Abstract
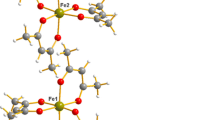
The crystal structure of synthetic stepanovite, Na[Mg(H2O)6][Fe(C2O4)3]·3H2O, and zhemchuzhnikovite, Na[Mg(H2O)6][Al0.55Fe0.45(C2O4)3]·3H2O, has been determined by single-crystal X-ray diffraction methods. The compounds are isotypic to each other and to the previously reported Na[Mg(H2O)6][M(C2O4)3]·3H2O (M: Cr, Al). They crystallize in the trigonal P3c1 space group with Z = 6 molecules per unit cell and (hexagonal axes) a = 17.0483(4), c = 12.4218(4) Å for the iron compound, and a = 16.8852(5), c = 12.5368(5) Å for the Al/Fe solid solution. Comparison of our crystallographic results with previous X-ray diffraction and chemical data of type stepanovite and zhemchuzhnikovite minerals provides compelling evidence that these natural materials possess the same crystal and molecular structure as their synthetic counterparts. It is shown that the originally reported unit cell for stepanovite represents a pronounced sub-cell and that the correct unit cell and space group are based on weak superstructure reflections. The infrared and Raman spectra of both synthetic analogs were also recorded and are briefly discussed.





Similar content being viewed by others
References
Anthony JW, Bideaux RA, Bladh KW, Nichols MC (eds) (2004) Handbook of mineralogy, mineralogical society of America, Chantilly, VA 20151-1110, USA; http://www.handbookofmineralogy.org/
Atencio D, Coutinho JMV, Graeser S, Matioli PA, Menezes Filho LAD (2004) Lindbergite, a new Mn oxalate dihydrate from Boca Rica mine, Galiléia, Minas Gerais, Brazil, and other occurrences. Am Mineral 89:1087–1091
Baran EJ (1995) Química bioinorgánica. McGraw-Hill Interamericana de España SA, Madrid
Baran EJ (2014) Review: natural oxalates and their analogous synthetic complexes. J Coord Chem 23–24:3734–3768
Baran EJ, Monje PV (2009) Oxalate biominerals. In: Sigel A, Sigel H, Sigel RKO (eds) Metal ions in life sciences, vol 4., Biomineralization, from nature to applicationsWiley, Chichester, pp 219–254
Chukanov NV (2014) Infrared spectra of mineral species, vol 1. Springer, Dordrecht
Chukanov NV, Pekov IV, Olysych LV, Massa W, Yakubovich OV, Zadov AE, Rastsvetaeva RK, Vigasina MF (2010) Kyanoxalite, a new cancrinite group mineral species with extraframework oxalate anion from the Lovezaro Alkaline Pluton, Kola Peninsula. Geol Ore Deposits 52:778–790
Clarke RM, Williams IR (1986) Moolooite, a naturally occurring hydrated copper oxalate from Western Australia. Mineral Mag 50:295–298
CrysAlisPro (2014) Oxford diffraction Ltd., Version 1.171.37.31 (release 14-01-2014 CrysAlis171.NET)
Echigo T, Kimata M (2010) Crystal chemistry and genesis of organic minerals: a review of oxalate and polycyclic aromatic hydrocarbon minerals. Canad Mineral 48:1329–1357
Edwards HGM, Russell NC (1998) Vibrational spectroscopic study of iron(II) and iron(III) oxalates. J Mol Struc 443:223–231
Farrugia LJ (1997) ORTPEP-3 for Windows-A version of ORTEP-III with a graphical user interface (GUI). J Appl Crystallogr 30:565
Fleischer M (1955) New mineral names: stepanovite. Am Mineral 40:551
Fleischer M (1956) New mineral names: minguzzite. Am Mineral 41:370
Fleischer M (1962) New mineral names: Zhemchuzhnikovite. Am Mineral 47:1482–1483
Fleischer M (1964) New mineral names: zhemchuzhnikovite, Stepanovite. Am Mineral 49:442
Fraústo da Silva JJR, Williams RJP (1991) The biological chemistry of the elements. Clarendon Press, Oxford
Frossard L (1956) Etude de trioxalatochrominate de sodium et de magnésium. Schweiz Mineral Petrograph Mitt 36:1–25
Fujita J, Martell AE, Nakamoto K (1962a) Infrared spectra of metal chelate compounds. VI. A normal coordinate treatment of oxalate metal complexes. J Chem Phys 36:324–331
Fujita J, Martell AE, Nakamoto K (1962b) Infrared spectra of metal chelate compounds. VII. Normal coordinate treatments on 1:2 and 1:3 oxalato complexes. J Chem Phys 36:331–338
Garavelli CL (1955a) Ritrovamento de oxalite tra i minerali secondari del giacimento de Capo Calamita. Rend Soc Mineral Ital 11:176–181
Garavelli CL (1955b) Un nuovo minerali tra i prodotti secondari del giacimento di Capo Calamita (Isola d’Elba). Atti Accad Naz Linzei 18:392–402
Junk PC (2005) Supramolecular interactions in the X-ray crystal structure of potassium tris(oxalato)ferrate(III) trihydrate. J Coord Chem 58:355–361
Khan SR (1995) Calcium oxalate in biological systems. CRC Press, Boca Raton
Knipovich YN, Komkov AI, Nefedov EI (1963) On stepanovite and the new mineral zhemchuzhnikovite. Trudy Vses Nauchno-Issled Geol Inst 96:131–135 (in Russian)
Krishnamurty KY, Harris GM (1961) The chemistry of the metal oxalate complexes. Chem Rev 61:213–246
Libowitzky E (1999) Correlation of O–H stretching frequencies and O–H···O hydrogen bond lengths in minerals. Monatsh Chem 130:1047–1059
Lipkowski J, Herbich J (1975) Preparation, composition and crystal growth of sodium magnesium tris(oxalato)aluminate nonahydrate containing ferric ions. Rocz Chem 49:853–857 (in Polish)
Matioli PA, Atencio DM, Coutinho JMV, Menezes Filho LAD (1997) Humboldtina de Santa Maria de Itabira, Minas Gerais, primeira ocorrência brasileira e primeira ocorrência mundial em fraturas de pegmatito. Anais Acad Bras Cien 69:431–432
Monje PV, Baran EJ (2004) Plant biomineralization. In: Hemantaranjan A (ed) Advances in plant physiology, vol 7. Scientific Publishers, Jodhpur, pp 395–410
Mortensen OS (1967) Vibronic spectra of transition-metal complexes. I. Polarized emission and absorption spectra of NaMg[Cr(C2O4)3]·9H2O. J Chem Phys 47:4215–4222
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds, 6th edn. Wiley, New York
Nefedov EI (1953) Report of new minerals discovered by him. Zapisky Vses Mineral Obshch 82:311–317 (in Russian)
Peacor DR, Rouse RC, Essene EJ, Lauf RJ (1999) Coskrenite-(Ce) (Ce, Nd, La)2 (SO4)2(C2O4)·8H2O, a new rare earth oxalate mineral from Alum Cave Bluff, Tennessee: characterization and crystal structure. Canad Mineral 37:1453–1462
Piper TS, Carlin RL (1960) Polarized visible spectra of crystalline trisoxalato-metallates: the source of intensity. J Chem Phys 33:608–609
Piper TS, Carlin RL (1961) Polarized visible spectra of crystalline trisoxalato-metallates. J Chem Phys 35:1809–1815
Piro OE, Echeverría GA, González-Baró AC, Baran EJ (2015) Crystallographic new light of an old complex: NaMg[Cr(C2O4)3]·9H2O and structure redetermination of the isomorphous aluminum(III) compound. J Coord Chem 38:3776–3787
Riesen H, Rae D (2008) Revisiting the crystal structure and thermal properties of NaMgAl(oxalate)3·9H2O/Cr(III): an extraordinary spectral hole-burning material. Dalton Trans 2008:4717–4722
Rouse RC, Peacor DR, Dunn PJ, Simmons WB, Newbury D (1986) Wheatleyite, Na2[Cu(C2O4)2]·2H2O, a natural sodium copper salt of oxalic acid. Am Mineral 71:1240–1242
Rouse RC, Peacor DR, Essene EJ, Coskren TD, Lauf RJ (2001) The new minerals levinsonite-(Y) [(Y, Nd, Ce)Al(SO4)2(C2O4)·12H2O] and zugshunstite-(Ce) [(Ce, Nd, La)Al(SO4)2(C2O4)·12H2O]: coexisting oxalates with different structures and differentiation of LREE and HREE. Geochim Cosmochim Acta 65:1101–1115
Saritha A, Raju B, Ramachary M, Raghavaiah P, Hussain KA (2012) Synthesis, crystal structure and characterization of chiral, three-dimensional anhydrous potassium tris(oxalato)ferrate(III). Phys B 407:4208–4213
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Sheldrick GM (2015) SHELXT-Integrated space group and crystal structure determination. Acta Crystallogr A 71:3–8
Siebert H (1966) Anwendungen der Schwingungsspektroskopie in der Anorganischen Chemie. Springer, Berlin
Strunz H, Nickel EH (2001) Strunz Mineralogical Tables. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart
Suh J-S, Shin J-Y, Yoon C, Lee K-W, Suh I-H, Lee J-H, Ryu B-Y, Lim S-S (1994) The crystal and molecular structure of sodium magnesium tris(oxalate) chromate(III) decahydrate, NaMg[Cr(C2O4)3]·10H2O. Bull Kor Chem Soc 15:245–249
Taylor D (1978) The crystal structures of potassium tris(oxalato)-chromate(III) and -aluminate(III) trihydrate: a reinvestigation. Austral J Chem 31:1455–1462
Truchanowicz T (1974) Thermal analysis and determination of x-ray powder data for sodium magnesium trioxalatoaluminate nonahydrate and sodium magnesium trioxalatochromate(III) nonahydrate crystals. Chem Anal (Warsaw) 19:1089–1094 (in Polish)
Truchanowicz T, Durski Z (1971) Determination of lattice constants and possible space groups of NaMg[Al(C2O4)3]·9H2O crystals. Rocz Chem 45:1777–1778 (in Polish)
van Niekerk JN, Schoening FRL (1952) The structure of potassium trioxalatochromate(III), K3[Cr(C2O4)3]·3H2O. Acta Crystallogr 5:196–202
Weiner S, Dove PM (2003) An overview of biomineralization processes and the problem of the vital effect. In: Dove PM, De Yoreo JJ, Weiner S (eds) Reviews in mineralogy and geochemistry, vol 54., Mineralogical SocAmerica/Geochemical Soc, Washington, DC, pp 1–29
Acknowledgments
We would like to thank Dr. Takuya Echigo and Dr. Uwe Kolitsch for their thorough revisions that helped to improve our article. This work was supported by the Universidad Nacional de La Plata, by CONICET (PIP 1529) and by ANPCyT (PME06 2804 and PICT06 2315) of Argentina. OEP, GAE and AGB are research fellows of CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piro, O.E., Echeverría, G.A., González-Baró, A.C. et al. Crystal and molecular structure and spectroscopic behavior of isotypic synthetic analogs of the oxalate minerals stepanovite and zhemchuzhnikovite. Phys Chem Minerals 43, 287–300 (2016). https://doi.org/10.1007/s00269-015-0793-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-015-0793-2