Hornera frondiculata ( Lamarck, 1816 )
|
publication ID |
https://doi.org/ 10.5252/zoosystema2020v42a27 |
|
publication LSID |
urn:lsid:zoobank.org:pub:08634080-E772-41F1-BDA7-D1AAD200A2BF |
|
DOI |
https://doi.org/10.5281/zenodo.4327913 |
|
persistent identifier |
https://treatment.plazi.org/id/2910754F-A809-272F-FF14-6BC58230F81F |
|
treatment provided by |
Felipe |
|
scientific name |
Hornera frondiculata ( Lamarck, 1816 ) |
| status |
|
Hornera frondiculata ( Lamarck, 1816) View in CoL
( Figs 1-4 View FIG View FIG View FIG View FIG ; Tables 1-3 View TABLE View TABLE View TABLE )
Retepora frondiculata Lamarck, 1816: 182 .
Hornera frondiculata Lamouroux, 1821: 41 View in CoL , pl. 26, fig. 1, pl. 74, figs 7-9 – Milne-Edwards 1838: 17, pl. 9, fig. 1 – Alder 1864: 109, pl. 5, fig. 7 – Heller 1867: 124 – Busk 1875: 17, pl. 20, figs 1-3, 6 – Calvet 1902: 43 – Waters 1904: 94, pl. 91, fig. 3 – Canu & Bassler 1930: 86, pl. 12, figs 15-16 – Neviani 1939: 69 – O’Donoghue & de Watteville 1939: 8 – Gautier 1955: 268, pl. 4, figs 31-32. – Lagaaij & Gautier 1965: chart 1 – Cook 1968: 238 (part: Mediterranean specimens) – Harmelin 1968: 1187; 1976: 223, table I, 229, table III – Mongereau 1972: 329 (part), pl. 5, figs 1-3 – Zabala 1986: 686, fig. 213 – Zabala 1993: 571, fig. 3 – Rosso 1987: 173, 175, 180-181, 188-189, fig. 6; 1996: 209, table 5, pl. 1c, g; 2005: 263, table 3; 2009: 134, 4 figs (not numbered) – Di Geronimo et al. 1988: 703, table 1; 1993: 89, 92, tables 2, 3, pl. IX, fig. C; 1994, table 3; 1997: 200, table 3; 1998: 248, table 1; 2003: 135, table 2; 2005: 73, table 4 – Zabala & Maluquer 1988: 182, pl. 36, figs 17-18 – Costa et al. 1991: 418, table 2 – Moissette & Spjeldnaes 1995: 786, pl. 2, figs 5-6 – Novosel 2005: 236, fig. 10 – Ballesteros 2006: 156, fig. 17A. – Smith et al. 2008: 371, 388, fig. 2A – Souto et al. 2010: 38 (list) – Belbacha et al. 2011: 46, fig. 53 – Weinberg 2013: 325 – Abdelsalam 2014: 271, fig. 2 – Rosso et al. 2013: 169, table 1 – Gerovasileiou & Rosso 2016: 36 View Cited Treatment (list) – Rosso & Di Martino 2016: 570 – Achilleos et al. 2020: 233, table 1.
Hornera lichenoides View in CoL (L., 1758) – Laubier 1966: 223: table – Argyrou et al. 2002, figs 5-29 (dubious identification).
Hornera violacea Sars, 1863 – Calvet 1902: 44 (erroneous identification).
Hornera caespitosa Busk, 1875 View in CoL – Calvet 1906: 478, pl. 30, figs 11- 12 (erroneous identification).
TYPE LOCALITY. — Mediterranean Sea.
MATERIAL EXAMINED. — France • several large colonies (up to 11 cm wide); Marseille, South Riou Island; Stn JGH-71.34; 70 m; 3.VII.1971; coarse DC; Dre; with H. mediterranea n. sp.; JGH leg . • 2 large colonies (up to 13 cm wide); Marseille , Grand Conglue Is.; 48 m; 7.IX.1968; COR; Div; JGH leg . • several colonies; Mar- seille, South Riou Island, Impérial du large; Stn JGH-68.33; 65 m; 11.VI.1968; COR; Div; JGH leg . • several colonies; Marseille, South Riou Is.; Stn JGH-72.8; 90 m; 2.III.1972; silted DC; Dre; JGH leg . • fragments; South Riou Is.; Stn JGH-73.9; 90-100 m; 6.IV.1973; Dre; with H. mediterranea n. sp.; JGH leg . • 1 colony; Marseille, North Mangespin ; 65 m; 18.IV.1972; with H. mediterranea n. sp.; JGH leg . • 1 colony; Hyères Islands, South Porquerolles Island ; 60-65 m; X.1996; A. Castric leg. • 1 large colony + UW photos; Corsica, Scandola, Palazzu Islet ; 42°23’09”N, 8°34’55”E; 35 m; X.1975; COR; Div; JGH leg GoogleMaps .
Italy • 11 small fragments; SE Sicilia; Gulf of Noto ; Stn PS /81 4C; 95- 86 m; VII-VIII.1981; A. Rosso leg. • 8 small fragments; SE Sicilia; Gulf of Noto ; Stn PS /81 4B; 65 m; VII-VIII.1981; A. Rosso leg. • 30 small fragments (thanatocenosis); North Sicilia; Ustica Island, Apollo Bank ; 70 m; VII.1986; A. Rosso leg. • 1 fragment; Ionian Sea ; 17 m; A. Tursi leg.
Tunisia • 2 colonies; northern coast, West Serrat Cape, Sidi Mech- rig, Kavensur ; 51-53 m; 28.VII.2006; COR; Div; S. Sartoretto leg. Greece • 1 colony; Corfou, Paleokastritsa; Stn JV-4c; 40 m; 5.VII.1958; Div; J. Vacelet leg. • 1 fragment; South Creta, Kalolimniones ; R/V Calypso , survey 1964; 80-125 m; 4.V.1964; Dre; JGH leg . • 7 fragments; Aegean Sea, Scarpanto Strait ; R/ V Jean Charcot, Stn 19.MO.67; 35°55’00”N, 27°28’30”E; 29-33 m; 29.VIII.1967; coarse DC; Dre; JGH leg GoogleMaps .
OTHER MATERIAL EXAMINED. — Specimens from the MNHN collection (examination on 05.II.2005). • Syntypes; two small colonies from Lamarck collection; labelled by Lamarck: ‘ Retepora frondiculata n., Millepora tubipora Solander et al. p. 139’; MNHN - M6 (R) 1867, no. 177b., presently MNHN-IB-2008-4691 and MNHN-IB-2008-4694 • 1 large colony depicted by Milne-Edwards (1838, pl. 9, fig. 1), labelled ‘ Hornera frondiculata Lamour’; MNHN 4690 - M6 (R) 1867 - no. 177e. • 1 colony ca. 3 cm wide; labelled ‘ Hornera frondiculata Lamouroux’, Bonifacio, R/V Travailleur, D 24, 40- 80 m, 15.VII.1881; coll. Jullien, 11t. 18. • 3 fragments, with gonozooid; Bonifacio, R/V Travailleur, no. 862, 55- 77 m. • 5 fragments; R/V Travailleur 1881, D.24 (2° sér.) 55-77 m, coll. Calvet 1892 11t. 18, no. 223. • Several fragments in two boxes; Marseille, coll. Jullien 1858, 2t. 18 no. 102 & 103 .
ADDITIONAL PHOTOGRAPHIC RECORDS. — • 1 large colony; France, Marseille, Riou Island, Impérial du large; 38 m; vertical rock; UW photo; E. Driancourt leg. • 2 large colonies; France, Marseille, Riou Island, Impérial du large; 30-35 m; vertical rocky; UW photos; D. Ader leg. • 1 large specimen; France, La Ciotat, Pierre du Le- vant; 43°09’19”N, 05°37’26.5”E, 65 m; VI.2008; COR; Div; photo of collected specimen; S. Sartoretto leg. GoogleMaps • 1 colony, SEM photos; Spain, Menorca Channel ; 39°51’1.541”N, 3°30’22.294”E; 60-80 m; VIII.2011; on maerl; INDEMARES IEO exped.; T. Madurell leg. ( MZB 2015-8368 , STUB 540) GoogleMaps . • 3 large fertile colonies; Croatia, Vis Island, Bisevo ; 30 m; rocky and sandy bottom with seaweeds; 22.IX.2014; UW photos; JGH leg . • 1 colony; Tunisia, Zembra Island ; 45 m; UW photo; P. Sánchez-Jérez leg. (as H. lichenoides in Argyrou et al. 2002 , Figs 5 View FIG -29) . • 1 colony; Algeria, Jijel, Aouana Island ; 43 m; UW photo; S. Belbacha leg. (in Belbacha et al. 2011, Fig. 53) . • 1 colony; Italy, Sicilia, Messina strait; 40 m, UW photo; S. Weinberg leg. (in Weinberg 2013: 325) .
DESCRIPTION
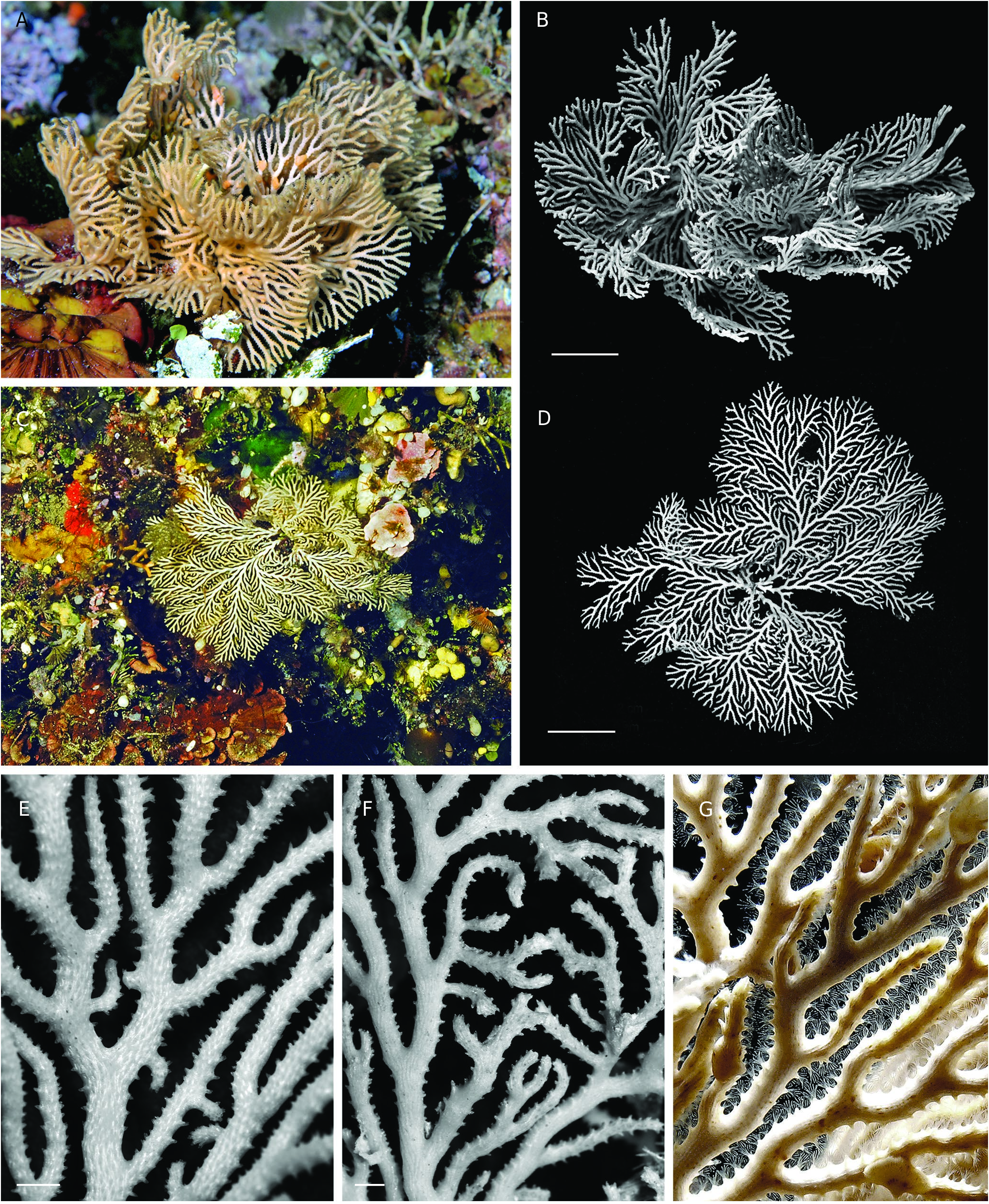
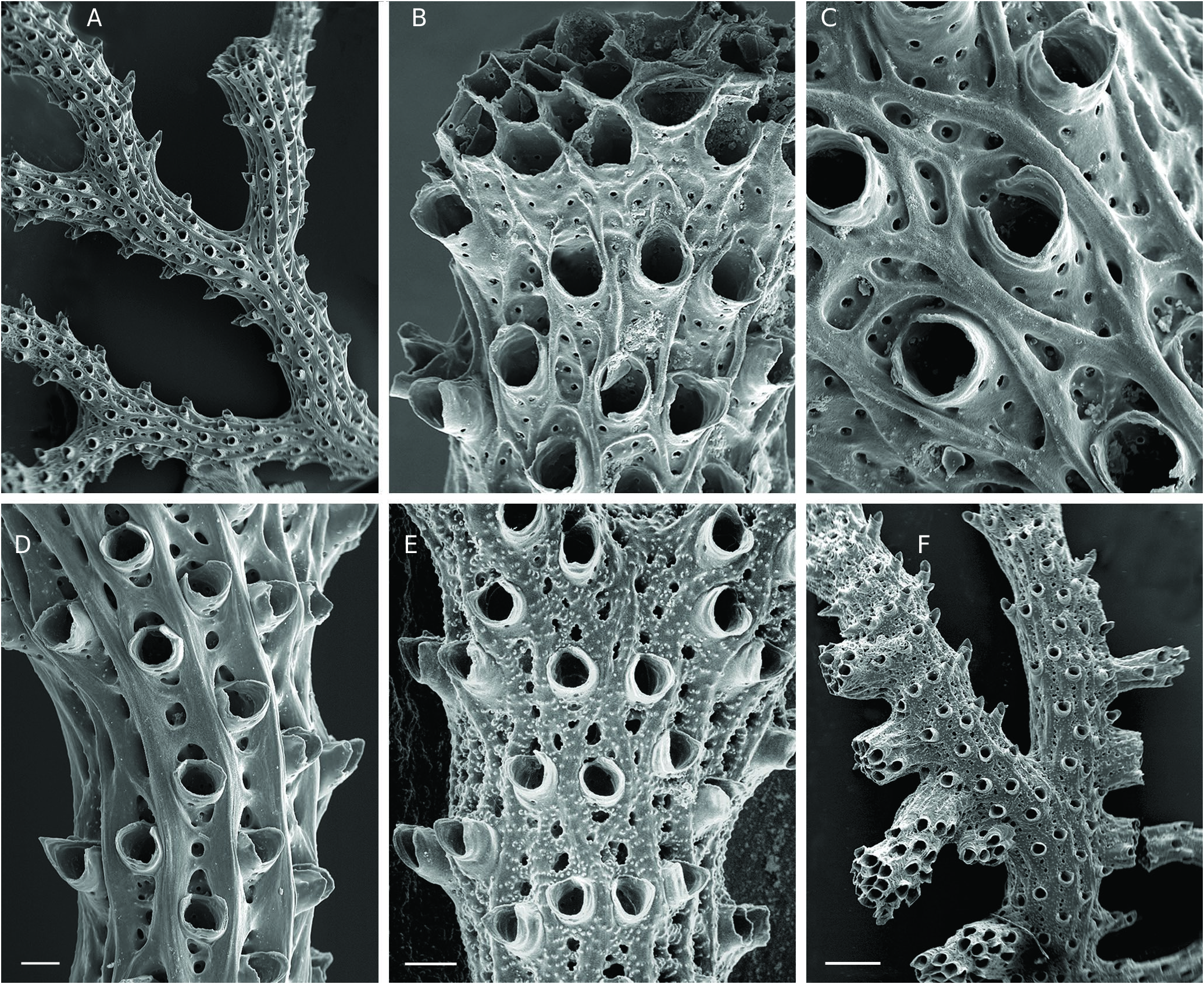
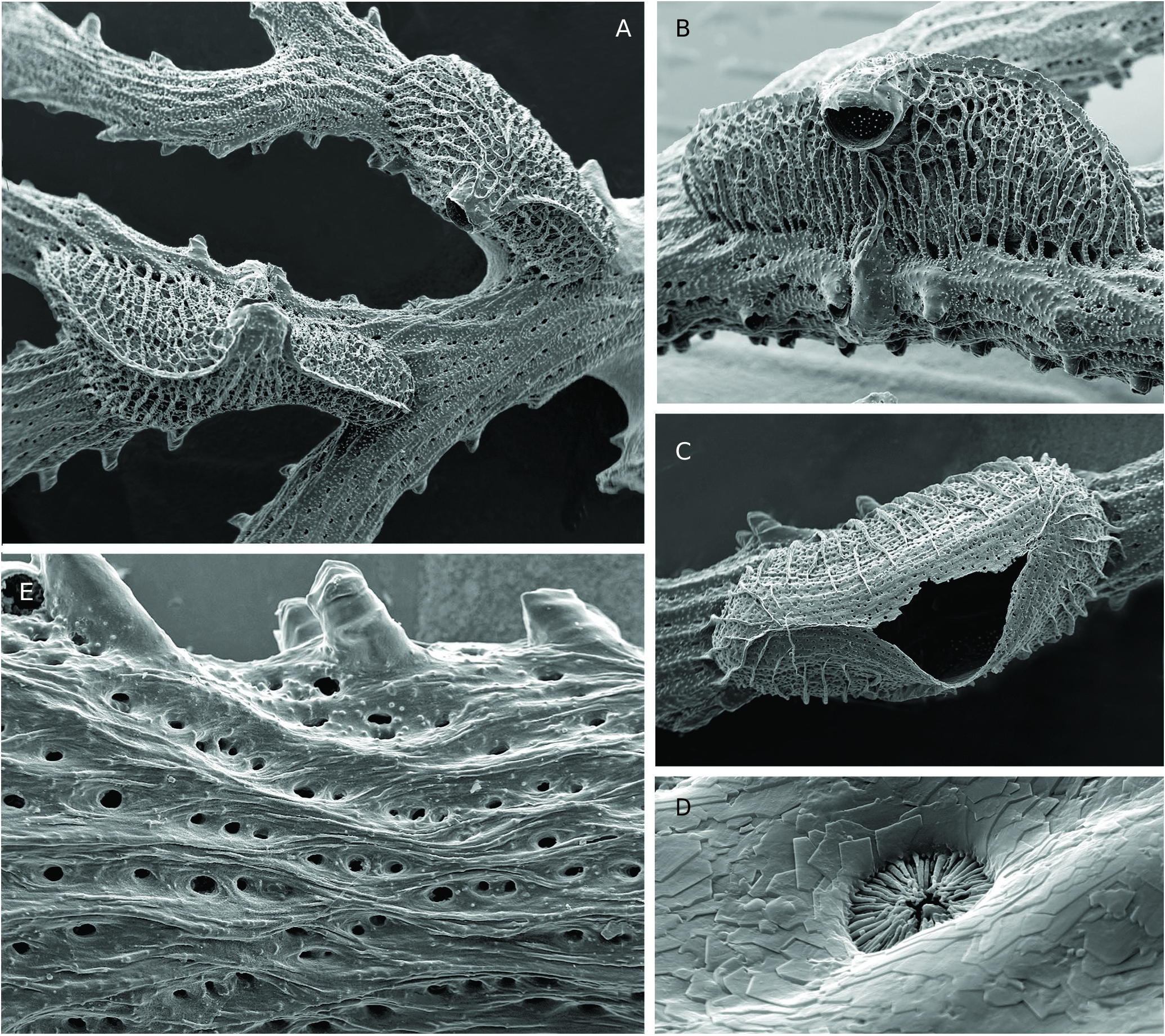
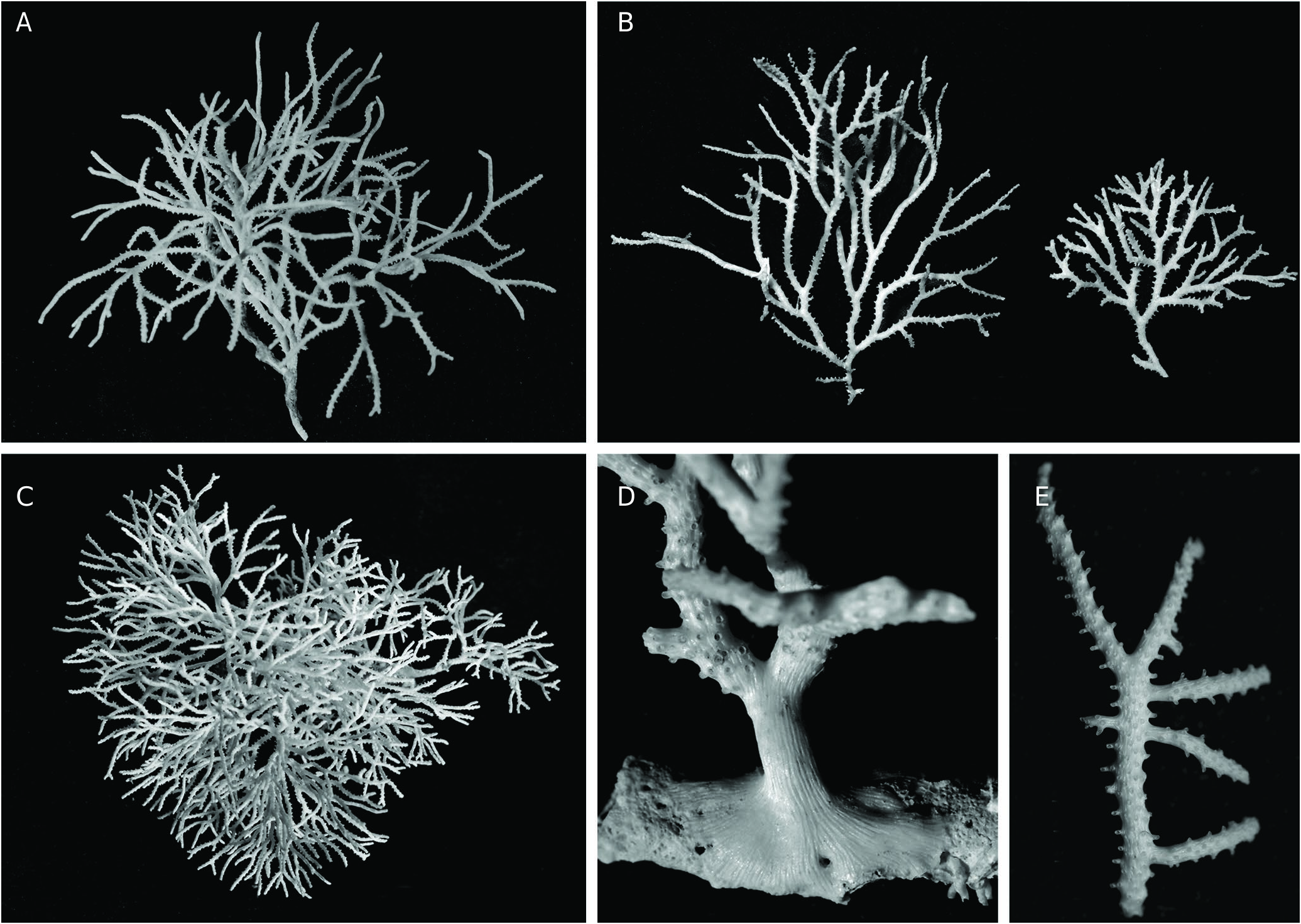
Colony erect, strongly calcified, firmly attached to a substrate by a broad expansion of secondary calcification, branching dichotomously many times with short, variously directed ramifications without anastomoses, and with the further addition of small, secondary lateral branches growing at right angle ( Fig. 3F View FIG ). Resulting growth-form varying from sub-planar to convoluted rosette shape, both reaching large size, up to ca. 15 cm in width and height, with narrow spacing between secondary branches, pale salmon pink in colour when alive ( Fig. 2A, G View FIG ). Autozooid apertures distributed on frontal side in 5-8 (6.7 in average) alternating longitudinal (linear) rows ( Fig. 3A, D, E View FIG ). Peristomes short, longer on lateral sides of branches, with distal edge typically lacking, leaving a U- or V-shaped notch, while lateral edges may be prominent and distinctly tapered, particularly in lateral rows ( Fig. 3C, D View FIG ). Horizontal part of autozooid tubes pierced with large, round pores (12.5-15 µm) down to the base of raised peristome, clearly visible in the apical zone of branches, where autozooid walls remain apparent in frontal view ( Fig. 3C View FIG ). Secondary calcification increasing greatly from the branch tips to the basal parts of the colony, rapidly masking the external features of autozooids. In an initial stage, secondary calcification forming thick longitudinal ridges surrounding the peristomes ( Fig. 3C View FIG ). These ridges (‘nervi’) increasing in thickness and soon joining with flat or convex transversal bridges of calcified layers which partially cover the autozooids, but leaving oblong or rounded windows (‘sulci’) within which some mural pores remain visible ( Fig. 3D View FIG ). In a further stage, frontal side, except for raised peristomes, entirely covered with a thick layer of secondary calcification densely punctuated with small, round pustules often aligned transversally and, proximally to each peristome, interrupted by 3-5 large, irregularly shaped holes (‘vacuoles’) ( Fig. 3E View FIG ). Dorsal side of branches convex, with surface structured by a network of longitudinal ridges branching and anastomosing to produce long, concave, spindle-shaped areas pierced with 2-6 large pores, covered with small pustules ( Fig. 4E View FIG ). Fertile colonies frequent, the large ones bearing a great number of gonozooids of the same colour as branches, but clearly denser ( Fig. 4A View FIG ). Gonozooid chamber large, developed on the dorsal side from an enlarged tube migrated from the frontal side ( Fig. 4B View FIG ), clearly longer than wide when placed between two bifurcations, or roughly triangular or heart-shaped when adjacent to a branch fork; a prominent crest along the upper midline of the chamber, extending on both sides of the ooeciostome ( Fig. 4A View FIG ). Brood chamber wall made of foliated crystallites overlapping according to the direction of wall growth, pierced with mural pores (10.6-14.4 µm), which are rapidly closed by pointed radial spines during the development of the gonozooid ( Fig. 4D View FIG ). External relief of the gonozooid formed by a dense network of small, reticulated ridges, spreading perpendicularly towards the upper crest, bearing a line of small, round pustules, and delimiting spaces (‘cancelli-like cavities’, Taylor & Jones 1993); ooeciostome large, much broader than the peristomes of autozooids (x 3.8 in average), placed at the middle of the upper crest, curved laterally, with a wide elliptical aperture opening towards the space delimited by the closest lateral branch ( Fig. 4A, B View FIG ). Ancestrula and early astogenetic stages not observed.
REMARKS
Taxonomic issues
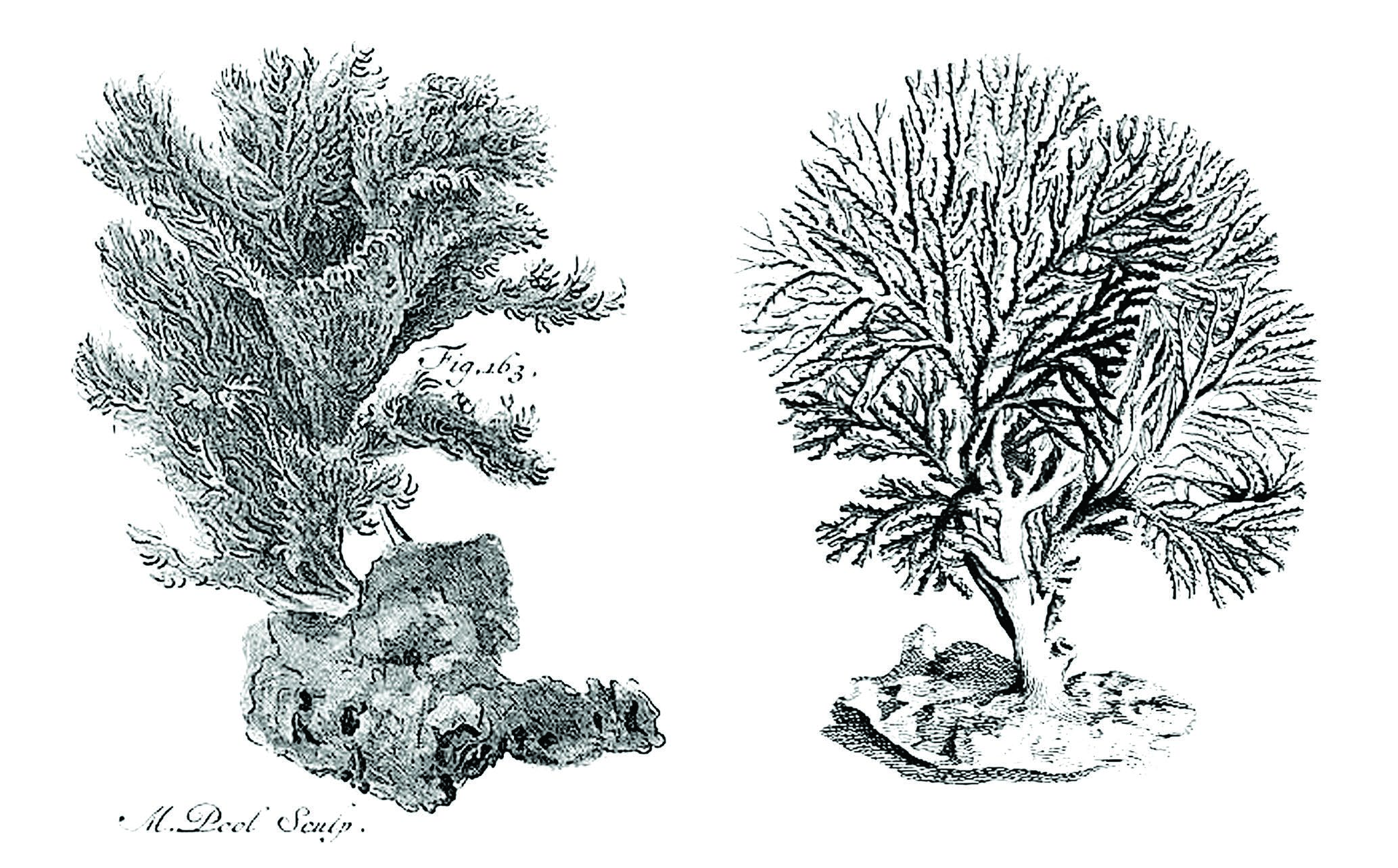
The authorship of H. frondiculata has long been attributed to Lamouroux (1821) and this designation was maintained by d’Hondt (1994: 302), though he noted that two specimens of this species kept at the MNHN had a handwritten label signed by Lamarck naming them ‘ Retepora frondiculata , Méditerranée’. There is no indication that Lamouroux had the opportunity to examine these specimens in Lamarck’s collection. However, his knowledge of the species Retepora frondiculata created by Lamarck (1816) is attested by the fact that he mentioned it in the synonymy of H. frondiculata ( Lamouroux 1821: 42, ‘Rétépore frondiculé; de Lam. Anim. Sans vert tom. 2, p. 182, no. 3’). In his revision of the European species of Hornera, Mongereau (1972) considered only fossil material, except for a Mediterranean specimen from d’Orbigny’s collection (MNHN.F.A15367, formerly MNHN no. 13773) designated as the neotype of H. frondiculata Auct. , arguing that the Lamouroux collection had been destroyed during World War II ( Mongereau 1972, see also d’Hondt 1991). As noted by Smith et al. (2008), this neotype is not valid. Moreover, Mongereau (1972) distinguished three morphotypes (‘ formes’) of H. frondiculata Auct. based on differences in the calcification of the frontal side of branches: frondiculata , lagaaiji and striata, the first one ranging from the Eocene to the Present, the two others being only fossil. The authorship of Lamarck is now admitted (e.g. Bock & Gordon 2019), and attested by high-definition photos (including SEM pictures and original Lamarck handwritten labels) of two syntypes of Retepora frondiculata Lamarck, 1816 (MNHN-IB-2008-4691 and MNHN-IB-2008-4694), available on the MNHN website (https://science.mnhn.fr/all/ list?originalCollection-Coll.+Lamarck). The assertion by Smith et al. (2008) that Lamouroux (1821) based his description of H. frondiculata on material from Kamtchatka is disputable. When indicating the distribution of H. frondiculata, Lamouroux indeed cited first ‘Kamtchatka, Tilesius’, then later ‘Océan indien et austral, Linné, Ellis’, and finally ‘Méditerranée, de Lamarck’. This succession of localities more probably follows a geographical order (farthest to nearest) rather than indicating that Kamtchatka was the type locality of the species, and a specimen from the Tilesius collection the type specimen of this species. It is more likely that the specimen illustrating the description of H. frondiculata ( Lamouroux 1821, pl. 74, figs 7-9) was from the Mediterranean.The same origin is highly probable for the large colony, beautifully illustrated, but left unnamed by J. Ellis ( Ellis & Solander 1786, pl. 26, fig. 1; here Fig. 1B View FIG ) and assigned to H. frondiculata by Lamouroux (1821 pl. 26, fig. 1), who reproduced Ellis’ plates.
The record in Corsica (Pietranera, North of Bastia, 35 m) by Calvet (1902) of H. violacea M. Sars , a species from the North Atlantic now classed in the Stimatoechidae Brood, 1972 [= Stigmatoechos violacea (M. Sars, 1863) ; Bock & Gordon 2019], is obviously a misidentification.The sampling depth of this occurrence suggests that it might be H. frondiculata . The specimens collected by Lagaaij & Gautier (1965) at 128 m and 145 m depth off the mouth of Rhône River may correspond either to H. frondiculata or to H. mediterranea n. sp. The record of H. caespitosa Busk, 1875 by Calvet (1906) at 445 m depth off Cape Sicié (East of Marseille) most likely matches colonies of H. frondiculata detached from their substratum and drifted down the slope of Sicié canyon. The report of H. lichenoides by Laubier (1966) without comment on coralligenous bottoms at Banyuls-sur-Mer at moderate depth (<40 m) is probably a misidentification of H. frondiculata .
Morphological features
The general shape and branching type of colonies of H. frondiculata are typical, and cursory examination, even underwater, allows a correct identification of the species. Old drawings of large colonies, such as that represented by Marsilli (1725 pl. 33, fig. 163; here Fig. 1A View FIG ), can therefore be assigned to this species with confidence. The shape of colonies shows a marked habitat-related plasticity, from nearly planar on rocky walls to strongly contorted on coarse detrital sandy bottoms ( Fig. 2 View FIG A-D, see below in Discussion). However, the detailed structure of the branches is similar in both growth-forms. Lateral branches growing at right angles are frequent in colonies of H. frondiculata ( Figs 2E, F View FIG , 3F View FIG ) regardless of their shape; they apparently appear to develop subsequently to the distal growth and bifurcation of the branches from which they are budded. Delayed budding of lateral branches is assumed to be an adaptive strategy to increase the fragmentation of empty spaces between laterally adjacent branches and improve the filtering activity of a colony according to its microenvironment (see below, Discussion). Lateral branches and typical notched peristomes are present on a colony of H. frondiculata collected by Abdelsalam (2014, fig. 2) on the Mediterranean coast of Egypt, but the stem and main branches are exceptionally thick and irregularly ramified. This unusual growth-form and remarkable calcification may be induced by peculiar features of the habitat, e.g. shallow depth (20-25 m) and proximity of the Nile delta and the mouth of the Suez Canal. The particular shape of peristomes characterized by a deep distal U-shaped notch ( Fig. 3C, D View FIG ), is a constant and highly discriminating trait of H. frondiculata . The main variability in the peristome shape concerns the lateral edges, more or less projecting and sometimes clearly triangular ( Fig. 3D View FIG ). The presence of notched peristomes in this species was noted in early publications (e.g. Busk 1875: 17, pl. 20, figs. 2, 3; Waters 1904: 94, pl. 9, fig. 3), but has not been systematically taken into account subsequently as a species-specific feature, leading to erroneous records, particularly of non-Mediterranean living specimens (e.g. Busk 1886), or of fossil material (e.g. Mongereau 1972; Moissette 1993; Moissette et al. 2007; ZágorŠek 2010). Besides having a distal notch, the peristomes of H. frondiculata differ from those of H. mediterranea n. sp. in their significantly smaller size ( Table 2 View TABLE ). The mural pores of the autozooid walls, visible in young parts of branches, are particularly large ( Fig. 3C View FIG ), clearly broader than in H. mediterranea n. sp. The frontal and dorsal sides of branches of H. frondiculata have a typical aspect, with calcified structures and hollows contributing to the distinctiveness of this species when compared to H. mediterranea n. sp. ( Fig. 3 View FIG ). However, the development of the secondary calcification on branches of H. frondiculata is similar to that observed in other Hornera species (e.g. H. antarctica: Borg 1926, 1944 , and below, H. mediterranea n. sp.). Schematically, the frontal side of the autozooid walls is first partially covered by longitudinal strips and transversal bars, that then merge to form a thick, complete cover between the raised peristomes, just interrupted by several small, irregularly shaped windows per zooid ( Fig. 3 View FIG B-E). The strengthening of the dorsal side by a cover of secondary calcification presents a distinctive aspect with spindle-shaped, longitudinal depressions ( Fig. 4E View FIG ) containing several pores, which are, like the holes of the frontal side, windows allowing communication between the hypostegal pseudo-coelom and the autozooids. Hornera frondiculata is characterized by high fertility, with a large proportion of fertile colonies ( Table 2 View TABLE ) and a large number of gonozooids per fertile colony. For example, 20 gonozooids were present in a medium-sized colony (H: 7.7 cm, W: 6.6 cm, Marseille, South Riou, 70 m; 3.VII.1971). On colonies with a contorted shape, gonozooids are generally placed on the convex parts of branches, probably for the efficient export of larvae. As noted by early authors (e.g. Alder 1864: 109, pl. 5, fig. 7), the carinated shape of the gonozooids, with a ridge along the upper side of the chamber (‘carina’, Borg 1926), is very typical. This prominent longitudinal ridge, which starts at the opposite sides of the long axis of the chamber and ends on both sides of the ooeciostome may result from the suture of two lateral valves, as suggested by stages in the development of the gonozooid ( Fig. 4C View FIG ). The tubular origin of the gonozooid from a zooid of the frontal side, well described by Borg (1926), is evident from SEM examination ( Fig. 4B View FIG ).
GEOLOGICAL DISTRIBUTION
Records of fossil material attributed to H. frondiculata are numerous (e.g. Mongereau 1972; Smith et al. 2008). In the list of Hornera species available at Bryozoa.net (see above), H. frondiculata is considered to span from the Palaeogene to the Recent. The overall appearance of fossil Hornera colonies can be misleading. For example, the holotype of Hornera affinis Milne Edwards, 1838 , from the Tertiary of Sicilia (MNHN-IB-2008-4416), looks like H. frondiculata . It is clear that the validity of these fossil occurrences cannot be evaluated if the raised peristomes are eroded (as in most cases), and therefore the presence or not of a distal notch, the most decisive criterion for this species, cannot be checked. This feature is not explicitly considered in the description of fossil material (e.g. Mongereau 1972; Moissette 1988; Moissette et al. 2007). The peristomes of a specimen from the Miocene of the Czech Republic assigned to Hornera cf. frondiculata by ZágorŠek (2010, pl. 26, fig. 12) are clearly not notched. However, notched peristomes can be recognised on a Plio- Pleistocene specimen from Rhodes illustrated by Moissette & Spjeldnaes (1995, pl. 2, fig. 5).
HABITAT DISTRIBUTION
As noted by Ballesteros (2006), H. frondiculata belongs to the assemblage of species from deep-water coralligenous habitats, together with other large rigidly erect bryozoans which form an intermediate stratum below that of large gorgonians ( Belbacha et al. 2011). However, H. frondiculata exhibits two frequency peaks among the Mediterranean coastal habitats ( Table 4 View TABLE ), (i) on dimly lit steep rocks of the coralligenous community, in some cases at its upper depth limit (30-35 m), but generally deeper (50-80 m), and (ii) on deep coarse detrital sand. In the coralligenous rocky habitat, H. frondiculata occupies only exposed microhabitats ( Novosel 2005), such as vertical walls shaded by large gorgonians. In contrast, it is absent from overhangs and cavities, or the entrance of caves. H ornera frondiculata can be abundant, forming large bushy colonies on detrital sand covered with coarse biogenic elements in the vicinity of steep rocks, (‘débris organogènes’, Harmelin 1976). These biogenic mineralized deposits are alive or dead (thanatocenose, Rosso 1996; Di Geronimo et al. 2001), coming in part from nearby coralligenous rocks, directly from drifted fragments, or indirectly from larval recruitment onto large erect cnidarians, empty bivalve shells ( Spondylus Linnaeus, 1758 , Arca Linnaeus, 1758 ) and large bryozoan colonies. In sites swept by bottom currents, the latter may form aggregations of erect colonies at the surface of coarse soft bottoms ( Marion 1883: ‘graviers à bryozoaires’; Picard & Bourcier 1976: ‘fonds détritiques côtiers à grands bryozoaires branchus’).
GEOGRAPHICAL DISTRIBUTION
There is no reliable indication that H. frondiculata occurs outside the Mediterranean. The records of this species at Cape Verde, West Africa by Busk (1886) and Cook (1968) are doubtful as the latter noted ‘tubular peristomes’, thus apparently without the characteristic distal indentation. The status of endemic to the Mediterranean for H. frondiculata is likely, but needs to be verified through the study of material from the near Atlantic. This species has been recorded in seven of the eight ecoregions of the Mediterranean ( Table 1 View TABLE ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hornera frondiculata ( Lamarck, 1816 )
| Harmelin, Jean-Georges 2020 |
Hornera lichenoides
| LAUBIER L. 1966: 223 |
Hornera caespitosa
| CALVET L. 1906: 478 |
Hornera violacea Sars, 1863
| CALVET L. 1902: 44 |
Hornera frondiculata
| ACHILLEOS K. & JIMENEZ C. & BERNING B. & PETROU A. 2020: 233 |
| GEROVASILEIOU V. & ROSSO A. 2016: 36 |
| ROSSO A. & DI MARTINO E. 2016: 570 |
| ABDELSALAM K. M. 2014: 271 |
| WEINBERG S. 2013: 325 |
| ROSSO A. & SANFILIPPO R. & TADDEI RUGGIERO E. & DI MARTINO E. 2013: 169 |
| BELBACHA S. & SEMROUD R. & ESPLA A. A. 2011: 46 |
| SOUTO J. & GIL O. & PULPEIRO E. 2010: 38 |
| SMITH A. M. & TAYLOR P. D. & SPENCER H. G. 2008: 371 |
| BALLESTEROS E. 2006: 156 |
| NOVOSEL M. 2005: 236 |
| MOISSETTE P. & SPJELDNAES N. 1995: 786 |
| ZABALA M. 1993: 571 |
| COSTA B. & ROSSO A. & SANFILIPPO R. & ZANINI A. 1991: 418 |
| DI GERONIMO I. & GIACOBBE S. & ROSSO A. & SANFILIPPO R. 1988: 703 |
| ZABALA M. & MALUQUER P. 1988: 182 |
| ROSSO A. 1987: 173 |
| ZABALA M. 1986: 686 |
| HARMELIN J. - G. 1976: 223 |
| MONGEREAU N. 1972: 329 |
| COOK P. L. 1968: 238 |
| HARMELIN J. - G. 1968: 1187 |
| GAUTIER Y. 1955: 268 |
| NEVIANI A. 1939: 69 |
| DE WATTEVILLE D. 1939: 8 |
| CANU F. & BASSLER R. S. 1930: 86 |
| WATERS A. W. 1904: 94 |
| CALVET L. 1902: 43 |
| BUSK G. 1875: 17 |
| HELLER C. 1867: 124 |
| ALDER J. 1864: 109 |
| LAMOUROUX J. V. F. 1821: 41 |
| Milne-Edwards 1838: 17 |
| Lagaaij & Gautier 1965 |
Retepora frondiculata
| LAMARCK J. B. & DE 1816: 182 |