Abstract
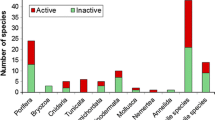
The Antarctic sea star Odontaster validus and the amphipod Cheirimedon femoratus are important predators in benthic communities. Some bryozoans are part of the diet of the asteroid and represent both potential host biosubstrata and prey for this omnivorous lysianassid amphipod. In response to such ecological pressure, bryozoans are expected to develop strategies to deter potential predators, ranging from physical to chemical mechanisms. However, the chemical ecology of Antarctic bryozoans has been scarcely studied. In this study we evaluated the presence of defenses against predation in selected species of Antarctic bryozoans. The sympatric omnivorous consumers O. validus and C. femoratus were selected to perform feeding assays with 16 ether extracts (EE) and 16 butanol extracts (BE) obtained from 16 samples that belonged to 13 different bryozoan species. Most species (9) were active (12 EE and 1 BE) in sea star bioassays. Only 1 BE displayed repellence, indicating that repellents against the sea star are mainly lipophilic. Repellence toward C. femoratus was found in all species in different extracts (10 EE and 12 BE), suggesting that defenses against the amphipod might be both lipophilic and hydrophilic. Interspecific and intraspecific variability of bioactivity was occasionally detected, suggesting possible environmental inductive responses, symbiotic associations, and/or genetic variability. Multivariate analysis revealed similarities among species in relation to bioactivities of EE and/or BE. These findings support the hypothesis that, while in some cases alternative chemical or physical mechanisms may also provide protection, repellent compounds play an important role in Antarctic bryozoans as defenses against predators.





Similar content being viewed by others
References
Al-Ogily SM, Knight Jones EW (1977) Anti-fouling role of antibiotics produced by marine algae and bryozoans. Nature 265:728–729
Amsler MO, McClintock JB, Amsler CD, Angus RA, Baker BJ (2009) An evaluation of sponge-associated amphipods from the Antarctic Peninsula. Antarct Sci 21:579–589
Anderson SA, Northcote PT, Page MJ (2010) Spatial and temporal variability of the bacterial community in different chemotypes of the New Zealand marine sponge Mycale hentscheli. FEMS Microbiol Ecol 72(3):328–342
Apeltans W, Ahyong ST, Anderson G, Angel MV, Artois T, Bailly N, Bamber R, Barber A et al (2012) The Magnitude of Global Marine Species Diversity. Curr Biol 22:2189–2202
Arntz WE, Gutt J, Klages M (1997) Antarctic marine biodiversity. In: Battaglia B, Valenica J, Walton DWH (eds) Antarctic communities: species, Structure and survival. Cambridge University Press, Cambridge, pp 3–14
Avila C, Iken K, Fontana A, Gimino G (2000) Chemical ecology of the Antarctic nudibranch Bathydoris hodgsoni Eliot, 1907: Defensive role and origin of its natural products. J Exp Biol Ecol 252:27–44
Avila C, Taboada S, Núñez-Pons L (2008) Antarctic marine chemical ecology: What is next? Mar Ecol 29:1–71
Barnes DKA, Kaiser S, Griffiths HJ, Linse K (2009) Marine, intertidal, freshwater and terrestrial biodiversity of an isolated polar archipelago. J Biogeogr 36:756–769
Best BA, Winston, JE (1984) Skeletal strength of encrusting cheilostome bryozoans. Biol Bull 167:390–409
Blackman AJ, Li CP (1994) New tambjamine alkaloids from the marine bryozoan Bugula dentata. Aust J Chem 47:1625–1629
Blackman AJ, Matthews DJ (1985) Amathamide alkaloids from the marine bryozoan Amathia wilsoni Kirkpatrick. Heterocycles 23:2829–2833
Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR (2012) Marine natural products. Nat Prod Rep 29(2):144–222
Bock PE, Gordon DP (2013) Phylum Bryozoa Ehrenberg, 1831. Zootaxa 3703(1):067–074
Brandt A, Gooday AJ, Brandao SN, Brix SB, Brökeland W, Cedhagen T, Choudhury M, Cornelius N, Danis B, De Mesel I, Diaz RJ, Gillan DC, Ebbe B, Howe J, Janussen D, Kaiser S, Linse K, Malyutina M, Pawlowski J, Raupach M, Vanreusel A (2007) First insights into biodiversity and biogeography of the Southern Ocean deep sea. Nature 447:307–311
Bregazzi PK (1972) Habitat selection by Cheirimedon femoratus (Pfeffer) and Tryphosella kergueleni (Miers) (Crustacea: Amphipoda). Br Antarct Surv Bull 31:21–31
Carter MC (2008) The Functional Morphology of Avicularia in Cheilostome Bryozoans. PhD dissertation, Victoria University of Wellington, Wellington
Carte B, Faulkner DJ (1983) Defensive metabolites from three nembrothid nudibranchs. J Org Chem 48:2314–2318
Carter MC, Gordon DP, Gardner JPA (2010) Polymorphism and variation in modular animals: morphometric and density analyses of bryozoan avicularia. Mar Ecol Prog Ser 399:117–130
Clarke A (1992) Is there a latitudinal diversity cline in the sea? Trends Ecol Evol 7:286–287
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143
Clarke KR, Green RH (1988) Statistical design and analysis for a ‘biological effects’ study. Mar Ecol Progr Ser 46:213–226
Clarke A, Johnston NM (2003) Antarctic marine benthic diversity. Oceanogr Mar Biol 41:47–114
Clarke A, Aronson RB, Crame JA, Gili JM, Blake DB (2004) Evolution and diversity of the benthic fauna of the Southern Ocean continental shelf. Antarct Sci 16:559–568
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80
Coleman (1989) Short note. Gnathiphimedia mandibularis K.H. Barnard 1930, an Antarctic amphipod feeding on Bryozoa. Antarct Sci 1:343–344
Cutignano A, Zhang W, Avila C, Cimino G, Fontana A (2011) Intrapopulation variability in the terpene metabolism of the Antarctic opisthobranch mollusc Austrodoris kerguelenensis. Eur J Org Chem 27:5383–5389
Dauby P, Scailteur Y, Chapelle G, De Broyer C (2001a) Potential impact of the main benthic amphipods on the eastern Weddell Sea shelf ecosystem (Antarctica). Polar Biol 24(9):657–662
Dauby P, Scailteur Y, De Broyer C (2001b) Trophic diversity within the eastern Weddell Sea amphipod community. Hydrobiologia 443:69–86
Davidson SK, Haygood MG (1999) Identification of sibling species of the bryozoan Bugula neritina that produce different anticancer bryostatins and harbor distinct strains of the bacterial symbiont “Candidatus Endobugula sertula”. Biol Bull 196:273–280
Dayton PK, Robilliard GA, Paine RT, Dayton LB (1974) Biological accommodation in the benthic community at McMurdo Sound, Antarctic. Ecol Monogr 44:105–128
Dayton PK, Morbida BJ, Bacon F (1994) Polar marine communities. Am Zool 34:90–99
Dearborn JH, Watling LE, Edwards KC, Fratt DB, Hendler GL (1983) Echinoderm biology and general benthic collecting along the Antarctic Peninsula. Antarct J US 17:162–164
De Broyer C, Rauschert M, Scailteur Y (1999) Structural and ecofunctional biodiversity of the benthic amphipod taxocoenoses. In: Arntz WE, Gutt J (eds) The expedition ANT XV/3 (EASIZ II) of RV “Polarstern” in 1998. Ber Polarforsch 301:163–174
De Broyer C, Scailteur Y, Chapelle G, Rauschert M (2001) Diversity of epibenthic habitats of gammaridean amphipods in the eastern Weddell Sea. Polar Biol 24:744–753
De Broyer C, Lowry JK, Jazdzewski K, Robert H (2007) Catalogue of the gammaridean and corophiidean Amphipoda (Crustacea) of the Southern Ocean with distribution and ecological data. Bull Inst R Sci Nat Belg Biol 77(1):1–135
De Broyer C, Danis B, 64 SCAR-MarBIN Taxonomic Editors (2011) How many species in the Southern Ocean? Towards a dynamic inventory of the Antarctic marine species. Deep-Sea Res Part II 58(1–2):5–17
Duckworth A, Battershill CN (2003) Sponge aquaculture for the production of biologically active metabolites: the influence of farming protocols and environment. Aquaculture 221:311–329
Figuerola B, Monleón-Getino T, Ballesteros M, Avila C (2012a) Spatial patterns and diversity of bryozoan communities from the Southern Ocean: South Shetland Islands, Bouvet Island and Eastern Weddell Sea. Syst Biodiv 10(1):109–123
Figuerola B, Núñez-Pons L, Vázquez J, Taboada S, Cristobo FJ, Ballesteros M, Avila C (2012b) Chemical interactions in Antarctic marine benthic ecosystems. In Cruzado A (ed) Marine ecosystems. http://www.intechopen.com/books/marine-ecosystems/chemical-interactions-in-antarctic-marine-benthic-ecosystems. ISBN: 978-953-51-0176-5, InTech
Figuerola B, Ballesteros M, Avila C (2013) Description of a new species of Reteporella (Bryozoa Phidoloporidae) from the Weddell Sea (Antarctica) and possible functional morphology of avicularia. Acta Zool 94(1):66–73
Forbes A (1938) Conditions affecting the response of the avicularia of Bugula. Biological Bulletin 65:469–479
Gray CA, McQuaid CD, Davies-Coleman MT (2005) A symbiotic shell-encrusting bryozoan provides subtidal whelks with chemical defense against rock lobsters. Afr J Mar Sci 27:549–556
Griffiths HJ (2010) Antarctic marine biodiversity—what do we know about the distribution of life in the Southern Ocean? PLoS ONE 5:e11683
Hayward PJ (1995) Antarctic cheilostomatous bryozoa. Oxford University Press, Oxford
Hayward PJ, Winston JE (2011) Bryozoa collected by the United States Antarctic research program: New taxa and new records. J Nat Hist 46(37–38):2259–2338
Hines DE, Pawlik JR (2012) Assessing the antipredatory defensive strategies of Caribbean non-scleractinian zoantharians (Cnidaria): Is the sting the only thing? Mar Biol 159(2):389–398
Huang YM, Amsler MO, McClintock JB, Amsler CD, Baker BJ (2007) Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctic Peninsula. Polar Biol 30:1417–1430
Huang JP, McClintock JB, Amsler CD, Huang YM (2008) Mesofauna associated with the marine sponge Amphimedon viridis: Do its physical and chemical attributes provide a prospective refuge from fish predation? J Exp Mar Biol Ecol 362:95–100
Iyengar EV, Harvell CD (2002) Specificity of cues triggering inducible spines in the bryozoan Membranipora membranacea. Mar Ecol Prog Ser 225:205–218
Kaufmann K (1971) The form and function of the avicularia of Bugula (Phylum Ectoprocta). Postilla 151:1–26
Koplovitz G, McClintock JB, Amsler CD, Baker BJ (2009) Palatability and chemical anti-predatory defenses in common ascidians from the Antarctic Peninsula. Aquat Biol 7:81–92
Krapp RH, Berge J, Flores H, Gulliksen B, Werner I (2008) Sympagic occurrence of Eusirid and Lysianassoid amphipods under Antarctic pack ice. Deep-Sea Res 55(II):1015–1023
Kuklinski P, Barnes DKA (2009) A new genus and three new species of Antarctic cheilostome Bryozoa. Polar Biol 32:1251–1259
Lebar MF, Heimbegner JL, Baker BJ (2007) Cold-water marine natural products. Nat Prod Rep 24(4):774–797
Legendre P, Legendre L (2012) Numerical ecology, 3rd edn. Elsevier, Amsterdam
Lidgard S (2008) Predation on marine bryozoan colonies: Taxa, traits and trophic groups. Mar Ecol Prog Ser 359:117–131
Lindquist N, Fenical W (1991) New tamjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators. Experientia 47:504–506
Lopanik N, Lindquist N, Targett N (2004) Potent cytotoxins produced by a microbial symbiont protect host larvae from predation. Oecologia 139:131–139
Mahon AR, Amsler CD, McClintock JB, Amsler MO, Baker BJ (2003) Tissue-specific palatability and chemical defenses against macro- predators and pathogens in the common articulate brachiopod Liothyrella uva from the Antarctic Peninsula. J Exp Mar Biol Ecol 290:197–210
McClintock JB (1994) Trophic biology of Antarctic echinoderms. Mar Ecol Prog Ser 111:191–202
McClintock JB, Baker BJ (1997) Palatability and chemical defense of eggs, embryos and larvae of shallow-water Antarctic marine invertebrates. Mar Ecol Prog Ser 154:121–131
McClintock JB, Slattery M, Heine J, Weston J (1992) Chemical defense, biochemical composition and energy content of three shallow-water Antarctic gastropods. Polar Biol 11:623–629
McClintock JB, Slattery M, Baker BJ, Heine JN (1993) Chemical ecology of Antarctic sponges from McMurdo Sound, Antarctica: Ecological aspects. Antarct J US 28:134–135
McClintock JB, Baker BJ, Slattery M, Heine JN, Bryan PJ, Yoshida W, Davies-Coleman MT, Faulkner DJ (1994) Chemical defense of common Antarctic shallow-water nudibranch Tritoniella belli Eliot (Mollusca: Tritonidae) and its prey, Clavularia frankliniana Rouel (Cnidaria: Octocorallia). J Chem Ecol 20:3361–3372
McClintock JB, Baker BJ, Amsler CD, Barlow TL (2000) Chemotactic tube-foot responses of the spongivorous sea star Perknaster fuscus to organic extracts of sponges from McMurdo Sound, Antarctica. Antarct Sci 12:41–46
McClintock JB, Amsler CD, Baker BJ (2010) Overview of the chemical ecology of benthic marine invertebrates along the Western Antarctic Peninsula. Integr Comp Biol 50:967–980
McKinney FK, Taylor PD, Lidgard S (2003) Predation on bryozoans and its reflection in the fossil record. In Kelley PH, Kowalewski M, Hansen TA (eds), Predator–prey interactions in the fossil record (pp 239–246)
Morris BD, Prinsep MR (1999) Amathaspiramides A–F, novel brominated alkaloids from the marine bryozoan Amathia wilsoni. J Nat Prod 62:688–693
Núñez-Pons L, Carbone C, Paris D, Melck D, Ríos P, Cristobo J, Castelluccio F, Gavagnin M, Avila C (2012a) Chemo-ecological studies on hexactinellid sponges from the Southern Ocean. Naturwissenschaften 99(5):353–368
Núñez-Pons L, Rodríguez-Arias M, Gómez-Garreta A, Ribera-Siguán A, Avila C (2012b) Feeding deterrence in Antarctic marine organisms: bioassays with the omnivore amphipod Cheirimedon femoratus. Mar Ecol Prog Ser 462:163–174
Orejas C, Gili JM, Arntz WE, Ros JD, López PJ, Teixido N, Filipe P (2000) Benthic suspension feeders, key players in Antarctic marine ecosystems? Contrib Sci 1:299–311
Oshel PE, Steele DH (1985) Amphipod Paramphithoe hystrix: A micropredator on the sponge Haliclona ventilabrum. Mar Ecol Prog Ser 23:307–309
Page MJ, West LM, Northcote PT, Battershill CN, Kelly-Shanks M (2005) Spatial and temporal variability of cytotoxic metabolites in populations of the New Zealand sponge Mycale hentscheli. J Chem Ecol 31:1161–1174
Paul V (1992) Ecological roles of marine natural products. Comstock Publications Association, Ithaca, NY
Pawlik JR (2012) Antipredatory defensive roles of natural products from marine invertebrates. In: Fattorusso E, Gerwick WH, Taglilatela-Scarfati (eds) Handbook of marine natural products. Springer, New York, p 1452
Peters KJ, Amsler CD, McClintock JB, van Soest RWM, Baker BJ (2009) Palatability and chemical defenses of sponges from the Western Antarctic Peninsula. Mar Ecol Prog Ser 385:77–85
Piel J, Butzke D, Fusetani N, Hui DQ, Platzer M, Wen GP, Matsunaga S (2005) Exploring the chemistry of uncultivated bacterial symbionts: antitumor polyketides of the pederin family. J Nat Prod 68:472–479
Puglisi MP, Paul VJ, Slattery M (2002) Biogeographic comparisons of chemical and structural defenses of the Pacific gorgonians Annella mollis and A. reticulata Mar Ecol Prog Ser 207:263–272
Roussis V, Vagias C, Tsitsimpikou C, Diamantopoulou N (2000) Chemical variability of the volatile metabolites from the Caribbean corals of the genus Gorgonia. Z Naturforsch C 55(5–6):431–441
Sharp JH, Winson MK, Porter JS (2007) Bryozoan metabolites: An ecological perspective. Nat Prod Rep 24:659–673
Silén L (1977) Polymorphism. In: Woollacott RM, Zimmer RL (eds) Biology of bryozoans. Academic, New York, pp 184–231
Slattery M, McClintock JB (1995) Population structure and feeding deterrence in three shallow-water Antarctic soft corals. Mar Biol 122:461–470
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of stadistics in biological research, Vol. Freeman WH and Co., NY
Stachowicz JJ, Lindquist N (2000) Hydroid defenses against predators: The importance of secondary metabolites versus nematocysts. Oecologia 124:280–288
Taboada T, Núñez-Pons L, Avila C (2013) Feeding repellence of Antarctic and sub-Antarctic benthic invertebrates against the omnivorous sea star Odontaster validus. Polar Biol 36(1):13–25
Teixidó N, Garrabou J, Arntz WE (2002) Spatial pattern quantification of Antarctic benthic communities using Landscape indices. Mar Ecol Prog Ser 242:1–14
Winston JE (1986) Victims of avicularia. Mar Ecol 7:193–199
Winston JE (1991) Avicularian behavior—a progress report. In Bigey FP (ed) Bryozoa living and fossil (pp 531–540). B Soc Sci Nat Ouest Fr Mem HS 1, Nantes, France
Winston JE (2009) Cold comfort: systematics and biology of Antarctic bryozoans. In: Krupnik I, Lang MA, Miller SE (eds) Smithsonian at the poles: contributions to international polar year science. Smithsonian Institute Scholar Press, Washington, DC, pp 205–221
Winston JE (2010) Life in the colonies: Learning the alien ways of colonial organisms. Integr Comp Biol 50(6):919–933
Winston JE, Bernheimer AW (1986) Hemolytic-activity in an Antarctic bryozoan. J Nat Hist 20:369–374
Acknowledgments
We are thankful to J. Vázquez, C. Angulo, F.J. Cristobo, and S. Taboada for their laboratory support. We are also very grateful for the helpful suggestions of the reviewers. In this work, we used extracts from previous projects (ECOQUIM and ACTIQUIM), and for this reason, we want to thank W. Arntz, the R/V Polarstern, and the BIO Hespérides crew. We would like to thank as well the Unidad de Tecnología Marina (UTM-CSIC) and the crew of Las Palmas vessel for all their logistic support. Special thanks are also given to the “Gabriel de Castilla BAE” crew for their help and to the participants of the 4th Australarwood Meeting in Townsville for their useful comments. This research was developed in the framework of the ACTIQUIM-I and II projects (CGL2007-65453/ANT, CTM2010-17415/ANT) with the financial support of the Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Figuerola, B., Núñez-Pons, L., Moles, J. et al. Feeding repellence in Antarctic bryozoans. Naturwissenschaften 100, 1069–1081 (2013). https://doi.org/10.1007/s00114-013-1112-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-013-1112-8