Abstract
The adhesion and aggregation are characteristic attributes of probiotic strains belonging to Lactobacillaceae genus. Due to these properties the host organisms can avoid colonisation of the intestinal tract by enteropathogenic bacteria. The presented research includes a comparison of the properties of various strains belonging to different Lactobacillaceae species and isolated from different sources The aim of this study was to investigate the ability of Lactocaseibacillus rhamnosus, Lactiplantibacillus plantarum, and Lactobacillus strains (L. acidophilus, L. gasseri, L. ultunensis) from probiotic products and clinical specimens to direct and competitive adherence to Caco-2 and HT-29 cell lines. Furthermore, the ability of lactobacilli and enteropathogenic bacteria, E. coli, E. faecalis, and S. Typhimurium, to auto- and co-aggregation was also investigated.
The results showed that all tested strains adhered to Caco-2 and HT-29 cell lines. Though, the factor of adhesion depended on the species and origin of the strain. L. rhamnosus strains showed a lowest degree of adherence as compared to L. plantarum and Lactobacillus sp. strains. On the other side both, L. rhamnosus and L. acidophilus strains reduced the pathogenic bacteria in competition adherence test most effectively. All tested lactobacilli strains were characterised by auto- and co-aggregation abilities, to various degrees. The properties of Lactobacillaceae strains analysed in this study, like adhesion abilities, competitive adherence, auto- and co-aggregation, may affect the prevention of colonisation and elimination of pathogenic bacteria in gastrointestinal tract.
Similar content being viewed by others
Introduction
According to the definition of the World Health Organization, probiotics are live microorganisms that, when administered in the appropriate amount, cause beneficial effects for the host organism (FAO/WHO 2002; Hill et al. 2014). Probiotic strains are applied in the production of various types of food products: fermented drinks, vegetables, and meats. The most common group of probiotics are lactic acid bacteria, especially strains from the family of Lactobacillaceae and Bifidobacterium, which belong to the gastrointestinal microbiota, and can be found most often in functional foods, medicinal products, dietary supplements, or medical devices (Monteagudo-Mera et al. 2019). The most important benefits of probiotic bacteria ingestion comprise stimulation of the immune system, production of antibacterial agents, regulation of the composition of the intestinal microbiota (Shehata et al. 2019), anti-mutagenic (eg. binding and transformation of mutagens or inhibition of the conversion of pro-mutagens to anti-mutagens) and anti-cancer properties (Prazdnova et al. 2022). Strains identified as a probiotic must demonstrate the ability to adhere to the mucous epithelial cells, cell lines, and should also be characterised by the ability to reduce the pathogenic microorganisms adhesion to the host cell surface (FAO/WHO 2002). The adhesion of microorganisms to the surface of intestinal cells is a way to extends the colonisation, which is important for the modulation of the immune response (Morita et al. 2002). Moreover, it may also influence the repair processes occurring in the injured intestinal mucosa (Morita et al. 2002). This behavior is one of the mechanisms that protect the host organism against pathogenic microorganism’s colonisation (Piątek et al. 2012).
Adhesion is a complicated process that enables microbes to adhere to other cells or surfaces (Duary et al. 2011; Paliwoda and Nowak 2017). The course of adhesion is influenced by many different factors, such as elements present in the cell wall, proteins, intestinal mucus, or environmental conditions (Paliwoda and Nowak 2017; Monteagudo-Mera et al. 2019). Initially, this process is based on physical interactions between surfaces, such as van der Waals forces or electrostatic interactions (Lewandowska et al. 2005; Behbahani et al. 2019). After attachment to the epithelial surface, the interaction between bacterial adhesins and receptors located in the epithelium plays an essential role (Lewandowska et al. 2005). The cell surface of the Lactobacillaceae bacteria contains capsular polysaccharides, teichoic and lipoteichoic acids, as well as various surface proteins (ex. mucin-binding protein, fibronectin-binding protein, collagen-binding protein) and lipoproteins. All of them allow the adhesion of these bacteria and the formation of biofilms on surfaces (Paliwoda and Nowak 2017; Archer et al. 2018; Monteagudo-Mera et al. 2019). On the other hand, structures and substances existing in the digestive tract, like mucin, extracellular matrix or lectin-like proteins, facilitate colonisation of probiotic strains (Grigoryan et al. 2018). The ability of Lactobacillaceae strains for auto-aggregation (an aggregation of bacteria belonging to the same strain) and co-aggregation (an aggregation of bacteria belonging to different species and strains) are related to the adhesion capacity (Kos et al. 2003; Collado et al. 2007; Hojjati et al. 2020). The natural colonisation process can be monitored and tested using cell lines. The colorectal adenocarcinoma cells, Caco-2 (non mucus secreting) and HT-29 (mucus secreting) are the most commonly used cell lines in the Lactobacillaceace in vitro adhesion studies (Sharma and Kanwar 2017). The Caco-2 cell line express morphological and functional differentiation in vitro and show characteristics of mature enterocytes. In turn, the HT-29 line shows a typical epithelial cell morphology, producing large amounts of mucus (Chauviere et al. 1992; Duary et al. 2011; Sharma and Kanwar 2017).
The resident gastrointestinal microbiota in vivo provides protection for the host against possible colonisation by the pathogenic bacteria (and play an important role in activating the immune system against these pathogens (Alp and Kuleasan 2019). Several reports have already documented the ability of probiotic lactobacilli and bifidobacteria to inhibit mucosa colonisation and invasion by pathogenic strains (Gopal et al. 2001; Ohashi and Ushida 2009). This may be associated with different mechanisms like: competition for nutrients and energy sources, which prevent pathogenic microorganisms’ growth and reproduction in the intestine (Cummings and Macfarlane 1997), production of antimicrobial substances by Lactobacillaceae strains (Chichlowski et al. 2007), competition for receptors of eukaryotic cells (Fonesca et al. 2021), immunomodulation (Fonesca et al. 2021), the intestinal barrier or co-aggregation abilities (Kos et al. 2003; Collado et al. 2007; Hojjati et al. 2020; Fonesca et al. 2021). If opportunities for pathogenic bacteria to adhere to host cells are reduced by the probiotic occupation of these sites, the incidence of infections may be reduced. According to Chapman et al. (2014), it is suggested that due to fewer sites being available to the pathogen, a greater reduction of infection occurrences is likely. Probably an application of probiotics as a method of prevention, can be more beneficial than medical treatment of infections. Many authors use three different variants of the study (competition assay, inhibition assay and displacement assay) to assess competitive exclusion. Li et al. (2008) showed that out of those three assays, the competition one showed the largest suppression of mucus adhesion both for the pathogens and lactobacilli. The displacement and inhibition assays exposed that, with respect to the addition order of the bacteria, those that were added latter had the predominance over bacteria that were already present. Many researchers have previously demonstrated protective effects against the attachment of a variety of enteric pathogenic bacteria, including E. coli, S. Typhimurium or E. faecalis, as the consequence of acidification with lactic acid (Ogawa et al. 2001; Markowiak and Śliżewska 2018), secreted nonacid products (Markowiak and Śliżewska 2017; Kerry et al. 2018), and interference with attachment to receptors or spaces, all of which may occur both directly and indirectly (Hirano et al. 2003).
Aggregation is associated with the surface of the bacterial cells and secreted substances, such as exopolysaccharides. These factors may play a significant role in the strength and speed of interactions between cells (Rajab et al. 2020). The ability to high auto-aggregation may also affect the longer duration of these strains in the digestive tract (Rajab et al. 2020). Rajab et al. (2020) suggest that auto- and co-aggregation properties may also depend on the length of bacterial cells. Longer cells present bigger surface areas, therefore, their aggregation is greater than in bacteria with short cells or spherical shape (Rajab et al. 2020). Some researchers have also reported that adherence and auto-aggregation of Lactobacillus cells are closely associated (Tuo et al. 2013; Celebioglu and Svennson 2018). On the other side, some scientists described, that strains with low ability to aggregation and co-aggregation may be characterised by a high degree of adhesion, which is opposite to the generally prevailing opinion (Alp and Kuleasan 2020).
The aim of the presented study was to investigate the possibility of protection of host organism against enteropathogenic bacteria colonisation, created by Lactobacillaceae strains derived from different sources. The characteristic of clinical strains that may have potential probiotic properties were compared with strains derived from available probiotic products and well characterized probiotic strain L. rhamnosus GG. The above goal was achieved through direct and competitive adherence of lactobacilli and selected standard enteropathogenic strains to enterocytes-like cell lines, the Caco-2 and HT-29. Moreover, auto- and co-aggregation of the lactobacilli and enteropathogenic bacteria, as processes impeding adherence, were investigated.
Materials and methods
Bacterial strains
The Lactobacillaceae strains used in this study were isolated from the probiotic products (dietary supplements, food for special medical purposes), present on the market in Poland, and from clinical material (swabs taken from cervix or anus of a healthy women, were collected at the Departament of Pharmaceutical Microbiology, Medical University of Warsaw, Poland). The following strains: Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum, L. acidophilus, L. gasseri and L. ultunensis were tested (Table 1). Moreover, L. rhamnosus GG (ATCC 53103), the most popular strain used in probiotic products (Capurso 2019), was used as a reference strain. All strains were identified by API 50 CHL tests (bioMérieux, France) and MALDI-TOF MS (ALAB Laboratory, Warsaw, Poland). The strains of the Lactobacillaceae family were grown on the De Man Rogosa and Sharpe Agar (MRS-Agar, Merck Millipore, Germany) in an atmosphere with 5% CO2 at 37 °C for 48–72 h.
As exemplary enteropathogenic strains E. coli ATCC 8739, E. faecalis ATCC 29212 and S. Typhimurium ATCC 14028 were used. All strains were cultivated on Tryptic Soy Agar (Difco, Thermo Fisher Scientific, USA) at 37 °C for 24 h.
Cell lines
The Caco-2 and HT-29 human colon carcinoma cell lines were purchased from American Type Culture Collection. Caco-2 cells were grown in Dulbeccoʼs modified Eagleʼs minimal essential medium DMEM (Gibco, Thermo Fisher Scientific, USA), supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 1% nonessential amino acids solution (Sigma–Aldrich, USA), penicillin (100 U/mL) (Sigma–Aldrich) and streptomycin (100 μg/mL) (Sigma–Aldrich). HT-29 cells were cultured in the DMEM medium supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 μg/mL). Incubation of both cell lines was carried out at 37 °C in a 95% (v/v) humidified atmosphere with 5% (v/v) CO2. Caco-2 and HT-29 cultures were incubated for 20 days, to promote differentiation, and the medium was replaced every 24–48 h.
Adhesion assay
For the adhesion assay Caco-2 cells and HT-29 cells, 20 days old cultures, were used. The cultures were grown until 85–95% of the surface areas covered. The medium was completely removed 24 h before adhesion assay with fresh DMEM medium without antibiotics. Prior to the assay, the cells were washed twice with phosphate-buffered saline (pH 7.4). Adhesion of various lactic acid bacteria (LAB) strains to Caco-2 and HT-29 cell lines was carried out with the method described by Lebeer et al. (2012) and Piątek et al. (2012) with necessary modifications. Acquired Caco-2 and HT-29 cells were grown at 37 °C in a humidified atmosphere of 5% CO2 in 12-well culture plates, starting from a density 4–5 × 104 cells/cm2. After 20 days and before adhesion assay, the cells were washed with PBS buffer (Gibco) and counted using a haematocytometer chamber for the three different cultures wells, and the results were statistically evaluated.
LAB strains were incubated on the De Man Rogosa and Sharpe Broth (MRS-Broth, Merck Millipore) in an atmosphere with 5% CO2 at 37 °C for 20 h. After the incubation, the microbial culture was centrifuged at 4500×g for 10 min and the precipitate was washed twice with 0.9% NaCl. Bacterial cells were suspended in 1 mL of DMEM without antibiotics and fetal bovine serum to the density 1 × 107 to 1 × 108 CFU per mL, at a multiplicity of infection (MOI) of 500:1. The bacteria were then incubated with Caco-2 or HT-29 cells for 90 min at 37 °C in a 5% CO2 atmosphere.
Non-adhering bacteria were removed from both the Caco-2 and HT-29 cells by rinsing three times with PBS buffer. To release attached bacterial cells, the Caco-2 and HT-29 cultures were treated with a solution of 1% Triton X-100 (Sigma–Aldrich). The lysis was performed on ice for 10 min. The lysates were centrifuged at 4500×g for 10 min and the precipitate was washed twice with PBS. Finally, the precipitate was suspended in 1 mL of 0.9% NaCl, inoculated, and the number of adhered bacteria quantified according to the serial dilution method.
Serial decimal dilutions ranging from (10– 1 to 10– 5 CFU per mL) were prepared and plated out on the solid MRS medium. After the incubation of plates with 5% CO2 at 37 °C for 72 h, the number of Lactobacillaceae colonies was counted. The dose of bacteria used for the adhesion process, the number of adhering bacteria for used inoculum, and the number of adhering bacteria per 100 Caco-2 and HT-29 cells were also calculated. Three independent experiments were conducted and the results were statistically evaluated.
Competitive adhesion of Lactobacilli and pathogenic bacteria
To study the competitive adhesion of pathogenic bacteria and Lactobacillaceae strains to Caco-2 and HT-29 cell lines, the competition assay tests were performed (Candela et al. 2008). Cell cultures and Lactobacillaceae strains with a density of 107–108 CFU/mL were prepared for the adhesion tests. In the case of pathogenic strains—E. coli, E. faecalis and S. Typhimurium, after an overnight cultivation (incubation in Tryptic Soy Broth, for 20 h, at 37° C in aerobic conditions), the suspensions with a density of 107–108 CFU mL (MOI 100:1) was prepared. For the competition adhesion assay, 1 mL of lactobacilli and 1 mL of pathogenic bacteria suspensions were added simultaneously to the same well of the plate with cell lines and then incubated at 37 °C with 5% CO2 for 90 min. After an incubation, all the non-adhered bacteria were removed by the method described above. Afterwards, the serial decimal dilutions ranging from (10– 1 to 10– 5 CFU/mL) were prepared and plated out on Tryptic Soy Agar for pathogenic bacteria. After incubation at 37 °C for 24 h in aerobic conditions, the number of bacterial colonies were calculated. The dose of bacteria used for the adhesion process, the number of adhering bacteria for used inoculum, and the reduction of adhering bacteria after incubation with Lactobacillaceae were calculated. Three independent experiments were conducted and the results were statistically evaluated.
Auto-aggregation and co-aggregation
Auto-aggregation and co-aggregation analysis were performed in accordance with Kos et al. (2003) and Tuo et al. (2013) with modifications. Lactobacillaceae strains were grown for 20 h in a MRS broth in atmosphere enriched 5% CO2 at 37 °C. The bacteria were centrifuged at 5000×g for 20 min, washed twice with PBS and then re-suspended in PBS. The level of absorbance (A600) has been adjusted to a value of 0.25 ± 0.05 to standardize the number of bacteria (107–108 CFU/mL). The suspension (4 mL) was vortexed (Ainitial) and then incubated for 2 h at 37 °C (A2h). Auto-aggregation was expressed with equation:
Bacterial suspensions for co-aggregation analysis were prepared with the same method as described for the auto-aggregation test. Two mL of both Lactobacillaceae (Aprob) and pathogenic strain (Apath) suspensions were mixed and then incubated at 37 °C without agitation. After two hours, the absorbance (Amix) was measured. Percentage of co-aggregation bacteria was determined as:
In the equation presented above, the factors Apath and Aprob represent the absorbance of separate strains before incubation and Amix represents the absorbance of mixed bacterial suspensions after 2 h of incubation.
Statistical analysis
All results were expressed as the mean and standard deviation of three independent experiments. The one-way analysis of variance (one-way ANOVA), followed by post-hoc Tukey`s test for multiple comparisons. Data that showed no normal distribution were analysed using the non-parametric Kruskal–Wallis test followed by a pairwise comparison. P values < 0.05 were considered significant. Pearson correlation analysis was performed for adhesion to both cell lines versus auto-aggregation, with statistical significance at p < 0.05. Statistical analysis was conducted using SPSS software (version 28.0.1.0, IBM, IL, USA).
Results
Lactobacillaceae adhesion
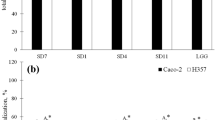
Adhesion of bacteria belonging to Lactobacillaceae family was performed on the Caco-2 and HT-29 tumor cell lines. The highest bacteria adhesion to the Caco-2 cell line (Fig. 1) was observed in the case of L. plantarum species (LpF—2800 CFU/100 cells Caco-2 and LpH—2000 CFU/100 cells Caco-2) and L. ultunensis (LaL—3150 CFU/100 cells Caco-2), the obtained values differed statistically (p < 0.05). In turn, the weakest adhesion was observed in the case of strains belonging to L. rhamnosus (LrA—15 CFU/100 cells Caco-2, LrB—45 CFU/100 cells Caco-2 and LrD—75 CFU/100 cells Caco-2), L. plantarum LpG—130 CFU/100 cells Caco-2 and L. acidophilus LaK—35 CFU/100 cells Caco-2. These strains did not show statistically significant differences compared to the control strain (LGG—62 CFU/100 cells Caco-2) (p < 0.05).
Adhesion of Lactobacillaceae strains to Caco- 2 and HT-29 cell lines, represents as average value and ± SD of adherent bacteria from 3 experiments per 100 Caco-2/HT-29 cells. Samples with different letters are significantly different (p < 0.05). Strain symbols are presented in Table 1
In the case of the second cell line, HT-29 (Fig. 1), the highest adhesion was observed with strains of L. plantarum (LpF—2200 CFU/100 cells HT-29, LpG—1850 CFU/100 cells HT-29 and LpH—1800 CFU/100 cells HT-29), L. gasseri (LaI—1420 CFU/100 cells HT-29), L. acidophilus (LaJ—1650 CFU/100 cells HT-29) and L. ultunensis (LaL—4500 CFU/100 cells HT-29), the obtained values differed statistically (p < 0.05), exception strains LpG and LpH (p > 0.05). The weakest adhesion was observed in the case of strains belonging to L. rhamnosus (LrA—30 CFU/100 cells HT-29, LrB—50 CFU/100 cells HT-29, LrC—40 CFU/100 cells HT-29 and LrD—50 CFU/100 cells HT-29). These strains did not show statistically significant differences compared to the control strain (LGG—64 CFU/100 cells HT-29) (p < 0.05).
All tested Lactobacillaceae strains were characterised by the ability to reduce the adherence of pathogenic microorganisms—S. Typhimurium, E. coli and E. faecalis (Figs. 2 and 3). Lactobacillus sp. mostly inhibits the growth of E. coli strain (1.3–1.5 log CFU/mL), result was statistically significant, p < 0.05, on the Caco-2 cell line (Fig. 2). Moreover Lacticaseibacillus strains LrA and LrD also inhibit the adhesion of E. coli (1.4 log CFU/mL and 1.3 log CFU/mL, respectively, p < 0.05). On the other hand, statistically significant (p < 0.05) inhibition of the growth of E. faecalis was observed after incubation with the Lacticaseibacillus strains (LrB—1.1 log CFU/mL, LrD—1.0 log CFU/mL), Lactiplantibacillus LpF—0.9 log CFU/mL, and Lactobacillus (LaK—1.0 log CFU/mL and LaL—1.4 log CFU/mL). The statistically significant reduction (p < 0.05) of S. Typhimurium strains was manifested by Lacticaseibacillus (LrA—1.0 log CFU/mL, LrC—0.9 log CFU/mL, LrD—0.7 log CFU/mL) and Lactobacillus sp. strains (LaI—0.9 log CFU/mL, LaJ—0.8 log CFU/mL, LaL—0.6 log CFU/mL). The weakest reduction of S. Typhimurium adhesion was observed after incubation with Lactiplantibacillus strains (growth reduction 0.2–0.4 log CFU/mL, not statistically significant, p > 0.05).
Competition adhesion test on Caco-2 cell line. Average value and ± SD of adherent bacteria from 3 experiments. *Significant differences among strains versus pathogenic bacteria. Strain symbols are presented in Table 1
Competition adhesion test on HT-29 cell line. Average value and ± SD of adherent bacteria from 3 experiments. *Significant differences among strains versus pathogenic bacteria. Strain symbols are presented in Table 1
In the studies focused on the HT-29 cell line (Fig. 3), the reduction of growth of E. coli about 1.0 log CFU/mL, statistically significant (p < 0.05), was observed after incubation with Lacticaseibacillus strains (LrA, LrB and LrD), Lactiplantibacillus LpE and Lactobacillus (LaI and LaK). The weakest reduction of adhesion was observed in the case of Lactiplantibacillus strains LpF, LpG, LpH—about 0.2–0.3 log CFU/mL (not statistically significant, p > 0.05). The reduction of E. faecalis was observed in case of Lacticaseibacillus (LrC—0.4 log CFU/mL and LrD—0.9 log CFU/mL), Lactiplantibacillus LpG—0.3 log CFU/mL and Lactobacillus (LaJ—0.5 log CFU/mL and LaL—0.8 log CFU/mL). In turn, statistically significant inhibition of the growth of S. Typhimurium strains was observed after incubation with strains of the genus Lactiplantibacillus (LpF—0.5 log CFU/mL, LpG—0.6 log CFU/mL, LpH—0.6 log CFU/mL) and Lactobacillus (LaI—0.8 log CFU/mL, LaJ—0.5 log CFU/mL, LaL—0.4 log CFU/mL), while Lacticaseibacillus strains did not inhibit the growth of S. Typhimurium at a statistically significant level.
Auto-aggregation and co-aggregation
The level of auto-aggregation of Lactobacillaceae strains (Table 2) and co-aggregation between Lactobacillaceae and pathogenic bacteria (Fig. 4) after two hours of incubation were related to tested strains. The auto-aggregation values were in the range from 8.4% in clinical L. plantarum (LpE) isolate to 21.4% in L. acidophilus strain isolated from probiotic product (LaK). The auto-aggregation of L. rhamnosus GG strain was 13.1%, which is a value on a similar level as Lacticaseibacillus (LrB, LrC), Lactiplantibacillus (LpF, LpH) and L. acidophilus LaJ (p < 0.05). Pathogenic strains in the conducted study showed a lower ability to auto-aggregation, at the level from 5.5% for E. faecalis to 12.2% for S. Typhimurium.
Co-aggregation of Lactobacillaceae strains with pathogenic bacteria after 2 h incubation at 37 °C. Average value and ± SD from 3 experiment. Different letters indicate the symbols of strains that differ statistically significantly (p < 0.05). Strain symbols are presented in Table 1
The highest co-aggregation was observed between Lactobacillaceae and E. faecalis strains (approx. 33.8%), whereas the lowest was noticed in tests conducted with S. Typhimurium (approx. 28.7%) (Fig. 4). The clinical L. plantarum (LpE) was characterised by the lowest auto-aggregation (8.4%) and expressed the strongest co-aggregation ability with all tested probiotic strains, with values ranged 37.7–38.6% (depending on the pathogenic strains). The LGG strain was characterised by co-aggregation at the level of about 22% to E. coli and S. Typhimurium, and 32% to E. faecalis. The lowest co-aggregation was observed in L. rhamnosus (LrB) clinical strain in test with E. coli-only 21.2%. This strain was also characterised by low co-aggregation with S. Typhimurium (22.4%), weak adhesion to both Caco-2 and HT-29 cell lines and the lowest ability to reduce E. coli in competition test.
There was no correlation between auto-aggregation and adhesion to both tested cell lines of the tested strains (p > 0.05).
Discussion
Probiotic strains, according to FAO/WHO guidelines, must present antagonistic properties against pathogenic microorganisms and present the ability to adhere to mucin and epithelial cells (FAO/WHO 2002). The pro-health effect of probiotic strains is manifested by hindering the colonisation of the gastrointestinal tract mucosa if it is caused by pathogenic strains. The most commonly used in adherence in vitro models, structurally and functionally similar to human enterocytes are colon adenocarcinoma cells (Duary et al. 2011). Good correlation between adhesion carried out in vitro, applying Caco-2 and HT-29 cell lines and adhesion in vivo, has been demonstrated in numerous studies conducted (Nowak and Motyl, 2017; Jose et al. 2017). Caco-2 cells have the ability to form a brush border, and create tight connections with each other (similarly to enterocytes). Those cell lines also have the ability to produce some enzymes (e.g., alkaline phosphatase, sucrase and aminopeptidase) and systems that transport substances from the lumen of the gastrointestinal tract directly into the bloodstream. As a result, cell lines used in this study show a functional similarity to the epithelium of the small intestine, imitating the natural in vivo conditions of the gastrointestinal tract (Hilgendorf et al. 2000). To be designated as probiotic, bacteria must adhere to mucosal epithelial cells lining the gut, which also depends on the number of bacteria added.
The value of adhesion L. rhamnosus GG strain obtained in this study (62 CFU/100 cells Caco-2 and 64 CFU/100 cells HT-29) was consistent with the results achieved by Gopal et al. (145 CFU/100 Caco-2 cell lines and 105 CFU/100 HT-29 cell lines) (Gopal et al. 2001). Deepika et al. (2009) conducted comparative studies of the adhesion of L. rhamnosus GG to Caco-2 cells under different growth conditions of cells. In the study, 20–200 bacteria per Caco-2 cell for 4–13 h cultures were used. Researchers suggested that differences in adhesion results may be due to different physiological conditions of bacterial cells, differences in bacterial cell culture and media conditions, and cell harvesting at different time points (Deepika et al. 2009). Moreover, the type of buffer and intensity of washing of non-adherent cells may also play a significant role (Hojjati et al. 2020). In our study L. plantarum strains were characterised by a much higher degree of adhesion than L. rhamnosus. The adhesion index of L. plantarum to Caco-2 cells depended on the strains (130–2820 CFU/100 cells Caco-2). The received results were on a similar level with the results achieved by Candela et al. (2008), amounting to 2530 CFU/100 Caco-2 cells for L. plantarum Bar10. The adhesion of Lactobacillus sp. strains, obtained in our study varied around the range of 517–4531 CFU/100 HT-29 cells, depending on the strains and their origin. Such variances in observed results was also seen in Lankaputhra and Shah (1998) research, where adherence of different L. acidophilus strains varied between 4 and 380 CFU/100 HT-29 cells.
In agreement with a previous study (Li et al. 2008), our results exposed that the adhesion ability of Lactobacillaceae strains to cell line was strains-specific and varied even within the same species, moreover, it could be associated with the strains origin, which is in line with the observations of other researchers (Sharma and Kanwar 2017; Rajab et al. 2020). These observations were aligned to the results seen in previous studies (Li et al. 2008; Mandal et al. 2016). Our observations also indicated the difference in adhesion index between two tested lines—Caco-2 and HT-29. The level of mucus secreted by the cells may play a significant role in adhesion (Sharma and Kanwar 2017).
The particular LAB strains in the present study were evaluated with regard to their ability to inhibit the adhesion of E. coli, S. Typhimurium and E. faecalis, to Caco-2 and HT-29 cells. The strongest inhibition of E. coli adherence was observed in the case of incubation with L. acidophilus strains on Caco-2 and HT-29 cells. Fonesca et al. (2021) reported inhibition of adhesion of E. coli in competition test by five different LAB strains on Caco-2 cell line at the significant level about 0.7 to 1.7 log CFU/mL. These authors reported also, that only two strains were able to reduce significantly E. coli strain on HT-29 cell line. Similar differences between the reduction of the number of pathogenic bacteria and the tested cell line were also noticeable in our work. In our study most of tested strains also reduce the adhesion of E. coli on Caco-2 cell line, at level about (0.5–1.5 log CFU/mL), only four strains probably were not able to reduce pathogenic strains (LrB, LrC, LpE, and LpH). In the case of HT-29 cell line only three strains of L. plantarum (LpF, LpG, and LpH) were characterised by very low reduction of E. coli cells adhesion.
Gopal et al. (2001) showed a 28–54% decrease of E. coli attachment to different epithelial cells with L. acidophilus; in the case of L. rhamnosus, the observed adherence reduction was in the range of 18–23%. All strains tested in our study demonstrated reduction of adhesion of pathogenic bacteria. The results suggest that the strains used in the presented study could prevent colonisation of the gastrointestinal tract by relevant pathogens such as E. coli, S. Typhimurium and E. faecalis.
The ability of Lactobacillaceae to aggregation can form a barrier and may exclude pathogenic strains from adhesion to gastrointestinal tract (Klopper et al. 2018). This property depends significantly on the incubation time (Piwat et al. 2015). Piwat et al. (2015) reported auto-aggregation of L. rhamnosus strains from the human oral cavity in the range more than 50% and L. plantarum about 50% after 24 h of incubation. Sophatha et al. (2015) also reported auto-aggregation of L. rhamnosus strains after 24 h incubation at the level of 55–60%. The results achieved by Grigoryan et al. (2018) were in the compartment of about 15% for L. delbrueckii subsp. bulgaricus to more than 70% to L. helveticus after 24 h incubation. The auto-aggregation of twenty LAB strains reported by Tuo et al. (2013) after 5 h incubation ranging from 24 to 41%, where the highest value has been reached by control L. rhamnosus GG strain. Collado et al. (2007) carried out auto-aggregation test focusing on to examine 2, 16, 20 and 24 h time periods. Results achieved in this test for L. plantarum was comparable to the results obtained in our study. The auto-aggregation of L. plantarum ATCC 14917 after 2 h incubation was reaching value about 25% (Wang et al. 2018) while for L. plantarum DM 69, after the same time of incubation, this value was 59% (Mohanty et al. 2019). In our study, for different L. plantarum strains this value ranged about 8.4–20.5%. D`Alessandro et al. (2021) tested auto-aggregation of vaginal lactobacilli strains. After 5 h of incubation some L. gasseri and L. crispatus strains achived the value of auto-aggregation above 90%, compared to the LGG control strain achieving auto-aggregation of 23% at the same time (D`Alessandro et al. 2021), while in our study, after 2 h incubation, this value for LGG strain reached about 13%. Kos et al. (2003) showed how the auto-aggregation of L. acidophilus strains were changing during 5 h of incubation, starting from about 25% after 1 h, to almost 70% after 5 h period. The same researchers tested also co-aggregation of L. acidophilus strains with pathogens—E. faecium, E. coli and S. Typhimurium. After 5 h of incubation the received results were as follow: 19%, 15% and 16%, respectively (Kos et al. 2003). Sophatha et al. (2020) tested co-aggregation of Lactobacillus strains after 24 h of incubation with pathogens such as enterotoxigenic E. coli, non-enterotoxigenic E. coli, S. enterica, ranges between 35 and 66%. Co-aggregation of different Lactobacillaceae strains with E. coli O157:H7 after 5 h incubation ranges between 21 and 32% (Sophatha et al. 2020). The co-aggregation results depend on the auto-aggregation properties between Lactobacillus and pathogen strains (Sophatha et al. 2020) and from the time of incubation (Piwat et al. 2015). In this study co-aggregation between L. rhamnosus and pathogenic strains, E. coli and S. Typhimurium, probable depends on the origin of the tested strain. In the case of clinical strains, the co-aggregation value ranged between 21–22% for E. coli and 22–23% for S. Typhimurium, in contrast to the strains isolated from probiotic products where these values were reached respectively 25–36% and 26–36%. Kowalska et al. (2020) reported that the co-aggregation rates may also depend on the source of pathogenic strains. The authors observed the highest co-aggregation for L. rhamnosus LOCK 1131 with S. Typhimurium ATCC 13311—84%, and the lowest for L. casei LOCK 1132 and S. Typhimurium ATCC 14028—11% (Kowalska et al. 2020).
Conclusions
In conclusion, it seems that most of the Lactobacillaceae sp. strains may play a role in the protection of the gastrointestinal mucosa against colonisation with pathogenic strains, such as E. coli, S. Typhimurium or E. faecalis. Studies on the adherence of Lactobacillaceae strains and their competitive adherence to cell lines indicate the protective function of these strains. Similarly, the phenomenon of bacterial auto-aggregation and co-aggregation may reduce the degree of mucosa colonisation by pathogenic strains.
Availability of data and material
All data generated and analysed during the study are included in the manuscript.
Code availability
Not applicable.
References
Alp D, Kuleaşan H (2019) Adhesion mechanisms of lactic acid bacteria: conventional and novel approaches for testing. World J Microbiol Biotechnol 35(10):156. https://doi.org/10.1007/s11274-019-2730-x
Alp D, Kuleasan H (2020) Determination of competition and adhesion abilities of lactic acid bacteria against gut pathogens in a whole-tissue model. Biosci Microbiota Food Health 39:250–258. https://doi.org/10.12938/bmfh.2020-033
Archer AC, Kurrey NK, Halami PM (2018) In vitro and anti-inflammatory properties of native Lactobacillus fermentum and Lactobacillus delbrueckii spp. J Appl Microbiol 125:243–256. https://doi.org/10.1111/jam.13757
Behbahani BA, Noshad M, Falah F (2019) Inhibition of Escherichia coli adhesion to human intestinal Caco-2 cells by probiotic candidate Lactobacillus plantarum strain L15. Microb Pathog 136:1–7. https://doi.org/10.1016/j.micpath.2019.103677
Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P (2008) Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol 125:286–292. https://doi.org/10.1016/j.ijfoodmicro.2008.04.012
Capurso L (2019) Thirty years of Lactobacillus rhamnosus GG A Review. J Clin Gastroenterol 53:S1–S41. https://doi.org/10.1097/MCG.0000000000001170
Celebioglu HU, Svensson B (2018) Dietary nutrients, proteomes, and adhesion of probiotic lactobacilli to mucin and host epithelial cells. Microorganisms 6(3):90. https://doi.org/10.3390/microorganisms6030090
Chapman CMC, Gibson GR, Rowland I (2014) Effects of single- and multi-strain probiotics on biofilm formation and in vitro adhesion to bladder cells by urinary tract pathogens. Anaerobe 27:71–76. https://doi.org/10.1016/j.anaerobe.2014.02.001
Chauviere G, Coconnier MH, Kerneis S, Fourniat J, Servin AL (1992) Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J Gen Microbiol 138:1689–1696. https://doi.org/10.1099/00221287-138-8-1689
Chichlowski M, Croom J, McBride BW, Havenstein GB, Koci MD (2007) Metabolic and physiological impact of probiotics or direct-fed-microbials on poultry: a brief review of current knowledge. Int J Poult Sci 6:694–704. https://doi.org/10.3923/ijps.2007.694.704
Collado MC, Surono I, Meriluoto J, Salminen S (2007) Indigenous dadih lactic acid bacteria: cell-surface properties and interactions with pathogens. J Food Sci 72:89–93. https://doi.org/10.1111/j.1750-3841.2007.00294.x
Cummings JH, Macfarlane GT (1997) Role of intestinal bacteria in nutrient metabolism. Clin Nutr 16:3–11. https://doi.org/10.1016/S0261-5614(97)80252-X
D’Alessandro M, Parolin C, Bukvicki D, Siroli L, Vitali B, De Angelis M, Lanciotti R, Patrignani F (2021) Probiotic and metabolic characterization of vaginal lactobacilli for a potential use in functional foods. Microorganisms 9(4):833. https://doi.org/10.3390/microorganisms9040833
Deepika G, Green RJ, Frazier RA, Charalampopoulos D (2009) Effect of growth time on the surface and adhesion properties of Lactobacillus rhamnosus. J Appl Microbiol 107:1230–1240. https://doi.org/10.1111/j.1365-2672.2009.04306.x
Duary RJ, Rajput YS, Batish VK, Grover S (2011) Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Indian J Med Res 134:664–671. https://doi.org/10.4103/0971-5916.90992
Fonesca HC, de Sousa MD, Ramos CL, Dias DR, Schwan RF (2021) Probiotic properties of Lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob Proteins 13:102–112. https://doi.org/10.1007/s12602-020-09659-2
Food and Agriculture Organization/World Health Organization (FAO/WHO) (2002) Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. Food and Agriculture Organization/World Health Organization, London
Gopal PK, Prasad J, Smart J, Gill HS (2001) In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int J Food Microbiol 67:207–216. https://doi.org/10.1016/s0168-1605(01)00440-8
Grigoryan S, Bazukyan I, Trchounian A (2018) Aggregation and adhesion activity of lactobacilli isolated from fermented products in vitro and in vivo: a potential probiotic strain. Probiotics Antimicrob Proteins 10:269–276. https://doi.org/10.1007/s12602-017-9283-9
Hilgendorf C, Spahn-Langguth H, Regard CG, Lipka E, Amidon GL, Langguth P (2000) Caco-2 versus Caco-2/HT-29-MTX co-cultured lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci 89:2548–2554. https://doi.org/10.1002/(SICI)1520-6017(200001)89:1%3c63::AID-JPS7%3e3.0.CO;2-6
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Hirano J, Yoshida T, Sugiyama T, Koide N, Mori I, Yokochi T (2003) The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro. Microbiol Immunol 47:405–409. https://doi.org/10.1111/j.1348-0421.2003.tb03377.x
Hojjati M, Behabahani BA, Falah F (2020) Aggregation, adherence, anti-adhesion and antagonistic activity properties relating to surface charge of probiotic Lactobacillus brevis gp104 against Staphylococcus aureus. Microb Pathog 147:1–9. https://doi.org/10.1016/j.micpath.2020.104420
Jose NM, Bunt CR, McDowell A, Chiu JZS, Hussain MA (2017) Short communication: a study of Lactobacillus isolates’ adherence to and influence on membrane integrity of human Caco-2 cells. Jl Dair Sci 100(10):7891–7896. https://doi.org/10.3168/jds.2017-12912
Kerry RG, Patra JK, Gouda S, Park Y, Shin HS, Das G (2018) Benefaction of probiotics for human health: a review. J Food Drug Anal 26(3):927–939. https://doi.org/10.1016/j.jfda.2018.01.002
Klopper KB, Deane SM, Dicks LMT (2018) Aciduric strains of Lactobacillus reuteri and Lactobacillus rhamnosus, isolated from human feces, have strong adhesion and aggregation properties. Probiotics Antimicrob Proteins 10:89–97. https://doi.org/10.1007/s12602-017-9307-5
Kos B, Suskovic J, Vukovic S, Simpraga M, Frece J, Matosic S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987. https://doi.org/10.1046/j.1365-2672.2003.01915.x
Kowalska JD, Nowak A, Śliżewska K, Stańczyk M, Łukasiak M, Dastych J (2020) Anti-Salmonella potential of new Lactobacillus strains with the application in the poultry industry. Pol J Microbiol 69:5–18. https://doi.org/10.33073/pjm-2020-001
Lankaputhra WEV, Shah NP (1998) Adherence of probiotic bacteria to human colonic cells. Biosci Microflora 17:105–113. https://doi.org/10.12938/bifidus1996.17.105
Lebeer S, Claes I, Tytgat HLP, Varhoeven TA, Marien E, Von Ossowski I, Reunanen J, Palva A, de Vos WM, De Keersmaecker SCJ, Vanderleyden J (2012) Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microb 78:185–193. https://doi.org/10.1128/AEM.06192-11
Lewandowska M, Olejnik A, Neuman M, Krepulec A, Piotrowska J, Teresiak A, Grajek W (2005) Comparative in vitro study on the adhesion of probiotic and pathogenic bacteria to different human intestinal cell lines. Biotechnologia 2:215–233
Li XJ, Yue LY, Guan XF, Qiao SY (2008) The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J Appl Microbiol 104:1082–1091. https://doi.org/10.1111/j.1365-2672.2007.03636.x
Mandal H, Jariwala R, Bagchi T (2016) Isolation and characterization of lactobacilli from human faeces and indigenous fermented foods for their potential application as probiotics. Can J Microbiol 62:349–359. https://doi.org/10.1139/cjm-2015-0576
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021. https://doi.org/10.3390/nu9091021
Mohanty D, Panda S, Kumar S, Ray P (2019) In vitro evaluation of adherence and anti-infective property of probiotic Lactobacillus plantarum DM 69 against Salmonella enterica. Microb Pathog 126:212–217. https://doi.org/10.1016/j.micpath.2018.11.014
Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103:6463–6472. https://doi.org/10.1007/s00253-019-09978-7
Morita H, He F, Fuse T, Ouwehand AC, Hashimoto H, Hosoda M, Mizumachi K, Kurisaki J (2002) Adhesion of lactic acid bacteria to Caco-2 cells and their effect on cytokine secretion. Microbiol Immunol 46:293–297. https://doi.org/10.1111/j.1348-0421.2002.tb02698.x
Nowak A, Motyl I (2017) In vitro anti-adherence effect of probiotic Lactobacillus strains on human enteropathogens. Biotechnol Food Sci 81(2):103–112
Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S (2001) Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecanoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl Environ Microb 67:1246–1252. https://doi.org/10.1128/AEM.67.3.1246-1252.2001
Ohashi Y, Ushida K (2009) Health-beneficial effects of probiotics: Its mode of action. Anim Sci J 80:361–371. https://doi.org/10.1111/j.1740-0929.2009.00645.x
Paliwoda A, Nowak A (2017) Factors determing the adhesive capacity of Lactobacillus bacteria. Post Mikrobiol 56:196–204. https://doi.org/10.21307/PM-2017.56.2.196
Piątek J, Gibas-Dorna M, Olejnik A, Krauss H, Wierzbicki K, Żukiewicz-Sobczak W, Glowacki M (2012) The viability and intestinal epithelial cell adhesion of probiotic strain combination- in vitro study. Ann Agr Env Med 19:99–102
Piwat S, Sophatha B, Teanpaisan R (2015) An assessment of adhesion, aggregation and surface charges of Lactobacillus strains derived from the human oral cavity. Lett Appl Microbiol 61:98–105. https://doi.org/10.1111/lam.12434
Prazdnova EV, Mazanko MS, Chistyakov VA, Bogdanova AA, Refeld AG, Kharchenko EY, Chikindas ML (2022) Antimutagenic activity as a criterion of potential probiotic properties. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-021-09870-9
Rajab S, Tabandeh F, Shahrky MK, Alahyaribeik S (2020) The effect of Lactobacillus cell size on its probiotic characteristics. Anaerobe 62:1–9. https://doi.org/10.1016/j.anaerobe.2019.102103
Sharma S, Kanwar SS (2017) Adherence potential of indigenous lactic acid bacterial isolates obtained from fermented foods of western himalayas to intestinal epithelial Caco-2 and HT-29 cell lines. J Food Sci Technol 54(11):3504–3511. https://doi.org/10.1007/s13197-017-2807-1
Shehata MG, Abu-Serie MM, El-Azoz NMA, El-Sohaimy SA (2019) In vitro assessment of antioxidant, antimicrobial and anticancer properties of lactic acid bacteria. Int J Pharmacol 15:651–663. https://doi.org/10.3923/ijp.2019.651.663
Sophatha B, Piwat S, Teanpaisan R (2020) Adhesion, anti-adhesion and aggregation properties relating to surface charges of selected Lactobacillus strains: study in Caco-2 and H357 cells. Arch Microbiol 202:1349–1357. https://doi.org/10.1007/s00203-020-01846-7
Tuo Y, Yu H, Ai L, Wu Z, Guo B, Chen W (2013) Aggregation and adhesion properties of 22 Lactobacillus strains. J Dairy Sci 96:4252–4257. https://doi.org/10.3168/jds.2013-6547
Wang W, He J, Daodong P, Wu Z, Guo Y, Zeng X, Lian L (2018) Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 13:1–16. https://doi.org/10.1371/journal.pone.0196231
Funding
Partial financial support was received from Medical University of Warsaw, project for young scientists FW15/PM1/16 and National Medicines Institute.
Author information
Authors and Affiliations
Contributions
AZR: designed the experiments, AZR, AK and KS: performed the experiments, AZR and AK: drafted the manuscript, AZR and ST: edited the manuscript and all authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performer by any of the authors.
Consent to participate
All participants agree with participation.
Consent for publication
All authors read and approved the final manuscript.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zawistowska-Rojek, A., Kośmider, A., Stępień, K. et al. Adhesion and aggregation properties of Lactobacillaceae strains as protection ways against enteropathogenic bacteria. Arch Microbiol 204, 285 (2022). https://doi.org/10.1007/s00203-022-02889-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02889-8