Abstract
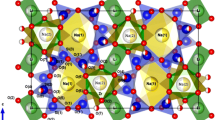
The minerals of the pearceite–polybasite group, general formula (Ag,Cu)16 M 2S11 with M = Sb, As, have been recently structurally characterized. On the whole, all the structures can be described as a regular succession of two module layers stacked along the c axis: a first module layer (labeled A), with general composition [(Ag,Cu)6(As,Sb)2S7]2−, and a second module layer (labeled B), with general composition [Ag9CuS4]2+. In detail, in the B layer of the pearceite structure silver cations are found in various sites corresponding to the most pronounced probability density function locations of diffusion-like paths. We have shown for the first time that the observed structural disorder in the B layer is strongly related to the fast ion conduction character exhibited by these minerals. This paper reports an integrated XREF, DSC, CIS and EPMA study on all the members of the pearceite–polybasite group. DSC and conductivity measurements pointed out that the 222 members show ionic-transitions at 340 K (arsenpolybasite-222) and 350 K (polybasite-222), whereas the 221 members have transitions at lower temperature, that is, 310–330 K (arsenpolybasite-221) and 335 K (polybasite-221). For the 111 members (pearceite and antimonpearceite), the transition occurs below room temperature at 273 K. In situ single-crystal X-ray diffraction experiments showed that these minerals present the same high temperature structure and are observed at room temperature either in their high temperature (HT) fast ion conductivity form or in one of the low temperature (LT) fully ordered (222), partially ordered (221) or still disordered (111) forms, with transition temperatures slightly above or below room temperature. The pearceite–polybasite group of minerals can be considered as a homogeneous series with the same aristotype, fast ion conducting form at high temperature. Depending upon the Cu content, an ordering occurs with transition temperatures related to that content: the lower the Cu content, the higher the transition temperature from the fast ion conducting HT form to the non-ion conducting form.









Similar content being viewed by others
References
Becker PJ, Coppens P (1974) Extinction within the limit of validity of the Darwin transfer equations. I. General formalism for primary and secondary extinction and their applications to spherical crystals. Acta Crystallogr A30:129–147
Belin R, Zerouale A, Pradel A, Ribes M (2001) Ion dynamics in the argyrodite compound Ag7GeSe5I: non-Arrhenius behavior and complete conductivity spectra. Solid State Ionics 143:445–455
Bindi L, Evain M, Menchetti S (2006a) Temperature dependence of the silver distribution in the crystal structure of natural pearceite, (Ag,Cu)16(As,Sb)2S11. Acta Crystallogr B62:212–219
Bindi L, Evain M, Menchetti S (2006b) Complex twinning, polytypism and disordered phenomena in the crystal structures of antimonpearceite and arsenpolybasite. Can Mineral (in press)
Boucher F, Evain M, Brec R (1993) Distribution and ionic diffusion path of silver in γ-Ag8GeTe6: a temperature dependent anharmonic single crystal structure study. J Solid State Chem 107:332–346
Brandenburg K (2001) Diamond version 3. Crystal impact GbR. Bonn, Germany
Edenharter A, Koto K, Nowacki W (1971) Uber Pearceit, Polybasit und Binnit. Neues Jahr Mineral Monat 1971:337–341
Evain M, Bindi L, Menchetti S (2006a) Structural complexity in minerals: twinning, polytypism and disorder in the crystal structure of polybasite, (Ag,Cu)16(Sb,As)2S11. Acta Crystallogr B62:447–456
Evain M, Bindi L, Menchetti S (2006b) Structure and phase transition in the Se-rich variety of antimonpearceite, (Ag14.67Cu1.20Bi0.01Pb0.01Zn0.01Fe0.03)15.93(Sb1.86As0.19)2.05(S8.47Se2.55)11.02. Acta Crystallogr B62:768–774
Frondel C (1963) Isodimorphism of the polybasite and pearceite series. Am Miner 48:565–572
Gaudin E, Boucher F, Evain M (2001) Some factors governing Ag+ and Cu+ low coordination in chalcogenide environments. J Solid State Chem 160:212–221
Hall HT (1967) The pearceite and polybasite series. Am Miner 52:1311–1321
Harris DC, Nuffield EW, Frohberg MH (1965) Studies of mineral sulphosalts: XIX-Selenian polybasite. Can Miner 8:172–184
Herrendorf W (1993) Habitus. Ph.D. dissertation, University of Karlsruhe, Germany
Hoppe R (1979) Effective coordination numbers (ECoN) and mean fictive ionic radii (MEFIR). Z Kristallogr 150:23–52
Johnson CK, Levy HA (1974) International tables for X-ray crystallography, vol IV. Kynoch Press, Birmingham, pp 311–336
Kuhs WF (1984) Site-symmetry restrictions on thermal-motion-tensor coefficients up to rank 8. Acta Crystallogr A40:133–137
Kuhs WF, Heger G (1979) In: Vashishta P, Mundy JN, Shenoy GK (eds) Fast ion transport in solids. Elsevier, Amsterdam, pp 233–236
Minčeva-Stefanova I, Bonev I, Punev L (1979) Pearceite with an intermediate unit cell—first discovery in nature (in Bulgarian). Geokh Miner I Petrol 11:13–34
Nespolo M (2004) Twin point groups and the polychromatic symmetry of twins. Z Kristallogr 219:57–71
Nespolo M, Ferraris G, Hoppe R (2001) Charge distribution analysis of ceramic materials. J Ceram Proc Res 2:38–44
Owens BB, Argue GR (1967) High-conductivity solid electrolytes: MAg4I5. Science 157:308–310
Padma Kumar P, Yashonath S (2006) Ionic conduction in the solid state. J Chem Sci 118:135–154
Peacock MA, Berry LG (1947) Studies of mineral sulphosalts: XII-Polybasite and Pearceite. Min Mag 28:2–13
Petricek V, Dusek M (2000) JANA2000, a crystallographic computing system. Institute of Physics, Academy of Sciences of the Czech Republic, Prague
Pfitzner A, Evain M, Petricek V (1997) Cu12Sb4S13: a temperature-dependent structure investigation. Acta Crystallogr B53:337–345
Reuter B, Hardel K (1965) Silbersulfidbromid Ag3SBr und Silbersulfidjodid Ag3SI. I. Darstellung, Eigenschaften und Phasenverhältnisse von Ag3SBr und Ag3SI. Z Anorg Allg Chem 340:158–167
Stoe & Cie (1996) X-shape (version 1.02). Stoe & Cie, Darmstadt, Germany
Sugaki A, Kitakaze A, Yoshimoto T (1983) Synthesized minerals of polybasite and pearceite series - synthetic sulfide minerals (XII). Science Reports of the Tohoku University, series 3: Mineralogy, petrology and economic geology 15, pp 461–469
West AR (1984) Solid state chemistry and its applications. Wiley, New York, pp 452–497
Acknowledgments
The authors are grateful to Professor Paul G. Spry (Iowa State University, USA) for his help in electron microprobe analyses. This work was funded by C.N.R. (Istituto di Geoscienze e Georisorse, sezione di Firenze) and by M.I.U.R., P.R.I.N. 2005 project “Complexity in minerals: modulation, modularity, structural disorder”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bindi, L., Evain, M., Pradel, A. et al. Fast ion conduction character and ionic phase-transitions in disordered crystals: the complex case of the minerals of the pearceite–polybasite group. Phys Chem Minerals 33, 677–690 (2006). https://doi.org/10.1007/s00269-006-0117-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0117-7