Carbon Monoxide Poisoning
Written by David Han, MD. Edited by Akshay (Sunny) Elagadhala, MD.
Background
Carbon monoxide is a colorless, odorless, tasteless, nonirritating gas formed by combustion of hydrocarbons. Fire related smoke inhalation is the leading cause of CO poisoning, but there is also an appreciable amount of exposure due to non-accidental and accidental/inadvertent non-fire related exposures. Typically these exposures peak in winter due to poorly ventilated/functioning heating systems, fuel-burning devices, and motor vehicles.
Non-fire related CO poisoning is responsible for up to 50,000 emergency department (ED) visits and 1200 deaths per year, making it one of the leading causes of poisoning death in the United States. Paint and varnish strippers and degreasing agents can also contain methylene chloride, or dichloromethane. These chemicals can be used in the production of photographic films, synthetic fibers, and circuit boards. Prolonged exposure to these can cause chemical burns as well as when inhaled, can be metabolized to formyl chloride, which is further metabolized to carbon monoxide. Additionally, sources of incomplete combustion can result in CO production. Sources of incomplete combustion include propane powered vehicles (Zamboni, power mowers, tractors), home heating, natural gas stoves, kerosene heaters, indoor hibachi grills, water heaters and radiators.
Morbidity, which is primarily related to late neurocognitive impairment, persists beyond initial stabilization in up to 40% of victims. As with most cardiorespiratory disorders, those at increased risk for morbidity and mortality include the elderly, infants less than 6 months, pregnant females, and those with coronary artery disease.
Pathophysiology
CO binds to hemoglobin with higher affinity than oxygen, approximately 240 times more. The level of exposure (duration, oxygen in the environment, minute ventilation) dictates the severity of the disease along with patient comorbidities. Because of this higher affinity, CO shifts the oxyhemoglobin dissociation curve leftwards and inhibits heme from off-loading oxygen to peripheral tissues.
In smoke inhalation there is often concomitant cyanide inhalation and can also contribute and be synergistic in the effects on peripheral oxygen utilization. The mechanism of delayed neurologic sequelae is poorly understood but may be attributed to lipid peroxidation by toxic oxygen species generated by xanthine oxidase.
What does it look like?
Symptoms can oftentimes be nonspecific and require a higher level of suspicion given the clinical picture. Typically patients will present complaining of headache, nausea, and dizziness. Blood samples may appear “cherry red” as opposed to “chocolate brown” in those with methemoglobinemia.
Diagnosis
Standard pulse oximetry CANNOT screen for CO, as it does not differentiate carboxyhemoglobin from oxyhemoglobin.
Elevated carboxyhemoglobin level measured by co-oximetry of an arterial blood gas sample. Venous samples may be used to determine the carboxyhemoglobin level, but they are less accurate in quantifying the associated acidosis.
Labs:
CoHb level: >3% in non smokers, >10% in smokers
Troponin: positive troponin indicates poorer prognosis
CMP for hyperglycemia and lactic acidosis (correlates with acute mortality rate)
CK for rhabdomyolysis
CBC for polycythemia (in chronic CO exposure)
Hcg, coags for DIC
Lactate from oxidative phosphorylation alterations
Consider co-exposures: acetaminophen, salicylates, cyanide (fire)
Imaging
Imaging is not typically indicated, but can be useful to rule out other causes of neurological decompensation.
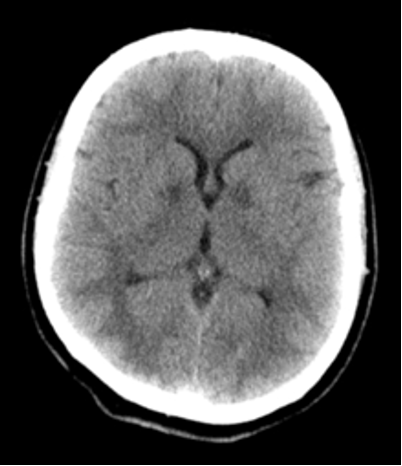
Neurologic sequelae
Within 12 hours of exposure as symmetric hypodense lesions in the basal ganglia (globus pallidus, putamen, and caudate nuclei).
Initial normal head CT scan usually predicts a favorable outcome,
Changes in the globus pallidus and subcortical white matter within 24 hours
associated with poor outcomes
Management
Never forget your ABCs
Remove from exposure
Telemetry
The half-life of CO is 4-5 hours on RA (21% FiO2)
60-90 minutes on 100% FiO2 (mask or ETT) at 1 atmosphere
15-30 minutes on 100% O2 at 3 atmospheres (hyperbaric oxygen therapy)
Hyperbaric Oxygen Therapy
Meta-analysis of seven studies prepared by Cochrane Review shows mixed results and currently shows no reduction of the incidence of adverse neurologic outcomes in HBO vs NBO [1].
In a study done by Weaver et al 2002, they noted “treatment of patients with acute, symptomatic carbon monoxide poisoning with three hyperbaric-oxygen sessions within a 24-hour period appear[ed] to reduce the rate of cognitive sequelae 6 weeks and 12 months later.” [4].
But this study was limited due to:
Patients in the control group, at baseline, had significantly higher rates of cerebellar dysfunction
Study was prematurely terminated, due to perceived benefit
8 patients lost to follow up (5 in the normobaric group, 3 in the hyperbaric group).
Change in primary endpoint between study start and end.
ACEP Clinical Policy
Level B Recommendations
Emergency physicians should use HBO2 therapy or high-flow normobaric therapy for acute CO-poisoned patients. It remains unclear whether HBO therapy is superior to normobaric oxygen therapy for improving long-term neurocognitive outcomes [2].
Follow up
Discharge home if: accidental, normal examination, normal ECG and labs, no end organ damage
Admit if: medical comorbidities, significant findings during workup
High risk features: LOC, neurologic dysfunction, metabolic acidosis, MI, age>55, pregnancy
Cardiac arrest: poor prognosis, infrequent survival to discharge, increased rates of neurologic injury
Neuropsych testing in 1-2 months if acute neuro findings
30% at 1 mo, 10% at 12 mo with long term sequelae
References
Buckley, N., Juurlink, D., Isbister, G., Bennett, M., & Lavonas, E. (2011, April 13). Hyperbaric oxygen for carbon monoxide poisoning - BUCKLEY, NA - 2011: Cochrane Library. Retrieved February 07, 2021, from https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002041.pub3/abstract
Carbon monoxide poisoning. (n.d.). Retrieved February 07, 2021, from https://www.acep.org/patient-care/clinical-policies/carbon-monoxide-poisoning/
Hampson, N. B. (n.d.). Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Retrieved February 07, 2021, from https://pubmed.ncbi.nlm.nih.gov/23087025/
Weaver, L. (2002, October 03). Hyperbaric oxygen for acute carbon monoxide poisoning: Nejm. Retrieved February 07, 2021, from https://www.nejm.org/doi/full/10.1056/nejmoa013121
Wu, P., & Juurlink, D. (2014, May 13). Carbon monoxide poisoning. Retrieved February 07, 2021, from https://www.cmaj.ca/content/186/8/611
Carbon Monoxide Poisoning. (n.d.). Retrieved February 07, 2021, from https://www.roshreview.com/