Abstract
Annelids and particularly polychaetes possess a great variety of sensory organs and respond to numerous sensory stimuli. Although eyes and nuchal organs are comparatively well studied, the so-called dorsal organs are among the lesser-known sense organs in aquatic annelids. Moreover, they are known to be restricted to only two out of approximately 80 families of polychaetes—Orbiniidae and Spionidae—which are not closely related. These organs have been regarded as segmentally repeated nuchal organs in the latter taxon, but in Orbiniidae, data are lacking, although it is known that the organs occur almost along the entire trunk except for the anterior-most segments. Furthermore, although the nuchal organ ultrastructure is known to be comparatively uniform for many polychaete species, a comparative investigation has not been conducted in Orbiniidae. To bridge this data gap, we examined an intertidal population of the widely distributed species Scoloplos armiger. Although not completely identical, nuchal and dorsal organs show a high degree of correspondence in the examined specimens. Moreover, both organs correspond to the general structure of nuchal organs. They comprise ciliated supportive cells and bipolar receptor cells and are innervated directly from the brain. The supportive cells form subcuticular spaces and olfactory chambers apically protected by specialized microvilli that house the sensory processes—cilia and microvilli—of the monociliated receptor cells. Therefore, it can be concluded that nuchal and dorsal organs are also identical in Orbiniidae. However, despite general correspondence with spionids, convergent evolution in the two taxa appears to be the most parsimonious interpretation.
Similar content being viewed by others
Introduction
Annelids possess a great variety of sensory structures and respond to numerous sensory stimuli (Mill 1978; Eakin and Hermans 1988; Storch and Schlötzer-Schrehardt 1988; Verger-Bocquet 1992; Purschke 2005, 2016). This variety notably applies to the more vagile and marine forms commonly known as polychaetes. Besides sensory cells not obviously related and associated with other cell types, a diversity of sense organs is usually present. Among the latter, eyes and nuchal organs are probably the best known sensory structures and are almost ubiquitously present (see references in Purschke 2016). In contrast, all other types of sensory organs are typically restricted to a certain group of taxa or species. The so-called dorsal organs belong to these less distributed and lesser-known sense organs, which are not even mentioned in certain reviews on sensory organs (Storch and Schlötzer-Schrehardt 1988; Verger-Bocquet 1992). Thus far, these structures are only known to occur in two out of approximately 80 so-called polychaete families, namely Spionidae and Orbiniidae (Söderström 1927; Orrhage 1964; Jelsing 2003; Blake et al. 2019; Bleidorn and Helm 2019). According to recent phylogenetic hypotheses, these two are not closely related or represent sister groups, although both are members of the large clade Sedentaria (Struck et al. 2015; Weigert and Bleidorn 2016; Struck 2019).
Dorsal organs are segmentally repeated structures originally regarded by Söderström (1927) to represent sense organs. Since that time, their similarity to nuchal organs has been emphasized, and the homology of these two organs was discussed. Formerly, nuchal organs were regarded as representing one of the most important apomorphic characters of polychaetes or even Annelida (Rouse and Fauchald 1997; Rouse and Pleijel 2001; Struck et al. 2011). However, this view should be challenged and revised based on recent observations (Beckers and Tilic 2021). The ultrastructure of nuchal organs is comparatively well known for several polychaete species from a variety of taxa within this group, enabling the reconstruction of a general structural plan (see references in Purschke 1997, 2016). Despite considerable external variability, their ultrastructure is rather uniform comprising bipolar primary sensory cells, ciliated supportive cells, and a direct innervation from the posterior part of the brain by the axons of the receptor cells. In addition, several substructures such as protective structures formed by microvilli of the supportive cells are also very common. Subsequent investigations in Spionidae confirmed that the dorsal and nuchal organs were similar or almost identical (Jelsing 2002, 2003; Jelsing and Eibye-Jacobsen 2010), contradicting initial investigations of a different species (Schlötzer-Schrehardt 1986, 1987, 1991). Similar investigations in Orbiniidae are lacking except for some preliminary observations (see Bleidorn and Helm 2019).
In Orbiniidae (as Ariciidae), Eisig (1914) first described these repetitive structures but interpreted them as segmental ciliary hillocks without sensory function. Söderström (1927) interpreted these structures as sensory organs and pointed out their similarity to nuchal organs. In Orbiniidae, Scoloplos armiger (O. F. Müller, 1776) is one of the most frequent species studied to date (e.g., Eisig 1914; Anderson 1959; Orrhage and Müller 2005; Wilkens and Purschke 2009a; Helm et al. 2015). This species was regarded to have a worldwide or even cosmopolitan distribution, but current analyses suggest that this name actually refers to a species complex (Kruse and Reise 2003; Bleidorn et al. 2006; Luttikhuizen et al. 2011). Nevertheless, most studies have been performed on the so-called “intertidalis clade” from Europe and thus most likely refer to the same species (see Helm et al. 2015).
Investigations of the nervous system have previously been performed in S. armiger (Orrhage and Müller 2005; Wilkens and Purschke 2009a; Helm et al. 2015). Among the sensory organs, only the photoreceptive organs have been investigated in more detail (Wilkens and Purschke 2009a); detailed structural analyses of the nuchal and segmental dorsal organs are still missing. In the present study, we describe the ultrastructure and innervation of the dorsal organs by applying scanning and transmission electron microscopy and confocal laser scanning microscopy. For comparison, nuchal organs are also included. The results demonstrate a striking structural similarity between these two types of sensory organs, finally confirming Söderström’s (1927) hypothesis. Furthermore, these organs follow the general structural pattern known for the nuchal organs in other polychaete species.
Material and methods
Adult and sub-adult specimens of S. armiger (O. F. Müller, 1776) were collected in the intertidal zone at the North Sea island of Sylt (List, Germany, 55.01543 N, 8.43778 E) and in front of the Station Marine Biologique at Roscoff (France, 48.72807 N, 3.98725 W) during summer, 2016. These animals represent the “intertidal clade” of the species complex referred to as S. armiger (see Kruse and Reise 2003; Bleidorn et al. 2006; Helm et al. 2015). Specimens were extracted from small sediment samples by the MgCl2 method, and animals were decanted through a 100 µm-mesh sieve. After revitalizing in seawater, specimens were sorted using a dissecting microscope or used for live observations under a compound microscope.
For transmission electron microscopy (TEM) and laser scanning microscopy (CLSM), small individuals comprising approximately 50–60 chaetigers (Fig. 1a) were chosen. For scanning electron microscopy (SEM), somewhat larger individuals were used. Prior to fixation, animals were relaxed in 8% magnesium chloride (MgCl2 × 6 H2O) isotonic with seawater and fixed immediately; for electron microscopy, a solution of picric acid, paraformaldehyde, and glutaraldehyde adjusted to the appropriate osmolality with sucrose [SPAFG, see Ermak and Eakin (1976); phosphate-buffered, 0.075 M, 2 h at 4 °C] was used. For CLSM, specimens were fixed in 4% paraformaldehyde in phosphate-buffered saline (PPS, 140 mM NaCl, 6.5 mM KCl, 2.5 mM Na2HPO4, 1.5 mM KH2PO4, and 12% sucrose, pH 7.4, 4 °C, 2.5 h).
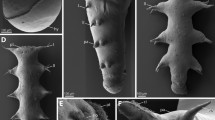
Scoloplos armiger. External morphology and position of dorsal organs. Small sub-adult individuals, trunk comprised approximately 50–60 chaetigers; a LM of living individual, b–h SEM micrographs. a Entire sub-adult individual showing tagmata. b Anterior end and transition zone between thorax and abdomen, with first visible branchiae on chaetiger 13. The inset areas are enlarged in c and d; arrowheads point to dorsal organs. c Dorsum of chaetigers 16 and 17 with the small dorsal organs marked on chaetiger 17 (arrowheads). d Dorsum of chaetiger 21 with the typical appearance of mid-body dorsal organs (arrowheads); scattered ciliated tufts represent sensory cell cilia (sci). e Two abdominal segments with paired dorsal organs (arrowheads) at the anterior end of each segment; flattened branchiae (br) with two ciliary bands (ci) on each narrow side emerge medially from the body beside the notopodia; lateral organs (lo) visible ventrally on the notopodial lobes. f Posterior end with newly forming segments; dorsal organs recognizable from the sixth to last segment (arrowheads). g Close-up of the posterior-most visible dorsal organ from the sixth to last segment. h Group of sensory cilia from ciliated tuft, scattered over the entire body. ac anal cirrus, br branchia, ch chaeta, ci cilia, ey eye, lo lateral organ, neu neuropodium, no nuchal organ, not notopodium, pa parapodium, po prostomium, pyg pygidium, sci sensory cilia
The TEM and SEM preparation protocol was as follows. After seven rinses in the same buffer adjusted with sucrose to the osmolarity of seawater used for fixation (10 min each), specimens were stored in this buffer containing 0.05% NaN3 at 4 °C until further processing, which was carried out in the Osnabrueck laboratory. Upon arrival, specimens were post-fixed in 1% OsO4 (phosphate-buffered) for 1 h at 4 °C. After being washed for 5 min in 0.075 M buffer, samples were dehydrated using an EtOH series [30%, 50%, 70%, 80%, 95% (2 ×), 100% (2 ×), for 5 or 10 min (≥ 70%), respectively]. Dehydration was carried out at 4 °C, and from 95% EtOH onward treatment was at RT. Specimens for SEM were then critical-point dried using CO2 (l), mounted with adhesive tabs on aluminum stubs, coated with platinum/iridium, and examined with a Zeiss Auriga (Oberkochen, Germany) scanning electron microscope.
Specimens for TEM were dissected into smaller parts representing the major body regions prior to further processing and embedding. These parts were transferred into a solution comprising ethanol and the intermedium propylene oxide (100% EtOH:propylene oxide, 1:1, 2 × 30 min), followed by pure propylene oxide (2 × 15 min). This solution was replaced by a mixture of the intermedium and the embedding medium [propylene oxide:Araldite/Epon (PolyBed 812) 3:1]. The intermedium was allowed to evaporate overnight. Embedding started with transferring the specimens into a drop of Araldite/Epon and placing them for 5 min at 60 °C. After two repetitions, specimens were brought into the embedding molds. Polymerization was carried out at 60 °C for 72 h. Specimens were cut in a series of semi-thin sections (1 µm) until the dorsal organs were detected and then a series of ultrathin sections (70 nm) were obtained, using a UC 6 Leica ultra-microtome (Wetzlar, Germany).
Semi-thin sections were stained with toluidine blue (0.5% toluidine blue in a 1% aqueous borax solution for 15 s at 60 °C), rinsed with H2O. Pictures were taken with a Zeiss Axioskop light microscope equipped with a CCD camera (Invisitron Systems) and Vision (Spot) software. Ribbons of ultrathin sections were placed on single-slot grids coated with pioloform support films, then contrasted at 20 °C with 2% uranyl acetate (30 min) and 0.5% lead citrate (20 min) in a Nanofilm Surface Analysis Ultrastainer (Göttingen, Germany). Finally, the sections were examined with Zeiss EM 902A and Libra 120 transmission electron microscopes (Oberkochen, Germany). Images were recorded using CCD cameras (Image SP®, 4 k, Mohrenweis, Germany).
For CLSM analysis, after fixation, specimens were rinsed in PBS and stored in the same buffer, containing a few crystals of NaN3 to avoid growth of bacteria and fungi. Prior to immunolabeling, specimens were incubated with PBT (9 ml PBS + 1 ml 1% Triton X-100) containing 6% BSA (bovine serum albumin) for 1 h. Finally, they were incubated with the primary antibody for 2–4 days at 4 °C.
The primary antibody was mouse anti-acetylated α-tubulin (monoclonal, clone 6-11-B-1; Sigma-Aldrich, Heidelberg, Germany, dilution 1:1000 in PBT). Following several washes (3 × in PBT, 20 min each), the secondary antibody was applied for 2–3 days at 4 °C (goat anti-mouse, Cy2 conjugated, Dianova, Hamburg, Germany, dilution 1:200). After being rinsed three times for 10 min in PBS, specimens were mounted in Fluoromount (Southern Biotech, Birmingham, AL, USA). The specificity of immunoreactivity was controlled by incubating specimens in the same manner but omitting the primary antibodies. Specimens were observed using a Zeiss Pascal 5 confocal laser scanning microscope (Zeiss, Jena, Germany). Z-stacks are displayed as maximum projections if not stated otherwise. All images were further processed using Adobe Photoshop® and Illustrator® to adjust brightness, contrast, and size for assembling and labeling the plates.
Results
Position and external appearance of the dorsal organs
The body of S. armiger is divided into two indistinct regions: thorax and abdomen, recognizable by a shift of the parapodia into a more dorsal position at chaetiger 19 (Fig. 1a, b). Additionally, the segments are somewhat more elongated in the abdomen. The prostomium is pointed without appendages, and the pygidium bears a pair of anal cirri. Two slit-like dorsolateral folds represent the nuchal organs at the border between the prostomium and the achaetigerous first segment (Fig. 1b). Externally visible branchiae start on chaetiger 13 as small papillae, and fully developed branchiae are observed from chaetiger 19 onward. The first branchiae are finger shaped and not ciliated. The subsequent branchiae are flattened with their broader axis perpendicular to the body axis, and they bear ciliary bands on their narrow sides (Fig. 1b, c, e). The dorsal organs are visible as small roundish ciliary and flat pits under the SEM. The first dorsal organs are externally visible from chaetiger 15 onward on each segment until the sixth to the last segment (Fig. 1b–g). The smallest anlagen of the branchiae are visible on the same posterior segment (Fig. 1f). Although the first and the last dorsal organs appear to be unpaired in the specimens investigated, all other organs are paired. They are situated close to the dorsal midline, in the abdomen shortly behind the furrow separating successive segments (Figs. 1e, 2a). On the thorax, the organs reside in the middle of the segments (Fig. 1c). They increase slightly in size from anterior to posterior, reaching the average diameter of approximately 25 µm on chaetiger 20 in the specimens investigated. In the posterior end, the developing organs decrease in size toward the posterior end; the smallest organ visible with SEM is 3.5 µm in diameter with only a few short cilia (Fig. 1g). In addition to the dorsal organs, numerous tufts comprising a few sensory cilia are distributed all over the body (Fig. 1h).
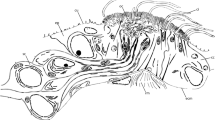
Scoloplos armiger. Position of dorsal and nuchal organs. Semi-thin sections, LM. a Cross section through the anterior segment of the abdomen (chaetiger 19) at the level of the dorsal organs, only right organ visible (boxed). b Prostomium with right nuchal organ (boxed) close to posterior somata (so) of the brain. coe coelom, dbv dorsal blood vessel, ep epidermis, iep intestinal epithelium, il intestinal lumen, lm longitudinal musculature, mo mouth cavity, rm retractor muscle, so somata of the brain, vbv ventral blood vessel, vnc ventral nerve cord
Dorsal organs
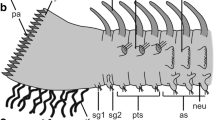
The dorsal organs are an integrative part of the epidermis, and the surrounding cells have no distinct border (Fig. 2a). The ciliated cells of the organ are somewhat thinner than the adjacent cells; thus, a small depression is formed on the epithelial surface. The organs rest on the thin extracellular matrix (ECM) separating the epidermis from the underlying muscle fibers. This ECM forms an almost perfect circle below the epidermis. The organs are close to the dorsal midline marked by the large dorsal blood vessel (Fig. 2a). No intrinsic musculature is attached to the dorsal organs; they are above the thick pair of longitudinal muscle bands. Between these fibers and the ECM, a few circular fibers are also present (Figs. 2a, 3a, b). The organs comprise unciliated supportive cells, two types of ciliated supportive cells, and sensory cells (Figs. 3a, b, 4). The sensory cells reach the epithelial surface somewhat irregularly and closer to the dorsal midline (Fig. 4). They are surrounded by supportive cells. The bodies of the supportive cells are positioned outside the ciliated area, and most are located underneath unciliated supportive cells adjacent to the organ. The same applies to the sensory cells, and their somata are also shifted to the dorsal midline, in the anterior region above a paired dorsal longitudinal nerve (Figs. 3b, 4). Thus, the internal dimensions of these organs are much larger than indicated by the dimension of the ciliary patches, and the epithelium has a pseudostratified appearance.
Scoloplos armiger. Dorsal organ of chaetiger 21. a Low power TEM micrograph showing entire dorsal organ; organ composed of ciliated supportive cells (suc) and sensory dendrites (sd); part of supportive cells bear specialized microvilli (smv) forming a dense apical cover above the olfactory chamber (oc) only penetrated by motile cilia (ci) of the supportive cells; marginal supportive cells bear regular microvilli (rmv) similar to surrounding epidermal supportive cells. Sensory processes of sensory dendrites (sd) are located in the center of the organ, and one reaches the surface of the olfactory chamber (oc). b Peripheral section, a bundle of sensory dendrites (sd) proceeding toward the neurite bundle (nb) of the dorsal nerve; sensory dendrites embedded in supportive cells (suc) and epidermal cells (ep). bb basal body, ci cilium, cm circular muscle, cr ciliary rootlet, cu cuticle, ecm ECM, ecu epicuticular projections, ep epidermis, g golgi apparatus, lm longitudinal muscle, mi mitochondrium, n nucleus, nb neurite bundle, oc olfactory chamber, rmv regular microvillus, sd sensory dendrite, smv specialized microvillus, suc supportive cell, ve vesicle. Insets in a and b show the approximate position of sections
Scoloplos armiger. Schematic representation of dorsal organ, combined from TEM observations on serial ultrathin sections, median to the right. The dorsal organ embedded between typical epidermal supportive cells (ep) forms a slight depression. The organ is composed of ciliated supportive cells (suc, shaded in dark gray) and sensory dendrites (sd, shaded in light gray) of sensory cells, the somata (sso, shaded in light gray) of which are situated medially above a neurite bundle (nb). More laterally positioned supportive cells bear regular microvilli (rmv) similar to epidermal cells, whereas the others bear specialized microvilli (smv), forming a cover above the olfactory chamber (oc). The olfactory chamber contains sensory cilia (sci) and microvillus-like processes of the sensory dendrites. Nuclei of all cells are positioned circularly around the center of the organ. Underlain predominantly longitudinal musculature omitted. ci (motile) cilium, cu cuticle, ecm ECM, ep epidermis, gc glial cell, nb neurite bundle, oc olfactory chamber, rmv regular microvilli, sci sensory cilium, sd sensory dendrite, smv specialized microvilli, sso soma of sensory cell, suc supportive cell
The unciliated supportive cells are identical to those of the surrounding epidermis (Figs. 2a, 3a, b, 4). They are characterized by a comparatively electron-lucent cytoplasm and numerous clear vesicles (diameter ~ 1.1 µm) accumulating apically. Between and below these vesicles, numerous mitochondria are found. These cells possess numerous branched microvilli that penetrate the cuticle and terminate in small knob-like epicuticular projections (diameter ~ 75 nm; Figs. 3b, 4). These projections form a dense layer reaching approximately 200–230 nm above the approximately 55-nm-thick epicuticle. The basal cuticle is divided into an upper layer comprising several layers of parallel collagen fibers embedded in a fibrillar matrix and a lower layer comprising minimal material and no collagen fibers.
The first type of ciliated supportive cells bears an unmodified cuticle and has a more electron-dense cytoplasm than the unciliated epidermal cells (Fig. 3a). These cells form a semicircle of approximately 2–3 cells around the center, excluding the median part. Each cell possesses numerous microvilli and cilia (numbers not determined), penetrating the cuticle and forming part of the ciliary brush (Figs. 3a, 4, 5a). The cilia have a 9 × 2 + 2 axoneme and rest on a basal body. These are anchored in the cell by long distal and short rostral rootlets, whereas the latter are oriented parallel to the apical membrane, the former extending deep into the cytoplasm. Numerous mitochondria are present between the rootlets. The cell bodies of the outermost cells are situated beneath unciliated supportive cells, and those of the innermost cells are situated under adjacent ciliated cells (Fig. 4). Both ciliated cell types regularly contain large irregularly shaped vesicles with dense contents, probably representing lysosomes (Figs. 3b, 4, 5c).
Scoloplos armiger. Dorsal organ. TEM cross-sections. a Supportive cells (suc) with specialized microvilli (smv) form dense cover above flat olfactory chamber (oc), sensory dendrite (sd) embedded in the epithelium, supportive cells bear motile cilia (ci) extending to the outside, specialized microvilli with specific branching pattern. b Group of sensory dendrites sheathed by supportive cells, sensory dendrites form a hairpin-like curve and run toward the dorsal nerve. c Apex of sensory dendrite giving rise to sensory cilium (sci) and sensory microvilli (semv). d Tangential section through microvillar cover above the olfactory chamber. e Olfactory chamber with a cover of specialized microvilli; olfactory chamber densely filled with sensory processes; sensory cilia and microvilli indistinguishable. aer agranular endoplasmic reticulum, bb basal body, ci cilium, cr ciliary rootlet, cu cuticle, ecm ECM, ecp epicuticular projection, ecu epicuticle, gc glial cell, m mitochondrium, n nucleus, oc olfactory chamber, rmv regular microvillus, sci sensory cilium, sd sensory dendrite, semv sensory microvillus, smv specialized microvillus, suc supportive cell, ve vesicle, za zonula adherens
The second type of ciliated supportive cells forms the dorsal organ’s central part, which is shifted to the dorsal midline (Figs. 3a, 4, 5a). The main difference from the other ciliated supportive cells is that they bear a modified cuticle and specialized microvilli (Fig. 5a, d, e). These type-2 ciliated cells sheath the dendrites of the sensory cells, which terminate between their apices (Figs. 4, 5a). Where these dendrites reach the epithelial surface, no cilia extend from the organ, and thus, a central part of the organs is without cilia (Fig. 1d, inset Fig. 3a, 4). As in the other supportive cells, the cilia have a 9 × 2 + 2 axoneme and penetrate the modified cuticle. Viewed externally, the cilia of the two cell types are indistinguishable. The ciliated cells are somewhat flatter than the adjacent cells, and their apical surface forms a slight depression under the layer of microvilli (Figs. 3a, 4, 5a). In this manner, an extracellular space is formed, occupied by the processes of the sensory cells. This space is called the olfactory chamber (Fig. 5a, c, e). The microvilli of these ciliated supportive cells originate as branched tube-like processes, pass the olfactory chamber and finally form a dense cover of flattened, somewhat ashlar-like shaped processes (Figs. 3a, 4, 5a, d, e). This cover is approximately 375–400 nm thick. Within this layer, the processes are only 45 nm apart. The space between them is filled with an electron-dense material except where cilia penetrate this layer (Fig. 5a, d). The microvilli project straight from the supportive cells, forming an angle of approximately 90° with the apical membrane, except for the areas where they border sensory dendrites. Here, the microvilli project obliquely from the supportive cells, and thus their apices form a continuous cover above the olfactory chamber (Figs. 3a, 4, 5a). The cuticle of this region is only half as thick as in adjacent regions; collagen fibers and an epicuticle are lacking. The cell bodies of these ciliated cells are situated outside the ciliated area and below unciliated cells anteriorly, medially, and posteriorly of the ciliated area (Figs. 3a, 4).
The dorsal organs are innervated from a paired dorsal nerve close to the dorsal midline (Fig. 6a). Each dorsal organ is innervated by two short neurite bundles emanating from these nerves (Fig. 6a–e). Two neurite bundles approach the dorsal organ: a more distinct anterior bundle and a somewhat faint and difficult to distinguish posterior neurite bundle (Fig. 6d, e). The anterior bundle approach the organ from its anterior side (Figs. 3b, 6d, e). These are bipolar primary sensory cells: their somata are located close to the pair of dorsal neurite bundles (Figs. 3b, 4). In LSM micrographs, this region is visible as a loose network of anti-acetylated α-tubulin signaling close to this longitudinal nerve (Fig. 6d). Some distance from the median, the dendrites adjoin each other, forming a discrete bundle entering the dorsal organ anteriorly (Figs. 3b, 5b, 6d). The posterior bundle approach the organ posterior laterally (Fig. 6e.
Scoloplos armiger. Innervation and development of dorsal organs as revealed with CLSM after staining with antibodies against anti-acetylated α-tubulin. Small sub-adult individuals, trunk comprising around 50–60 chaetigers; anterior to the left in all images. a Posterior end with newly formed segments in front of the pygidium (py). Posterior-most dorsal organs encircled on the left; first recognizable dorsal organ on the left side in the third last segment; the last segment borders the front of the proliferation zone, segment borders of posterior segments marked by arrows; segments numbered reversely, 1 = first posterior-most recognizable segment. Size of dorsal organs increases continuously toward anterior. Dorsal organs innervated by short branches of the dorsal nerve (dn), which nearby also give rise to a segmental nerve entering the parapodia. b Enlargement of the region with early developing dorsal organs; posterior three organs encircled; arrows point to segment borders. c Anterior-most dorsal organs (arrows) as revealed with immunostaining on chaetigers 16, 17; on the left organs start in chaetiger 16, on the right in chaetiger 17. d, e Mid-body dorsal organs at higher magnification. d After some distance from the intensely stained motile cilia, the neurite bundle fans out, forming a net-like arrangement and finally joins the dorsal nerve (arrows); the sensory cell somata are situated in this net-like region. Almost at the same position, the segmental nerve (sn) branches off, and the segmental nerves are dorsally connected to those of the other side of the body. The cilia (ci) stained on the body surface likely belong to tufts of other sensory cells. e In addition to these neurites, an additional, smaller bundle proceeds toward the dorsal organ (arrows). Abbreviations: ci cilium, dn dorsal nerve, do dorsal organ, nac nerve of anal cirrus, py pygidium, pyn pygidial nerve, sn segmental nerve
The sensory dendrites are comparatively electron lucent, contain several clear vesicles, cisterns of smooth ER, and apically, numerous mitochondria (Fig. 5a–c). In addition, longitudinally arranged microtubules are present. Somewhat closer to the cell bodies, lysosome-like inclusions also occur. The dendrites are integrated into the epithelium and connected to their neighbors by typical junctional complexes, i.e., a zonula adherens followed by a septate junction. Most apices are approximately 1.1–1.3 µm wide, but some are up to 2 µm wide (Fig. 5a–c). These apices form the bottom of the olfactory chamber. Each of them gives rise to microvilli and a single cilium (Fig. 5c). The cilia arise from basal bodies without rootlets. The cilia have a 9 × 2 + 0 axoneme and extend into the olfactory chamber, where they bend in various directions (Fig. 5a, c, e). After some distance, they branch into microvillus-like structures resulting in an olfactory chamber densely occupied by sensory cell processes. Finally, microvilli and ciliary branches are indistinguishable, and they extend laterally above the adjacent type-2 ciliated supportive cells. The dendrites and neurite bundles are accompanied by glial cell processes recognizable by their ovoid gliosomes and bundles of intermediate filaments.
In the posterior end, the first anlagen of dorsal organs are found in the third to last segment (Fig. 6a, b). They are visible as an intense anti-acetylated α-tubulin-like immune-reactive feature resembling a dot of antigen situated close to the dorsal longitudinal nerve and attached to it by a thin process. These dots give rise to a few short processes conferring on them a star-like appearance. Their size increases continuously from posterior to anterior, and after approximately ten segments, they reach their final size and structure (Fig. 6a). Simultaneously, when they grow, their position shifts somewhat more laterally; and consequently their innervation elongates, comprises more fibers and becomes more clearly visible. The paired dorsal longitudinal nerve not only innervates the dorsal organs, but also builds up connections to the segmental nerves, which form circles in each segment underneath the parapodia and branchiae (Fig. 6a–e). Three ring-like nerves are found in each segment, the segmental or parapodial nerve being the largest. Close to the dorsal organs, two additional circular nerves are observed (Fig. 6c): and ultimately, these neurite bundles are connected to the ventral nerve cord. This segmental nerve also connects the two dorsal longitudinal neurite bundles. Anteriorly, the organs are smaller and, as observed in the LSM preparations (Fig. 6c), begin unpaired; i.e., only one organ is visible in the first segment with dorsal organs, but two organs are visible from the following segment onward.
Nuchal organs
The nuchal organs are situated laterally in small depressions at the posterior part of the prostomium (Fig. 2b). They are slit-like with their longer axis perpendicular to the body’s longitudinal axis; in this axis, they span an area of more than 50 µm. The organs primarily comprise ciliated supportive cells and sensory cells (Fig. 7a–d). These cells border typical unciliated epidermal supportive cells on all sides. Basally, at the ventral border, a retractor muscle attaches to the organ, which proceed transversely toward the mid-body region (Fig. 2b). This muscle comprises several muscle fibers and is separated by an ECM from the epidermal tissues (Fig. 7a). Dorsally, above the retractor muscles, the supportive cells of the nuchal organs directly border the somata of the primary sensory cells without being separated by an ECM (Fig. 7a). The axons of these cells form the nuchal nerve and dorsally extended into the brain.
Scoloplos armiger. Nuchal organ. TEM. a Nuchal organ in cross-section; dorsal side to the left. The nuchal organ mainly consists of numerous ciliated supportive cells (suc) which surround sensory dendrites (sd) terminating between supportive cells, the latter with processes extending above the epithelial surface and thereby forming the spacious olfactory chamber (oc). Nuchal organ separated from the outside by dense cover above the cuticle, which is traversed by motile cilia of supportive cells. In the center, processes of sensory dendrites forming a neurite bundle (nb). Epithelial cells rest on an ECM (ecm, arrowheads) to which the retractor muscle (rm) attaches; somata of the brain (so) are in direct contact with the dorsal (left) part of the nuchal organ. b Tangential section through dense cover above nuchal organ formed by flattened processes of specialized microvilli (smv) regularly penetrated by cilia of the supportive cells. c Two adjacent sensory dendrites terminating at the olfactory chamber, dendrites forming junctional complexes (za, sj) with neighboring cells, dendrites give rise to a single cilium (sci) and sensory microvilli (semv). d Supportive cells form mushroom-like processes (sucp), the caps bearing the motile cilia and specialized microvilli, the stalks housing ciliary rootlets a (cr) and mitochondria (m) and shaping the olfactory chamber (oc). aer agranular endoplasmic reticulum, bb basal body, bv blood vessel, ci cilium, cr ciliary rootlet, cu cuticle, ecm ECM, m mitochondrion, n nucleus, nb neurite bundle, oc olfactory chamber, rm retractor muscle, sci sensory cilium, sd sensory dendrite, semv sensory microvillus, sj septate junction, smv specialized microvillus, so soma of neuron, suc supportive cell, sucp supportive cell process, ve vesicle, za zonula adherens
The supportive cells are multiciliated and bear a modified cuticle. Additionally, their microvilli form a specialized apical layer (Fig. 7a, b, d). In the nuchal organ, the cuticle is somewhat thinner than on the adjacent epidermal cells. Grid-like layers of strong collagen fibers are absent, but a network of small fibers crossing at approximately 90° is present below the microvillar tips (Fig. 7b). The supportive cells building up the transition to the regular epidermis are of the same height as the other epidermal cells, whereas those forming the central part terminate deeper and leave a subcuticular space, the olfactory chamber, below the cuticle (Fig. 2a, d). The olfactory chamber is approximately 5 µm high, and each supportive cell possesses a mushroom-like process traversing the chamber. There are gaps between the upper shield-like parts; these gaps connect the olfactory chamber with the subcuticular space. The supportive cells form junctional complexes (zonulae adherentes followed by septate junctions) below the mushroom-like processes. These processes bear motile cilia and microvilli; additional microvilli originate from the apical surface below the processes. The cilia possess a 9 × 2 + 2 axoneme and arise from a basal body equipped with ciliary rootlets extending into the stalk-like parts of the processes. The microvilli extend apically, traverse the cuticle, and form an apical cover of electron-dense tips (Fig. 7a, b). These tips are flattened, somewhat ashlar-shaped, and form a cover approximately 375–400 nm thick. Within this cover, the microvillar processes are only 45 nm apart and attached to each other by electron-dense material except where cilia penetrate this layer (Fig. 5a, d).
Most mitochondria occur in the stalk-like processes and the apical regions of the supportive cells. In addition, these cells contain large clear vesicles and pinocytic vesicles, lysosomes, and usually a well-developed Golgi complex and rough ER. The nuclei are situated in the basal half of the cells. In the center, a comparatively large neurite bundle is found, and in this area, the cell bodies of the supportive cells are pushed aside (Fig. 7a).
The majority of these neurites belong to bipolar primary sensory cells and are accompanied by a few glial cell processes. Single or small groups of neurites then proceed apically and terminate between the supportive cells. These dendritic processes become wider and can be distinguished from the supportive cells by their electron-lucent cytoplasm. Apically, they contain smooth ER cisterns, various types of small vesicles, and a few mitochondria (Fig. 7a, c). Each dendrite gives rise to a single cilium and multiple microvilli (Fig. 7c). Cilia and microvilli proceed into the olfactory chamber in various directions but remain below the cuticle proper and the microvillar cover (Fig. 7a). The cilia rest on a basal body possessing a short shaft comprising a 9 × 2 + 0 axoneme. The cilia branch and form microvillus-like structures devoid of microtubules after short distances, making both types of cell processes indistinguishable after some distance. Finally, these processes form large vesicle-like swellings. The somata of the sensory cells are situated above the retractor muscle below the supportive cells and form a ganglion-like cluster (Figs. 2b, 7a).
Discussion
General aspects
Ciliated structures on the dorsum of various polychaetes, particularly those present in Spionidae and Orbiniidae, have generally been termed dorsal organs and mostly regarded as sensory (Eisig 1914; Söderström 1927; Orrhage 1964; Jelsing 2003; Purschke 2005; Jelsing and Eibye-Jacobsen 2010). Due to their similar appearance and innervation by dorsal nerves, these organs have been considered to represent segmentally repeated nuchal organs (Söderström 1927; Jelsing 2003; Jelsing and Eibye-Jacobsen 2010). Other authors preferred to distinguish these two organs, irrespective of their probable similar function and structure (Rullier 1951; Orrhage 1964; Bleidorn and Helm 2019). It must be considered that, especially in Spionidae, some of these ciliated structures may not be sensory and may serve different functions (Purschke 2005; Blake et al. 2019). For instance, certain ciliated structures may simply generate water currents, aiding in continuously providing oxygen-rich water for the branchiae, or they may serve in spermatophore transfer (Orrhage 1964; Schlötzer-Schrehardt 1987, 1991). Although Orrhage (1964) explicitly distinguished between dorsal sensory organs and non-sensory ciliary bands (“Flimmerbänder”), this has somehow been subsequently overlooked, finally leading to ultrastructural analyses in various spionid species (Schlötzer-Schrehardt 1987, 1991; Jelsing 2002, 2003; Jelsing and Eibye-Jacobsen 2010). Although the first of such investigations in Pygospio elegans Claparède, 1863 could not corroborate a sensory function (Schlötzer-Schrehardt 1987, 1991), such a function could be demonstrated for several other species of Spionidae (Jelsing 2002, 2003; Jelsing and Eibye-Jacobsen 2010; Blake et al. 2019) finally validating Orrhage’s (1964) view. After their first description in Orbiniidae by Eisig (1914), the dorsal organs were not regarded as representing sensory organs; this hypothesis was put forward by Söderström (1927). Preliminary ultrastructural observations in S. armiger also provide possible evidence for a sensory function and similarity to nuchal organs (Bleidorn and Helm 2019). Therefore, to evaluate and validate these observations, ultrastructural analysis and comparison with nuchal organs are essential.
Nuchal organs
Nuchal organs belong to the most important sensory organs of Annelida. Regarded to be chemosensory, they are found in almost every polychaete species: only a few taxa lack these organs, among them Palaeoannelida, Chaetopteriformia, Pisionidae, Clitellata, and Siboglinidae (Purschke 1997, 2005, 2016; Beckers et al. 2019; Beckers and Tilic 2021). Mainly based on their structure, these organs are considered chemoreceptive (Storch and Schlötzer-Schrehardt 1988; Verger-Bocquet 1992; Purschke 1997, 2005; Lindsay 2009). Although physiological evidence for their function is scarce, investigations in Platynereis dumerilii (Audouin and Milne Edwards, 1833) clearly support chemoreception besides other chemosensory structures (palps, antennae, and tentacular cirri) (Chartier et al. 2018). These authors hypothesized that nuchal organs are especially involved in inter-individual communication and the detection of pheromones. Due to their widespread occurrence, nuchal organs were regarded to represent an important annelid or even polychaete apomorphy (Rouse and Fauchald 1997; Rouse and Pleijel 2001; Purschke 2005; Purschke et al. 2014). However, according to current phylogenetic hypotheses, these organs are absent in the two basal-most annelid lineages, challenging this view (Beckers et al. 2019; Beckers and Tilic 2021). Considering this, they are currently regarded as having evolved within Annelida and constitute a synapomorphy of a clade comprising Amphinomida/Sipuncula and Pleistoannelida (Beckers and Tilic 2021).
Usually situated at the posterior end of the prostomium, nuchal organs show considerable external variability. Mostly they represent ciliary patches or bands of different forms, but they may also be completely internalized in certain species (Fauchald and Rouse 1997; Purschke 1997, 2000, 2005; Purschke and Hessling 2002). The largest continuous nuchal organs occur in certain Amphinomidae and Spionidae, where they are elongated and may extend posteriorly over several segments, usually as doubled ciliary bands (Purschke 1997; Jelsing 2002, 2003; Jelsing and Eibye-Jacobsen 2010). In these cases, a bulging dorsal sensory area is often formed, commonly called a caruncle (Fauchald and Rouse 1997; Purschke 2005). Other examples of large nuchal organs are found in Poecilochaetidae, certain Syllidae and the phyllodocid taxon Notophyllum Örsted, 1843 (Purschke 1997, 2005). In Sabellariidae, with their strongly modified anterior end, parts of the median organ are regarded to represent the nuchal organs (Helm et al. 2018a). Despite their external variability, nuchal organs are characterized by bipolar primary sensory cells, ciliated supporting cells, an olfactory chamber, a retractor muscle, and a direct innervation from the brain (Rullier 1951; Purschke 1997, 2005, 2016; Jelsing and Eibye-Jacobsen 2010; Schmidtberg and Dorresteijn 2010; Helm et al. 2018a). Diverging descriptions are usually due to incomplete, preliminary, or erroneous interpretations of the cellular composition (e.g., Storch and Welsch 1969).
The cilia of the supportive cells are motile and generate water currents, enabling a rapid exchange of sensory stimuli. Generally, only one type of such supportive cells has been described, but sometimes peripheral epidermal cells or other additional, usually unciliated, supportive cells are mentioned showing a different morphology than other epidermal cells (West 1978; Rhode 1990; Purschke 1997, 2000). The ciliated supportive cells form one or several subcuticular spaces called olfactory chambers, apically often separated from the exterior by specialized covers. These covers are formed by the cuticle and microvilli of the supportive cells. In species with internalized nuchal organs, these covers may be reduced or even absent (Purschke 1986, 2000; Purschke and Hessling 2002), whereas they are usually present in species with externally exposed nuchal organs (Schlötzer-Schrehardt 1986, 1987; Rhode 1990; Lewbart and Riser 1996; Purschke 1997, 2005; Jelsing 2002; Jelsing and Eibye-Jacobsen 2010; Helm et al. 2018a; Beckers and Tilic 2021). However, several species that possess external nuchal organs without specialized microvilli also exist (Whittle and Zhaid 1974; Purschke 1997; Schmidtberg and Dorresteijn 2010). Frequently, the microvilli are apically widened and thus form a layer of closely apposed microvilli, which are only penetrated by the cilia of the supportive cells. Several forms of protective layers have been distinguished (Purschke 1997). Sometimes these parts of the microvilli show a paving stone-like arrangement (Schlötzer-Schrehardt 1986, 1987; Purschke 1990; Rhode 1990; Purschke and Jouin-Toulmond 1994; Jelsing 2002, 2003); in other species these microvilli somehow demonstrate other profiles (Lewbart and Riser 1996; Rhode 1990; Wilkens and Purschke 2009b). Whereas formerly regarded to be of high phylogenetic value (Purschke 1990, 2005), this view cannot be maintained due to current phylogenetic hypotheses and the widespread occurrence of this feature in many distantly related polychaete taxa (Struck et al. 2011, 2015; Weigert et al. 2014; Weigert and Bleidorn 2016; Helm et al. 2018b; Beckers and Tilic 2021).
The bipolar sensory cells send dendritic processes toward the olfactory chambers, thereby terminating isolated or in clusters between the supportive cells (Purschke 1997, 2005; Schmidtberg and Dorresteijn 2010). The dendrites are usually monociliated, sprouting a single modified cilium and multiple microvilli. Both types of processes extend into the olfactory chamber, intermingle and form a more or less dense network occupying the space in the olfactory chamber underneath the ciliated region of the nuchal organ. These processes never penetrate the cuticle or the protective cover. The sensory cilia have a short shaft and generally an axoneme with a 9 × 2 + 0 pattern of microtubules. Basal bodies are generally well developed, but rootlets are either lacking or reduced. Typically, these cilia ramify shortly after emergence and form microvillus-like branches indistinguishable from microvilli also arising from the sensory cells. The cell bodies of the receptor cells are located outside the area of the supportive cells. They often form one or a few clusters of somata close to the brain and have been termed nuchal ganglia (Purschke 1990, 2005, 2016; Wilkens and Purschke 2009a; Schmidtberg and Dorresteijn 2010), although not constituting a ganglion in the strict sense (see Richter et al. 2010). Their axons unite as a neurite bundle entering the brain dorsally, often called the nuchal nerve. The nuchal nerves are closely associated with the dorsal roots of the circumesophageal connectives, either joining their dorsal commissure or forming a separate nuchal commissure in the brain (Orrhage 1964; Purschke 1997, 2016; Orrhage and Müller 2005).
Nuchal and dorsal organs in S. armiger
When comparing the structures observed for the nuchal organ of S. armiger, it becomes obvious that these structures are nuchal organs undoubtedly exhibiting the characters outlined above. Their innervation and somata position have already been described in a previous investigation and correspond to the pattern generally observed (Wilkens and Purschke 2009a). From the olfactory chambers, the dendritic processes unite and run anteriorly toward the brain. Thus, a few neurite bundles are formed that emanate separately from the nuchal organ, unite to form the paired nuchal ganglion and enter the brain dorsally. Originating together with the nuchal nerve from the brain, one branch of these neurite bundles passes the nuchal organ and continues posteriorly as the so-called dorsolateral nerve running throughout the entire trunk (Fig. 13C in Orrhage and Müller 2005). The large olfactory chambers in S. armiger house a high number of sensory processes, which may indicate the importance of these organs for this species. These enlarged olfactory chambers are formed by the mushroom-like apices (especially their stalks) of the inner supportive cells. Similar structures have been found in other polychaete species such as, e.g., the phyllodocids Phyllodoce mucosa Örsted, 1843 (as Anaitides mucosa), Eteone longa (Fabricius, 1780), and Eulalia viridis (Linnaeus, 1767), the nereidids Hediste diversicolor (O.F. Müller, 1776) (as Nereis diversicolor) and Platynereis dumerilii, the dorvilleid Protodorvillea kefersteini (McIntosh, 1869) and the fauveliopsid Fauveliopsis cf. adriatica Katzmann & Laubier, 1974 (Whittle and Zahid 1974; Rhode 1990; Purschke 1997; Schmidtberg and Dorresteijn 2010). Since these species belong to different clades, this observation favors an adaptive and functional significance rather than the phylogenetic importance of these enlarged olfactory chambers.
The dorsal organs of S. armiger show striking similarities to its nuchal organs, although some differences between the two organs are obviously present as well. The similarities of the dorsal organs with the nuchal organs are displayed in their composition of bipolar primary sensory cells and ciliated supportive cells, an olfactory chamber, a modified cuticle, and an identical microvillar cover. The sensory dendrites show similarly structured cilia and microvilli, and both cell processes occupy the olfactory chamber and do not penetrate the cuticle. The primary sensory cells are connected to the dorsolateral nerves, which have a common origin with the nuchal nerves at the dorsal side of the brain (Wilkens and Purschke 2009a). The anterior neurite bundle most likely represents the dendrites of the sensory cells, whereas the posterior bundle presumably represents the afferent innervation of the ciliated cells. This placement also corresponds to nuchal organs where efferent and afferent innervation is always separate (Purschke 1997). The efferent innervation most likely modulates the activity of the ciliated supportive cells. At least the division of the anterior neurite bundle close to the dorsolateral nerve represents the somata of the primary sensory cells in all probability due to their corresponding position in whole mounts and ultrathin sections, respectively. In both organs, the ciliated supportive cells forming the margin of the organ are not involved in constructing the olfactory chamber. A similar arrangement of supportive cells has also been observed in the nuchal organs of dorvilleids (Purschke 1997).
However, ciliated cells without a specialized cover are only present in the dorsal organ and were not found in the nuchal organ. In contrast, supportive cells with mushroom-like extensions bearing cilia and thus forming comparatively large olfactory chambers are only present in the nuchal organ of S. armiger. The same applies to the musculature: in contrast to Eisig (1914), we could not find intrinsic musculature related to the dorsal organs. Instead, they are situated above the muscle fibers of the body wall. So, according to the general correspondence and despite the differences mentioned, it can be concluded that the dorsal organs are sensory and have a similar function to the nuchal organs. As concluded by Jelsing (2002, 2003) and Jelsing and Eibye-Jacobsen (2010) for Spionidae, dorsal and nuchal organs may also be regarded as homologous for Orbiniidae, finally confirming Söderström’s (1927) ideas. However, future investigations must show whether the same genes are expressed in the respective regions during the development of nuchal and dorsal organs.
In contrast to Spionidae with either dorsally prolonged nuchal organs or separate nuchal and dorsal organs, there is a distinct gap of several segments devoid of dorsal organs in the thorax of S. armiger. Our observations of their first occurrence in the thorax corresponds to Eisig’s (1914) description. Such a gap has been observed in all species examined for this character so far, although their first appearance differs between species (Eisig 1914). However, the organs always start in the posterior thorax region. Currently, the functional significance is difficult to evaluate; a hypothesis might be that this arrangement allows a better orientation in gradients of sensory stimuli.
Conclusions
The ultrastructural analysis of the two types of sensory organs in S. armiger corresponds to the general structure of nuchal organs. This correspondence applies to their cellular composition, their fine structure, and their innervation. Thus, the present findings strongly suggest that nuchal organs and dorsal organs respond to the same sensory stimuli and the dorsal organs in S. armiger are, in fact, metameric nuchal organs. Their restriction to two distantly related taxa, Spionidae and Orbiniidae, speaks in favor of a derived situation that evolved independently in the two lineages. Moreover, in contrast to Orbiniidae, their non-uniform occurrence in Spionidae further corroborates this view (Eisig 1914; Orrhage 1964; Jelsing 2003; Bleidorn and Helm 2019).
References
Anderson DT (1959) The embryology of the polychaete Scoloplos armiger. Q J Microsc Sci 100:89–166
Beckers P, Tilic E (2021) Fine structure of the brain in Amphinomida (Annelida). Acta Zool. https://doi.org/10.1111/azo.12383
Beckers P, Helm C, Purschke G, Worsaae K, Hutchings P, Bartolomaeus T (2019) The central nervous system of Oweniidae (Annelida) and its implications for the structure of the ancestral annelid brain. Front Zool 16:6
Blake JA, Maciolek NJ, Meißner K (2019) Spionidae Grube, 1850. In: Purschke G, Böggemann M, Westheide W (eds) Handbook of zoology. Annelida. Pleistoannelida, Sedentaria II, vol 2. DeGruyter, Berlin, pp 1–103
Bleidorn C, Helm C (2019) Orbiniidae Hartman, 1942. In: Purschke G, Böggemann M, Westheide W (eds) Handbook of zoology. Annelida. Annelida, Basal Groups and Pleistoannelida, Sedentaria I, vol 1. DeGruyter, Berlin, pp 251–269
Bleidorn C, Kruse I, Albrecht S, Bartolomaeus T (2006) Mitochondrial sequence data expose the putative cosmopolitan polychaete Scoloplos armiger (Annelida, Orbiniidae) as a species complex. BMC Evol Biol 6:47
Chartier TF, Deschamps J, Dürichen W, Jékely G, Arendt D (2018) Whole-head recording of chemosensory activity in the marine annelid Platynereis dumerilii. Open Biol 8:180139
Eakin RM, Hermans CO (1988) Eyes. In: Westheide W, Hermans CO (eds) The ultrastructure of Polychaeta. Microfauna marina, vol 4. Gustav Fischer, Stuttgart New York, pp 135–156
Eisig H (1914) Zur Systematik, Anatomie und Morphologie der Ariciiden nebst Beiträgen zur generellen Systematik. Mitt Zool Stn Neapel 21:153–593
Ermak TH, Eakin RM (1976) Fine structure of the cerebral and pygidial ocelli in Chone ecaudata (Polychaeta: Sabellidae). J Ultrastruct Res 54:243–260
Fauchald K, Rouse G (1997) Polychaete systematics: past and present. Zool Scr 26:71–138
Helm C, Krause A, Bleidorn C (2015) Immunohistochemical investigations of the development of Scoloplos armiger (“intertidalis clade”) indicate a paedomorphic origin of Proscoloplos cygnochaetus (Annelida, Orbiniidae). Invertebr Biol 134:214–230
Helm C, Bok MJ, Hutchings P, Kupriyanova E, Capa M (2018a) Developmental studies provide new insights into the evolution of sense organs in Sabellariidae (Annelida). BMC Evol Biol 18:149
Helm C, Beckers P, Bartolomaeus T, Drukewitz SH, Kourtesis I, Weigert A, Purschke G, Worsaae K, Struck TH, Bleidorn C (2018b) Convergent evolution of the ladder-like ventral nerve cord in Annelida. Front Zool 15:36
Jelsing J (2002) Ultrastructural investigations on the cephalic and metameric nuchal organs of Spio cf. filicornis (Polychaeta, Spionidae). Zoomorphology 121:213–220
Jelsing J (2003) Ultrastructural studies of dorsal ciliated organs in Spionidae (Annelida: Polychaeta). Hydrobiologia 496:241–251
Jelsing J, Eibye-Jacobsen D (2010) Ultrastructure of the extensively developed nuchal organs in Laonice bahusienis (Annelida: Canalipalpata: Spionidae). J Morph 271:376–382
Kruse I, Reise K (2003) Reproductive isolation between intertidal and subtidal Scoloplos armiger (Polychaeta, Orbiniidae) indicates sibling species in the North Sea. Mar Biol 143:511–517
Lewbart GA, Riser NW (1996) Nuchal organs of the polychaete Parapionosyllis manca (Syllidae). Inv Biol 115:286–298
Lindsay SM (2009) Ecology and biology of chemoreception in polychaetes. Zoosymposia 2:339367
Luttikhuizen PC, Bol A, Cardoso JFMF, Dekker R (2011) Overlapping distributions of cryptic Scoloplos cf. armiger species in the western Wadden Sea. J Sea Res 66:231–237
Mill PJ (1978) Sense organs and sensory pathways. In: Mill PJ (ed) Physiology of annelids. Academic Press, New York, pp 63–114
Orrhage L (1964) Anatomische und morphologische Studien über die Polychaetenfamilien Spionidae, Disomidae und Poecilochaetidae. Zool Bidr Uppsala 36:335–405
Orrhage L, Müller MCM (2005) Morphology of the nervous system of Polychaeta (Annelida). Hydrobiologia 535(536):79–111
Purschke G (1986) Ultrastructure of the nuchal organ in the interstitial polychaete Stygocapitella subterranea (Parergodrilidae). Zool Scr 16:13–20
Purschke G (1990) Comparative electron-microscopic investigation of the nuchal organs in Protodriloides, Protodrilus, and Saccocirrus (Annelida, Polychaeta). Can J Zool 68:325–338
Purschke G (1997) Ultrastructure of nuchal organs in polychaetes (Annelida)—new results and review. Acta Zool (stockh) 78:123–143
Purschke G (2000) Sense organs and central nervous system in an enigmatic terrestrial polychaete, Hrabeiella periglandulata (Annelida)—implications for annelid evolution. Invertebr Biol 119:329–341
Purschke G (2005) Sense organs in polychaetes (Annelida). Hydrobiologia 535(536):53–78
Purschke G (2016) Annelida: Basal groups and Pleistoannelida. In: Schmidt-Rhaesa A, Harzsch S, Purschke G (eds) Structure and evolution of invertebrate nervous systems. Oxford University Press, Oxford, pp 254–312
Purschke G, Hessling R (2002) Analysis of the central nervous system and sense organs in Potamodrilus fluviatilis (Annelida: Potamodrilidae). Zool Anz 241:19–35
Purschke G, Jouin-Toulmond C (1994) Ultrastructure of sense organs and the central nervous system in Parenterodrilus taenioides and their phylogenetic significance in the taxon Protodrilida (Annelida, Polychaeta). Mém Mus Hist Nat 162:119–128
Purschke G, Bleidorn C, Struck T (2014) Systematics, evolution and phylogeny of Annelida—a morphological perspective. Mem Mus Vic 71:247–269
Rhode B (1990) Ultrastructure of nuchal organs in some marine polychaetes. J Morphol 206:95–107
Richter S, Loesel R, Purschke G, Schmidt-Rhaesa A, Scholtz G, Stach T, Vogt L, Wanninger A, Brenneis G, Döring C, Faller S, Fritsch M, Grobe P, Heuer CM, Kaul S, Möller OS, Müller CHG, Rieger V, Rothe BH, Stegner MEJ, Harzsch S (2010) Invertebrate neurophylogeny: suggested terms and definitions for a neuroanatomical glossary. Front Zool 7:29
Rouse GW, Fauchald K (1997) Cladistics and Polychaetes. Zool Scr 26:139–204
Rouse GW, Pleijel F (2001) Polychaetes. Oxford University Press, Oxford, pp 1–354
Rullier F (1951) Étude morphologique, histologique et physiologique de l’organe nuchal chez les annélides polychètes sédentaires. Ann Inst Oceanogr Monaco 25:207–341
Schlötzer-Schrehardt U (1986) Ultrastructural investigation of the nuchal organs of Pygospio elegans (Polychaeta). I. Larval nuchal organs. Helgol Meeresunters 40:397–417
Schlötzer-Schrehardt U (1987) Ultrastructural investigation of the nuchal organs of Pygospio elegans (Polychaeta). II. Adult nuchal and dorsal organs. Zoomorphology 107:169–179
Schlötzer-Schrehardt U (1991) Ultrastructural differentiation of nuchal and dorsal organs during postembryonic and sexual development of Pygospio elegans Claparéde (Polychaeta: Spionidae). Ophelia 5:633–640
Schmidtberg H, Dorresteijn A (2010) Ultrastructure of the nuchal organs in the polychaete Platynereis dumerilii (Annelida, Nerididae). Invertebr Biol 129:252–265
Söderström A (1927) Über segmental wiederholte „Nuchalorgane“ bei Polychäten. Zool Bidr Uppsala 12:1–18
Storch V, Schlötzer-Schrehardt U (1988) Sensory structures. In: Westheide W, Hermans CO (eds) The Ultrastructure of Polychaeta. Microfauna marina, Gustav Fischer, vol 4. Stuttgart New York, pp 121–133
Storch V, Welsch U (1969) Zur Feinstruktur des Nuchalorgans von Eurythoe complanata (Pallas) (Amphinomidae, Polychaeta). Z Zellforsch Mikrosk Anat 110:411–420
Struck TH (2019) Phylogeny. In: Purschke G, Böggemann M, Westheide W (eds) Handbook of zoology. Annelida. Annelida, Basal Groups and Pleistoannelida, Sedentaria I, vol 1. DeGruyter, Berlin, pp 37–68
Struck TH, Paul C, Hill N, Hartmann S, Hoesel C, Kube M, Lieb B, Meyer A, Tiedemann R, Purschke G, Bleidorn C (2011) Phylogenomic analyses unravel annelid evolution. Nature 471:95–98
Struck TH, Golombek A, Weigert A, Franke FA, Westheide W, Purschke G, Bleidorn C, Halanych KM (2015) The evolution of annelids reveals two adaptive routes to the interstitial realm. Curr Biol 25:1993–1999
Verger-Bocquet M (1992) Polychaeta: sensory structures. In: Harrison FW, Gardiner SL (eds) Microscopic anatomy of invertebrates. Vol 7 Annelida. Wiley-Liss, New York, pp 181–196
Weigert A, Bleidorn C (2016) Current status of annelid phylogeny. Org Divers Evol 16:345–362
Weigert A, Helm C, Meyer M, Nickel B, Arendt D, Hausdorf B, Santos SR, Halanych KM, Purschke G, Bleidorn C, Struck TH (2014) Illuminating the base of the annelid tree using transcriptomics. Mol Biol Evol 31:1391–1401
West DL (1978) Comparative ultrastructure of juvenile and adult nuchal organs of an annelid (Polychaeta, Opheliidae). Tissue Cell 10:243–257
Whittle AC, Zhaid ZR (1974) Fine structure of nuchal organs in some errant polychaetous annelids. J Morphol 144:167–184
Wilkens V, Purschke G (2009a) Pigmented eyes, photoreceptor-like sense organs and central nervous system in the polychaete Scoloplos armiger (Orbiniidae, Annelida) and their phylogenetic importance. J Morphol 270:1–15
Wilkens V, Purschke G (2009b) Central nervous system and sense organs, with special reference to photoreceptor-like sensory elements, in Polygordius appendiculatus (Annelida), an interstitial polychaete with uncertain phylogenetic affinities. Invertebr Biol 128:46–64
Acknowledgements
We are grateful to the head of our department, Professor Dr. A. Paululat, Osnabrück, for abundant support and discussion. Thanks are also due to K. Etzold and W. Mangerich, Osnabrück, for various kinds of technical assistance, particularly for introducing JSB to electron microscopy techniques. C. Meyer and Dr. H. Meyer, Osnabrück, kindly introduced JSB to LSM techniques.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
We collected neither endangered species nor animals from protected areas. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buhre, J.S., Purschke, G. Ultrastructure and functional morphology of the dorsal organs in Scoloplos armiger (Annelida, Sedentaria, Orbiniida). Zoomorphology 140, 437–452 (2021). https://doi.org/10.1007/s00435-021-00545-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-021-00545-1