habitat fragmentation and degradation on flocks of African ant-following birds. Ecological Applications 18: 847–858. doi: 10.1890/07-1295.1
- Powell S, Clark E (2004) Combat between large derived societies: a subterranean army ant established as a predator of mature leaf-cutting ant colonies. Insectes Sociaux 51: 342–351. doi: 10.1007/s00040-004-0752-2
- Powell S, Franks NR (2007) How a few help all: living pothole plugs speed prey delivery in the army ant Eciton burchellii. Animal Behaviour 73: 1067–1076. doi: 10.1016/j.anbehav.2006.11.005
- Quiroz-Robledo LN (2003) First record of genus Cylindromyrmex Mayr for Mexico. Folia Entomologica Mexicana 42(2): 295–296.
- Radchenko AG (1993) New ants of the subfamily Cerapachyinae (Hymenoptera, Formicidae) from Vietnam. Zhurnal Ukrains’koho Entomolohichnoho Tovarystva 1(1): 43–47.
- Radchenko AG, Alipanah H (2004) The first record of the subfamily Aenictinae (Hymenoptera, Formicidae) from Iran. Vestnik Zoologii 38(4): 75–78.
- Raignier A (1972) Sur l’origine des nouvelles sociétés des Fourmis voyageuses africaines (Hyménoptères formicidae, Dorylinae). Insectes Sociaux 19: 153–170. doi: 10.1007/BF02226624
- Raignier A, Boven JKA (1955) Étude taxonomique, biologique et biométrique des Dorylus du sous-genre Anomma (HymenopteraFormicidae). Annales du Musée Royal du Congo Belge. Nouvelle Série in Quarto. Sciences Zoologiques 2: 1–359.
- Ravary F, Jahyny B, Jaisson P (2006) Brood stimulation controls the phasic reproductive cycle of the parthenogenetic ant Cerapachys biroi. Insectes Sociaux 53: 20–26. doi: 10.1007/s00040-005-0828-7
- Ravary F, Jaisson P (2002) The reproductive cycle of thelytokous colonies of Cerapachys biroi Forel (Formicidae, Cerapachyinae). Insectes Sociaux 49: 114–119. doi: 10.1007/s00040-002-8288-9
- Ravary F, Jaisson P (2004) Absence of individual sterility in thelytokous colonies of the ant Cerapachys biroi Forel (Formicidae, Cerapachyinae). Insectes Sociaux 51: 67–73. doi: 10.1007/s00040-003-0724-y
- Ravary F, Lecoutey E, Kaminski G, Châline N, Jaisson P (2007) Individual experience alone can generate lasting division of labor in ants. Current Biology 17: 1308–1312. doi: 10.1016/j.cub.2007.06.047
- Rettenmeyer CW (1963) Behavioral studies of army ants. University of Kansas Science Bulletin 44: 281–465.
- Rettenmeyer CW, Watkins JFII (1978) Polygyny and monogyny in army ants (Hymenoptera: Formicidae). Journal of the Kansas Entomological Society 51: 581–591.
- Rettenmeyer CW, Chadab-Crepet R, Naumann MG, Morales L (1983) Comparative foraging by Neotropical army ants. In: Jaisson P (Ed.) Social insects in the tropics, vol.2. Université Paris-Nord, Paris, 59–73.
- Rettenmeyer CW, Rettenmeyer ME, Joseph J, Berghoff SM (2011) The largest animal association centered on one species: the army ant Eciton burchellii and its more than 300 associates. Insectes Sociaux 58: 281–292. doi: 10.1007/s00040-010-0128-8
- Roger J (1861) Die Ponera-artigen Ameisen (Schluss). Berliner Entomologische Zeitschrift 5: 1–54.
- Roger J (1862) Einige neue exotische Ameisen-Gattungen und Arten. Berliner Entomologische Zeitschrift 6: 233–254. doi: 10.1002/mmnd.47918620118
- Roger J (1863) Die neu aufgeführten Gattungen und Arten meines Formiciden-Verzeichnisses nebst Ergänzung einiger früher gegebenen Beschreibungen. Berliner Entomologische Zeitschrift 7: 131–214. doi: 10.1002/mmnd.18630070116
- Roonwal ML (1975) Plant-pest status of root-eating ant, Dorylus orientalis, with notes on taxonomy, distribution and habits (Insecta: Hymenoptera). Journal of the Bombay Naural History Society 72: 305–313.
- Rościszewski K, Maschwitz U (1994) Prey specialization of army ants of the genus Aenictus in Malaysia andrias 13: 179–187.
- Sánchez-Peña SR, Mueller UG (2002) A nocturnal raid of Nomamyrmex army ants on Atta leafcutting ants in Tamaulipas, Mexico. Southwestern Entomologist 27: 221–224.
- Santschi F (1910a) Nouvelles fourmis de Tunisie (suite). Bulletin de la Société d’Histoire Naturelle de l’Afrique du Nord 1: 61–64.
- Santschi F (1910b) [‘1909’] Formicides nouveaux ou peu connus du Congo français. Annales de la Société Entomologique de France 78: 349–400.
- Santschi F (1910c) Nouveaux dorylines africains. Revue Suisse de Zoologie 18: 737–759. doi: 10.5962/bhl.part.16674
- Santschi F (1911a) Nouvelles fourmis du Congo et du Benguela. Revue Zoologique Africaine (Brussels) 1: 204–217.
- Santschi F (1911b) Une nouvelle espèce d’Eciton. Berliner Entomologische Zeitschrift 56: 113. doi: 10.1002/mmnd.48019110710
- Santschi F (1912) Fourmis d’Afrique et de Madagascar. Annales de la Société Entomologique de Belgique 56: 150–167. doi: 10.5962/bhl.part.5816
- Santschi F (1913) Glanures de fourmis africaines. Annales de la Société Entomologique de Belgique 57: 302–314.
- Santschi F (1914a) Voyage de Ch. Alluaud et R. Jeannel en Afrique Orientale, 1911–1912. Résultats scientifiques. Insectes Hyménoptères. II. Formicidae. Libr. A. Schulz, Paris, 41–148.
- Santschi F (1914b) Formicides de l’Afrique occidentale et australe du voyage de Mr. le Professeur F. Silvestri. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d’Agricoltura. Portici 8: 309–385.
- Santschi F (1915) Nouvelles fourmis d’Afrique. Annales de la Société Entomologique de France 84: 244–282.
- Santschi F (1916) Formicides sudaméricains nouveaux ou peu connus. Physis (Buenos Aires) 2: 365–399.
- Santschi F (1917) Note sur Dorylusaffinis Shuckard mâle et ses variétés. Bulletin de la Société d’Histoire Naturelle de l’Afrique du Nord 8: 18–21.
- Santschi F (1919a) Fourmis nouvelles éthiopiennes. Revue Zoologique Africaine (Brussels) 6: 229–240. doi: 10.5962/bhl.part.19457
- Santschi F (1919b) Fourmis nouvelles du Congo. Revue Zoologique Africaine (Brussels) 6: 243–250. doi: 10.5962/bhl.part.19458
- Santschi F (1920a) Formicides africains et américains nouveaux. Annales de la Société Entomologique de France 88: 361–390.
- Santschi F (1920b) Fourmis d’Indo-Chine. Annales de la Société Entomologique de Belgique 60: 158–176.
- Santschi F (1921a) Quelques nouveaux Formicides africains. Annales de la Société Entomologique de Belgique 61: 113–122.
- Santschi F (1921b) Ponerinae, Dorylinae et quelques autres formicides néotropiques. Bulletin de la Société Vaudoise des Sciences Naturelles 54: 81–103.
- Santschi F (1923a) Solenopsis et autres fourmis néotropicales. Revue Suisse de Zoologie 30: 245–273. doi: 10.5962/bhl.part.117787
- Santschi F (1923b) Descriptions de nouveaux Formicides éthiopiens et notes diverses. I. Revue Zoologique Africaine (Brussels) 11: 259–295.
- Santschi F (1923c) Descriptions de quelques nouvelles fourmis du Brésil. Revista do Museu Paulista 13: 1255–1264.
- Santschi F (1924) Descriptions de nouveaux Formicides africains et notes diverses. II. Revue Zoologique Africaine (Brussels) 12: 195–224.
- Santschi F (1925) [‘1924’] Nouvelles fourmis brésiliennes. Annales de la Société Entomologique de Belgique 64: 5–20.
- Santschi F (1926a) Description de nouveaux Formicides éthiopiens (IIIme partie). Revue Zoologique Africaine (Brussels) 13: 207–267.
- Santschi F (1926b) Quelques fourmis nord-africaines. Bulletin de la Société d’Histoire Naturelle de l’Afrique du Nord 17: 229–236.
- Santschi F (1928) Fourmis de Sumatra, récoltées par Mr. J. B. Corporaal. Tijdschrift voor Entomologie 71: 119–140.
- Santschi F (1929) [‘1928’] Sur quelques nouvelles fourmis du Brésil (Hym. Form.). Deutsche Entomologische Zeitschrift 1928: 414–416.
- Santschi F (1930) Description de Formicides éthiopiens nouveaux ou peu connus. V. Bulletin et Annales de la Société Entomologique de Belgique 70: 49–77.
- Santschi F (1931) La reine du Dorylus fulvus Westw. Bulletin de la Société d’Histoire Naturelle de l’Afrique du Nord 22: 401–408.
- Santschi F (1932) Formicides sud-africains. In: Jeannel R (Ed.) Société Entomologique de France.Livre du centenaire. Paris: Société Entomologique de France, 381–392.
- Santschi F (1933) Contribution à l’étude des fourmis de l’Afrique tropicale. Bulletin et Annales de la Société Entomologique de Belgique 73: 95–108.
- Santschi F (1937a) Glanure de fourmis éthiopiennes. Bulletin et Annales de la Société Entomologique de Belgique 77: 47–66.
- Santschi F (1937b) Résultats entomologiques d’un voyage au Cameroon. Formicides récoltés par Mr. le Dr. F. Zumpt. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 17: 93–104.
- Santschi F (1938) [‘1937’] Quelques nouvelles fourmis d’Egypte. Bulletin. Société Entomologique d’Egypte 21: 28–44.
- Sanz CM, Schöning C, Morgan DB (2010) Chimpanzees prey on army ants with specialized tool set. American Journal of Primatology 72: 17–24. doi: 10.1002/ajp.20744
- Sarnat EM, Economo EP (2012) The ants of Fiji. University of California Publications in Entomology 132: 1–384.
- Savage TS (1847) On the habits of the ‘Drivers’ or visiting ants of West Africa. Transactions of the Entomological Society of London 5: 1–15. doi: 10.1111/j.1365-2311.1847.tb01686.x
- Savage TS (1849) The driver ants of western Africa. Proceedings of the Academy of Natural Sciences of Philadelphia 4: 195–200.
- Schmidt CA, Shattuck SO (2014) The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817(1): 1–242. doi: 10.11646/zootaxa.3817.1.1
- Schneirla TC (1971) Army ants. A study in social organization.(Edited by H. R. Topoff.).W. H. Freeman and Co., San Francisco, xx + 349 pp.
- Schneirla TC, Reyes AY (1966) Raiding and related behaviour in two surface-adapted species of the old world doryline ant, Aenictus. Animal Behavior 14: 132–148. doi: 10.1016/S0003-3472(66)80022-2
- Schneirla TC, Reyes AY (1969) Emigrations and related behaviour in two surface-adapted species of the old-world doryline ant, Aenictus. Animal Behavior 17: 87–103. doi: 10.1016/0003-3472(69)90116-X
- Schöning C, Ellis D, Fowler A, Sommer V (2007) Army ant availability and consumption by chimpanzees (Pan troglodytes vellerosus) at Gashaka / Nigeria. Journal of Zoology 271: 125–133. doi: 10.1111/j.1469-7998.2006.00177.x
- Schöning C, Kinuthia W, Franks NR (2005a) Evolution of allometries in the worker caste of Dorylus army ants. Oikos 110: 231–240. doi: 10.1111/j.0030-1299.2005.13672.x
- Schöning C, Njagi W, Franks NR (2005b) Temporal and spatial patterns in the emigrations of the army ant Dorylus (Anomma) molestus at Mt Kenya. Ecological Entomology 30: 532–540. doi: 10.1111/j.0307-6946.2005.00720.x
- Schöning C, Moffett M (2007) Driver ants invading a termite nest – why do the most catholic predators of all seldom take this abundant prey? Biotropica 39: 663–667. doi: 10.1111/j.1744-7429.2007.00296.x
- Schöning C, Njagi W, Kinuthia W (2008) Prey spectra of two swarm-raiding army ant species in East Africa. Journal of Zoology 274: 85–93.
- Schulz WA (1906) Spolia hymenopterologica. Junfermannsche Buchhandlung, Paderborn, 355 pp. doi: 10.5962/bhl.title.59757
- Sharaf MR (2007) [Untitled. New species attributed to Sharaf.]. Pp. 237–244 In: Fadl HH, Bakr RF, Badawy RM, Sharaf MR (2007) Six new species of ants (Insecta: Hymenoptera: Formicidae) from Egypt. Pp. 235–249 In: Entomological Society of Egypt2007. Proceedings of the Second International Conference of Economic Entomology. Volume 1. Entomological Society of Egypt, Cairo.
- Sharaf MR, Aldawood SA (2012) Aenictus arabicus Sharaf and Aldawood, sp. n. Pp. 42–45 in: Sharaf MR, Aldawood SA, El-Hawagry MS (2012) First record of the ant subfamily Aenictinae (Hymenoptera, Formicidae) from Saudi Arabia, with the description of a new species. ZooKeys 228: 39–49. doi: 10.3897/zookeys.228.3559
- Sharaf MR, Aldawood SA, El-Hawagry MS (2012) First record of the ant subfamily Aenictinae (Hymenoptera, Formicidae) from Saudi Arabia, with the description of a new species. ZooKeys 228: 39–49. doi: 10.3897/zookeys.228.3559
- Shattuck SO (2008) Review of the ant genus Aenictus (Hymenoptera: Formicidae) in Australia with notes on A. ceylonicus (Mayr). Zootaxa 1926: 1–19.
- Shuckard WE (1840a) Monograph of the Dorylidae, a family of the Hymenoptera Heterogyna. Annals of Natural History 5: 188–201. doi: 10.1080/00222934009496804
- Shuckard WE (1840b) Monograph of the Dorylidae, a family of the Hymenoptera Heterogyna. (Continued from p. 201.). Annals of Natural History 5: 258–271. doi: 10.1080/00222934009496821
- Shuckard WE (1840c) Monograph of the Dorylidae, a family of the Hymenoptera Heterogyna. (Concluded from p. 271.). Annals of Natural History 5: 315–328. doi: 10.1080/00222934009496836
- Shuckard WE (1840d) Appendix to Mr. Shuckard’s monograph of the Dorylidae, containing a description of two new species of Labidus. Annals of Natural History 5: 396–398. doi: 10.1080/00222934009496860
- Silva RR, Feitosa RM, Brandão CRF, Freitas AVL (2013) The first Leptanilloides species (Hymenoptera: Formicidae: Leptanilloidinae) from eastern South America. Journal of Natural History 47: 2039–2047. doi: 10.1080/00222933.2012.763058
- Smith AA, Haight KL (2008) Army ants as research and collection tools. Journal of Insect Science 8: 71, 5 pp.
- Smith F (1855) Descriptions of some species of Brazilian ants belonging to the genera Pseudomyrma, Eciton and Myrmica (with observations on their economy by Mr. H. W. Bates). Transactions of the Entomological Society of London (2)3: 156–169. doi: 10.1111/j.1365-2311.1855.tb02668.x
- Smith F (1857) Catalogue of the hymenopterous insects collected at Sarawak, Borneo; Mount Ophir, Malacca; and at Singapore, by A. R. Wallace. [part]. Journal and Proceedings of the Linnean Society of London. Zoology 2: 42–88.
- Smith F (1858) Catalogue of hymenopterous insects in the collection of the British Museum. Part VI. Formicidae. British Museum, London, 216 pp.
- Smith F (1859) Catalogue of hymenopterous insects in the collection of the British Museum. Part VII. Dorylidae and Thynnidae. British Museum, London, 76 pp.
- Smith F (1865) Descriptions of new species of hymenopterous insects from the islands of Sumatra, Sula, Gilolo, Salwatty, and New Guinea, collected by Mr. A. R. Wallace. Journal and Proceedings of the Linnean Society of London. Zoology 8: 61–94.
- Smith MR (1942b) The males of two North American cerapachyine ants. Proceedings of the Entomological Society of Washington 44: 62–64.
- Smith MR (1942b) The legionary ants of the United States belonging to Eciton subgenus Neivamyrmex Borgmeier. American Midland Naturalist 27: 537–590. doi: 10.2307/2420913
- Smith MR (1943) A new male legionary ant from the Mojave Desert, California. Lloydia 6: 196–197.
- Snelling RR (1981) Systematics of social Hymenoptera. In: Hermann HR (Ed.) 1981.Social insects. Volume 2. Academic Press, New York, 369–453.
- Snelling GC, Snelling RR (2007) New synonymy, new species, new keys to Neivamyrmex army ants of the United States. Memoirs of the American Entomological Institute 80: 459–550.
- Snodgrass RE (1984) The anatomy of the honey bee. Cornell Univerity Press, Ithaca, 334 pp.
- Souza JL, Moura CA (2008) Predation of ants and termites by army ants, Nomamyrmex esenbeckii (Formicidae, Ecitoninae) in the Brazilian Amazon. Sociobiology 52:399.
- Spinola M (1851) Compte rendu des Hyménoptères inédits provenants du voyage entomologique de M. Ghiliani dans le Para en 1846. Extrait des Mémoires de l’Académie des Sciences de Turin (2)13: 3–78.
- Staab M (2014a) A new species of the Aenictus wroughtonii group (Hymenoptera, Formicidae) from South-East Asia. ZooKeys 391: 65–73. doi: 10.3897/zookeys.391.7213
- Staab M (2014b) The first observation of honeydew foraging in army ants since 1933: Aenictus hodgsoni Forel, 1901 tending Eutrichosiphum heterotrichum (Raychaudhuri, 1956) in Southeast China. Asian Myrmecology 6: 115–118. doi: 10.3897/zookeys.516.9927
- Staab M (2015) Aenictus hoelldobleri sp. n., a new species of the Aenictus ceylonicus group (Hymenoptera, Formicidae) from China, with a key to the Chinese members of the group. ZooKeys 516: 137–155.
- Stitz H (1910) Westafrikanische Ameisen. I. Mitteilungen aus dem Zoologischen Museum in Berlin 5: 125–151. doi: 10.1002/mmnz.4830050108
- Stitz H (1911) Formicidae. Wissenschaftliche Ergebnisse der Deutschen Zentral-Afrika-Expedition 3: 375–392.
- Stitz H (1917) Ameisen aus dem westlichen Mittelmeergebiet und von den Kanarischen Inseln. Mitteilungen aus dem Zoologischen Museum in Berlin 8: 333–353.
- Swainson W, Shuckard WE (1840) On the history and natural arrangement of insects. London: Longman, Brown, Green and Longman’s, 406 pp.
- Swartz MB (1998) Predation on an Atta cephalotes colony by an army ant Nomamyrmex esenbeckii. Biotropica 30: 682–684. doi: 10.1111/j.1744-7429.1998.tb00110.x
- Tang J, Li S (1982) Hymenoptera: Formicidae. In: Academy of Science Survey Team1982. Insects of Tibet. Vol. 2. Academy of Science Publishing House, Beijing, 371–374.
- Taylor RW (1965) New Melanesian ants of the genera Simopone and Amblyopone (Hymenoptera-Formicidae) of zoogeographic significance. Breviora 221: 1–11.
- Taylor RW (1966) [‘1965’] Notes on the Indo-Australian ants of genus Simopone Forel (Hymenoptera-Formicidae). Psyche (Cambridge) 72: 287–290. doi: 10.1155/1965/21567
- Teles Da Silva M (1977a) Behavior of the army ant Eciton burchelli Westwood (Hymenoptera: Formicidae) in the Belém region. I. Nomadic-statary cycles. Animal Behaviour 25: 910–923. doi: 10.1016/0003-3472(77)90041-0
- Teles Da Silva M (1977b) Behavior of the army ant Eciton burchelli Westwood (Hymenoptera: Formicidae) in the Belém region. II. Bivouacs. Boletim de Zoologia, Universidade de São Paulo 2: 107–128.
- Terayama M (1984) A new species of the army ant genus Aenictus from Taiwan (Insecta; Hymenoptera; Formicidae). Bulletin of the Biogeographical Society of Japan 39: 13–16.
- Terayama M (1996) Taxonomic studies on the Japanese Formicidae, part 2. Seven genera of Ponerinae, Cerapachyinae and Myrmicinae. Nature and Human Activities 1: 9–32.
- Terayama M (2009) A synopsis of the family Formicidae of Taiwan (Insecta: Hymenoptera). Research Bulletin of Kanto Gakuen University. Liberal Arts 17: 81–266.
- Terayama M, Kubota S (1993) The army ant genus Aenictus (Hymenoptera: Formicidae) from Thailand and Viet Nam, with descriptions of three new species. Bulletin of the Biogeographical Society of Japan 48: 68–72.
- Terayama M, Yamane S (1989) The army ant genus Aenictus (Hymenoptera, Formicidae) from Sumatra, with descriptions of three new species. Japanese Journal of Entomology 57: 597–603.
- Terayama M, Kubota S, Sakai H, Kawazoe A (1988) Rediscovery of Cerapachys sauteri Forel, 1913 (Insecta: Hymenoptera: Formicidae) from Taiwan, with notes on the Taiwanese species of the genus Cerapachys. Bulletin of the Biogeographical Society of Japan 43: 35–38.
- Teseo S, Kronauer DJC, Jaisson P, Châline N (2013) Enforcement of reproductive synchrony via policing in a clonal ant. Current Biology 23: 328–332. doi: 10.1016/j.cub.2013.01.011
- Topoff H, Lawson K (1979) Orientation of the army ant Neivamyrmex nigrescens: Integration of chemical and tactile information. Animal Behaviour 27: 429–433. doi: 10.1016/0003-3472(79)90179-9
- Topoff H, Mirenda J (1980) Army ants do not eat and run: Influence of food supply on emigration behaviour in Neivamyrmex nigrescens. Animal Behaviour 28: 1040–1045. doi: 10.1016/S0003-3472(80)80093-5
- Topoff H, Mirenda J, Droual R, Herrick S (1980) Behavioural ecology of mass recruitment in the army ant Neivamyrmex nigrescens. Animal Behaviour 28: 779–789. doi: 10.1016/S0003-3472(80)80138-2
- Tsuji K, Yamauchi K (1995) Production of females by parthenogenesis in the ant Cerapachys biroi. Insectes Sociaux 42: 333–336. doi: 10.1007/BF01240430
- Varela Hernández F, Castaño-Meneses G (2011) Neivamyrmex albacorpus, a new species of ant (Hymenoptera: Formicidae: Ecitoninae) from Metztitlán, Hidalgo, México. Sociobiology 58: 579–584.
- Vidal-Riggs JM, Chaves-Campos J (2008) Method review: estimation of colony densities of the army ant Eciton burchellii in Costa Rica. Biotropica 40: 259–262. doi: 10.1111/j.1744-7429.2007.00347.x
- Viehmeyer H (1913) Ameisen aus dem Kopal von Celebes. Stettiner Entomologische Zeitung 74: 141–155.
- Viehmeyer H (1914) [‘1913’] Neue und unvollständig bekannte Ameisen der alten Welt. Archiv für Naturgeschichte (A) 79(12): 24–60.
- Viehmeyer H (1916) [‘1915’] Ameisen von Singapore. Beobachtet und gesammelt von H. Overbeck. Archiv für Naturgeschichte (A) 81(8): 108–168.
- Vilhelmsen L, Miko I, Krogmann L (2010) Beyond the wasp-waist: structural diversity and phylogenetic significance of the mesosoma in apocritan wasps (Insecta: Hymenoptera). Zoological Journal of the Linnean Society 159(1): 22–194. doi: 10.1111/j.1096-3642.2009.00576.x
- Voessler J (1905) Die ostafrikanische Treiberameise (Siafu). Pflanzer 1: 289–302.
- Walker F (1860) Characters of some apparently undescribed Ceylon insects. [part]. Annals and Magazine of Natural History (3)5: 304–311.
- Wang W (2006) A new species of the genus Aenictus Shuckard from China (Hymenoptera, Formicidae). Acta Zootaxonomica Sinica 31: 637–639. [In Chinese]
- Ward PS (1990) The ant subfamily Pseudomyrmecinae (Hymenoptera: Formicidae): generic revision and relationship to other formicids. Systematic Entomology 15: 449–489. doi: 10.1111/j.1365-3113.1990.tb00077.x
- Ward PS (2007) The ant genus Leptanilloides: discovery of the male and evaluation of phylogenetic relationships based on DNA sequence data. Memoirs of the American Entomological Institute 80: 637–649.
- Ward PS (2014) The phylogeny and evolution of ants. Annual Review of Ecology, Evolution, and Systematics 45: 23–43. doi: 10.1146/annurev-ecolsys-120213-091824
- Ward PS, Brady SG (2009) Rediscovery of the ant genus Amyrmex Kusnezov (Hymenoptera: Formicidae) and its transfer from Dolichoderinae to Leptanilloidinae. Zootaxa 2063: 46–54.
- Wasmann E (1904) Zur Kenntniss der Gäste der Treiberameisen und ihrer Wirthe am obern Congo, nach den Sammlungen und Beobachtungen von P. Herm. Kohl C,S.S.C. bearbeitet. Zoologische Jahrbücher, Supplement 7: 611–682.
- Wasmann E (1911) Zur Kenntnis der Termiten und Termitengäste vom belgischen Congo. [part]. Revue Zoologique Africaine (Brussels) 1: 91–117.
- Wasmann E (1917) Neue Anpassungstypen bei Dorylinengästen Afrikas (Col. Staphylinidae). Zeitschrift für Wissenschaftliche Zoologie 117: 257–360.
- Watkins JFII (1969) [‘1968’] A new species of Neivamyrmex (Hymenoptera: Formicidae) from Louisiana. Journal of the Kansas Entomological Society 41: 528–531.
- Watkins JFII (1972) The taxonomy of Neivamyrmex texanus, n. sp., N. nigrescens and N. californicus (Formicidae: Dorylinae), with distribution map and keys to the species of Neivamyrmex of the United States. Journal of the Kansas Entomological Society 45: 347–372.
- Watkins JFII (1973) Neivamyrmex baylori, n. sp. (Formicidae: Dorylinae) from Waco, Texas U,S.A. Journal of the Kansas Entomological Society 46: 430–433.
- Watkins JFII (1974) Neivamyrmex angulimandibulatus, new species (Formicidae: Dorylinae) from Cordoba, Mexico. The Southwestern Naturalist 19: 309–312. doi: 10.2307/3669936
- Watkins JFII (1975a) Neivamyrmex cornutus, n. sp. (Formicidae: Dorylinae) from Oaxaca, Mexico. Journal of the Kansas Entomological Society 48: 92–95.
- Watkins JFII (1975b) Neivamyrmex digitistipus, n. sp. (Formicidae: Dorylinae) from Costa Rica. Texas Journal of Science 26: 203–206.
- Watkins JFII (1975c) Neivamyrmex quadratoocciputus, n. sp. (Formicidae: Dorylinae) from El Salvador. Texas Journal of Science 26: 207–211.
- Watkins JFII (1976) The identification and distribution of New World army ants (Dorylinae: Formicidae). Baylor University Press, Waco, Texas, 102 pp.
- Watkins JFII (1977) Neivamyrmex nyensis, n. sp. (Formicidae: Dorylinae) from Nye County, Nevada U,S.A. The Southwestern Naturalist 22: 421–425. doi: 10.2307/3670143
- Watkins JFII (1986) Neivamyrmex chamelensis, n. sp. (Hymenoptera: Formicidae: Ecitoninae) from Jalisco, Mexico. Journal of the Kansas Entomological Society 59: 361–366.
- Watkins JFII (1990) Neivamyrmex crassiscapus, n. sp. (Hymenoptera: Formicidae: Ecitoninae) from Mexico. Journal of the Kansas Entomological Society 63: 348–350.
- Watkins JFII (1994) [‘1993’] Neivamyrmex curvinotus, n. sp. (Hymenoptera: Formicidae: Ecitoninae) from South America. Journal of the Kansas Entomological Society 66: 411–413.
- Weber NA (1939) New ants of rare genera and a new genus of ponerine ants. Annals of the Entomological Society of America 32: 91–104. doi: 10.1093/aesa/32.1.91
- Weber NA (1941) The rediscovery of the queen of Eciton (Labidus) coecum Latr. (Hym.: Formicidae). American Midland Naturalist 26: 325–329. doi: 10.2307/2420962
- Weber NA (1942) New doryline, cerapachyine and ponerine ants from the Imatong Mountains, Anglo-Egyptian Sudan. Proceedings of the Entomological Society of Washington 44: 40–49.
- Weber NA (1949a) New African ants of the genera Cerapachys, Phryacaces, and Simopone. American Museum Novitates 1396: 1–9.
- Weber NA (1949b) A new Panama Eciton (Hymenoptera, Formicidae). American Museum Novitates 1441: 1–8.
- Weissflog A, Sternheim E, Dorow WHO, Berghoff S, Maschwitz U (2000) How to study subterranean army ants: a novel method for locating and monitoring field populations of the South East Asian army ant Dorylus (Dichthadia) laevigatus Smith, 1857 (Formicidae, Dorylinae) with observations on their ecology. Insectes Sociaux 47: 317–324. doi: 10.1007/PL00001723
- Westwood JO (1835) [Untitled. Introduced by: ‘Specimens were exhibited, partly from the collection of the Rev. F. W. Hope, and partly from that of Mr. Westwood, of various Hymenopterous insects, which Mr. Westwood regarded as new to science.’]. Proceedings of the Zoological Society of London 3: 68–72.
- Westwood JO (1839) An introduction to the modern classification of insects; founded on the natural habits and corresponding organisation of the different families. Volume 2. Part XI. Longman, Orme, Brown, Green and Longmans, London, 193–224.
- Westwood JO (1842) Monograph of the hymenopterous group, Dorylides. In: Westwood JO (1842) Arcana entomologica; or illustrations of new, rare, and interesting insects. Volume 1, No. 5. W. Smith, London, 73–80, pl. 17–20.
- Westwood JO (1845) Description of a new species of the Hymenopterous genus Aenictus, belonging to the Dorylidae. Journal and Proceedings of the Entomological Society of London1840–1846: 85.
- Westwood JO (1847) Description of the ‘Driver’ ants, described in the preceding article. Transactions of the Entomological Society of London 5: 16–18. doi: 10.1111/j.1365-2311.1847.tb01687.x
- Wetterer JK, Kronauer DJC, Borowiec ML (2012) Worldwide spread of Cerapachys biroi (Hymenoptera: Formicidae: Cerapachyinae). Myrmecological News 17: 1–4.
- Wheeler GC (1943) The larvae of the army ants. Annals of the Entomological Society of America 36: 319–332. doi: 10.1093/aesa/36.2.319
- Wheeler GC, Wheeler J (1964a) The ant larvae of the subfamily Cerapachyinae: supplement. Proceedings of the Entomological Society of Washington 66: 65–71.
- Wheeler GC, Wheeler J (1964b) The ant larvae of the subfamily Dorylinae: supplement. Proceedings of the Entomological Society of Washington 66: 129–137.
- Wheeler GC, Wheeler J (1973) Supplementary studies on ant larvae: Cerapachyinae, Pseudomyrmecinae and Myrmicinae. Psyche (Cambridge) 80: 204–211. doi: 10.1155/1973/67836
- Wheeler GC, Wheeler J (1984) The larvae of the army ants: a revision. Journal of the Kansas Entomological Society 57: 263–275.
- Wheeler GC, Wheeler J (1990) [‘1989’] Notes on ant larvae. Transactions of the American Entomological Society 115: 457–473.
- Wheeler WM (1902) An American Cerapachys, with remarks on the affinities of the Cerapachyinae. Biological Bulletin (Woods Hole) 3: 181–191. doi: 10.2307/1535872
- Wheeler WM (1903a) A decad of Texan Formicidae. Psyche (Cambridge) 10: 93–111. doi: 10.1155/1903/67840
- Wheeler WM (1903b) Some notes on the habits of Ceraphachys augustae. Psyche (Cambridge) 10: 205–209. doi: 10.1155/1903/64524
- Wheeler WM (1908) The polymorphism of ants. Annals of the Entomological Society of America 1: 39–69. doi: 10.1093/aesa/1.1.39
- Wheeler WM (1913) Zoological results of the Abor Expedition, 1911–1912, XVII. Hymenoptera, II: Ants (Formicidae). Records of the Indian Museum 8: 233–237.
- Wheeler WM (1914) Ants collected by W. M. Mann in the state of Hidalgo, Mexico. Journal of the New York Entomological Society 22: 37–61.
- Wheeler WM (1915a) Some additions to the North American ant-fauna. Bulletin of the American Museum of Natural History 34: 389–421.
- Wheeler WM (1915b) [‘1914’] The ants of the Baltic amber. Schriften der Physikalisch-Ökonomischen Gesellschaft zu Königsberg 55: 1–142.
- Wheeler WM (1916) The Australian ants of the genus Onychomyrmex. Bulletin of the Museum of Comparative Zoology 60: 45–54.
- Wheeler WM (1918) The Australian ants of the ponerine tribe Cerapachyini. Proceedings of the American Academy of Arts and Sciences 53: 215–265. doi: 10.2307/25129989
- Wheeler WM (1919) The ants of Borneo. Bulletin of the Museum of Comparative Zoology 63: 43–147.
- Wheeler WM (1920) The subfamilies of Formicidae, and other taxonomic notes. Psyche (Cambridge) 27: 46–55. doi: 10.1155/1920/14961
- Wheeler WM (1921) Observations on army ants in British Guiana. Proceedings of the American Academy of Arts and Sciences 56: 291–328. doi: 10.2307/20025856
- Wheeler WM (1922a) Ants of the American Museum Congo expedition. Bulletin of the American Museum of Natural History, New York, 1139 pp.
- Wheeler WM (1922b) The ants of Trinidad. American Museum Novitates 45: 1–16.
- Wheeler WM (1924a) The Formicidae of the Harrison Williams Galapagos Expedition. Zoologica (New York) 5: 101–122.
- Wheeler WM (1924b) On the ant-genus Chrysapace Crawley. Psyche (Cambridge) 31: 224–225. doi: 10.1155/1924/94380
- Wheeler WM (1925a) Zoological results of the Swedish Expedition to Central Africa 1921. Insecta. 10. Formicidae. Arkiv för Zoologi 17A(25): 1–3.
- Wheeler WM (1925b) The finding of the queen of the army ant Eciton hamatum Fabricius. Biological Bulletin (Woods Hole) 49: 139–149. doi: 10.2307/1536459
- Wheeler WM (1927) Chinese ants collected by Professor S. F. Light and Professor N. Gist Gee. American Museum Novitates 255: 1–12.
- Wheeler WM (1929) Ants collected by Professor F. Silvestri in Formosa, the Malay Peninsula and the Philippines. Bollettino del Laboratorio di Zoologia Generale e Agraria della Reale Scuola Superiore d’Agricoltura. Portici 24: 27–64.
- Wheeler WM (1930) Philippine ants of the genus Aenictus with descriptions of the females of two species. Journal of the New York Entomological Society 38: 193–212.
- Wheeler WM (1934) Neotropical ants collected by Dr. Elisabeth Skwarra and others. Bulletin of the Museum of Comparative Zoology 77: 157–240.
- Wheeler WM (1936) Ecological relations of ponerine and other ants to termites. Proceedings of the American Academy of Arts and Sciences 71: 159–243. doi: 10.2307/20023221
- Wheeler WM (1937) Ants mostly from the mountains of Cuba. Bulletin of the Museum of Comparative Zoology 81: 439–465.
- Wheeler WM, Chapman JW (1925) The ants of the Philippine Islands. Part I, Dorylinae and Ponerinae. Philippine Journal of Science 28: 47–73.
- Wheeler WM, Chapman JW (1930a) Aenictus (Typhlatta) alticola Wheeler and Chapman, sp. n. Pp. 205–206 in: Wheeler WM (1930) Philippine ants of the genus Aenictus with descriptions of the females of two species. Journal of the New York Entomological Society 38: 193–212.
- Wheeler WM, Chapman JW (1930b) Aenictus (Aenictus) aratus Forel subsp. nesiotis Wheeler and Chapman subsp. n. Pp. 208–209 In: Wheeler WM (1930) Philippine ants of the genus Aenictus with descriptions of the females of two species. Journal of the New York Entomological Society 38: 193–212.
- Wheeler WM, Chapman JW (1930c) Aenictus (Aenictus) aratus subsp. nesiotis var fraterculus Wheeler and Chapman, var. n. P. 209 In: Wheeler WM (1930) Philippine ants of the genus Aenictus with descriptions of the females of two species. Journal of the New York Entomological Society 38: 193–212.
- Wheeler WM, Chapman JW (1930d) Aenictus (Aenictus) piercei Wheeler and Chapman, sp. n. Pp. 209–210 in: Wheeler WM (1930) Philippine ants of the genus Aenictus with descriptions of the females of two species. Journal of the New York Entomological Society 38: 193–212.
- Wheeler WM, Chapman JW (1930e) Aenictus (Aenictus) powersi Wheeler and Chapman, sp. n. Pp. 210–212 In: Wheeler WM (1930) Philippine ants of the genus Aenictus with descriptions of the females of two species. Journal of the New York Entomological Society 38: 193–212.
- Wild AL (2007) A catalogue of the ants of Paraguay (Hymenoptera: Formicidae). Zootaxa 1622: 1–55.
- Willson SK, Sharp R, Ramler IP, Sen A (2011) Spatial movement optimization in Amazonian Eciton burchellii army ants. Insectes Sociaux 58: 325–334. doi: 10.1007/s00040-011-0171-0
- Willis EO, Oniki Y (1978) Birds and army ants. Annual Review of Ecology and Systematics 9: 243–263. doi: 10.1146/annurev.es.09.110178.001331
- Wilson EO (1957) The discovery of cerapachyine ants on New Caledonia, with the description of new species of Phyracaces and Sphinctomyrmex. Breviora 74: 1–9.
- Wilson EO (1958) Observations on the behavior of the cerapachyine ants. Insectes Sociaux 5: 129–140. doi: 10.1007/BF02222432
- Wilson EO (1959) Studies on the ant fauna of Melanesia. VI. The tribe Cerapachyini. Pacific Insects 1: 39–57.
- Wilson EO (1964) The true army ants of the Indo-Australian area (Hymenoptera: Formicidae: Dorylinae). Pacific Insects 6: 427–483.
- Wilson EO (1985) Ants of the Dominican amber (Hymenoptera: Formicidae). 2. The first fossil army ants. Psyche (Cambridge) 92: 11–16. doi: 10.1155/1985/63693
- Wiwatwitaya D, Jaitrong W (2011) The army ant Aenictus hottai (Hymenoptera: Formicidae: Aenictinae) and related species in Southeast Asia, with a description of a new species. Sociobiology 58: 557–565.
- Wong MKL, Guénard B (2016) Aenictus seletarius, a new species of hypogaeic army ant from Singapore, with an updated key to the Aenictus minutulus species group (Hymenoptera: Formicidae: Dorylinae) from Southeast Asia. Annales Zoologici (Warsaw) 66(1): 35–42. doi: 10.3161/00034541ANZ2016.66.1.002
- Xu Z-H (2000) Two new genera of ant subfamilies Dorylinae and Ponerinae (Hymenoptera: Formicidae) from Yunnan, China. Zoological Research 21: 297–302.
- Yamane S, Hashimoto Y (1999) A remarkable new species of the army ant genus Aenictus (Hymenoptera, Formicidae) with a polymorphic worker caste. Tropics 8: 427–432. doi: 10.3759/tropics.8.427
- Yek SH, Mueller UG (2011) The metapleural gland of ants. Biological Reviews 86: 774–791. doi: 10.1111/j.1469-185X.2010.00170.x
- Yoder MJ, Miko I, Seltmann KC, Bertone MA, Deans AR (2010) A gross anatomy ontology for Hymenoptera. PLoS ONE 5(12): e15991. doi: 10.1371/journal.pone.0015991
- Zettel H, Sorger DM (2010) Three new species of the army ant genus Aenictus Shuckard, 1840 (Hymenoptera: Formicidae: Aenictinae) from Borneo and the Philippines. Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 62: 115–125.
- Zhang W (1995) [‘1994’] A new species of Aenictus from Sichuan Province (Hymenoptera: Formicidae). In: Xi G, Liu Z, Xu S (Eds) Entomological research.First issue. A collection of research papers commemorating 40 years of teaching by Professor Zheng Zhemin. Shaanxi Normal University Press, Xi’an, 101–102. [In Chinese]
- Zhou S (2001) Ants of Guangxi. Guangxi Normal University Press, Guilin, China, 255 pp. [In Chinese]
- Zhou S, Chen Z (1999) The ant genus Aenictus Shuckard from Guangxi (Hymenoptera: Formicidae). Guangxi Sciences 6(1): 63–64. [In Chinese]
Appendix
Anatomical terms used here as annotated by Hymenoptera Anatomy Ontology (HAO). The first column provides the name of the term, the second is the HAO definition, and the third column provides a Uniform Resource Identifier (URI) that is a link to concept definition.
| Term | Definition | URI |
|---|---|---|
| abdomen | The tagma that is located posterior to the thorax. | http://purl.obolibrary.org/obo/HAO_0000015 |
| abdominal segment | The segment that is located posterior to the head and does not bear legs. | http://purl.obolibrary.org/obo/HAO_0000016 |
| abscissa | The wing vein that is delimited by its intersection with two other abscissae. | http://purl.obolibrary.org/obo/HAO_0000076 |
| anal vein | The wing vein that articulates proximally with the third axillary sclerite and is located just posteriorly of the claval fold. | http://purl.obolibrary.org/obo/HAO_0000093 |
| annulus | The sclerite that is ring-like and is not attached to muscles. | http://purl.obolibrary.org/obo/HAO_0000096 |
| antenna | The appendage that is composed of ringlike sclerites and the anatomical structures encircled by these sclerites and that is articulated with the cranium. | http://purl.obolibrary.org/obo/HAO_0000101 |
| antennal scrobe | The scrobe that is located dorsally of the antennal foramen and is for the reception of the antenna. | http://purl.obolibrary.org/obo/HAO_0001432 |
| antennal segment | The antennomere that is an appendage segment. | http://purl.obolibrary.org/obo/HAO_0000104 |
| apodeme | The process that is internal. | http://purl.obolibrary.org/obo/HAO_0000142 |
| basitarsus | The tarsomere that is the proximal most annulus of the tarsus, connected proximally with the tibia and distally with the second tarsomere via membranous conjunctivae. | http://purl.obolibrary.org/obo/HAO_0000178 |
| body | The anatomical cluster that is composed of the whole organism but which excludes the antennae, legs and wings. | http://purl.obolibrary.org/obo/HAO_0000182 |
| carina | The process that is elongate and external. | http://purl.obolibrary.org/obo/HAO_0000188 |
| cell | The wing membrane that is delimited by wing veins. | http://purl.obolibrary.org/obo/HAO_0001091 |
| cercus | The sense organ that located apicolaterally on one of the apicalmost abdominal terga and attaches to a large nerve cord. | http://purl.obolibrary.org/obo/HAO_0000191 |
| clypeus | The area that corresponds to the site of origin of the clypeo-epipharyngeal muscle. | http://purl.obolibrary.org/obo/HAO_0000212 |
| costa | The wing vein that is anterior to the subcosta and is connected to the humeral plate. | http://purl.obolibrary.org/obo/HAO_0000225 |
| coxa | The leg segment that is connected to the body and to the trochanter via conjunctivae and muscles. | http://purl.obolibrary.org/obo/HAO_0000228 |
| crest | The projection that is lamella-like and is located on a rim, carina, apodeme or edge. | http://purl.obolibrary.org/obo/HAO_0000344 |
| cubital vein | The longitudinal vein that is posterior to the marginal vein. | http://purl.obolibrary.org/obo/HAO_0000237 |
| cupula | The sclerite that is connected via conjunctiva and attached via muscles to abdominal tergum 9 and the gonostyle/volsella complex. | http://purl.obolibrary.org/obo/HAO_0000238 |
| cuticle | The acellular anatomical structure that is the external layer of the integument (covers the entire body surface as well as lines ectodermal invaginations such as the stomodeum, proctodeum and tracheae) and produced by the epidermal cells. | http://purl.obolibrary.org/obo/HAO_0000240 |
| depression | The area that is external, concave, point-like and does not correspond to an apodeme. | http://purl.obolibrary.org/obo/HAO_0000241 |
| edge | The margin that extends along the border of two areas that are oriented differently. | http://purl.obolibrary.org/obo/HAO_0000285 |
| eye | The compound organ that is composed of ommatidia. | http://purl.obolibrary.org/obo/HAO_0000217 |
| flange | The projection that is lamella-like and is located on a rim, carina, apodeme or edge. | http://purl.obolibrary.org/obo/HAO_0000344 |
| foramen | The anatomical space that is surrounded by sclerites and allows for the passage of haemolymph, nerves and tracheae. | http://purl.obolibrary.org/obo/HAO_0000345 |
| fore wing | The wing that is located on the mesothorax. | http://purl.obolibrary.org/obo/HAO_0000351 |
| gaster | The anatomical cluster that is composed of all abdominal segments posterior to abdominal segment 2. [note: in ants, this definition is applied to all segments posterior to segment 2 or to all segments posterior to segment 3 if the latter is small and differentiated into a postpetiole] | http://purl.obolibrary.org/obo/HAO_0000369 |
| gena | The area that is delimited by the intersection of the interorbital plane, the margin of the compound eye, the margin of the oral foramen, the occipital carina and the malar sulcus. | http://purl.obolibrary.org/obo/HAO_0000371 |
| genitalia | The anatomical system that is involved in copulation, fertilization and/or oviposition. | http://purl.obolibrary.org/obo/HAO_0000374 |
| groove | The line that is located on the sclerite and is impressed. | http://purl.obolibrary.org/obo/HAO_0001525 |
| head | The tagma that is located anterior to the thorax. | http://purl.obolibrary.org/obo/HAO_0000397 |
| head capsule | The anatomical cluster that is composed of the cranium and compound eye cuticle. | http://purl.obolibrary.org/obo/HAO_0000398 |
| hind wing | The wing that is located on the metathorax. | http://purl.obolibrary.org/obo/HAO_0000400 |
| hypopygium | The abdominal sternum that is located on abdominal segment 7. | http://purl.obolibrary.org/obo/HAO_0000044 |
| jugal lobe | The wing region that is delimited by the plica jugalis. | http://purl.obolibrary.org/obo/HAO_0000445 |
| labial palp | The anatomical structure that is distal to the proximal margin of the first sclerite of the labial palp. | http://purl.obolibrary.org/obo/HAO_0000450 |
| labium | The appendage that is encircled by the area that is proximally delimited by the lateral margins of the cardo and the posterior stipital sclerite laterally, and the anatomical line that is tangential to the salivary duct and traverses the salivary orifice anteriorly. | http://purl.obolibrary.org/obo/HAO_0000453 |
| labrum | The sclerite that is situated along the distal margin of the clypeus and is connected along its proximal margin with the distal margin of the epipharyngeal wall. | http://purl.obolibrary.org/obo/HAO_0000456 |
| lamella | The process that is elongate and external. | http://purl.obolibrary.org/obo/HAO_0000188 |
| leg | The appendage that is composed of the coxa and all distal leg segments and is connected to the pectus. | http://purl.obolibrary.org/obo/HAO_0000494 |
| line | The anatomical structure that is linear. | http://purl.obolibrary.org/obo/HAO_0001586 |
| lobe | The evagination that is mostly membranous. | http://purl.obolibrary.org/obo/HAO_0001587 |
| longitudinal vein | The anatomical cluster that is composed of abscissae extending along the long axis of the wing. | http://purl.obolibrary.org/obo/HAO_0000501 |
| mandible | The appendage that is encircled by one sclerite that is connected to the cranium proximolaterally and to the maxillo-labial complex proximomedially via conjunctivae and articulates with the cranium via the anterior and posterior cranio-mandibular articulations. | http://purl.obolibrary.org/obo/HAO_0000506 |
| maxilla | The appendage that is encircled by the area that is proximally delimited by the hypostoma posteriorly, the median margin of the mandible laterally, the labrum anterolaterally and the labium medially. | http://purl.obolibrary.org/obo/HAO_0000513 |
| maxillary palp | The anatomical structure that is distal to the proximal margin of the first sclerite of the maxillary palp. | http://purl.obolibrary.org/obo/HAO_0000515 |
| mesonotum | The area that is limited anteriorly by the pronotum, laterally by the basalare, axillary sclerites, subalare and the mesopectus and posterolaterally by the mesopostnotum and the metanotum. | http://purl.obolibrary.org/obo/HAO_0000556 |
| mesopleuron | The lateral (vertical) area that is anterior to the mesometapleural sulcus and posterior to the pronotum. | http://purl.obolibrary.org/obo/HAO_0002363 |
| mesosoma | The anatomical cluster that is composed of the prothorax, mesothorax and the metapectal-propodeal complex. | http://purl.obolibrary.org/obo/HAO_0000576 |
| metafurca | The furca that is located medioventrally on the metapectus. | http://purl.obolibrary.org/obo/HAO_0000592 |
| metanotal depression | The transverse, dorsal groove on the mesonoto-metanoto-mesopecto-metapecto-propodeal complex posterior to the pronotal spiracle and anterior to the propodeal spiracle. | http://purl.obolibrary.org/obo/HAO_0002362 |
| metapleuron | The lateral (vertical) area that is posterior to the mesometapleural sulcus and anterior to the metapleural carina. | http://purl.obolibrary.org/obo/HAO_0002360 |
| metasoma | The tagma that is connected anteriorly to the metapectal-propodeal complex at the propodeal foramen and consists of abdominal segments. | http://purl.obolibrary.org/obo/HAO_0000626 |
| metathorax | The thoracic segment that is located between the mesothorax and the first abdominal tergum and is delimited by the metanotum and the metapectus. | http://purl.obolibrary.org/obo/HAO_0000630 |
| mouthparts | The anatomical cluster that is composed of the labrum, epipharyngeal wall, hypopharyngeal wall (including the sitophore), mandibles, maxillae, labium and conjunctivae connecting them. | http://purl.obolibrary.org/obo/HAO_0000639 |
| notch | The part of the margin of a sclerite that is concave. | http://purl.obolibrary.org/obo/HAO_0000648 |
| occipital foramen | The foramen that is delimited dorsally by the postocciput. | http://purl.obolibrary.org/obo/HAO_0000347 |
| ocellus | The multi-tissue structure that is located on the top of the head, composed of the corneal lens, pigment cell, rhabdoms and synaptic plexus. | http://purl.obolibrary.org/obo/HAO_0000661 |
| ommatidium | One of the facets of the compound eye. | http://purl.obolibrary.org/obo/HAO_0000666 |
| palp | The anatomical cluster that is composed of the palpal segments. | http://purl.obolibrary.org/obo/HAO_0000683 |
| patch | The area that is round and differs from surrounding regions in sculpture, setae, and/or pigmentation. | http://purl.obolibrary.org/obo/HAO_0000704 |
| penisvalva | The sclerite that is in the middle of the external male genitalia, surrounds the distal part of the ductus ejaculatorius and the endophallus. | http://purl.obolibrary.org/obo/HAO_0000707 |
| posttergite | The area that corresponds to the narrow postcostal lip of a postnotal thoracic plate. | http://purl.obolibrary.org/obo/HAO_0000797 |
| prementum | The sclerite that is median, is connected via conjunctiva along its proximolateral margins to the stipites, is articulated with the labial palps, is continuous along its distal margin with the ligula and distolateral margins with the distal hypopharynx and receives the site of attachments of the extrinsic labial palp muscles. | http://purl.obolibrary.org/obo/HAO_0000804 |
| process | The area on the sclerite that is raised. | http://purl.obolibrary.org/obo/HAO_0000822 |
| pronotum | The notum that is located in the prothorax. | http://purl.obolibrary.org/obo/HAO_0000853 |
| propodeal spiracle | The abdominal spiracle that is the anteriormost in the body. | http://purl.obolibrary.org/obo/HAO_0000329 |
| propodeum | The tergum that is located on abdominal segment 1. | http://purl.obolibrary.org/obo/HAO_0000051 |
| prora | The carina that is transverse and is located anterior to the antecostal sulcus on the abdominal sternum 3. | http://purl.obolibrary.org/obo/HAO_0001341 |
| pterostigma | The patch on the wing that is sclerotized and is on the anterior margin of the fore wing. | http://purl.obolibrary.org/obo/HAO_0000957 |
| pulvillum | The area that is located laterally on the pretarsus and arises beneath the proximal part of the tarsal claw. | http://purl.obolibrary.org/obo/HAO_0000884 |
| ridge | The process that is elongate and external. | http://purl.obolibrary.org/obo/HAO_0000188 |
| row | The anatomical cluster that is composed of repeated units of anatomical structures. | http://purl.obolibrary.org/obo/HAO_0000901 |
| scape | The antennal segment that is proximal to the pedicel and is connected to the head via the radicle. | http://purl.obolibrary.org/obo/HAO_0000908 |
| sclerite | The area of the cuticle that is strongly sclerotised, with thick exocuticle and is surrounded by conjunctivae. | http://purl.obolibrary.org/obo/HAO_0000909 |
| scrobe | The area that is impressed and is for the reception or concealment of another sclerite. | http://purl.obolibrary.org/obo/HAO_0000912 |
| segment | An anatomical structure that is metameric and is connected to other metameric subdivisions by muscles and is delimited by its sclerites. | http://purl.obolibrary.org/obo/HAO_0000929 |
| spiracle | The anatomical cluster that is composed of the distal end of the trachea and the margin of the sclerite or conjunctiva surrounding the spiracular opening. | http://purl.obolibrary.org/obo/HAO_0000950 |
| spur | The process that is surrounded by conjunctiva and evaginated and that is basally sclerotized. | http://purl.obolibrary.org/obo/HAO_0000951 |
| sternite | The sclerite that is located on the sternum. | http://purl.obolibrary.org/obo/HAO_0000955 |
| stipes | The appendage that is connected posteroproximally to the hypostoma, anteroproximally and lateroproximally to the mandible and medioproximally to the labium and the hypopharynx via conjunctiva, is connected to the cranium via muscles and that bears the maxillary palp. | http://purl.obolibrary.org/obo/HAO_0000958 |
| tarsus | The leg segment that is apical to the tibia. | http://purl.obolibrary.org/obo/HAO_0000992 |
| tegula | The sclerite that is located laterally of the preaxilla and obscures the anterior mesonoto-first axillary articulation and the mesopleuro-second axillary sclerite joints. | http://purl.obolibrary.org/obo/HAO_0000993 |
| tergite | The sclerite that is located on the tergum. | http://purl.obolibrary.org/obo/HAO_0001005 |
| tibia | The leg segment that is proximal to the tarsus and distal to the femur. | http://purl.obolibrary.org/obo/HAO_0001017 |
| tooth | The projection that is located distally on the mandible. | http://purl.obolibrary.org/obo/HAO_0001019 |
| venation | The anatomical cluster that is composed of abscissae. | http://purl.obolibrary.org/obo/HAO_0001096 |
| volsella | The anatomical cluster that is composed of the sclerites on the ventral part of the male genitalia that are not connected to the cupula via muscles. | http://purl.obolibrary.org/obo/HAO_0001084 |
| wing | The appendage that is between the notum and the pectus and is connected to the body by the axillary sclerite muscles. | http://purl.obolibrary.org/obo/HAO_0001089 |