ERYTHROPOIESIS
It is believed that the
pluripotential hematopoietic stem cell is induced by certain microenvironmental
influences to become the committed erythroid progenitor cell which is sensitive
to erythropoeitic stimulation. In response to a hormone, erythropoietin, the
committed progenitor cells in the marrow undergoes mitosis and one or both
daughter cells enter the erythroid maturation sequence.
The committed progenitor
cell gives rise by mitosis to immature erythroblasts which are capable of
further division and at the same time mature into semi–mature erythroblasts.
These in turn develop into mature erythroblast which on losing their nuclear
substance by dissolution from reticulocytes. The maturation sequence from
rubriblast to reticulocyte in the bone marrow takes approximately seven days.
The reticulocyte then remains another three and a half days or go into the bone
marrow before issuing into the bloodstream where it remains recognizable as a
reticulocyte for one or more day before discharging its oxygen–transporting
function as a normal erythrocyte.
ERYTHRON
The term “erythron” has
been applied to the single functional entity composed of red cells and their
precursors. This includes the normoblasts at all stage of maturation, the
reticulocytes as well as the erythroid–committed stem cells and the circulating
erythrocytes. The interstitial tissue of the erythron is represented by the
plasma and the fat and reticulum of the bone marrow.
Nutritional requirements
for red cell production
a. Proteins and
amino acids
b. Vitamins –
Vitamin B12, Folic acid, Vitamin B6, riboflavin,
panthotenic acid, nicotinic acid, ascorbic acid.
c. Minerals
Iron metabolism
The metabolism of iron is
dominated by its role in hemoglobin synthesis. When synthesis is complete, the
iron now in the form of hemoglobin in mature red cells is delivered to the
circulation. At the end of the 120 days life span, the red cells are engulfed
by macrophages of the reticuloendothelial system (RES) where the iron is
extracted from the hemoglobin. Some of this iron may remain stored in the RES
as ferritin of hemosiderin, but most is delivered to the plasma where it
becomes bound to transferrin, completing the iron cycle.
Control of
erythropoiesis
Alterations in the
concentration of hemoglobin in the blood lead to charges in tissue oxygen
tension within the kidney. In response to hypoxia, the kidney secretes a factor
that interacts with plasma substrate to produce a hormone, erythropoietin. This
hormone induces primitive marrow cells to differentiate into pronormoblasts,
thereby bringing about expansion of the erythroid marrow and an increase in red
cell production. This in turn leads to an increase in the size of the erythron
and increase in tissue oxygen levels.
Synthesis of Hemoglobin
– Heme (iron) plus Globin (protein)
a. Heme synthesis
occurs in most red cells of the body, except the mature erythrocyte, but most
abundantly in the erythroid precursor.
b. Globin synthesis
occurs in the cytoplasm of the erythroblast and reticulocyte.
Hemoglobinization
occurs while the erythrocyte is developing in the bone marrow and still
possesses a nucleus. It usually begins during the latter half of the
polychromatophilic normoblast stage. Hemoglobinization is completed while the
cell still possesses a nucleus. However, it can continue in the reticulocyte
stage, although the rate of synthesis is greatly reduced because there is no
nucleus.
Structure of
erythrocytes
The mature erythrocytes are
6 to 8 micrometers in diameter. They have a thickness of 1.5 to 2.5 micrometers
(average of 2), a corpuscular volume of 75 to 95 femtoliters (average is 87)
and a surface area of 130 to 150 square micrometers (average is 135).
Under the microscope,
unstained red cells have light greenish yellow color. Red cells stain a buff of
reddish color. Their shape is that of a biconcave disc, thus called discocyte
causing the cells to appear lighter in the center than in the periphery. Some
of the cells may have cup or spherical shapes. The shape of erythrocytes can
change tremendously as they pass through the capillaries. Actually, red blood
cells look like a “bag” that can be deformed into almost any shape.
Composition of
erythrocytes
1. 60% water and 40%
solids
2. Hemoglobin – iron
bearing protein which serves as the most important agent in the erythrocyte
3. Red cell membrane
which is composed of:
a. Stroma – the innermost structure of the erythrocyte
which is composed primarily of protein and lipids. The hemoglobin attaches
itself in an interlacing manner to the stroma.
b. Membrane – composed of lipoproteins. This membrane is
extremely thin and pliable. It is a dynamic semi–permeable one, retaining
potassium in high concentration within the cell and excluding sodium, while
allowing hydrogen, chloride and bicarbonate ions to pass freely into or out of
the cell in proportion to the ionic gradient.
4. Red cell enzymes
a. Carbonic anhydrase
b. Methemoglobin reductase
c. Catalase
d. Glucose–6–phosphate dehydrogenase
e. Pyruvate kinase
f.
Adenosine
deaminase
g. Aldolase
h. Lactic dehydrogenase
i.
Glutathione
reductase
j.
Nucleoside
phosphorylase
k. Acetylcholinesterase
Functions of
erythrocytes
1. To mediate the
exchange of respiratory gases, oxygen and carbon dioxide between the lungs and
tissues.
2. To control the
blood pH to assist in the maintenance of acid–base equilibrium
Erythrocyte destruction
The erythrocyte gradually
undergoes metabolic changes over the course of its 120 day life span, at which
time the less viable senescent cell is removed from the circulation. Certain
glycolytic enzymes diminish in activity as the cell ages. Older red cells have
a smaller surface area and in increased MCHC compared with younger cells.
Changes in the cell surface may render the cell more liable to phagocytosis.
The exact mechanism by which senescent erythrocytes are recognized and removed
by the reticuloendothelial system is unknown. The process may be phagocytosis
of whole erythrocytes or fragmenting senescent cells.
Mechanism of red cell
destruction
1. Fragmentation
– loss of a portion of the
erythrocytes membrane, accompanied by loss of cellular contents, including
hemoglobin
2. Osmotic
lysis – the passing of water into the
red cell on such a scale as to ultimately burst it (hemolysis)
3. Electrophagocytosis – ingestion of whole red cells by circulating
monocytes or neutrophils or by fixed macrophages of the reticuloendothelial
system (RES)
4. Complement
induced cytolysis – complement has
the ability to attach to the cells and induce lysis. This is the usual event
that triggers cellular fixation of complement, that is the reaction between the
cellular antigen and a humoral factor.
5. Hemoglobin
denaturation – when erythrocytes are
exposed to oxidant stress and the mechanism to protect the cell from such
damage fails to work, denatured hemoglobin precipitates forming inclusion
bodies known as Heinz bodies.
Site of erythrocyte
destruction
1. Intravascular
hemolysis – lysis of erythrocytes
occurs within the circulation, through the classic pathway. It is the usual outcome
of sensitization of erythrocytes with complement
2. Extravascular
hemolysis – lysis of erythrocyte
outside the circulation but in the reticuloendothelial cells of the liver and
spleen. This usually happens through phagocytosis
Intra and extracellular
hemolysis
1. Intracellular
or intracorpuscular hemolysis – lysis
of the red cells is due to intracorpuscular defects like abnormalities in RBC
membrane, deficiency in enzymes, abnormalities in synthesis of hemoglobin.
2. Extracellular
or extracorpuscular hemolysis – lysis
of the red cells is due to extracorpuscular defects or factors like infectious
agents, chemicals, drugs, antibodies, venous factors, physical agents, etc.
Erythrocyte count
The erythrocytes occupy
the largest function of the formed elements of the blood. As the body functions
normally, the blood count remains stable, but physiologic as well as pathologic
conditions alter the red blood cell count.
Adult male 4,500,000 – 6,500,000 4.5 – 6.5 x 1012/L
Adult female 4,000,000 – 5,000,000 4 – 5 x 1012/L
Infants 4,000,000 – 6,000,000 4 – 6 x 1012/L
Children 4,000,000 – 5,700,000 4 – 5.7 x 1012/L
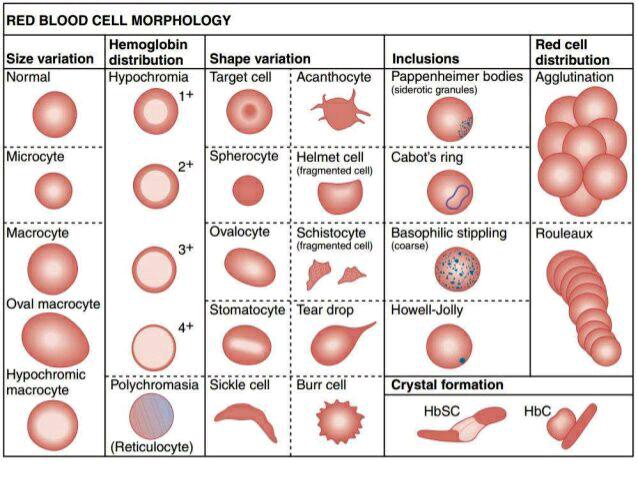
RED CELL ABNORMALITIES
In diseases, erythrocytes
vary in their hemoglobin content, size, shape, staining properties and
structure
A. Variation
in hemoglobin content
Irregularities
in hemoglobin distribution are usually due to the shape of the cell and to
degenerative changes or abnormalities of cell formation particularly hemoglobin
synthesis
1. Normochromic
cell – refers to erythrocyte with
normal amount of hemoglobin
2. Hypochromic cell
– refers to erythrocyte wherein the central light area of the cell is larger
and paler than normal
a. Hypochromia in erythrocytes with normal size indicates
that there is less than the normal amount of hemoglobin present in the cell.
b. Hypochromia in erythrocytes with larger than normal
size (macrocytes) indicates that a normal amount of hemoglobin may be present
due to the increased size of the cells, a hypochromic effect is still produced.
Hypochromia is found in
Iron deficiency anemia In parasitism
Hemorrhages In
case of malignancy
Thalassemia Sideroblastic
anemia
3. Hyperchromic
cell – refers to erythrocyte which
has an increased hemoglobin content and wherein the central light area is
smaller than normal or non–existent.
In
spherocytosis, the cells are hyperchromic, though the hemoglobin content is
normal, the hemoglobin concentration is increased due to reduced surface /
volume ratio.
Hyperchromia is seen in:
Megaloblastic anemia Severe
diarrhea
Hereditary spherocytosis Heart
disease
4. Anisochromia
– a condition wherein both
hypochromic and normochromic cells are present in the same blood film. It is
sometimes called dimorphic anemia. This is found in:
Sideroblastic anemia
Iron deficiency anemia after iron therapy
B. Variation
in staining property
1. Polychromatophilia
or polychromasia or diffuse basophilia
This
is a condition wherein the red cells are stained with various shades of blue
with tinges of pink. This is due to the combination of the affinity of
hemoglobin to acid stain and the affinity of ribonucleic acid (RNA) to the
basic dye. This is a characteristic of young or immature red cells with
residual RNA; cells are larger than normal and correspond to reticulocytes.
This condition is found in:
Reticulocytosis Acute
blood loss
Hemolysis Pernicious
anemia and other anemia
Punctuate basophilia or basophilic stippling of RBC
This
is a special form of polychromasia in which basophilic granules usually
isolated either fine or sometimes coarse appear in the cytoplasm of
erythrocytes. This is due to vacuolar degeneration of the polychromatic
substance of the cytoplasm; the stippling probably represents aggregated RNA.
This is found in:
Toxic anemia Lead
poisoning
Hemolytic anemia Thalassemia
Leukemia Other
congenital forms of anemia
2. Hypochromasia – a condition wherein the red cells stain usually
palely. There are two possible causes: a lowered hemoglobin concentration and
abnormal thinness of the red cells. This is found in:
Iron deficiency anemia Sideroblastic
anemia
Thalassemia
3. Hyperchromasia – a condition wherein the red cells are stained
deeply due to abnormal thickness of the cells. This is found in:
Macrocytosis Megaloblastic anemia
Spherocytosis
4. Anisochromia – a condition wherein a proportion only of the red
cells stains palely, and can be found in:
Iron deficiency anemia responding to
iron therapy
Sideroblastic anemia
5. Anulocyte
or “ring” cells – these are thin
cells with low hemoglobin content and are represented by thin stain ring
surrounding a large central space.
C. Variation
in size
1. Anisocytosis – a condition wherein the red cells vary in size,
both macrocytes and microcytes coexist in the same smear
2. Normocyte – 6 – 8 micrometers in diameter; this is an
erythrocyte with a normal size
3. Macrocyte – cell with 10 – 12 micrometers in diameter;
characteristic of young red cell of skipped generation with early loss of
nucleus. The cell is well filled with hemoglobin.
This is found in: pernicious anemia, aplastic anemia,
cirrhosis of the liver
4. Microcyte – cell with less than 5 micrometers in diameter; this
is small and round red cell, and is formed as such, or results from
fragmentation. Microcytosis is found in:
Iron deficiency anemia Hemolytic anemia
Thalassemia Sideroblastic anemia
5. Megalocyte – large oval–shaped red cell with over 12 micrometers
in diameter with impaired DNA synthesis.
It is found in:
Megaloblastic anemias like pernicious anemia or
Vitamin B12 deficiency anemia,
Folic acid deficiency anemia
D.
Variation
in shape
1. Poikilocytosis
– condition wherein the red cells
exhibit variation in shape
2. Discocyte – normal cell with biconcave disc shape
3. Target
cell – also known as leptocyte or
Mexican hat cell or cell with a Bull’s eye appearance
–
this is an erythrocyte with a distinct peripheral and central zone of
hemoglobin and annular area or
pallor in between.
– target cells are thought to result
from cells having a surface which is
disproportionately large
compared with their volume. This
leptocytosis is found in
thalassemia, after splenectomy, chronic liver
disease, iron deficiency anemia, certain
hemoglobinopathies
4.
Elliptocyte – also known as ovalocyte. Found in:
Healthy persons
Megaloblastic anemia
Hypochromic anemia
5.
Sickle cell – crescent shaped cell due to abnormal aggregation of
HbS which gives
a tendency for the cell
to assume a sickle shape in deprivation of
oxygen. Also known as Drepanocyte
or meniscocyte. Found in sickle
cell anemia and sickle
cell trait which is common among Negroes.
6.
Spherocyte – a special form of microcyte approximately 6
micrometer in
diameter which is more spheroidal
than normal red cell. This results
from genetic defects of
the red cell membrane. It is found in
hereditary hemolytic
anemia
7.
Schistocytes – fragmented or greatly distorted red cells which are
helmet or
triangular or irregular
in shape; red blood cells lose fragments after
impact with fibrin strands,
walls of diseased blood vessel and artificial
surfaces in the
circulation
8.
“Tear
drop” cell – red blood cell which has
a shape resembling a drop, a type of
distorted or
Fragmented RBC; found in myelofibrosis and
thalassemia
9.
Fragmentocytes
– fragmented red cells which are
sometimes called “eggshells”;
found in diseases
involving intravascular clotting.
10. Stomatocytes
– red cells in which the central biconcave area appears slit–like in
dried films. In Wet preparations, the cells are cup–shaped;
particularly present in blood smears of Australian or
Mediterranean
origin; found in alcoholic cirrhosis and
other liver
diseases, hereditary hemolytic anemia
11. Pyknocyte –
distorted and contracted red blood cell similar to echinocyte; found
in infantile
pinocytosis.
12. Spiculed cells
a. Crenated
cells – red cells which develop many
or numerous projections from their surface; crenation can result from many
causes, e.g., by washing red cells free from plasma and suspending them in NSS
between glass surfaces; crenation may also result from suspension of cells in
hypertonic solution; can be found in uremia.
b. Echinocytes – also called “sea–urchin” cells or burr cells; small
cells or cell fragments bearing 10 to 30 spines or spicules evenly distributed
over the surface of the cells; echinocytes may be caused by accumulation of
fatty acid or lyso– lecithin on RBC surface; found in uremia, hemolytic anemia,
post splenectomy, pyruvate kinase deficiency.
c. Acanthocytes – also known as spur cells; red blood cells with
spiny projections of various lengths and irregular spacing; have 5 to 10
spicules; abnormality is due to increased ratio of cholesterol/lecithin on the
RBC membrane; found in liver disease, hemolytic anemia, post splenectomy,
pyruvate kinase deficiency.
E. Variation
due to presence of erythrocytic inclusion bodies
1. Malarial
stippling – fine granular appearance
of erythrocytes that harbor malarial parasites
Schuffner’s granules (P.vivax)
Maurer’s dots (P. falciparum)
Zieman’s dots (P. malariae)
2. Punctuate
basophilia – refers variation in
staining
3. Siderocyte – mature red cells with iron particles in the ferric
form that give a positive reaction to Perl’s reaction or Prussian blue
reaction; indicative of faulty iron utilization in the synthesis of hemoglobin;
found in hemolytic anemia and chemical poisoning
4. Sideroblast – nucleated red cell containing siderotic granules;
sometimes the granules may be arranged around the nucleus in the form of a ring
and the cells are then called “ringed sideroblast”
5. Semi–lunar
bodies – also known as achromocyte or
crescent bodies; pale bluish–pink non–granular structures which are half-moon
shaped; these bodies are degenerative remains or smudges of the RBC stroma;
maybe found in malaria or hemolytic anemia
6. Howell–Jolly
bodies – single or double bud bodies
of varying sizes; these bodies are nuclear remnants containing DNA; these are
indicative of rapid blood regeneration; maybe found in megaloblastic anemia,
hemolytic anemia, leukemia, after splenectomy
7. Magraliano
body – vacuole–like or elliptical
body found in the center or periphery of the cell
8. Cabot
rings – looped or figure of 8 shape
or ring with red–purple color; ring is composed of fine granules arranged in a
linear pattern; represent particles of DNA probably attached to endoplasmic
reticulum; indicative of defective of regenerative activity
9. Heinz or
Ehrlich bodies – intracorpuscular
aggregates of denatured hemoglobin (especially globin residue); small round
inclusions with intense blue color located close to the cell membrane or eve
outside it; need supravital staining with methylene blue, brilliant cresyl blue
or crystal violet; indicative of disturbed hemoglobin synthesis and breakdown
10. Pappenheimer bodies – basophilic iron containing granules found in red cells stained with
a Romanowsky dye, e.g. Wright’s stain; indicative of abnormal utilization of
iron in the synthesis of hemoglobin
11. Hemoglobin H inclusions – caused by instability of HbH
12. Hemoglobin Zurich inclusions – found in drug induced conditions; infections
F. Miscellaneous
variation
1. Rouleaux
formation – alignment of red cells
one on top of the other forming an arrangement resembling stacks of coins.
2. Crenated
erythrocytes – cells with puckered
outer edges found in blood smears which dry slowly
3. Partially hemolyzed
RBC – slightly colored and malformed
RBC caused by moisture on the slide prior to smear making

No comments:
Post a Comment